Abstract
The aba4-1 mutant completely lacks neoxanthin but retains all other xanthophyll species. The missing neoxanthin in light-harvesting complex (Lhc) proteins is compensated for by higher levels of violaxanthin, albeit with lower capacity for photoprotection compared with proteins with wild-type levels of neoxanthin. Detached leaves of aba4-1 were more sensitive to oxidative stress than the wild type when exposed to high light and incubated in a solution of photosensitizer agents. Both treatments caused more rapid pigment bleaching and lipid oxidation in aba4-1 than wild-type plants, suggesting that neoxanthin acts as an antioxidant within the photosystem II (PSII) supercomplex in thylakoids. While neoxanthin-depleted Lhc proteins and leaves had similar sensitivity as the wild type to hydrogen peroxide and singlet oxygen, they were more sensitive to superoxide anions. aba4-1 intact plants were not more sensitive than the wild type to high-light stress, indicating the existence of compensatory mechanisms of photoprotection involving the accumulation of zeaxanthin. However, the aba4-1 npq1 double mutant, lacking zeaxanthin and neoxanthin, underwent stronger PSII photoinhibition and more extensive oxidation of pigments than the npq1 mutant, which still contains neoxanthin. We conclude that neoxanthin preserves PSII from photoinactivation and protects membrane lipids from photooxidation by reactive oxygen species. Neoxanthin appears particularly active against superoxide anions produced by the Mehler's reaction, whose rate is known to be enhanced in abiotic stress conditions.
INTRODUCTION
Carotenoids form a class of compounds synthesized in all photosynthetic organisms and in several species of etherotrophic bacteria and fungi (Britton et al., 1998). Carotenoids participate in coloring fruits, flowers, and leaves; they represent an important nutritional source for mammals, as precursor animal cell cofactors, like vitamin A. Due to their strong antioxidant activity, carotenoids are commercialized as human diet supplements and chemicals directed against the action of free radicals (Krinsky, 1979; Demmig-Adams and Adams, 2002). Both zeaxanthin (Zea) and lutein (Lute) are major antioxidant compounds in several human tissues, namely, in the eye (Krinsky et al., 2003). Neoxanthin (Neo) has been shown to induce apoptosis in prostate cancer cells (Kotake-Nara et al., 2005). Carotenoids play an irreplaceable role as components of the photosynthetic apparatus (Frank and Cogdell, 1996). In higher plants and algae, β-carotene binds to chloroplast-encoded subunits of reaction center core complexes, while xanthophylls are chromophores of the peripheral antenna system composed of light-harvesting complexes (Lhc) (Bassi et al., 1993; Jansson, 1999). These xanthophylls, involved in light harvesting, are structural determinants of pigment protein architecture (Formaggio et al., 2001) and are essential for quenching triplet chlorophyll (3Chl*). Moreover, it has been proposed that Zea scavenges singlet oxygen (1O2) produced by photosynthetic activity when free in the lipid phase of thylakoids (Havaux and Niyogi, 1999).
Carotenoids form a class of compounds with very similar absorption characteristics in the visible region, both in term of molar extinction value and absorption wavelengths. Nevertheless, nature maintains a remarkably high degree of conservation in xanthophyll composition across a wide range of plant taxa: virtually all higher plants share the same xanthophyll species in very similar relative amounts (Frank and Cogdell, 1996; Cunningham and Gantt, 1998).
The few exceptions, like Cuscuta reflexa that lacks Neo, are found in parasitic plants with ill-functional photosynthetic apparatus (Bungard et al., 1999). The above data suggest that each xanthophyll species plays a specific role in photosynthetic function.
In recent years, much effort has been devoted to gaining new insight about the specific role of each xanthophyll using multiple approaches: (1) in vitro analysis of recombinant Lhc with different carotenoid contents (Croce et al., 1999b; Formaggio et al., 2001) and (2) isolation and in vivo characterization of mutants lacking specific xanthophylls (Pogson et al., 1996; Havaux and Niyogi, 1999; Lokstein et al., 2002; Baroli et al., 2003). Together, these efforts have improved our understanding of xanthophyll biology, suggesting that the function of individual xanthophyll species can be understood within the framework of their binding to Lhc proteins. Nevertheless, the function of Neo remains essentially unknown. Initial information has only been obtained from the analysis of recombinant Lhc proteins, while a mutant plant only lacking Neo has never been isolated. Neo depletion has to date only been obtained together with the accumulation of Zea at the expense of violaxanthin (Viola) (Rock and Zeevaart, 1991; Niyogi et al., 1998), thus obscuring the specific Neo-associated phenotype. Neo is an allenic monoepoxy-carotenoid (Goldsmith and Krinsky, 1960) accumulated in leaf tissue in the 9-cis conformation and produced from Viola in a reaction catalyzed by the neoxanthin synthase enzyme, which had been tentatively identified (Al Babili et al., 2000; Bouvier et al., 2000); it represents the final step in the so-called β-branch of the carotenoid biosynthetic pathway. Finally, in vivo Neo represents an ultimate precursor of the plant growth regulator abscisic acid (ABA) (Milborrow, 2001).
Neo, like Viola, accounts for 14% of total carotenoids in low light conditions. Neo is mostly bound to the N1 binding site of the major LHCII complex (Croce et al., 1999a). The N1 site has been located in between helix C and the helix A/B cross by mutation analysis (Croce et al., 1999b), which was recently confirmed by x-ray crystallography (Liu et al., 2004). Minor amounts of Neo also bind to the minor Lhc proteins CP29 and CP26 at a different binding site, namely L2, in competition with Viola (Bassi et al., 1999; Gastaldelli et al., 2003). Neo is the carotenoid species that appears to be most strongly bound to Lhc proteins, its concentration in the lipid phase being the lowest among carotenoid species (Caffarri et al., 2001). In vitro, Neo is not essential for the folding of recombinant Lhcb proteins in the presence of Lute, Viola, or Zea (Formaggio et al., 2001), and attempts to fold any antenna complex in the presence of Neo alone have been unsuccessful (Giuffra et al., 1996; Hobe et al., 2000).
In this work, we have studied aba4-1, a mutant lacking Neo. The physiological and biochemical phenotype has been investigated in vivo for performance on growth and photoprotection; in vitro, Lhcb proteins have been purified and compared with those from the wild type by biochemical and spectroscopic methods. We found that Neo plays a specific role in the protection of Lhc proteins, the photosystem II (PSII) reaction center, and thylakoids from photooxidative stress and that its action is effective against the damaging effect of reactive oxygen species (ROS), particularly superoxide anion.
RESULTS
Pigment Composition
The mutant aba4-1 was isolated in a screen of an Arabidopsis thaliana Wasijliewska-2 accession T-DNA insertion collection described by North et al. (2007). Wild-type and aba4-1 plants grown in low light (120 μmol photons m−2 s−1) for 4 weeks were phenotypically indistinguishable, showing similar organ size and chlorophyll content per leaf surface. HPLC analysis of dark-adapted leaf pigment composition showed that Neo was absent in the mutant genotype, while an increase in Viola content was recorded (from 3.3 to 7.6 mol per 100 mol of chlorophylls a+b) that compensated for the missing Neo. Like the wild type, aba4-1 leaf tissue also contained the trans-isomer of Viola and had detectable amounts of 9-cis-Viola (up to ∼5.5% of the total xanthophyll pool) identified by HPLC as previously described (Snyder et al., 2004). Lute and β-carotene content were essentially the same in both genotypes (Figure 1, Table 1). The aba4-1 mutant phenotypes were mild compared with previously identified ABA-deficient mutants that exhibit vegetative tissue phenotypes, with no significant difference in growth characteristics. Indeed, ABA levels in unstressed leaves and seeds of aba4-1 mutants were higher than those of mutants npq2 (ABA-deficient1) (North et al., 2007) and aba3 (ABA-deficient3) (Schwartz et al., 1997). Most of the experiments described in this work were also performed with aba4-1 plants that had been supplemented with ABA by daily spraying (Llorente et al., 2000), and the results obtained with or without exogenous ABA treatment were similar.
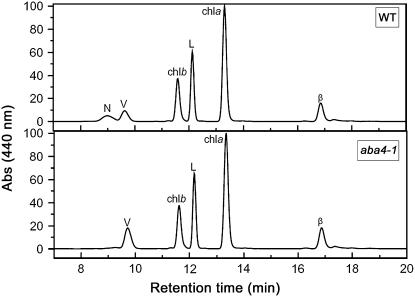
Figure 1.
Analysis of Pigment Content in the Leaves of 4-Week-Old Wild-Type and aba4-1 Plants.
Separation of lipid-soluble pigments was based on HPLC analysis. Each chromatogram represents absorbance (Abs) at 440 nm of pigments extracted from a leaf disc. Note the missing peak corresponding to Neo in aba4-1. N, Neo; V, Viola; L, Lute; β, β-carotene; chla, chlorophyll a; chlb, chlorophyll b.
Table 1.
Pigment Composition of Dark-Adapted Leaf Tissue from the Wild Type and aba4-1
| Genotype | Chlorophyll a/b | Chlorophyll/ Carotenoid | μg Chlorophyll/ cm2 | Neo | cis-Viola | trans-Viola | Anthera | Lute | Zea | β-Carotene |
|---|---|---|---|---|---|---|---|---|---|---|
| Wild type | 2.9 ± 0.1 | 3.7 ± 0.1 | 21.3 ± 0.4 | 4.0 ± 0.1 | – | 3.3 ± 0.1 | – | 13.1 ± 1.0 | – | 6.1 ± 0.3 |
| aba4-1 | 2.9 ± 0.1 | 3.8 ± 0.1 | 21.3 ± 0.8 | – | 1.1 ± 0.1 | 6.5 ± 0.1 | – | 13.3 ± 1.2 | – | 6.8 ± 0.7 |
| npq1 | 3.0 ± 0.1 | 3.8 ± 0.4 | 20.2 ± 1.5 | 3.9 ± 0.1 | – | 3.2 ± 0.3 | – | 13.4 ± 0.5 | – | 6.9 ± 0.2 |
| aba4-1 npq1 | 3.0 ± 0.1 | 4.0 ± 0.3 | 21.8 ± 0.4 | – | 1.2 ± 0.1 | 6.6 ± 0.1 | – | 13.7 ± 0.1 | – | 6.9 ± 0.2 |
Data are normalized to 100 chlorophyll a+b molecules and are expressed as means ± sd; n = 3. Pigment content was quantified by spectral deconvolution of acetone extracts and by HPLC.
Photosynthetic Characteristics and Excess Energy Dissipation
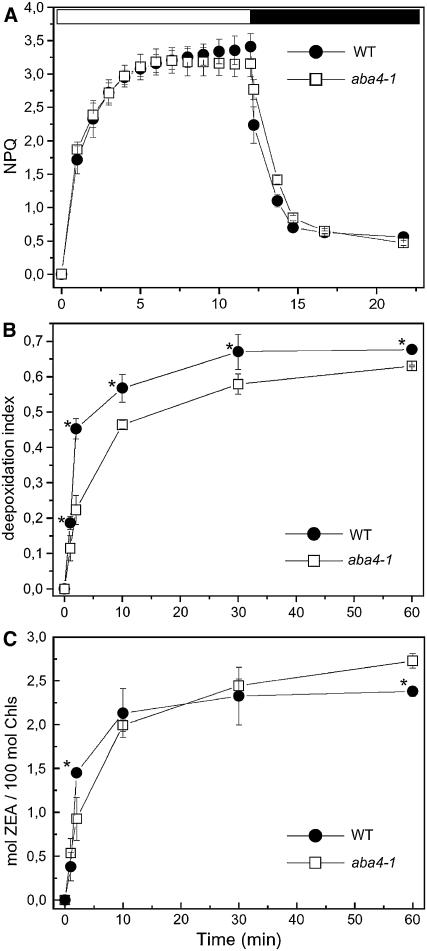
On illumination of dark-adapted samples, a rise in nonphotochemical quenching (NPQ), which indicates the capacity for excess energy dissipation, occurs in two distinct phases: a rapid phase, within 1 min, that is largely independent of Zea formation; and a slower phase that is inhibited by blocking Zea synthesis (Horton, 1996). Quenching kinetics in wild-type and aba4-1 plants (Figure 2A) were essentially the same: when exposed to strong light (1200 μmol photons m−2 s−1) for 15 min, aba4-1 leaves showed an NPQ kinetic identical to that of control plants, both in terms of NPQ rise and fluorescence recovery in the dark (Figure 2A). Accumulation of antheraxanthin (Anthera) + Zea during the light treatment was measured at different time intervals (Figures 2B and 2C): the increase of deepoxidation index observed over time was slightly slower than for wild-type leaves (Figure 2B) possibly because, although more Viola was present in the aba4-1 mutant, a larger pool was protein-bound and not readily available for the violaxanthin deepoxidase enzyme; nevertheless, after longer incubation, high-light treatment resulted in the accumulation of a higher level of Zea (Figure 2C) with respect to wild-type plants, thus showing that aba4-1 leaves were able to draw from a larger supply of deepoxidable Viola in response to long overexcitation events.
Figure 2.
Activation of NPQ and Zea Synthesis Rate in Wild-Type and aba4-1 Leaves.
(A) Induction of NPQ was measured during exposure of dark-adapted leaves to actinic light at 1200 μmol m−2 s−1.
(B) Kinetics of deepoxidation index as measured on intact leaves during high-light (1200 μmol m−2 s−1) treatment at room temperature. Deepoxidation index was calculated as (1/2Anthera + Zea)/(Viola + Anthera + Zea).
(C) Rate of accumulation of Zea in intact leaves exposed to high light at room temperature. The curve was normalized to 100 mol chlorophyll. Mean values (±sd) of three to five independent measurements are shown. Significantly different values (evaluated by Student's t test, P < 0.05) in comparison with the wild type are marked by an asterisk.
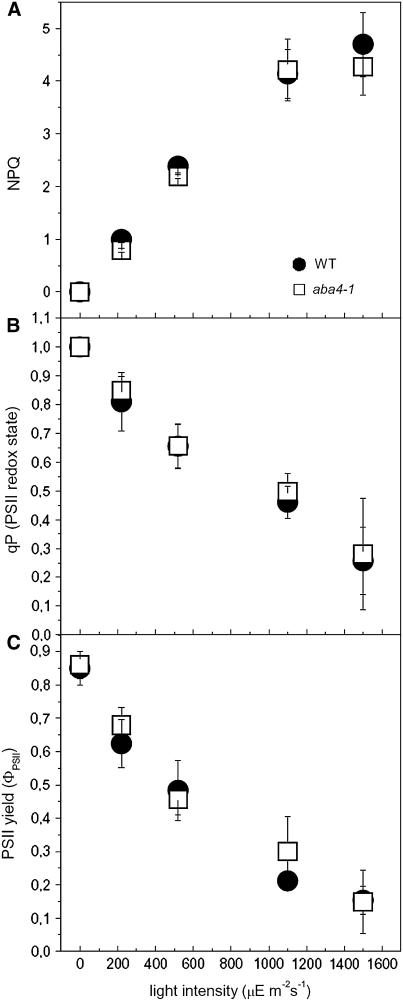
PSII activity during photosynthesis was investigated further by chlorophyll fluorescence (Figure 3). aba4-1 plants showed no changes in either the PSII oxidation state (qP versus actinic light intensity; Figure 3B) or in PSII photochemical efficiency (φPSII versus. actinic light intensity; Figure 3C) compared with control plants. Also, the amplitude of NPQ was comparable in the wild type and the aba4-1 genotype (Figure 3A) at all light intensities. Therefore, we conclude that Neo is not involved in short-term adaptation of either photochemical or nonphotochemical mechanisms upon dark-to-light transition.
Figure 3.
Analysis of Room Temperature Chlorophyll Fluorescence during Photosynthesis.
Fluorescence was monitored in intact, dark-adapted leaves (wild type, circles; aba4-1, squares). Thermal energy dissipation (NPQ; [A]), PSII redox state (qP; [B]), and photosynthetic efficiency (ΦPSII; [C]) during photosynthesis. Leaves were illuminated for 20 min and parameters determined during steady state photosynthesis. Symbols and error bars show means ± sd (n ≥ 3).
Effect of Short-Term Light Stress on Leaf Photoprotection
Treatment of leaf discs with strong light produces a rapid decrease in the Fv/Fm ratio, bleaching of pigments, and extensive oxidation of thylakoid lipids (Havaux, 2003): indeed, CO2 uptake is reduced in leaf discs floating on water, which restricts photosynthesis. High-light treatments therefore cause a higher level of photooxidative stress on leaf discs compared with the same treatment on whole plants. This experimental setup is thus effective in measuring the level of resistance to photoinhibitory treatments on leaf tissue.
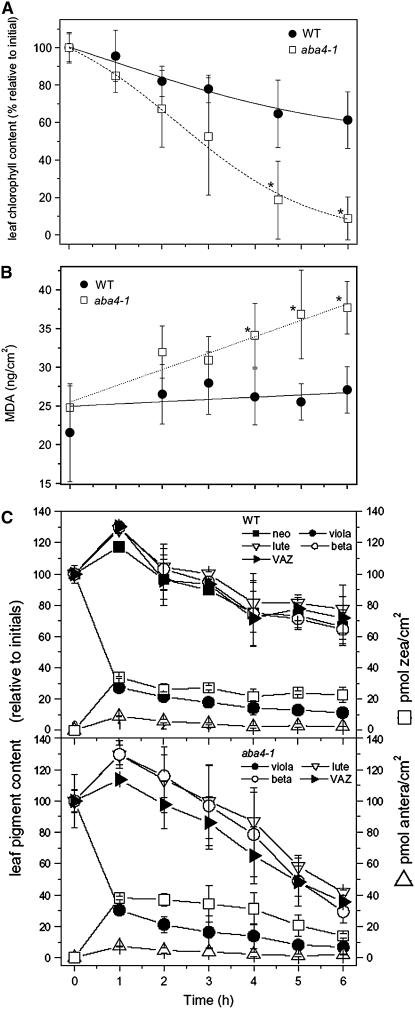
To estimate if Neo content affects photoprotection, leaf discs from the wild type and aba4-1, floated on water, were illuminated with strong light (1800 μmol m−2 s−1) at room temperature; light-dependent bleaching of chlorophyll was followed by sampling discs at different times and quantifying their pigment content and lipid peroxidation level. aba4-1 leaf discs were clearly more sensitive to light stress than the wild type (Figure 4). After 6 h of treatment, 90% of chlorophyll was bleached in aba4-1 versus 35% in the wild type (Figure 4A). To investigate the level of membrane lipid peroxidation, the same leaf discs were analyzed for malondialdehyde content by HPLC (Havaux et al., 2005). In aba4-1 leaves, high-light treatment resulted in a higher level of lipid peroxidation with respect to the wild type (Figure 4B). Rates of carotenoid photoxidation were measured by HPLC analysis of leaf pigment content (Figures 4C and 4D). Results show that while carotenoid content decreased rapidly in aba4-1 leaves with respect to the wild type, no carotenoid species was preferentially destroyed.
Figure 4.
Photoprotection in Leaf Discs of the Wild Type and aba4-1.
Leaf discs from the wild type and aba4-1 were treated with strong white light (1800 μmol m−2 s−1) at room temperature for different times, and the amount of chlorophylls, carotenoids, and lipid peroxides were evaluated. Data shown are the means ± sd from at least four independent experiments. Data sets with a significance level of P < 0.05 according to Student's t test are marked by an asterisk.
(A) Kinetic of chlorophylls bleaching.
(B) Time course of lipid peroxidation. Lipid peroxides were quantified by HPLC as thiobarbituric reactive substances. MDA, malondialdehyde.
(C) Kinetics of carotenoids bleaching.
Since aba4-1 has a reduced steady state ABA content (45% of wild-type content), we could not exclude that higher photosensitivity of aba4-1 is related to ABA reduced content. We thus measured kinetics of pigment bleaching and lipid peroxidation on the mutants aba3-1 and aba4-3 (ecotype Col-0): aba3-1 shows a wild-type level of Neo but is impaired in the conversion of ABA-aldehyde to ABA; thus, the mutant of Arabidopsis is without ABA. The behavior of this mutant was compared with that of the wild type (see Supplemental Figure 1 online), thus showing that ABA deficiency is not the reason for the differential sensitivity of aba4-1 and supporting the view that pigment composition is the only motive for higher photoxidation of aba4-1 leaf discs.
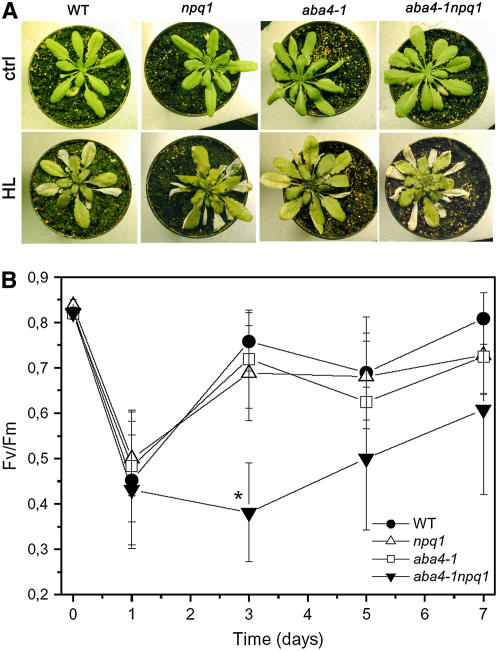
The sensitivity to photoxidative stress was also assessed on whole plants. Since aba4-1 plants have a larger pool of deepoxidable Viola (see above), higher levels of Zea are accumulated in plants under stress conditions with respect to wild-type plants (Figure 2C). Zea provides effective photoprotection through two mechanisms: (1) by the modulation of the thermal energy dissipation mechanism when it is bound to PSII subunits and (2) by acting as an efficient scavenger for ROS when it is free in the lipid phase. Previous reports have demonstrated that the photoprotective function mediated by Zea overlaps with other antioxidant mechanisms of the chloroplast, like tocopherols (Havaux et al., 2005), ascorbate (Muller-Moule et al., 2003), or other xanthophylls, like Lute (Niyogi et al., 2001). To avoid interference by different levels of Zea on the analysis of Neo contribution to photoprotection, we compared npq1 (nonphotochemical quenching1) with the double mutant aba4-1 npq1, which are both unable to accumulate Zea. Plants were kept under control conditions (24°C, 120 μmol m−2 s−1) or transferred to high light at low temperature (15°C, 1600 μmol m−2 s−1) and then analyzed for 6 d. The results are summarized in Figure 5. Although aba4-1 npq1 plants did not show a strong photosensitive phenotype (although older leaves were more damaged than those of the wild type) (Figure 5A), more detailed analysis was performed after 6 d of treatment by determining the leaf content of molecules that are a target or indicator of photooxidative stress. The mutant aba4-1 npq1 underwent a significant reduction in leaf chlorophyll content and a strong accumulation of the antioxidant molecule tocopherol with respect to npq1 (Table 2). aba4-1 did not, however, show a similar increase in tocopherol content with respect to the wild type, although it accumulated twice as much Zea than the wild type. These results showed that the Neo-depleted aba4-1 npq1 genotype was more sensitive to photooxidative stress with respect to its Neo-containing reference npq1, while in aba4-1, the effects of Neo depletion were compensated for by an overaccumulation of Zea with respect to the wild type.
Figure 5.
Effect of High Light and Low Temperature on Wild-Type and Mutant Plants.
(A) Wild-type, npq1, aba4-1, and aba4-1 npq1 plants were grown at 1600 μmol m−2 s−1 and 15°C for 6 d. HL, high light.
(B) Photoinhibition of the same genotypes was measured daily as Fv/Fm during growth in high light at low temperature. During this treatment, plants were sprayed daily with 10 μM ABA to compensate for the reduced hormone content of the aba4-1 genotypes. Data are expressed as means ± sd; n = 7. Significantly lower Fv/Fm values (evaluated by Student's t test, P < 0.05) compared with the wild type are marked by an asterisk.
Table 2.
Analysis of High-Light Stress Effect on Mutants with Concomitant Suppression of Neo and Zea Accumulation
| Total Chlorophyll (nmol cm−2)
|
Tocopherol (pmol cm−2)
|
Zea (nmol cm−2)
|
||||
|---|---|---|---|---|---|---|
| Genotype | Control | Stress | Control | Stress | Control | Stress |
| Wild type | 26.2 ± 1.9 | 38.1 ± 6.6 | 0.46 ± 0.03 | 2.65 ± 0.17 | – | 0.84 ± 0.11 |
| aba4-1 | 25.2 ± 1.3 | 35.7 ± 9.2 | 0.44 ± 0.02 | 2.78 ± 0.13 | – | 1.96 ± 0.54* |
| npq1 | 24.9 ± 1.8 | 36.4 ± 10.5 | 0.57 ± 0.10 | 2.29 ± 0.62 | – | – |
| aba4-1 npq1 | 26.9 ± 0.5 | 19.3 ± 1.8* | 0.40 ± 0.03 | 7.56 ± 1.75* | – | – |
Wild-type, npq1, aba4-1, and aba4-1 npq1 plants were grown for 6 d at high light and low temperature. Chlorophylls, Zea, and tocopherol leaf content were measured before and after stress as described in Methods. Data are means ± sd; n = 4. Significantly different values (Student's t test, P < 0.05) in comparison with the wild type are marked by an asterisk.
We also measured the level of PSII photoinhibition over a 7-d period of high-light and cold treatment (Figure 5B). The first day of treatment caused a decrease of Fv/Fm from 0.82 to 0.5 in all genotypes. Wild-type, aba4-1, and npq1 mutants recovered most of their PSII activity through acclimation in the following 2 d, while aba4-1 npq1 was photoinhibited further and had only recovered to similar levels as the other genotypes after 6 d. A more detailed time-course analysis of plant pigment content during the stress period (see Supplemental Table 1 online) showed that most of the damaging effect, measured as pigment destruction, was observed after 3 d of treatment accompanied by a decrease of the chlorophyll/carotenoid ratio and an increase in tocopherol content; both effects were much stronger in aba4-1 npq1 with respect to the other genotypes. Significant differences were observed over the following days, during which plants exhibited signs of recovery. This recovery was impaired in the aba4-1 npq1 genotype, which further increased its tocopherol content, increased chlorophyll a/b ratio, and decreased chlorophyll/carotenoid ratio with respect to the others genotypes. Also worth noting is the Zea content, which was 5 times higher than that of the wild type in aba4-1 after 3 d and still twice as much after 6 d.
Effect of Exogenous Prooxidants on Leaf Chlorophyll Bleaching
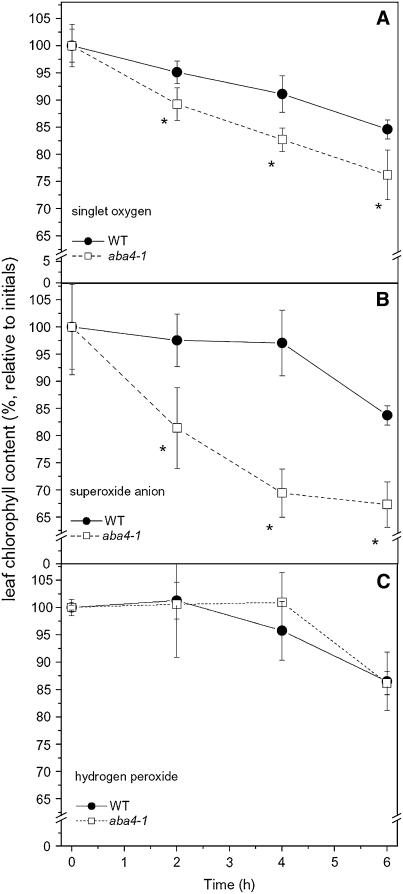
The above results clearly showed that Neo-deficient plants were more sensitive to photooxidative stress than wild-type plants. We further analyzed the aba4-1 mutant to verify if Neo-dependent protection was directed against all ROS or was specific to a particular ROS species. ROS were either artificially produced in intact leaves by the photosensitizing compounds Rose Bengal (RB; a singlet oxygen generator) or Metronidazole (MZ; a superoxide anion generator), or leaves were directly treated with H2O2. Wild-type and aba4-1 leaves were vacuum-infiltrated with the above-mentioned chemicals, and thylakoid sensitivity was measured by quantifying the reduction of chlorophyll content over time (Figures 6A to 6C). The sensitivity of the aba4-1 mutant and the wild type to H2O2 treatment was the same (Figure 6C), while aba4-1 was more sensitive than the wild type to singlet oxygen (produced by RB; Figure 6A) and, to a greater extent, to superoxide anion (produced by MZ; Figure 6B).
Figure 6.
Sensitivity of Wild-Type and aba4-1 Leaf Discs to Different ROS Species.
Leaf discs were infiltrated with either 50 μM RB (A), 250 μM MZ (B), 250 mM hydrogen peroxide (C), or water and then floated on the same solution. Singlet oxygen was produced exogenously by illuminating samples with green light (photon flux density 100 μmol m−2 s−1); superoxide anion radicals were generated by illuminating with a fluorescence lamp (50 μmol m−2 s−1), and discs infiltrated with hydrogen peroxide were kept in the dark. ROS-mediated bleaching was followed by quantification of disc chlorophyll content. The gas phase during the experiment was air. Data are means ± sd; n = 4. Data sets with a significance level of P < 0.05 according to Student's t test are marked by an asterisk.
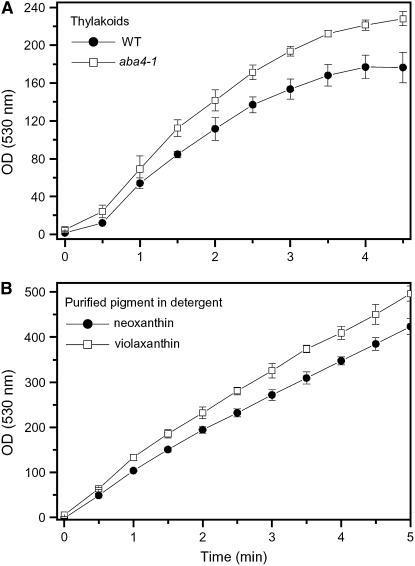
The major source of superoxide in the chloroplast is the Mehler's reaction (Wise and Naylor, 1987a, 1987b), which is enhanced in conditions where the electron acceptor NADP+ is overreduced, thus diverting electrons to molecular oxygen. Superoxide is scavenged by superoxide dismutase (SOD), whose inhibition can thus mimic some of the effects of environmental stress in plants (Noctor and Foyer, 1998). These results suggest that Neo is a scavenger of both ROS, with a preference for superoxide. This conclusion was further supported by measurements of superoxide scavenging ability of both free pigments and thylakoid membrane using a NADH/phenazine methosulfate superoxide generating system in which the antioxidant effect was monitored through the inhibition of nitroblue tetrazolium oxidation (see Methods for details). Viola free in a detergent solution (Figure 7B) appeared to be less efficient than free Neo in detoxifying superoxide anions. When wild-type and Neo-depleted membranes (Figure 7A) were used in this assay, similar results were obtained. We thus conclude that Neo exerts an efficient antioxidant activity both as a free pigment and when inserted in its physiological binding site(s) within the photosynthetic membrane.
Figure 7.
Capacity for Scavenging Superoxide Anions.
ROS were generated by the reaction of NADH and phenazine methosulfate (see Methods for details). The  scavenging ability was measured as the inhibition of the increase in absorbance recorded at 530 nm, corresponding to nitroblue tetrazolium oxidation. Measurements were performed on thylakoids isolated from wild-type and aba4-1 plants (A) and purified Neo and Viola (B). Values are the mean of three measurements ± sd. All data sets for aba4-1 are significantly higher than the wild type according to Student's t test statistical analysis (P < 0.05).
scavenging ability was measured as the inhibition of the increase in absorbance recorded at 530 nm, corresponding to nitroblue tetrazolium oxidation. Measurements were performed on thylakoids isolated from wild-type and aba4-1 plants (A) and purified Neo and Viola (B). Values are the mean of three measurements ± sd. All data sets for aba4-1 are significantly higher than the wild type according to Student's t test statistical analysis (P < 0.05).
These results were confirmed by measuring chlorophyll bleaching rates in leaves infiltrated with 4 mM diethyl-dithio-carbamic acid, an inhibitor of SOD (Auh and Murphy, 1995). Leaf discs were floated on the solution and illuminated with moderately high light (700 μmol m−2 s−1) for 6 h at room temperature. Light treatment alone caused stronger bleaching in aba4-1 leaf discs (Table 3), as had been previously observed (see Figure 6B); however, SOD inhibition led to a strong and specific enhancement of photobleaching in aba4-1 leaves with respect to the wild type. To obtain information on the mechanism of superoxide anion scavenging, we studied the fate of Neo when reacting with superoxide anion: is it preferentially destroyed by ROS during the reaction? To this aim, a time-course analysis of carotenoid content was performed on (1) wild-type and aba4-1 leaves vacuum-infiltrated with MZ (superoxide anion generators) and (2) on wild-type and aba4-1 thylakoids incubated in the superoxide generation/detection system above described in Figure 7. Rates obtained are reported in Supplemental Figure 2 online and show that (1) β-β-xanthophylls are less resistant than Lute in superoxide anion-mediated carotenoid destruction, and (2) no specific loss of Neo was observed under the effect of superoxide. These data suggest that the photoprotection mechanism mediated by Neo does not involve a preferential destruction of this xanthophyll with respect to other leaf carotenoids.
Table 3.
Neo Plays a Role in Preventing Chlorophyll Bleaching by Superoxide Anion
| Leaf Chlorophyll Content (μg cm−2)
|
|||
|---|---|---|---|
| Genotype | t0 | t6 h (control) | t6 h (+DDC) |
| Wild type | 19.8 ± 0.8 | 17.8 ± 1.4 | 13.8 ± 1.0 |
| aba4-1 | 18.8 ± 0.3 | 12.7 ± 0.6* | 5.5 ± 0.3* |
Leaf discs form the wild type and aba4-1 were infiltrated with 4 mM diethyl-dithio-carbamic acid (DDC) or water and then floated on the same solution. Endogeneous superoxide anion was produced by high-light illumination (photon flux density 700 μmol m−2 s−1) at room temperature; ROS-mediated bleaching was followed by quantification of disc content after 6 h of stress. Data are means ± sd; n = 4. Significantly lower values (Student's t test, P < 0.05) in comparison with the wild type are marked by an asterisk.
Photoprotection Function of Neo in Isolated Lhc Proteins
Excited chlorophyll can react with 3O2 to form the highly reactive chemical species singlet oxygen (1O2), which rapidly oxidizes any organic compounds, including chlorophyll itself. Thus, Lhcb photoprotection by xanthophylls can be evaluated from their ability to prevent chlorophyll photobleaching through thermal deactivation of 3Chl* and/or scavenging of 1O2 under strong white light illumination in the presence of oxygen (Formaggio et al., 2001).
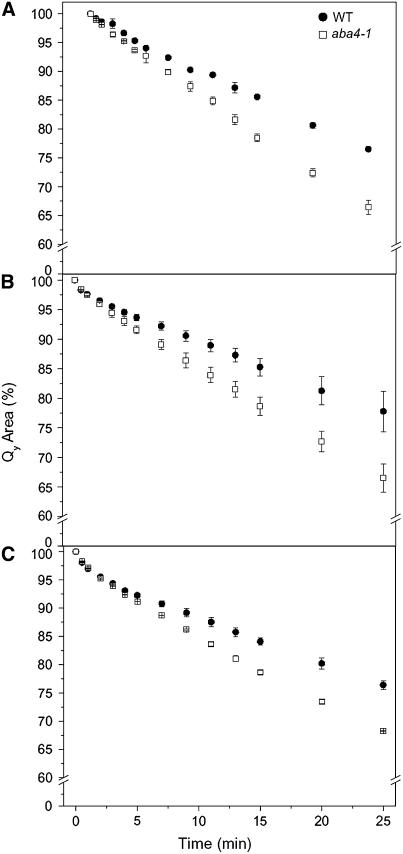
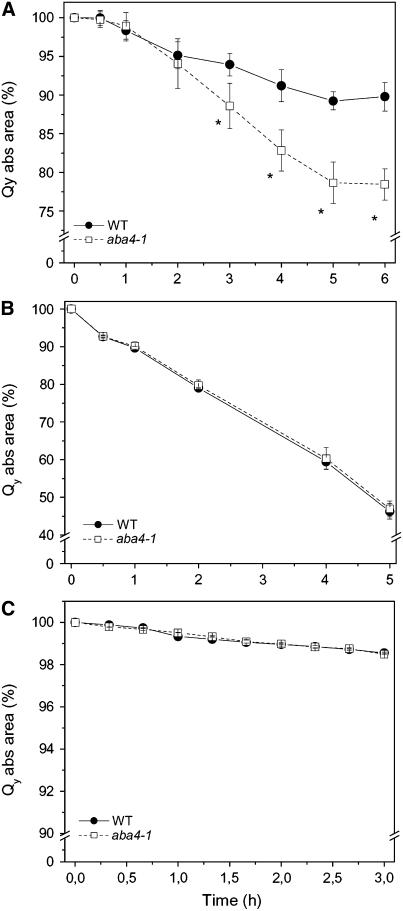
To identify a possible effect of the altered pigment composition on the photoprotection of Lhcb proteins, we measured the photobleaching rates of Lhcb proteins purified from the wild type and aba4-1 by native flatbed isoelectric focusing (IEF). Different Lhcb proteins can be fractionated according to their pI as Lhcb1 + Lhcb2 (fraction 2), Lhcb4 (fraction 4), and Lhcb5 (fraction 5). In each case, the proteins purified from the Neo-deficient aba4-1 showed a corresponding increase in Viola and were more sensitive to photobleaching than the corresponding proteins from the wild type (Figure 8).
Figure 8.
Photobleaching Behavior of Purified Monomeric Lhcb.
Flatbed IEF-purified Lhcb subunits from aba4-1 and wild-type plants. Photobleaching kinetics of decay of Qy transition absorbance was recorded for LHCII (A), CP29 (B), and CP26 (C) as described in Methods. Lhcb chlorophyll concentrations were adjusted to 8 μg/mL, and samples were cooled to 10°C during measurements. Values are the mean of three measurements ± sd. All aba4-1 data sets corresponding to times >8 min had significantly lower values (evaluated by Student's t test, P < 0.05) compared with the wild type. Closed symbols, wild-type Lhc; open symbols, aba4-1 Lhc.
Fractions containing the same Lhcb apoproteins showed the same heat denaturation temperature, thus implying that the substitution of Neo by Viola had no effect on protein stability (see Supplemental Table 2 online).
Effect of Exogenous Prooxidants on Photoprotection of Isolated LHCs
The results obtained above (Figure 7, Table 3) indicated that Neo plays a role as a scavenger of ROS, such as singlet oxygen and superoxide anions. To verify if the selectivity to certain ROS could be ascribed to protein-bound Neo rather than to the small fraction (<1%) found free in the lipid phase (Caffarri et al., 2001), we extended the study to isolated major light-harvesting complex of PSII (LHCII). Trimeric LHCII, isolated by sucrose gradient ultracentrifugation from the wild type and aba4-1, underwent chemical bleaching of bound chlorophylls when exposed to the ROS generators used above for leaf infiltration. Prooxidant concentrations were chosen that resulted in a significant bleaching of wild-type trimers. In the wild type, both singlet oxygen (produced by RB) and hydrogen peroxide caused a progressive reduction in the Qy absorption region of LHCII, without showing evidence of a differential contribution of bound Neo to the photoprotection ability of trimeric LHCII (Figures 9B and 9C). By contrast, LHCII from aba4-1 was much more sensitive than the wild type to the superoxide anion produced by MZ (Figure 9A). The specificity for superoxide scavenging found in leaves was therefore reproduced with purified LHCII, whereas that for singlet oxygen was not. Sampling of LHCII during MZ treatment was performed at different times to measure the destruction rate of each xanthophyll species. Results obtained (see Supplemental Figure 3 online) are distinct with respect to those described on whole leaves vacuum-infiltrated with MZ (see Supplemental Figure 2 online): in this in vitro system, Neo in wild-type LHCII was destroyed more rapidly than the other carotenoids.
Figure 9.
Sensitivity of LHCII from the Wild Type and aba4-1 to Different ROS.
LHCII isolated from solubilized thylakoids of the wild type and aba4-1 were analyzed by following the Qy transition absorbance decay when prooxidants were added to the solution: 250 μM MZ (A); 50 μM RB (B); and 1 M hydrogen peroxide (C). For details, see Methods. Lhcb chlorophyll concentrations were adjusted to 8 μg/mL. Data are expressed as means ± sd; n = 3. Data sets with a significance level of P < 0.05 according to Student's t test are marked by an asterisk.
Supramolecular Organization of Pigment Binding Complexes
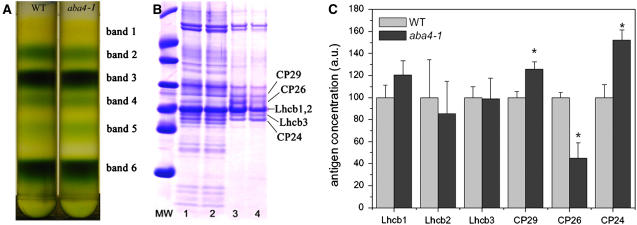
Neo binds to a specific site within LHCII complexes, named the N1 site, in a stoichiometry 1:1 with apoproteins (Croce et al., 1999a; Liu et al., 2004). Furthermore, Neo is a chromophore bound to the minor antenna complexes CP29 (Lhcb4) and CP26 (Lhcb5) but does not bind to CP24 (Lhcb6) or light-harvesting complex of photosystem I (Lhca) (Bassi et al., 1993; Pagano et al., 1998). In principle, we could not exclude that the increased photosensitivity of aba4-1 plants could be due to a disruption of the molecular organization of the antenna system. To verify this possibility, we compared the distribution of pigments in aba4-1 versus wild-type thylakoids by fractionating dodecyl-α-d-maltoside (α-DM)–solubilized thylakoids by sucrose gradient ultracentrifugation (Figure 10A). Six bands were obtained in each gradient: band 1 contained free carotenoid pigments; band 2 contained the minor antenna complexes CP29, CP26, and CP24 as well as LHCII in monomeric state; band 3 contained LHCII trimers; band 4 contained the LHCII-CP24-CP29 complex; and PSII core complex and photosystem I (PSI-LHCI) were found in bands 5 and 6, respectively. The band pattern, as well as the chlorophyll distribution along the gradient (data not shown), were essentially the same in both samples, indicating that no major changes in the organization of PSII antenna system was induced by the Neo depletion. Nevertheless, SDS-PAGE analysis of thylakoids and Lhcb-containing fractions showed that wild type and aba4-1 polypeptide composition was different (Figure 10B): both thylakoids and band 2 (monomeric Lhcb proteins) have a marked reduction in CP26 levels. Quantification of Lhcb proteins from the wild type and aba4-1 thylakoids by protein gel blotting (Figure 10C) confirmed the strong decrease in CP26 content and showed a compensatory increase in CP29 and CP24.
Figure 10.
Sucrose Density Gradient Fractionation of the Wild Type and aba4-1 Solubilized Thylakoids and SDS-PAGE Analysis of Protein Content.
(A) Thylakoid membranes from wild-type and aba4-1 plants were solubilized with α-DM and loaded on sucrose gradients; for each gradient, fractions harvested are indicated.
(B) Tris-Tricine SDS-PAGE analyses of thylakoid (lanes 1 and 2) and monomeric Lhcb (lanes 3 and 4) from wild-type (lanes 1 and 3) and aba4-1 (lanes 2 and 4) plants. MW, molecular weight marker.
(C) Results of quantitative immunoblotting analysis of Lhcb proteins in wild-type versus aba4-1 thylakoid membranes. An antibody against CP47 was used as an internal standard. Significantly different values (P < 0.05) than the control genotype are marked by an asterisk. Data are expressed as means ± sd; n = 3. a.u., absorbance units.
Viola Substitutes for Neo in Xanthophyll Binding Site N1 of LHCII
Interpretation of the aba4-1 phenotype requires the determination of the Viola binding site, which compensates for the missing Neo in aba4-1. Neo, all-trans-Viola, and Lute were associated with Lhcb proteins in the wild type, whereas Lhcb from aba4-1 had more Viola, compensating for the lack of Neo (Table 4). Part (∼25%) of the Viola bound to LHCII subunits from aba4-1 had a 9-cis configuration. Trimeric complexes from both genotypes bind four xanthophylls per monomer, while the chlorophyll a/b ratio of aba4-1 LHCII was slightly lower with respect to the wild type.
Table 4.
Pigment Composition of Monomeric Lhcb and Trimeric LHCII from the Wild Type and aba4-1
| Genotype | Chlorophyll a/b | Chlorophyll/Carotenoid | cis-Neo | cis-Viola | trans-Viola | Anthera | Lute | Zea |
|---|---|---|---|---|---|---|---|---|
| Wild-type band 2 | 1.85 ± 0.01 | 3.65 ± 0.01 | 5.2 ± 0.1 | – | 5.1 ± 0.1 | – | 17.1 ± 0.4 | – |
| aba4-1 band 2 | 1.73 ± 0.02 | 3.64 ± 0.04 | – | 0.9 ± 0.1 | 9.4 ± 0.1 | – | 17.1 ± 0.1 | – |
| Wild-type band 3 | 1.44 ± 0.01 | 3.54 ± 0.01 | 7.2 ± 0.1 | – | 2.1 ± 0.1 | – | 18.9 ± 0.1 | – |
| aba4-1 band 3 | 1.31 ± 0.01 | 3.59 ± 0.01 | – | 1.9 ± 0.1 | 6.7 ± 0.1 | – | 19.2 ± 0.1 | – |
Bands 2 (monomeric Lhcb proteins) and 3 (trimeric LHCII) were isolated from solubilized thylakoid membranes by sucrose gradient ultracentrifugation as shown in Figure 10. Data were normalized to 100 chlorophyll a+b and mean ± sd; n = 3.
Analysis of the spectral forms of chlorophylls and carotenoids in the Lhc protein environment (Cinque et al., 2000; Croce et al., 2000) allows the assignment of the different pigments to each binding site. In this case, we investigated whether Viola does indeed occupy the site normally taken by Neo in the wild-type complex. For spectral deconvolution analysis, we used trimeric LHCII purified by IEF, which contains only three bound xanthophylls (Caffarri et al., 2001): V1, the LHCII external site removed by IEF, does not bind Neo (Caffarri et al., 2001); thus, we simplify deconvolution without introducing artifacts. The results obtained are summarized in Table 5 and in Supplemental Figure 4 online. The best fit for LHCII from the wild type was obtained with two different spectral forms of lute at 490.5 and 507 nm, one Neo form at 487 nm, and one Viola spectral form, with low amplitude, at 491 nm. The best fit for LHCII from aba4-1 was obtained with two spectral forms of Lute with similar amplitudes and shifts with respect to the wild type (492 and 507 nm) and a spectral form of Viola peaking at 487 nm. Thus, the additional Viola in aba4-1 LHCII binds to the N1 site, peaking at the same wavelength as Neo in wild-type LHCII.
Table 5.
Xanthophyll Spectral Forms and Efficiency of Energy Transfer to Chlorophyll a in LHCII Trimeric Subunit Purified by Nondenaturing IEF from Wild-Type and aba4-1 Thylakoids
| Genotype | Lute 1 | Lute 2 | Viola | Neo | |
|---|---|---|---|---|---|
| LHCII wild type | Spectral form | 486.5 nm | 503 nm | 491 nm | 487 nm |
| Efficiency | 79% | 90% | – | 87% | |
| LHCII aba4-1 | Spectral form | 488 nm | 503 nm | 491 nm | 487 nm |
| Efficiency | 77% | 90% | 77% | – |
Spectral deconvolution analysis and calculation of energy transfer efficiency were as in Croce et al. (1999b). The data, normalized to the wild type, are relative to a 100% chlorophyll a–to–chlorophyll a energy transfer efficiency. The error in the energy transfer efficiency was <4%, with the exception of Viola in the wild type and Neo in aba4 (>10%). Xanthophyll absorption maxima in ethanol are 477.2 and 468.4 nm for Viola and Neo, respectively. Binding to site N1 shifts Neo from 468.4 to 486.9 nm. In aba4 LHCII, Viola binds to site N1 with a shift that gives an absorbance spectrum overlapping that of Neo in N1.
Energy transfer efficiency can be calculated by comparing the amplitude of components deconvoluted from fluorescence excitation and 1-T spectra. This analysis showed that both Neo and Lute have a high energy transfer efficiency (80 to 90%) to chlorophyll a in wild-type LHCII, while Viola in N1 had a somewhat lower efficiency of energy transfer in aba4-1 samples with respect to Neo in the same site in wild-type protein (77 versus 87%). Lack of Neo also affected the Qy absorption region of the LHCII spectrum: the chlorophyll b absorption components were increased in LHCII complexes (see Supplemental Figure 5 online). In conclusion, substitution of Neo with Viola in site N1 increased the protein affinity for chlorophyll b and produced an enrichment of red-shifted chlorophyll a absorption forms (683 nm). This suggests that LHCII undergoes a small conformational change upon substitution of Neo with Viola.
DISCUSSION
Neo Deficiency in Arabidopsis Results in a Compensatory Increase in Viola
Neo, together with Lute and Viola, is one of the three major xanthophylls of leaves: it is a mono-epoxy carotenoid, bound to the N1 site of LHCII as 9-cis-Neo localized between the helix A/helix B cross and helix C as determined by mutation analysis of recombinant proteins (Croce et al., 1999a, 1999b) and later confirmed by x-ray crystallography (Liu et al., 2004). Two additional pigment protein complexes also bind Neo (CP29 and CP26), while CP24 and Lhca1-4 do not bind Neo (Bassi et al., 1993). CP26 and CP29 do not have a N1 binding site; they bind Neo in site L2 in competition with Viola (Bassi et al., 1999; Gastaldelli et al., 2003).
Recombinant proteins, when refolded in vitro in the absence of Neo, bind an additional corresponding amount of Viola (Giuffra et al., 1996; Hobe et al., 2000). The same effect occurred in vivo in the aba4-1 mutant. As in LHCII purified from mutant leaves, one additional molecule of Viola was bound compared with LHCII from the wild type (Table 4). The thylakoid chlorophyll/carotenoide and chlorophyll a/b ratios, however, were not affected, which would not be expected due to the decrease in CP26 and, possibly, in the LHCII trimer connected to CP26. This apparent contradiction was reconciled by the observation that LHCII folded without Neo has a higher affinity for chlorophyll b, thus compensating for the reduced CP26 content of thylakoid membranes. A decrease in CP26 was reported in Zea accumulating genotypes npq2 and npq2 lut2 (lutein deficient2) (Havaux et al., 2004), which also lack Neo. The results presented here suggest that CP26 is destabilized in vivo by the lack of Neo rather than by the accumulation of Zea. By contrast, the major LHCII is maintained in the npq2 mutant (Connelly et al., 1997) in spite of the complete lack of Neo.
Depletion of Neo has been reported for C. reflexa, a parasitic plant with low photosynthetic activity (Bungard et al., 1999), where it is substituted by 9-cis-Viola in LHCII (Snyder et al., 2004). A similar situation occurred in aba4-1 LHCII. Spectral deconvolution of LHCII absorption spectra from aba4-1 revealed that a good fit can be obtained using the Viola spectral form shifted to the same absorption wavelength as Neo in the wild type (487 nm); since 9-cis-Neo (the stereoisomer bound to wild-type LHCII) and 9-cis-Viola have the same absorption spectra in organic solvents (Snyder et al., 2004), this indicates that Viola can bind to the N1 site in LHCII from aba4-1 in its 9-cis configuration. Since the 9-cis stereoisomer of Viola is not stable in organic solvents, even after heat-induced isomerization of all-trans xanthophylls (Niedzwiedzki et al., 2005), the low amounts of 9-cis-Viola that were detected probably derive from Viola bound to the N1 site, whereas xanthophyll bound to the L2 site would be in the all-trans configuration (Liu et al., 2004). The aba4-1 mutant therefore represents a useful tool for the determination of the physiological function of Neo and whether this function is different from that of Viola.
It has been suggested that Neo plays a role in stabilizing LHCII trimers (Ruban and Horton, 1999) due to the trimer/monomer ratio in npq2 mutants, which accumulate Zea and are depleted for Neo (Tardy and Havaux, 1996; Lokstein et al., 2002). As trimeric organization appeared to be unaltered in aba4-1 (Figure 10A), this suggests that monomerization is due to the accumulation of Zea with more than one Zea molecule per LHCII monomer (Connelly et al., 1997). This implies that a fraction of LHCII molecules has Zea in the L1 site, which must be occupied by Lute for trimerization (Lokstein et al., 2002) and not Zea or Viola (Havaux et al., 2004).
PSII function and overall photosynthetic rate were not affected in the aba4-1 mutant, suggesting that Neo was not essential for plant survival in mild environmental conditions. The only noticeable effect was the decrease in CP26 content mentioned above, which was compensated for by a corresponding increase in CP29 and CP24 similar to that previously observed for LHCII (Ruban et al., 2003). Nevertheless, CP26 antisense plants did not have a photosensitive phenotype (Andersson et al., 2001).
Resistance to Photooxidative Stress Is Decreased in aba4-1 with Respect to the Wild Type
Carotenoids are essential for the protection of the chloroplast from photoxidative stress (Niyogi, 1999). Previous work with recombinant proteins suggested that Neo might function in the scavenging of ROS generated from the incomplete quenching of chlorophyll triplets (Croce et al., 1999a). Nevertheless, this hypothesis could not be verified in vivo since the only plants available to date depleted in Neo also undergo substitution of Viola by Zea. Zea strongly affects PSII function by decreasing chlorophyll fluorescence lifetime through a conformational change of Lhc proteins (Moya et al., 2001; Dall'Osto et al., 2005) and also prevents photooxidative damage (Havaux and Niyogi, 1999; Baroli et al., 2003, 2004); therefore, the contribution of Neo to photoprotection function could easily be masked by the accumulation of Zea in npq2 (Niyogi et al., 1998; Baroli et al., 2003; Dall'Osto et al., 2005). The aba4-1 mutant specifically lacks Neo and appears to be more sensitive to stress caused by high-light treatment, as leaf discs excised from aba4-1 bleached faster under high light than discs from the wild type (Figure 4A). This effect was due to a higher sensitivity to the ROS produced by the photosynthetic machinery, as proven from increased lipid peroxidation (Figure 4B), and cannot be ascribed to the reduced content in ABA (see Supplemental Figure 1 online). Whole plants were more resistant to high-light treatment than excised leaf discs. aba4-1 plants do not have a strong in vivo phenotype compared with the wild type (Figure 5A). Nevertheless, the high-light treatment of aba4-1 plants induced a twofold increase in Zea synthesis (Table 2) with respect to wild-type plants. To eliminate the photoprotective effect of Zea, we compared aba4-1 npq1 with npq1 during high-light treatment. In this situation, aba4-1 npq1 plants were more sensitive than npq1 based on the rate of chlorophyll photobleaching, PSII photoinhibition, and overproduction of tocopherol. Moreover, while the Neo- or Zea-containing genotypes rapidly recovered from the initial damage, the double mutant did not fully recover photosynthetic efficiency, even after 1 week, despite the accumulation of higher amounts of the antioxidant tocopherol. Alternatively, the photosensitive phenotype of aba4-1 could, in principle, be related to reduced content in ABA. However, this hypothesis seems rather unlikely due to the following reasons: (1) leaf discs from aba3-1 do not show higher sensitivity to high light with respect to wild-type leaves, implying that photoxidation measured on aba4-1 leaves cannot be related to deficiency in ABA or any other metabolite of Neo; (2) it appears unlikely that the effects of ABA deficiency, absent on detached leaves, would appear on whole plants grown in high-humidity conditions and sprayed daily with ABA. This view is supported by several reports (e.g., Ruggiero et al., 2004) that demonstrated the efficiency of ABA supplementation on restoring the wild-type phenotype.
We conclude that the substitution of Neo with Viola in the chloroplasts of aba4-1 affects the capacity of the photosynthetic apparatus to face oxidative stress. This is compensated for by the synthesis of Zea and tocopherol, in the absence of which, the increased sensitivity of Neo-minus genotypes to photooxidative stress becomes evident in either leaf discs or whole plants.
What Can Neo Do That Viola Cannot?
In the aba4-1 mutant, the replacement of the missing Neo by Viola yielded a decrease in stress resistance, while the overaccumulation of Viola, in the presence of Neo, was shown to increase photoprotection in vivo (Davison et al., 2002). This suggests a distinct role for the different xanthophyll species in photoprotection. Cooperative effects on photoprotection by different xanthophyll species has been suggested in early experiments using recombinant Lhcb proteins reconstituted with different xanthophylls (Croce et al., 1999b). These showed that Lhcb1 reconstituted with Lute + Viola + Neo was more resistant to photobleaching in the presence of oxygen than proteins reconstituted with any single xanthophyll, implying a specific role for each.
This hypothesis has been confirmed in measurements of chlorophyll photobleaching in leaves challenged with either singlet oxygen, superoxide anion, or H2O2, which shows that Neo-containing leaves were selectively more resistant to damage from superoxide and singlet oxygen (Figure 7). The selective photoprotection against superoxide was reproduced in isolated LHCII, while the damage by hydrogen peroxide and singlet oxygen was identical in Neo and Viola binding proteins (Figure 9). Such evidence supports the idea that one function of Neo is to confer higher resistance to superoxide anions, a ROS species mainly produced by the Mehler's reaction when PSI electron acceptors are overreduced (Asada, 1999). Chloroplasts activate an antioxidant network to reduce damage by singlet oxygen and superoxide anions: these mechanisms involve (1) direct scavenging by carotenoids and (2) enzymatic detoxification of  mediated by SOD and ascorbate peroxidase (Sano and Asada, 1994; Gomez et al., 2004). Our results with MZ in vivo clearly show that in high light, Viola, which substitutes for Neo in aba4-1, cannot effectively counteract the higher rate of superoxide anion or singlet oxygen production, leading to pigment bleaching. This conclusion for
mediated by SOD and ascorbate peroxidase (Sano and Asada, 1994; Gomez et al., 2004). Our results with MZ in vivo clearly show that in high light, Viola, which substitutes for Neo in aba4-1, cannot effectively counteract the higher rate of superoxide anion or singlet oxygen production, leading to pigment bleaching. This conclusion for  was supported further by the effects of SOD inhibition in aba4-1 leaves: leaf infiltration with the SOD inhibitor diethyl-dithio-carbamic acid, without direct addition of exogenous superoxide, increased the level of photoxidative bleaching of leaf pigments (Table 3). Measurements of the scavenging capacity of either thylakoid membranes or purified pigments (Figure 7) confirmed that Neo molecules were more efficient as ROS scavengers than Viola. These results suggest an overlapping function between Neo and enzymatic scavenger systems in the chloroplast. In this respect, it is of interest to note that the double mutant aba4-1 npq1, depleted in both Zea and Neo, showed an enhancement of the phenotype. Zea has previously been shown to have a ROS scavenger effect when present as a free pigment released in the lipid phase (Havaux and Niyogi, 1999; Baroli et al., 2003, 2004). Thus, depletion of Zea and Neo simultaneously would deactivate two components of the photoprotective system against ROS in the lipid phase. Therefore, Neo would appear to be an effective ROS scavenger that forms part of a diverse group of antioxidant molecules that act in the chloroplast, like Zea (Havaux and Niyogi, 1999), ascorbate (Muller-Moule et al., 2002), and tocopherol (Havaux et al., 2005). In principle, we cannot exclude the possibility that the results described above are due to some kind of disruption of the normal Lhcb protein structure and function induced by binding of Viola to site N1, normally occupied by Neo, which could increase sensitivity to ROS, rather than to the deficiency in Neo. To distinguish between these two hypotheses is anything but easy: ROS scavenging measurements both on isolated pigments and thylakoids clearly support the antioxidant role of Neo; on the other hand, the higher sensitivity of aba4-1 detached leaves to high light could be (at least in part) related to Lhc conformational change due to substitution of Neo with Viola. It should be noted, however, that Neo is bound to LHCII by one side only. It happens that Neo and Viola differ by the part of the molecule that protrudes out from the LHCII protein structure, while the bound end has the same structure (Liu et al., 2004). This explains why Viola can substitute for Neo in LHCII structure and why the differences in the absorption spectra induced by the exchange are very small (see Supplemental Figure 5 online). We note that substitution of other xanthophylls in sites L2 or L1 yield much stronger effects (Holt et al., 2003), and even the emptiness of site N1 does not affect LHCII function (Connelly et al., 1997). Thus, although disruption of LHCII structure by binding of Viola to the site cannot be excluded, we do not favor this hypothesis.
was supported further by the effects of SOD inhibition in aba4-1 leaves: leaf infiltration with the SOD inhibitor diethyl-dithio-carbamic acid, without direct addition of exogenous superoxide, increased the level of photoxidative bleaching of leaf pigments (Table 3). Measurements of the scavenging capacity of either thylakoid membranes or purified pigments (Figure 7) confirmed that Neo molecules were more efficient as ROS scavengers than Viola. These results suggest an overlapping function between Neo and enzymatic scavenger systems in the chloroplast. In this respect, it is of interest to note that the double mutant aba4-1 npq1, depleted in both Zea and Neo, showed an enhancement of the phenotype. Zea has previously been shown to have a ROS scavenger effect when present as a free pigment released in the lipid phase (Havaux and Niyogi, 1999; Baroli et al., 2003, 2004). Thus, depletion of Zea and Neo simultaneously would deactivate two components of the photoprotective system against ROS in the lipid phase. Therefore, Neo would appear to be an effective ROS scavenger that forms part of a diverse group of antioxidant molecules that act in the chloroplast, like Zea (Havaux and Niyogi, 1999), ascorbate (Muller-Moule et al., 2002), and tocopherol (Havaux et al., 2005). In principle, we cannot exclude the possibility that the results described above are due to some kind of disruption of the normal Lhcb protein structure and function induced by binding of Viola to site N1, normally occupied by Neo, which could increase sensitivity to ROS, rather than to the deficiency in Neo. To distinguish between these two hypotheses is anything but easy: ROS scavenging measurements both on isolated pigments and thylakoids clearly support the antioxidant role of Neo; on the other hand, the higher sensitivity of aba4-1 detached leaves to high light could be (at least in part) related to Lhc conformational change due to substitution of Neo with Viola. It should be noted, however, that Neo is bound to LHCII by one side only. It happens that Neo and Viola differ by the part of the molecule that protrudes out from the LHCII protein structure, while the bound end has the same structure (Liu et al., 2004). This explains why Viola can substitute for Neo in LHCII structure and why the differences in the absorption spectra induced by the exchange are very small (see Supplemental Figure 5 online). We note that substitution of other xanthophylls in sites L2 or L1 yield much stronger effects (Holt et al., 2003), and even the emptiness of site N1 does not affect LHCII function (Connelly et al., 1997). Thus, although disruption of LHCII structure by binding of Viola to the site cannot be excluded, we do not favor this hypothesis.
Neo-Dependent Photoprotection in Leaves and in Purified Lhc Proteins
As increased photoprotection of wild type versus aba4-1 was observed not only in leaves but also in purified Lhc proteins exposed to the action of different ROS species produced by sensitizers, this suggests that PSII antenna proteins in particular require protection against superoxide anions. In vivo, this ROS species is mainly produced in the Mehler's reaction by PSI upon overreduction of electron acceptors, while overexcitation of PSII essentially yields singlet oxygen, which is the major cause of PSII photoinhibition (Krieger-Liszkay, 2005).
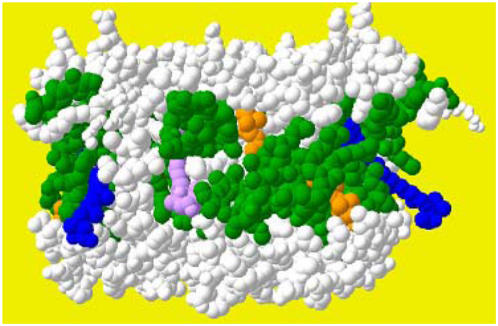
Previous work with cold-stressed barley (Hordeum vulgare) has shown that treatments increasing the Mehler's reaction and/or suppressing SOD activity not only induce PSI damage but also elicit PSII photoinhibition, implying that Qa reduction might be only one source for photoinhibition (Tjus et al., 1998). These reports suggest two possible scenarios: (1) the effect of Neo might be directed against ROS originating outside the PSII chlorophyll binding proteins and diffusing toward PSII reaction centers, namely, superoxide anion originated from Mehler's reaction; and (2) Viola binding complexes could be structurally more sensitive to the ROS action. When observing the structure of the LHCII trimer (Figure 11) (Liu et al., 2004), it is striking to note that Lute molecules bound to L1 and L2 sites are buried deep inside the protein structure, and Viola in V1 site is shielded by the interaction of a neighbor LHCII monomer. Neo in N1, instead, protrudes out from the structure by most of its length and is thus exposed to the surrounding lipid phase and to reactive species diffusing in it. Due to the short lifetime of singlet oxygen, it is unlikely that scavenging of this ROS would be the major target of Neo action at the periphery of the PSII supercomplex, while the superoxide anions produced by PSI at grana margins are long-lived and may diffuse toward PSII reaction centers; furthermore, radical chain reactions triggered by ROS attack to membrane lipids could easily propagate through the double layer. An alternative hypothesis is that superoxide is produced in PSII. In effect, it has been reported that superoxide anions are produced by the PSII acceptor side in purified PSII preparations (Pospisil et al., 2004, 2006). At present, it is not clear if superoxide anions are a product of PSII in vivo.
Figure 11.
Localization of Neo Molecules in Higher Plant Antennae.
LHCII structure is based on data from Liu et al. (2004). The position of Neo molecules, protruding with allenic group exposed outside the complex, is highlighted in blue. Other cofactors bound are indicated as follows: chlorophylls (green), Lute in L2 (orange), and Lute in L1 (purple).
PSI and Neo
Our results show that Neo is particularly effective in photoprotection from superoxide anions/hydroxyl radicals. It is surprising that PSI, which is the major site of superoxide production, does not bind Neo (Bassi and Simpson, 1987). This might be because PSII is in fact more sensitive to ROS than PSI due to the presence of a plastoquinone binding site accessible to lipophilic molecules (Loll et al., 2005) that allows access to superoxide anions from PSI. Although it has been reported that PSII is very sensitive to singlet oxygen (Krieger-Liszkay, 2005), no data are available concerning its sensitivity to other ROS species.
Possible Mechanism of Action for Neo Protection from ROS
It is known that the structural features of different carotenoids are important for their antioxidant activity, and we show here that Neo is more active than Viola in scavenging superoxide anions (Figure 7). The allenic function of Neo, which is exposed in the lipid phase from the LHCII structure (Liu et al., 2004), is the distinctive feature with respect to Viola, while the rest of the molecule is identical and might be responsible for increased reactivity. We are presently unable to distinguish between the two major pathways produced by the reaction of carotenoids with radical species, namely, the formation of a carotenoid radical adduct or the production of a carotenoid radical cation by electron transfer (Burton and Ingold, 1984; Woodall et al., 1997a, 1997b). Nevertheless, the observation that exposure of Neo to superoxide generators leads to preferential destruction of Neo in purified LHCII but not in leaves (see Supplemental Figures 2 and 3 online) suggests that Neo might be involved in the formation of radicals that are then detoxified by a partner molecule, absent in the purified LHCII sample. A candidate for this function is tocopherol, whose synergistic action with carotenoids in photoprotection has been previously highlighted (Havaux et al., 2005). Construction of an aba4 vte1 double mutant is in progress as a tool for clarifying the mechanism of Neo action.
Conclusion
We have shown a specific effect for Neo in scavenging superoxide anions, which could account for the aba4-1 higher sensitivity to high-light stress and to inhibitors of chloroplastic SOD. Superoxide anions are thought to be mainly produced by the Mehler's reaction in conditions leading to overreduction of electron acceptors to PSI, which is located in distinct membrane domains with respect to PSII; thus, we propose that the binding site of Neo, protruding outwards from LHCII trimers and located at the periphery of PSII supercomplexes, could be particularly effective in protecting PSII from superoxide anions diffusing from stroma membranes into grana partitions. Neo is also effective in forming Lhc proteins that are better protected from superoxide damage, thus supporting the view that different xanthophyll species play complementary roles in the photoprotection of the chloroplast. We also reported evidence of a limited conformational change of Lhc subunits due to higher Viola content. It cannot be excluded that these structural changes, although of small amplitude, could in principle contribute to the ROS-specific sensitivity of aba4-1. This effect would add to the well-demonstrated superoxide scavenging ability of Neo. It can be asked why plants would need protection against ROS, in addition to that provided by Zea, which has been shown to be effective (Havaux and Niyogi, 1999; Baroli et al., 2003; Dall'Osto et al., 2005) and to become available when stress is induced in wild-type plants. A constitutive accumulation of Zea induces strong thermal degradation by shortening 1Chl* lifetime and decreasing the probability of excitation trapping by PSII (Moya et al., 2001; Polivka et al., 2002), thus leading to reduced plant growth in limiting light (Dall'Osto et al., 2005). In fact, Zea accumulating genotypes lut2 npq2 and npq2 are phototolerant but dwarf (Havaux et al., 2004). It is clear that plants pay off photoprotection by Zea with a reduction in growth or even arrest. Photoprotection by Lute, Viola, and Neo can be more selective and/or less effective than that of Zea without preventing plant growth.
METHODS
Plant Material
The npq1 mutant was a kind gift of K. Niyogi (Berkeley, CA). aba3-1 (Col-0) was provided by Institut National de la Recherche Agronomique. aba4-1 (Wass-2) and aba4-3 (Col-0) were isolated as described by North et al. (2007). The genotype aba4-1 npq1 was obtained by crossing single mutant plants and selecting progeny by pigment analysis after high-light treatment.
Wild-type (accessions Wassilijewska-2 and Col-0) and mutant plants were grown on compost in a growth chamber for 4 weeks under controlled conditions (∼120 μmol photons m−2 s−1, 24°C, 8 h light/16 h dark, 80% relative humidity).
Stress Conditions
For high-light treatments, light was provided by 150-W halogen lamps (Focus 3; Prisma). Short-term high-light treatment was performed for 1 h at 1200 μmol photons m−2 s−1 at room temperature (24°C) to measure Zea accumulation on detached leaves. Photooxidative stress was induced in whole plants by a strong light treatment at low temperature. Whole Arabidopsis thaliana plants (40 d old) were exposed to high light (1600 μmol photon m−2 s−1 with a day/night photoperiod of 12 h/12 h) at low temperature (15°C/10°C, day/night air temperature, 80% relative humidity) for 7 d. Plants were daily sprayed with 100 μM ABA.
Light stress was also imposed on 1-cm diameter leaf discs. The discs were harvested from mature leaves. Leaf discs were vacuum-infiltrated with water and kept floating on water and then exposed to white light (photon flux density 1800 μmol m−2 s−1 for chlorophyll bleaching) at room temperature.
In Vivo Fluorescence and NPQ Measurements
NPQ of chlorophyll fluorescence, photochemical quenching (qP), and PSII yield (ΦPSII) was measured with a PAM 101 fluorimeter (Walz). Minimum fluorescence (F0) was measured with a 0.15 μmol m−2 s−1 beam, Fm was determined with a 1-s light pulse (5000 μmol m−2 s−1), and white continuous light (1200 μmol m−2 s−1) was supplied by a KL1500 halogen lamp (Schott). NPQ was calculated according to the following equation (Van Kooten and Snel, 1990): NPQ = (Fm −F′m)/F′m, where Fm is the maximum chlorophyll fluorescence from dark-adapted leaves and F′m is the maximum chlorophyll fluorescence under actinic light exposition. Measurements of room temperature fluorescence during photosynthesis with illumination from a model KL1500 lamp (Schott) and calculation of fluorescence parameters and NPQ of chlorophyll fluorescence were performed as described previously (Walters and Horton, 1995). Leaf discs were given 20 min of illumination, and PSII redox state and photosynthetic efficiency were determined during steady state photosynthesis. After fluorescence determination, leaves were weighed and leaf area measured, and then chlorophyll was extracted with 85% (v/v) acetone buffered with Na2CO3; chlorophyll concentration was determined according to Porra et al. (1989).
Thylakoid Isolation and Thylakoid Protein Preparation
Unstacked thylakoids were isolated from leaves as previously described (Bassi et al., 1988). Membranes corresponding to 500 μg of chlorophylls were washed with 5 mM EDTA and then solubilized in 1 mL of 0.6% α-DM and 10 mM HEPES, pH 7.5. Solubilized samples were then fractionated by ultracentrifugation in a 0.1 to 1 M sucrose gradient containing 0.06% α-DM and 10 mM HEPES, pH 7.5 (22 h at 280,000g, 4°C).
Monomeric Lhcb proteins were further fractionated by flatbed IEF at 4°C as previously described (Dainese et al., 1990). Green bands were harvested and eluted from a small column with 10 mM HEPES, pH 7.5, and 0.06% α-DM and further fractionated on a 0.1 to 1 M sucrose gradient containing 0.06% α-DM and 10 mM HEPES, pH 7.5, for 23 h at 280,000g at 4°C.
Pigment Analysis
Pigments were extracted from thylakoids, leaf discs, or isolated complexes with 80% acetone (v/v) and then separated and quantified by HPLC (Gilmore and Yamamoto, 1991) and by comparison of the spectrum of the sample acetone extract with the spectra of individual pigments (Croce et al., 2002). Tocopherol content per leaf area was quantified as previously described (Havaux et al., 2005).
Gel Electrophoresis and Immunoblotting
SDS-PAGE analysis was performed with the Tris-Tricine buffer system as previously described (Schägger and von Jagow, 1987). For immunotitration, thylakoid samples corresponding to 1, 2, 4, and 8 μg of chlorophylls were loaded for each sample and electroblotted on nitrocellulose membranes. Filters were incubated with the antibody raised against Lhcb1, Lhcb2, Lhcb3, CP29 (Lhcb4), CP26 (Lhcb5), CP24 (Lhcb6), or CP47 (PsbB) and were revealed with alkaline phosphatase–conjugated antibody according to Towbin et al. (1979). Signal amplitude was quantified using GelPro 3.2 software (Bio-Rad).
Spectroscopy
Spectra were obtained using samples in 10 mM HEPES, pH 7.5, 0.06% α-DM, and 0.2 M sucrose. Absorption measurements were performed using an SLM-Aminco DW-2000 spectrophotometer at room temperature. Circular dichroism spectra were recorded with a Jasco 600 spectropolarimeter at 10°C. Fluorescence excitation spectra were measured at room temperature using a Jasco FP-777 spectrofluorimeter exciting from 350 to 550 nm and measuring emission at 680 nm, with a 5-nm bandwidth beam for excitation and 3 nm for emission. The chlorophyll concentration was 0.1 μg/mL.
Protein Stability
This was analyzed as described (Croce et al., 2002) by following the decrease of the 492-nm circular dichroism signal detected by a Jasco 600 spectropolarimeter at increasing temperatures from 20 to 80°C (scan rate 1°C/min and step 0.2°C).
Photobleaching Assay
The kinetics of antennae photobleaching was measured as described (Croce et al., 1999b) but with a higher light intensity of ∼6000 μmol m−2 s−1 and sample cooling at 10°C. Initial and maximal absorbance was 0.6.
Determination of the Sensitivity to Different ROS on Either Whole Leaves or Isolated LHCII
Bleaching of leaf chlorophyll in leaf discs was induced by (1) singlet oxygen produced by RB photosensitizer, (2) superoxide anion produced by MZ, or (3) hydrogen peroxide. For bleaching mediated by singlet oxygen, leaf discs were vacuum-infiltrated with a 50 μM solution of RB; leaf discs floating in the same RB solution were exposed for 6 h to green light (500 nm < λ < 550 nm, room temperature, 100 μmol photons m−2 s−1). The wavelength range was chosen to both excite the dye, whose absorption maximum is 520 nm, and minimize light absorption by leaves. For bleaching mediated by superoxide anions, a similar experimental procedure was followed, infiltrating leaf discs with a 250 μM MZ solution and lighting with a fluorescence lamp (100 μmol photons m−2 s−1). For bleaching mediated by hydrogen peroxide, leaf discs were infiltrated with a 250 mM H2O2 solution and experiments were performed in the dark. At the end of treatments, leaf discs were frozen in liquid nitrogen. The experimental setup for measurements of ROS-mediated bleaching of trimeric LHCII was similar to that of whole leaves. Trimeric LHCII isolated by sucrose gradient ultracentrifugation from the wild type and aba4-1 thylakoids was incubated with RB (50 μM), MZ (250 μM), and hydrogen peroxide (1 M) into a microtiter well and treated as described for the whole-leaf experiment. Rates of bleaching were measured by following the decay of absorption area in the Qy transition region.
Chemical Detection of ROS Superoxide Anion
The capacity for scavenging superoxide anions either by thylakoids or purified carotenoids was measured with a NADH/phenazine methosulfate-superoxide–generating system (Peng et al., 2006); the inhibition of nitroblue tetrazolium oxidation was measured recording absorbance at 530 nm (Murphy and Auh, 1996). The chlorophyll concentration of thylakoids was 30 μg/mL; concentration of purified xanthophylls was 10 μg/mL.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: U44133 (npq1), AF117256 (lut2), AB026640 (npq2), AY034895 (aba3), and At1g67080 (aba4-1 and aba4-3).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Photoprotection in Leaf Discs of the Wild Type, aba4-3, and aba3-1.
Supplemental Figure 2. Time Course of Carotenoid Oxidation by Superoxide Anion.
Supplemental Figure 3. Sensitivity of LHCII-Bound Xanthophylls to Superoxide Anion.
Supplemental Figure 4. Spectral Deconvolution Analysis in LHCII Trimeric Subunits Purified by IEF from Wild-Type and aba4 Thylakoids.
Supplemental Figure 5. Spectral Deconvolution Analysis of Wild-Type and aba4 LHCII Trimeric Subunits Purified by Sucrose Gradient Ultracentrifugation.
Supplemental Table 1. Time Course of Leaf Pigment Composition of Wild-Type and Mutant Plants Grown for 6 d in High Light and Low Temperature (1600 μmol m−2 s−1, 15°C).
Supplemental Table 2. Thermal Stability of Lhcb Proteins Purified by IEF from Wild-Type and aba4 Thylakoids.
Supplementary Material
Acknowledgments
We thank R. Croce (University of Groeningen, Holland) for help with deconvolution analyses of isolated LHCII, S. Zorzan (University of Verona, Italy) for protein structure analysis, and Salvatore Sortino (University of Catania, Italy) for valuable discussions on photochemistry of MZ. This work was financed by Ministero dell'Università e della Ricerca FIRB Grant RBLA0345SF002.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Roberto Bassi (bassi@sci.univr.it).
Online version contains Web-only data.
References
- Al Babili, S., Hugueney, P., Schledz, M., Welsch, R., Frohnmeyer, H., Laule, O., and Beyer, P. (2000). Identification of a novel gene coding for neoxanthin synthase from Solanum tuberosum. FEBS Lett. 485 168–172. [DOI] [PubMed] [Google Scholar]
- Andersson, J., Walters, R.G., Horton, P., and Jansson, S. (2001). Antisense inhibition of the photosynthetic antenna proteins CP29 and CP26: Implications for the mechanism of protective energy dissipation. Plant Cell 13 1193–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada, K. (1999). The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu.Rev.Plant Physiol Plant Mol.Biol. 50 601–639. [DOI] [PubMed] [Google Scholar]
- Auh, C.K., and Murphy, T.M. (1995). Plasma membrane redox enzyme is involved in the synthesis of O2- and H2O2 by phytophthora elicitor-stimulated rose cells. Plant Physiol. 107 1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroli, I., Do, A.D., Yamane, T., and Niyogi, K.K. (2003). Zeaxanthin accumulation in the absence of a functional xanthophyll cycle protects Chlamydomonas reinhardtii from photooxidative stress. Plant Cell 15 992–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroli, I., Gutman, B.L., Ledford, H.K., Shin, J.W., Chin, B.L., Havaux, M., and Niyogi, K.K. (2004). Photo-oxidative stress in a xanthophyll-deficient mutant of Chlamydomonas. J. Biol. Chem. 279 6337–6344. [DOI] [PubMed] [Google Scholar]
- Bassi, R., Croce, R., Cugini, D., and Sandona, D. (1999). Mutational analysis of a higher plant antenna protein provides identification of chromophores bound into multiple sites. Proc. Natl. Acad. Sci. USA 96 10056–10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi, R., Pineau, B., Dainese, P., and Marquardt, J. (1993). Carotenoid-binding proteins of photosystem-II. Eur. J. Biochem. 212 297–303. [DOI] [PubMed] [Google Scholar]
- Bassi, R., Rigoni, F., Barbato, R., and Giacometti, G.M. (1988). Light-harvesting chlorophyll a/b proteins (LHCII) populations in phosphorylated membranes. Biochim. Biophys. Acta 936 29–38. [Google Scholar]
- Bassi, R., and Simpson, D. (1987). Chlorophyll-protein complexes of barley photosystem I. Eur. J. Biochem. 163 221–230. [DOI] [PubMed] [Google Scholar]
- Bouvier, F., D'Harlingue, A., Backhaus, R.A., Kumagai, M.K., and Camara, B. (2000). Identification of neoxanthin synthase as a carotenoid cyclase paralog. Eur. J. Biochem. 267 6346–6352. [DOI] [PubMed] [Google Scholar]
- Britton, G., Liaaen-Jensen, S., and Pfander, H. (1998). Carotenoids. 3. Biosynthesis and Metabolism. (Basel, Switzerland: Birkhauser Verlag).
- Bungard, R.A., Ruban, A.V., Hibberd, J.M., Press, M.C., Horton, P., and Scholes, J.D. (1999). Unusual carotenoid composition and a new type of xanthophyll cycle in plants. Proc. Natl. Acad. Sci. USA 96 1135–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton, G.W., and Ingold, K.U. (1984). β-Carotene: An unusual type of lipid antioxidant. Science 224 569–573. [DOI] [PubMed] [Google Scholar]
- Caffarri, S., Croce, R., Breton, J., and Bassi, R. (2001). The major antenna complex of photosystem II has a xanthophyll binding site not involved in light harvesting. J. Biol. Chem. 276 35924–35933. [DOI] [PubMed] [Google Scholar]
- Cinque, G., Croce, R., and Bassi, R. (2000). Absorption spectra of chlorophyll a and b in Lhcb protein environment. Photosynth. Res. 64 233–242. [DOI] [PubMed] [Google Scholar]
- Connelly, J.P., Müller, M.G., Bassi, R., Croce, R., and Holzwarth, A.R. (1997). Femtosecond transient absorption study of carotenoid to chlorophyll energy transfer in the light harvesting complex II of photosystem II. Biochemistry 36 281–287. [DOI] [PubMed] [Google Scholar]
- Croce, R., Canino, G., Ros, F., and Bassi, R. (2002). Chromophore organization in the higher-plant photosystem II antenna protein CP26. Biochemistry 41 7334–7343. [DOI] [PubMed] [Google Scholar]
- Croce, R., Cinque, G., Holzwarth, A.R., and Bassi, R. (2000). The soret absorption properties of carotenoids and chlorophylls in antenna complexes of higher plants. Photosynth. Res. 64 221–231. [DOI] [PubMed] [Google Scholar]
- Croce, R., Remelli, R., Varotto, C., Breton, J., and Bassi, R. (1999. a). The neoxanthin binding site of the major light harvesting complex (LHC II) from higher plants. FEBS Lett. 456 1–6. [DOI] [PubMed] [Google Scholar]
- Croce, R., Weiss, S., and Bassi, R. (1999. b). Carotenoid-binding sites of the major light-harvesting complex II of higher plants. J. Biol. Chem. 274 29613–29623. [DOI] [PubMed] [Google Scholar]
- Cunningham, F.X., and Gantt, E. (1998). Genes and enzymes of carotenoid biosynthesis in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49 557–583. [DOI] [PubMed] [Google Scholar]
- Dainese, P., Hoyer-hansen, G., and Bassi, R. (1990). The resolution of chlorophyll a/b binding proteins by a preparative method based on flat bed isoelectric focusing. Photochem. Photobiol. 51 693–703. [DOI] [PubMed] [Google Scholar]
- Dall'Osto, L., Caffarri, S., and Bassi, R. (2005). A mechanism of nonphotochemical energy dissipation, independent from Psbs, revealed by a conformational change in the antenna protein CP26. Plant Cell 17 1217–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison, P.A., Hunter, C.N., and Horton, P. (2002). Overexpression of beta-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature 418 203–206. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams, B., and Adams, W.W., III. (2002). Antioxidants in photosynthesis and human nutrition. Science 298 2149–2153. [DOI] [PubMed] [Google Scholar]
- Formaggio, E., Cinque, G., and Bassi, R. (2001). Functional architecture of the major light-harvesting complex from higher plants. J. Mol. Biol. 314 1157–1166. [DOI] [PubMed] [Google Scholar]
- Frank, H.A., and Cogdell, R.J. (1996). Carotenoids in photosynthesis. Photochem. Photobiol. 63 257–264. [DOI] [PubMed] [Google Scholar]
- Gastaldelli, M., Canino, G., Croce, R., and Bassi, R. (2003). Xanthophyll binding sites of the CP29 (Lhcb4) subunit of higher plant photosystem II investigated by domain swapping and mutation analysis. J. Biol. Chem. 278 19190–19198. [DOI] [PubMed] [Google Scholar]
- Gilmore, A.M., and Yamamoto, H.Y. (1991). Zeaxanthin formation and energy-dependent fluorescence quenching in pea chloroplasts under artificially mediated linear and cyclic electron transport. Plant Physiol. 96 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffra, E., Cugini, D., Croce, R., and Bassi, R. (1996). Reconstitution and pigment-binding properties of recombinant CP29. Eur. J. Biochem. 238 112–120. [DOI] [PubMed] [Google Scholar]
- Goldsmith, T.H., and Krinsky, N.I. (1960). The epoxide nature of the carotenoid neoxanthin. Nature 198 491–493. [DOI] [PubMed] [Google Scholar]
- Gomez, J.M., Jimenez, A., Olmos, E., and Sevilla, F. (2004). Location and effects of long-term NaCl stress on superoxide dismutase and ascorbate peroxidase isoenzymes of pea (Pisum sativum cv. Puget) chloroplasts. J. Exp. Bot. 55 119–130. [DOI] [PubMed] [Google Scholar]
- Havaux, M. (2003). Spontaneous and thermoinduced photon emission: New methods to detect and quantify oxidative stress in plants. Trends Plant Sci. 8 409–413. [DOI] [PubMed] [Google Scholar]
- Havaux, M., Dall'Osto, L., Cuine, S., Giuliano, G., and Bassi, R. (2004). The effect of zeaxanthin as the only xanthophyll on the structure and function of the photosynthetic apparatus in Arabidopsis thaliana. J. Biol. Chem. 279 13878–13888. [DOI] [PubMed] [Google Scholar]
- Havaux, M., Eymery, F., Porfirova, S., Rey, P., and Dormann, P. (2005). Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell 17 3451–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux, M., and Niyogi, K.K. (1999). The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc. Natl. Acad. Sci. USA 96 8762–8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobe, S., Niemeier, H., Bender, A., and Paulsen, H. (2000). Carotenoid binding sites in LHCIIb - Relative affinities towards major xanthophylls of higher plants. Eur. J. Biochem. 267 616–624. [DOI] [PubMed] [Google Scholar]
- Holt, N.E., Kennis, J.T.M., Dall'Osto, L., Bassi, R., and Fleming, G.R. (2003). Carotenoid to chlorophyll energy transfer in light harvesting complex II from Arabidopsis thaliana probed by femtosecond fluorescence upconversion. Chem. Phys. Lett. 379 305–313. [Google Scholar]
- Horton, P. (1996). Nonphotochemical quenching of chlorophyll fluorescence. In Light as an Energy Source and Information Carrier in Plant Physiology, R.C. Jennings, ed (New York: Plenum Press), pp. 99–111.
- Jansson, S. (1999). A guide to the Lhc genes and their relatives in Arabidopsis. Trends Plant Sci. 4 236–240. [DOI] [PubMed] [Google Scholar]
- Kotake-Nara, E., Asai, A., and Nagao, A. (2005). Neoxanthin and fucoxanthin induce apoptosis in PC-3 human prostate cancer cells. Cancer Lett. 220 75–84. [DOI] [PubMed] [Google Scholar]
- Krieger-Liszkay, A. (2005). Singlet oxygen production in photosynthesis. J. Exp. Bot. 56 337–346. [DOI] [PubMed] [Google Scholar]
- Krinsky, N.I. (1979). Biological Roles of Singlet Oxygen. (New York: Academic Press).
- Krinsky, N.I., Landrum, J.T., and Bone, R.A. (2003). Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 23 171–201. [DOI] [PubMed] [Google Scholar]
- Liu, Z., Yan, H., Wang, K., Kuang, T., Zhang, J., Gui, L., An, X., and Chang, W. (2004). Crystal structure of spinach major light-harvesting complex at 2.72 A resolution. Nature 428 287–292. [DOI] [PubMed] [Google Scholar]
- Llorente, F., Oliveros, J.C., Martinez-Zapater, J.M., and Salinas, J. (2000). A freezing-sensitive mutant of Arabidopsis, frs1, is a new aba3 allele. Planta 211 648–655. [DOI] [PubMed] [Google Scholar]
- Lokstein, H., Tian, L., Polle, J.E., and DellaPenna, D. (2002). Xanthophyll biosynthetic mutants of Arabidopsis thaliana: Altered nonphotochemical quenching of chlorophyll fluorescence is due to changes in photosystem II antenna size and stability. Biochim. Biophys. Acta 1553 309–319. [DOI] [PubMed] [Google Scholar]
- Loll, B., Kern, J., Saenger, W., Zouni, A., and Biesiadka, J. (2005). Towards complete cofactor arrangement in the 3.0 A resolution structure of photosystem II. Nature 438 1040–1044. [DOI] [PubMed] [Google Scholar]
- Milborrow, B.V. (2001). The pathway of biosynthesis of abscisic acid in vascular plants: A review of the present state of knowledge of ABA biosynthesis. J. Exp. Bot. 52 1145–1164. [PubMed] [Google Scholar]
- Moya, I., Silvestri, M., Vallon, O., Cinque, G., and Bassi, R. (2001). Time-resolved fluorescence analysis of the photosystem II antenna proteins in detergent micelles and liposomes. Biochemistry 40 12552–12561. [DOI] [PubMed] [Google Scholar]
- Muller-Moule, P., Conklin, P.L., and Niyogi, K.K. (2002). Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol. 128 970–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Moule, P., Havaux, M., and Niyogi, K.K. (2003). Zeaxanthin deficiency enhances the high light sensitivity of an ascorbate-deficient mutant of Arabidopsis. Plant Physiol. 133 748–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, T.M., and Auh, C.K. (1996). The superoxide synthases of plasma membrane preparations from cultured rose cells. Plant Physiol. 110 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedzwiedzki, D., Krupa, Z., and Gruszecki, W.I. (2005). Temperature-induced isomerization of violaxanthin in organic solvents and in light-harvesting complex II. J. Photochem. Photobiol. B 78 109–114. [DOI] [PubMed] [Google Scholar]
- Niyogi, K.K. (1999). Photoprotection revisited: Genetic and molecular approaches. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50 333–359. [DOI] [PubMed] [Google Scholar]
- Niyogi, K.K., Grossman, A.R., and Björkman, O. (1998). Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10 1121–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi, K.K., Shih, C., Chow, W.S., Pogson, B.J., DellaPenna, D., and Bjorkman, O. (2001). Photoprotection in a zeaxanthin- and lutein-deficient double mutant of Arabidopsis. Photosynth. Res. 67 139–145. [DOI] [PubMed] [Google Scholar]
- Noctor, G., and Foyer, C.H. (1998). Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49 249–279. [DOI] [PubMed] [Google Scholar]
- North, H.M., De Almeida, A., Boutin, J.P., Frey, A., To, A., Botran, L., Sotta, B., and Marion-Poll, A. (2007). The Arabidopsis ABA-deficient mutant aba4 demonstrate that the major route for stress-induced ABA accumulation is via neoxanthin isomers. Plant J., in press. [DOI] [PubMed]
- Pagano, A., Cinque, G., and Bassi, R. (1998). In vitro reconstitution of the recombinant photosystem II light-harvesting complex CP24 and its spectroscopic characterization. J. Biol. Chem. 273 17154–17165. [DOI] [PubMed] [Google Scholar]
- Peng, C.-L., Lin, Z.-F., Su, Y.-Z., Lin, G.-Z., Dou, H.-Y., and Zhao, C.-X. (2006). The antioxidative function of lutein: Electron spin resonance studies and chemical detection. Funct. Plant Biol. 33 839–846. [DOI] [PubMed] [Google Scholar]
- Pogson, B., McDonald, K.A., Truong, M., Britton, G., and DellaPenna, D. (1996). Arabidopsis carotenoid mutants demonstrate that lutein is not essential for photosynthesis in higher plants. Plant Cell 8 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polivka, T., Zigmantas, D., Sundström, V., Formaggio, E., Cinque, G., and Bassi, R. (2002). Carotenoid S-1 state in a recombinant light-harvesting complex of photosystem II. Biochemistry 41 439–450. [DOI] [PubMed] [Google Scholar]
- Porra, R.J., Thompson, W.A., and Kriedemann, P.E. (1989). Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 975 384–394. [Google Scholar]
- Pospisil, P., Arato, A., Krieger-Liszkay, A., and Rutherford, A.W. (2004). Hydroxyl radical generation by photosystem II. Biochemistry 43 6783–6792. [DOI] [PubMed] [Google Scholar]
- Pospisil, P., Snyrychova, I., Kruk, J., Strzalka, K., and Naus, J. (2006). Evidence that cytochrome b 559 is involved in superoxide production in photosystem II: Effect of synthetic short-chain plastoquinones in a cytochrome b 559 tobacco mutant. Biochem. J. 397 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock, C.D., and Zeevaart, J.A. (1991). The aba mutant of Arabidopsis thaliana is impaired in epoxy-carotenoid biosynthesis. Proc. Natl. Acad. Sci. USA 88 7496–7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruban, A.V., and Horton, P. (1999). The xanthophyll cycle modulates the kinetics of nonphotochemical energy dissipation in isolated light-harvesting complexes, intact chloroplasts, and leaves of spinach. Plant Physiol. 119 531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruban, A.V., Wentworth, M., Yakushevska, A.E., Andersson, J., Lee, P.J., Keegstra, W., Dekker, J.P., Boekema, E.J., Jansson, S., and Horton, P. (2003). Plants lacking the main light-harvesting complex retain photosystem II macro-organization. Nature 421 648–652. [DOI] [PubMed] [Google Scholar]
- Ruggiero, B., Koiwa, H., Manabe, Y., Quist, T.M., Inan, G., Saccardo, F., Joly, R.J., Hasegawa, P.M., Bressan, R.A., and Maggio, A. (2004). Uncoupling the effects of abscisic acid on plant growth and water relations. Analysis of sto1/nced3, an abscisic acid-deficient but salt stress-tolerant mutant in Arabidopsis. Plant Physiol. 136 3134–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano, S., and Asada, K. (1994). cDNA cloning of monodehydroascorbate radical reductase from cucumber: A high degree of homology in terms of amino acid sequence between this enzyme and bacterial flavoenzymes. Plant Cell Physiol. 35 425–437. [PubMed] [Google Scholar]
- Schägger, H., and von Jagow, G. (1987). Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166 368–379. [DOI] [PubMed] [Google Scholar]
- Schwartz, S.H., Leon-Kloosterziel, K.M., Koornneef, M., and Zeevaart, J.A. (1997). Biochemical characterization of the aba2 and aba3 mutants in Arabidopsis thaliana. Plant Physiol. 114 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder, A.M., Clark, B.M., Robert, B., Ruban, A.V., and Bungard, R.A. (2004). Carotenoid specificity of light-harvesting complex II binding sites. J. Biol. Chem. 279 5162–5168. [DOI] [PubMed] [Google Scholar]
- Tardy, F., and Havaux, M. (1996). Photosynthesis, chlorophyll fluorescence, light-harvesting system and photoinhibition resistance of a zeaxanthin-accumulating mutant of Arabidopsis thaliana. J. Photochem. Photobiol. B 34 87–94. [DOI] [PubMed] [Google Scholar]
- Tjus, S.E., Moller, B.L., and Scheller, H.V. (1998). Photosystem I is an early target of photoinhibition in barley illuminated at chilling temperatures. Plant Physiol. 116 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin, H., Staehelin, T., and Gordon, J. (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 76 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kooten, O., and Snel, J.F.H. (1990). The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth. Res. 25 147–150. [DOI] [PubMed] [Google Scholar]
- Walters, R.G., and Horton, P. (1995). Acclimation of Arabidopsis thaliana to the light environment: Changes in photosynthetic function. Planta 197 306–312. [DOI] [PubMed] [Google Scholar]
- Wise, R.R., and Naylor, A.W. (1987. a). Chilling-enhanced photooxidation: Evidence for the role of singlet oxygen and superoxide in the breakdown of pigments and endogenous antioxidants. Plant Physiol. 83 278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise, R.R., and Naylor, A.W. (1987. b). Chilling-enhanced Photooxidation: The peroxidative destruction of lipids during chilling injury to photosynthesis and ultrastructure. Plant Physiol. 83 272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodall, A.A., Britton, G., and Jackson, M.J. (1997. a). Carotenoids and protection of phospholipids in solution or in liposomes against oxidation by peroxyl radicals: Relationship between carotenoid structure and protective ability. Biochim. Biophys. Acta 1336 575–586. [DOI] [PubMed] [Google Scholar]
- Woodall, A.A., Lee, S.W., Weesie, R.J., Jackson, M.J., and Britton, G. (1997. b). Oxidation of carotenoids by free radicals: Relationship between structure and reactivity. Biochim. Biophys. Acta 1336 33–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.