Abstract
We explore the roles of gibberellin (GA) signaling genes SLEEPY1 (SLY1) and RGA-LIKE2 (RGL2) in regulation of seed germination in Arabidopsis thaliana, a plant in which the hormone GA is required for seed germination. Seed germination failure in the GA biosynthesis mutant ga1-3 is rescued by GA and by mutations in the DELLA gene RGL2, suggesting that RGL2 represses seed germination. RGL2 protein disappears before wild-type seed germination, consistent with the model that GA stimulates germination by causing the SCFSLY1 E3 ubiquitin ligase complex to trigger ubiquitination and destruction of RGL2. Unlike ga1-3, the GA-insensitive sly1 mutants show variable seed dormancy. Seed lots with high seed dormancy after-ripened slowly, with stronger alleles requiring more time. We expected that if RGL2 negatively controls seed germination, sly1 mutant seeds that germinate well should accumulate lower RGL2 levels than those failing to germinate. Surprisingly, RGL2 accumulated at high levels even in after-ripened sly1 mutant seeds with 100% germination, suggesting that RGL2 disappearance is not a prerequisite for seed germination in the sly1 background. Without GA, several GA-induced genes show increased accumulation in sly1 seeds compared with ga1-3. It is possible that the RGL2 repressor of seed germination is inactivated by after-ripening of sly1 mutant seeds.
INTRODUCTION
The seed is the main mechanism of plant propagation, and the proper regulation of seed dormancy and germination is of critical importance to plant survival. Seeds that fail to germinate under favorable environmental conditions are said to be dormant (reviewed in Bewley and Black, 1994). Dormant seeds acquire the ability to germinate following a period of dry storage called after-ripening. Dormancy helps to ensure the survival of a species by allowing some ungerminated seeds to survive natural catastrophes. Seed dormancy is also important from the agronomic viewpoint because the absence of dormancy in several cereal grains causes preharvest sprouting when cool humid conditions persist close to harvest. There are two classes of seed dormancy: coat-imposed and embryo dormancy. In coat-imposed dormancy, radicle emergence is blocked by the physical barrier of the testa, aleurone, or endosperm layer. Seed coat–imposed dormancy, which is commonly found in Arabidopsis thaliana, can be overcome by cutting the seed coat (Debeaujon and Koornneef, 2000). Conversely, seeds with embryo dormancy will not germinate when the seed coat is cut (Bewley and Black, 1994). Germination of dormant seeds can also be stimulated by a period of cold imbibition called stratification prior to imbibition under conditions that stimulate germination.
Genetic analysis of seed dormancy is complicated by the fact that the degree of dormancy is dependent on both environmental and genetic factors. However, some genes influencing the degree of seed dormancy have been cloned. For example, quantitative trait loci analysis of a cross between highly dormant Arabidopsis ecotype Cvi (Cape Verde Islands) and the less dormant ecotype Landsberg erecta (Ler) has identified seven quantitative trait loci associated with seed dormancy (Alonso-Blanco et al., 2003). Loss of the COMATOSE gene encoding a putative ATP binding cassette transporter results in strong seed dormancy and reduced germination potential without affecting seed or plant morphology (Russell et al., 2000; Footitt et al., 2002). Loss of the DOF AFFECTING GERMINATION1 gene encoding a putative zinc finger transcription factor results in reduced seed dormancy as a maternal effect without affecting seed coat structure (Papi et al., 2000, 2002). Other examples of genes involved in the establishment and maintenance of seed dormancy include ABSCISIC ACID INSENSITIVE3, LEAFY COTYLEDON1 (LEC1), LEC2, and FUSCA3 (Ooms et al., 1993; Keith et al., 1994; Nambara et al., 1995; Parcy and Giraudat, 1997).
Seed dormancy is subject to hormonal control. The plant hormone abscisic acid (ABA) is needed to induce seed dormancy during embryo maturation, whereas the hormone gibberellin (GA) is needed to stimulate seed germination. Mutants with decreased ABA biosynthesis or sensitivity show decreased seed dormancy, whereas mutants with decreased ABA catabolism or increased ABA sensitivity show increased seed dormancy (reviewed in Finkelstein and Rock, 2002; Kushiro et al., 2004; Okamoto et al., 2006).
Several lines of evidence demonstrate the importance of GA biosynthesis and signaling for stimulating seed germination in Arabidopsis. The role of GA in promoting various stages in Arabidopsis growth and development is demonstrated by the phenotypes of GA biosynthesis mutants like ga1-3, including severe dwarfism, male sterility, and strong coat-imposed seed dormancy (reviewed in Olszewski et al., 2002). GA application rescues all of these defects. GA does not appear to directly control dormancy per se but acts as an antagonist to ABA by stimulating radicle emergence (reviewed in Bewley, 1997). Many GA biosynthetic genes are upregulated in seed tissues during seed germination and in the cold (Yamaguchi et al., 2001; Penfield et al., 2006). GA signaling is also required for efficient seed germination in Arabidopsis. Mutations in the SLEEPY1 (SLY1) GA signaling gene lead to increased seed dormancy, and a triple knockout of the GA receptors GID1A, GID1B, and GID1C leads to failure to germinate (Steber et al., 1998; Griffiths et al., 2006).
Our current understanding of the control of Arabidopsis seed germination by GA signaling is derived mostly from studies of the GA signaling genes SLY1 and the DELLA gene RGA-LIKE2 (RGL2) (Steber et al., 1998; Lee et al., 2002; Bassel et al., 2004; Tyler et al., 2004). DELLA proteins are a subfamily of the GRAS (for GAI, RGA, and Scarecrow) family of putative transcription factors that are nuclear-localized and negatively regulate GA signaling (Peng et al., 1999; Pysh et al., 1999). There are five DELLA proteins in Arabidopsis with partially overlapping functions in GA signaling. Consistent with the negative role of DELLA proteins, loss-of-function mutations in DELLA genes result in a decreased requirement for GA in plant growth and development. Based on phenotypic analysis, the main negative regulator of stem elongation is the DELLA protein RGA, although GAI and RGL1 also function in stem elongation. DELLA proteins RGA, RGL2, and RGL1 are the main regulators of flowering (reviewed in Thomas et al., 2005). No function has yet been attributed to RGL3. Finally, RGL2 is the main negative regulator of seed germination, whereas RGA, GAI, and RGL1 play lesser roles (Cao et al., 2005).
GA relieves DELLA inhibition of seed germination, stem elongation, and transition to flowering by triggering DELLA protein destruction via the ubiquitin-proteasome pathway. It has been demonstrated that the GA signal in rice (Oryza sativa) is perceived by a GA receptor with homology to human hormone-sensitive lipase, GID1 (for GA-INSENSITIVE DWARF1) (Ueguchi-Tanaka et al., 2005). Recently, three homologs of the rice GID1 gene (GID1a, GID1b, and GID1c) have been shown to function in Arabidopsis GA signaling (Nakajima et al., 2006). The fact that GID1 shows GA-dependent interaction with the five DELLA proteins suggests that Arabidopsis GID1 controls DELLA function. On binding GA, GID1 binds to DELLA protein and appears to stimulate ubiquitination of DELLA proteins by promoting binding to the SCFSLY1 E3 ubiquitin ligase (Griffiths et al., 2006; Hartweck and Olszewski, 2006). DELLA proteins have been shown to directly interact with SLY1 and are believed to disappear rapidly from the nucleus once ubiquitinated due to proteolytic degradation by the 26S proteasome pathway. The SLY1 gene is a positive regulator of GA signaling encoding the F-box subunit that determines the substrate specificity of an SCF (for Skp1, Cullin, F-box) E3 ubiquitin ligase complex (McGinnis et al., 2003; Dill et al., 2004; Fu et al., 2004). Mutations in SLY1 were isolated in screens for decreased germination capacity (Steber et al., 1998; Steber and McCourt, 2001). The sly1 mutants show increased seed dormancy and increased sensitivity to inhibition of seed germination by ABA (Strader et al., 2004). By contrast, DELLA genes are negative regulators of GA signaling, and mutations in the DELLA gene RGL2 result in increased capacity to germinate (Lee et al., 2002; Tyler et al., 2004). Thus, it appears that SCFSLY1 may positively regulate GA signaling by causing the destruction of the DELLA protein RGL2 during seed germination.
Several lines of evidence indicate that RGL2 is a critical negative regulator of seed germination. The strongest evidence is that mutations in RGL2 restore seed germination in the ga1-3 background (Lee et al., 2002; Tyler et al., 2004). The pattern of RGL2 mRNA expression is consistent with a regulatory role in seed germination. In wild-type seeds, the RGL2 transcript level rises during a stratification treatment of seed imbibition at low temperature (4°C) and rapidly declines after the seeds are transferred to 23°C, a temperature that favors seed germination (Lee et al., 2002). In the ga1-3 mutant, however, RGL2 mRNA levels increase during imbibition at low temperature and remain high even after the seeds are transferred to 23°C. If GA is added, the RGL2 transcript level rapidly declines and germination capacity increases. Thus, RGL2 transcript levels negatively correlate with seed germination, suggesting that downregulation of RGL2 transcript is necessary for seed germination in Arabidopsis (Lee et al., 2002; Tyler et al., 2004). Subsequent work by Bassel et al. (2004) showed that the RGL2 mRNA does not completely disappear prior to radicle emergence, indicating that disappearance of RGL2 mRNA is not absolutely required for seed germination. Interestingly, protein gel blot analysis showed that RGL2 protein disappears within 5 h of addition of GA to incubating ga1-3 seeds (Tyler et al., 2004). RGL2 protein did not disappear, however, within 5 h of adding GA to imbibing sly1-10 seeds. Thus, the current model is that disappearance of RGL2 protein is required for seed germination and that rapid disappearance of RGL2 protein requires the SCFSLY1 E3 ubiquitin ligase (Tyler et al., 2004). Based on genetic evidence, it is clear that RGL2 negatively regulates seed germination. The question that remains is whether the decreases in RGL2 transcript and protein levels are the sole mechanisms controlling GA-stimulated seed germination.
To address this question, this study examines whether the pattern of RGL2 protein accumulation in sly1 mutants is consistent with the current model for control of seed germination by RGL2. This study shows that the dormant seed phenotype of sly1 mutants is variable between different seed lots. Those seed lots showing high seed dormancy eventually after-ripen. Stronger alleles show a stronger seed germination phenotype and require more time to after-ripen. For example, the sly1-2 allele can require up to 2 years to fully after-ripen. Based on the current model, we expected that the level of RGL2 protein accumulation should be higher in those sly1 lines that show lower germination capacity. Surprisingly, we found that RGL2 protein accumulated at high levels in sly1 mutants regardless of the degree of seed dormancy. RGL2 protein accumulated to the same level in after-ripened and dormant sly1-2 seeds prior to radicle emergence. Indeed, after-ripened sly1-2 seeds that were capable of 91% germination expressed high levels of RGL2 protein. This suggests that SCFSLY1 is the main E3 ubiquitin ligase controlling RGL2 protein accumulation and that in the sly1 mutant background, RGL2 protein disappearance is not a prerequisite for seed germination. The possibility that the RGL2 protein accumulating in after-ripened sly1 mutant seeds is either inactive or controlled by other factors is considered.
RESULTS
Isolation of a New SLY1 Allele
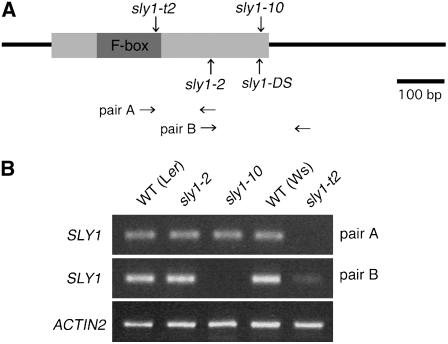
Here, we compare two new mutant alleles to the two recessive alleles of sly1 in the Ler background previously described (Figure 1A; Steber et al., 1998; Steber and McCourt, 2001; McGinnis et al., 2003). The sly1-2 allele is a 2-bp deletion causing a frame shift and the predicted loss of the last 40 out of 151 amino acids. The sly1-10 allele contains a 23-bp deletion followed by an ∼8-kb insertion, causing the predicted loss of the last eight amino acids and the addition of 46 random amino acids (Figure 1A). The new allele sly1-DS in the Ler background resembles sly1-10 and contains a transposon insertion resulting in loss of the last 12 amino acids (Sundaresan et al., 1995). The new sly1-t2 allele in the Wassilewskija (Ws) background was identified in the Sussman lines (Krysan et al., 1999). It contains a T-DNA insertion within the F-box motif (at +219 bp) and is predicted to result in loss of the last 78 amino acids. This is the most severe lesion identified in the SLY1 gene thus far. The sly1-t2 allele showed severe defects in plant development, such as dwarfism and reduced fertility (see Supplemental Table 1 online). The effect of each allele on SLY1 mRNA levels was examined by RT-PCR using SLY1 gene-specific primers (Figure 1B). When the SLY1-specific primers were employed, no significant change in mRNA accumulation was observed in sly1-10 and sly1-2, whereas a marked decrease was seen in sly1-t2. These results indicate that SLY1 transcript accumulation is reduced by the T-DNA insertion in sly1-t2, whereas the other two alleles, sly1-10 and sly1-2, do not alter transcript levels compared with the wild type.
Figure 1.
Structure of the SLY1 Gene and the SLY1 mRNA Expression Detected by RT-PCR in Three Mutant Alleles.
(A) The mutation sites are shown in three alleles. The sly1-10 mutant has an 8-kb insertion at +429 nucleotides from translational start. The sly1-2 mutant has a 2-bp deletion at the +333-nucleotide position. The T-DNA is inserted at the +219-nucleotide position in sly1-t2.
(B) The top panel shows the SLY1 expression in three mutant alleles and the wild type using the sly1-2f and sly1-2r primers (pair A) that flank the insertion in sly1-t2 giving no product. The middle panel shows the expression using the 2-63f and sly1-r primers (pair B) that flank the sly1-10 rearrangement giving no product. The bottom panel shows the ACTIN2 control.
The sly1 Germination Phenotype Is Variable but Dependent on the Allele and the Age of the Seed
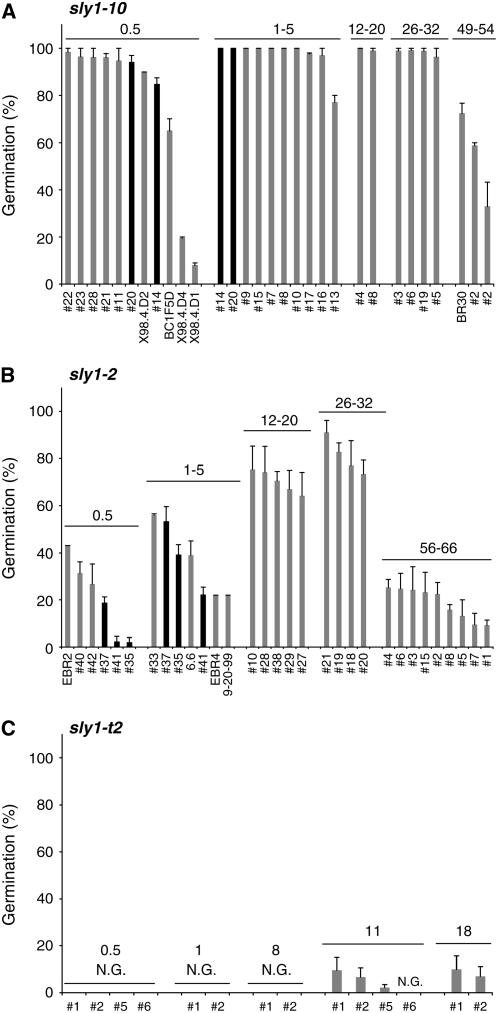
Because GA synthesis is required for seed germination, it was expected that GA signaling would also be required for seed germination. Consistent with this, mutants in the GA signaling gene SLY1 cause increased seed dormancy and increased sensitivity to ABA in seed germination (Strader et al., 2004). Subsequently, anecdotal evidence showed that the sly1 seed dormancy phenotype was variable. To characterize the sly1 seed dormancy phenotype, the percentage of germination of many independent sly1 seed lots of different ages were compared after 7 d of incubation at 22°C (Figure 2). While the germination capacity of independent seed lots varied significantly, it did correlate with allele and with the period of seed dry storage.
Figure 2.
Germination Rates of sly1 Mutant Seeds Varied but Were Dependent on Allele and Age of Seeds.
Seeds plated on MS-agar were incubated 3 d at 4°C followed by 7 d at 22°C. Percentage of germination was calculated based on cotyledon emergence for independent seed lots of: sly1-10 (A), sly1-2 (B), and sly1-t2 (C). Duration of dry storage or age of seed is indicated above the bars (months). N.G., none of the seeds germinated. Error bars show se (n = 3). Seed lot numbers (#) are indicated on the x axis.
In general, sly1-2 seeds showed stronger dormancy than sly1-10 seeds. The sly1-t2 allele showed the highest degree of seed dormancy. However, the germination capacity of sly1-t2 seeds cannot be directly compared with sly1-2 and sly1-10 because it is in a different genetic background. In sly1-10, the germination rate of the 0.5-month-old seed lots ranged from 8.0 to 98.4% (Figure 2A). Less variation in germination rate was seen after seeds were subjected to 1 to 5 months (77.1 to 100%) and 12 to 31 months (96.3 to 100%) of room temperature dry storage. The sly1-2 allele showed more severe seed dormancy (Figure 2B). In sly1-2, the germination rate of the 0.5-month-old seed lots ranged from 2.0 to 43.0%. The germination rate increased following longer periods of dry storage for 1 to 5 months (22.0 to 56.1%), 12 to 20 months (64.2 to 75.4%), and 26 to 28 months (73.4 to 91.1%). Unlike sly1-10, sly1-2 seeds never reached 100% germination. The sly1-t2 seeds showed the most severe dormancy phenotype. No seed germination was observed in 16 independent seed lots stored for 8 months or less, and seeds stored for 11 to 18 months showed between 0 and 9.6% seed germination (Figure 2C; data not shown). It is not known whether sly1-t2 seeds ever fully after-ripen. These results indicate that the degree of seed dormancy is dependent on the severity of the sly1 allele. However, other factors, possibly environmental, may cause the variation in dormancy.
The data in Figures 2A and 2B show that both sly1-2 and sly1-10 seeds lose their capacity to germinate following long periods of dry storage. In sly1-10, seed lots stored for 49 to 54 months showed 32.9 to 72.3% seed germination, whereas sly1-2 seeds stored for 56 to 66 months showed 9.2 to 25.2% germination. It is possible that this loss of germination capacity is due to seed deterioration and that this loss of viability is more severe in the stronger allele.
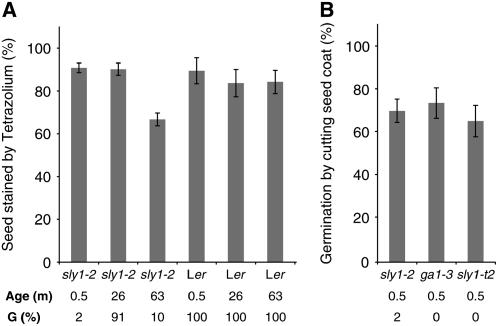
To clarify if dormancy or poor viability caused seeds to show low percentage of germination, intact seeds were stained with tetrazolium solution, a vital stain (Debeaujon et al., 2000). In sly1-2, a similar staining pattern was seen in a 0.5-month-old seed lot (#35) showing 2% germination as in a 26-month-old seed lot (#21) showing 91.1% seed germination (Figures 2A, 2B, and 3A). A similar staining pattern was also seen in Ler (wild-type), ga1-3, and sly1-10 seeds of comparable age (data not shown). By contrast, reduced staining (66.8% stained) was seen in sly1-2 seeds stored for 63 months (#7) and giving 10% germination (Figures 2B and 3A). By contrast, 63-month-old Ler seeds showed 100% germination and 84.3% tetrazolium-stained seeds. These data suggest that the low percentage of germination in 0.5-month-old sly1-2 seeds is due to seed dormancy, whereas the reduction in germination capacity of older sly1-2 seeds is due, at least in part, to loss of longevity. Consistent with this, germination of 0.5-month-old sly1-2 seeds is rescued by cutting the seed coat as is the germination of 0.5-month-old ga1-3 seeds and sly1-t2 (Figure 3B). Failure to achieve 100% germination is probably due to inadvertent damage to the embryo.
Figure 3.
Determination of Seed Viability by Tetrazolium Staining and Germination Rescue by Cutting the Seed Coat.
(A) Seeds of the wild type (0.5, 26, and 63 months old), after-ripened sly1-2 (26 months old, seed lot #21), and dormant sly1-2 (0.5 and 63 months old, seed lots #35 and #7, respectively) were stained with 0.5% tetrazolium for 10 h. Fully stained seeds were counted as viable.
(B) To examine seed viability, dormant sly1-2 (seed lot #35, 0.5 months old) and sly1-t2 (seed lot #12, 0.5 months old) seed coats were nicked with forceps to stimulate seed germination. ga1-3 (0.5 months old) seeds were used as a positive control for seed germination rescue.
Age of each seed stock is shown below the graph (months). Germination rates (G) are shown below the figure (%). Error bars show se (n = 30).
sly1 Mutants After-Ripen Slowly
The fact that sly1 seed lots subjected to longer periods of dry storage show better seed germination suggests that dormant sly1 seeds are able to after-ripen slowly (Figure 2). Ler (wild-type) seeds are fully after-ripened and show 100% germination after 0.5 months of dry storage (Figure 4). During an after-ripening time course, a single sly1-2 seed lot (#37) showed 18.7% germination after 0.5 months of dry storage, 53.2% germination after 1 month of dry storage, and 64.2% after 8 months of dry storage (Figure 2B; see Supplemental Figure 1 online). The period required to after-ripen also appears to depend on the severity of the allele. The sly1-10 seeds reached ∼97.0% germination after 1 to 5 months after-ripening, whereas sly1-2 seeds reached 73.4 to 91.1% germination after 26 to 28 months after-ripening (Figure 2).
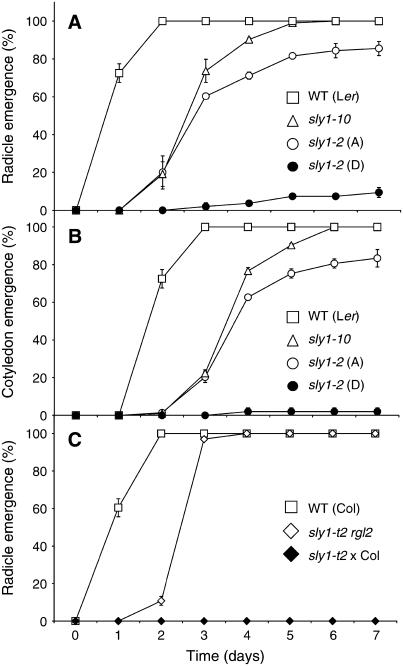
Figure 4.
sly Mutant Seeds Germinate More Slowly Than the Wild Type, and Germination Is Rescued by a Mutation in RGL2.
Seeds were plated on MS-agar and the number of germinated seeds scored daily after transfer to 22°C. The wild type (28 months old), sly1-10 (27 months old, #3), after-ripened (A) sly1-2 (26 months old, #21), dormant (D) sly1-2 (0.5 months old, #35), 1-month-old wild type (Col), sly1-t2 rgl2-13 (1 month old), and sly1-t2 (1 month old, crossed to wild-type Col) were used. Error bars show se (n = 3).
(A) Percentage of radicle emergence based on radicle protrusion from seed coat.
(B) Percentage of cotyledon emergence based on appearance of green cotyledons outside the seed coat.
(C) Rescue of sly1-t2 seed germination by the rgl2-13 mutation based on percentage of radicle emergence.
Segregation analysis was used to examine the dormant seed phenotype of sly1-t2. The mutant was crossed with Ws (wild-type), and F2 progeny were sown to check the segregation ratio of geminated-to-ungerminated seeds. Of 133 F2 progeny, 29 failed to germinate, giving a 3:1 segregation ratio (χ2 = 0.72; 1.0 < P < 0.5). These results suggested that the seed dormancy phenotype in sly1-t2 was caused by a single recessive mutation. When germination was rescued by cutting the seed coat, all of the ungerminated seeds gave rise to dwarf sly1 plants (data not shown). None of the 104 germinated seedlings gave rise to dwarves. The sly1-t2 allele shows 0 to 9.9% seed germination following 18 months of after-ripening. Thus, this severe truncation of the SLY1 gene results in strong seed dormancy.
sly1 Seeds Germinate Slowly and Show Increased Sensitivity to Polyethylene Glycol and Paclobutrazol in Germination
After-ripened sly1 mutant seed lots showed reduced germination capacity compared with wild-type Ler seeds. This was first evaluated based on percentage of germination over time. To accurately examine the effect of the sly1 mutations on germination rate, both the percentage of radicle emergence (Figure 4A) and percentage of cotyledon emergence (Figure 4B) were determined over time comparing wild-type Ler with sly1-10 (#3) and dormant and after-ripened sly1-2 seeds (#35 and #21, respectively). Similar curves were obtained using percentage of radicle emergence and cotyledon emergence, except that cotyledon emergence occurred ∼1 d (24 h) after radicle emergence. Wild-type Ler and Ws (data not shown) reached 100% radicle emergence within 2 d. Germination was considerably slower in sly1 mutants. The sly1-10 seeds required 5 d to reach 100% radicle emergence, whereas after-ripened sly1-2 seeds (#21) required 7 d to reach a final 85.6% radicle emergence.
Plating experiments using increasing concentrations of polyethylene glycol (PEG) 8000 were used to examine the effect of increasing media osmotic potential on sly1 mutant and wild-type seed germination. We have observed that when sly1-10 and sly1-2 seeds are sown directly on moistened soil, virtually no seeds germinated (data not shown). This observation suggested that the sly1 mutant seeds have difficulty imbibing water. To examine this, after-ripened wild-type and sly1 mutant seeds were sown on Murashige and Skoog (MS)–agar media containing various low concentrations of PEG 8000. This experiment showed that sly1 seeds were more sensitive to PEG 8000 than the wild type and that sly1-2 showed a more severe germination phenotype than sly1-10 (see Supplemental Figure 2 online). Next, the sensitivity of sly1 seeds to GA biosynthesis inhibitor paclobutrazol (PAC) was examined. After-ripened wild-type, sly1, and ga1-3 mutant seeds were first incubated in 100 μM PAC solution for 3 d at 4°C, and then these seeds were sown on different concentrations of GA3. This experiment showed that sly1 seeds were more sensitive to PAC than the wild type and that sly1-2 showed a more severe germination phenotype than sly1-10 (see Supplemental Figure 3 online).
A sly1-t2 rgl2-13 double mutant was constructed to determine the effect of the rgl2-13 mutation on the sly1 seed germination phenotype. Although the germination of double mutant seeds was somewhat slower than the wild type, the 1-month-old sly1-t2 rgl2-13 seeds reached 100% germination by day 4, whereas the 1-month-old sly1-t2 seeds crossed into the Columbia (Col) background never germinated (Figure 4C). Thus, it appears that the RGL2 mutation is sufficient to rescue sly1-t2 germination. Similar suppression of the sly1-2 germination phenotype was seen in a sly1-2 rgl2-13 double mutant (data not shown). This result suggests that the reduced germination in sly1 mutants is due to overaccumulation of RGL2 protein. This is consistent with data of Tyler et al. (2004) showing that RGL2 protein disappears within 5 h of GA treatment in ga1-3 seeds but not in sly1 mutant seeds.
RGL2 Does Not Disappear during sly1 Mutant Seed Germination
This section examines the hypothesis that the decreased germination capacity of GA mutants is due to increased RGL2 protein accumulation. The expression of RGL2 protein was examined over a time course of seed imbibition in wild-type, ga1-3, and sly1 mutant seeds to determine whether RGL2 disappears prior to wild-type seed germination and to determine whether RGL2 protein levels correlate with the severity of the sly1 seed germination phenotype (Figure 5). This experiment was conducted using polyclonal antibody to RGL2 that was found to be specific using double mutant analysis (Hussain et al., 2005; see Supplemental Figure 4 online)
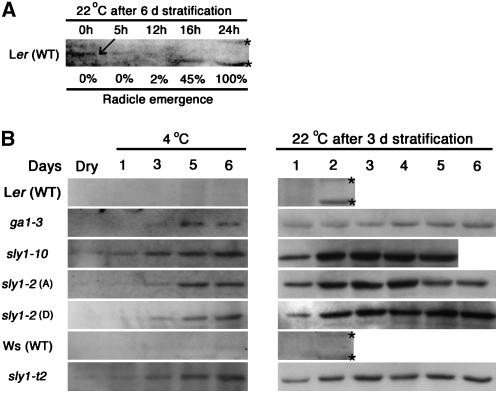
Figure 5.
Accumulation of RGL2 Protein during a Time Course of Seed Imbibition.
RGL2 protein was detected by protein gel blot analysis.
(A) Ler seeds were incubated for 6 d at 4°C and then time points were at indicated intervals after transfer to 22°C (blot exposure >30 min). The correlation between RGL2 protein accumulation and radicle emergence (%) was determined. RGL2 protein disappeared within 12 h, before radicle emergence.
(B) Time-course analysis of RGL2 protein in wild-type and mutant backgrounds. Protein gel blot analysis (exposure time 5 to 10 min) of protein isolated from seeds imbibed at 4°C for 1, 3, 5, and 6 d and from seeds first imbibed for 3 d at 4°C then transferred to 22°C for indicated number of days. Protein from dry seeds was also examined. Seed samples used were Ler (28 months old), ga1-3 (25 months old), sly1-10 (27 months old, #3), after-ripened (A) sly1-2 (26 months old, #21), dormant (D) sly1-2 (0.5 months old, #35), Ws (0.5 months old), and sly1-t2 (0.5 months old, #12). Asterisks indicate nonspecific bands.
It was expected that if RGL2 is a major negative regulator of seed germination, RGL2 protein should disappear well before wild-type radicle emergence. Following 6 d of imbibition at 4°C, Ler (wild-type) seeds were transferred to 22°C, and time points were taken for protein blot analysis using RGL2 antibody (Figure 5A; see Supplemental Figure 4C online). RGL2 protein was present at low levels immediately after transfer to 22°C, persisted at very low levels through 5 h of incubation, and disappeared by 12 h of incubation. At 12 h of incubation, only 2% of seeds showed radicle emergence. Thus, RGL2 protein disappeared well before completion of radicle emergence at 24 h incubation at 22°C. This is consistent with data from previous studies suggesting that RGL2 protein must disappear prior to wild-type seed germination (Tyler et al., 2004).
If the seed germination phenotype of GA mutants is due to overaccumulation of RGL2 protein, then we expect the severity of the germination phenotype to positively correlate with the level of RGL2 protein accumulation. To test this hypothesis, a time course was performed examining RGL2 protein accumulation during imbibition of wild-type, ga1-3, and sly1 mutant seeds (1) over 6 d of stratification at 4°C and (2) over 2 to 6 d of imbibition at 22°C following 3 d of stratification at 4°C (Figure 5B). In both Ler and Ws seeds, RGL2 protein accumulation was barely detected within 5 to 6 d of seed imbibition at 4°C (Figure 5; see Supplemental Figure 4C online). When wild-type seeds were transferred to 22°C following 3 d of stratification, RGL2 protein was not detected after 24 h of transfer. In the ga1-3 seeds, RGL2 protein was detected earlier and at higher levels than in Ler during stratification at 4°C and did not decrease during 6 d of incubation at 22°C. During this time, ga1-3 seeds did not germinate.
RGL2 protein accumulation in after-ripened sly1-10 (seed lot #3, 27 months old) and sly1-2 (seed lot #21, 26 months old) seeds known to show efficient seed germination were compared with dormant sly1-2 (seed lot #35, 0.5 months old) and sly1-t2 seeds (seed lot #12, 0.5 months old) (Figures 2 and 5B). The percentage of radicle emergence for each time point in this experiment can be found in Figure 4A. When sly1 mutant seeds were stratified at 4°C, the RGL2 protein gradually accumulated to high levels similar to the pattern of expression seen in ga1-3 (Figure 5B). Following transfer of seeds to 22°C, RGL2 protein accumulated at higher levels in sly1 than in ga1-3 mutants, regardless of allele, degree of seed dormancy, or seed lot (Figure 5B; see Supplemental Figures 4B and 4D online). Interestingly, although the sly1-10 seeds showed 73.7% radicle emergence after 3 d of incubation at 22°C, RGL2 protein levels remained high. Rather than decreasing as germination approached, RGL2 protein levels gradually increased, reaching a maximum at day 3, and remained high after 100% radicle emergence was reached at day 5 (Figures 4 and 5B). It was expected that since sly1-2 shows a more severe germination phenotype than sly1-10, the RGL2 protein levels would be higher than in sly1-10. However, the accumulation of RGL2 protein in the after-ripened sly1-2 seeds was similar to or less than that seen in sly1-10. It was expected that RGL2 protein would accumulate at higher levels in dormant sly1-2 seeds than after-ripened. However, almost no difference was seen between after-ripened and dormant sly1-2 through 4 d of incubation at 22°C. At 5 and 6 d of incubation at 22°C, slightly higher RGL2 levels were seen in dormant sly1-2 than in after-ripened sly1-2. At day 6, dormant sly1-2 seeds showed 7.4% radicle emergence, whereas after-ripened sly1-2 seeds showed 85.6%. Thus, higher germination levels may partly correlate with reduced RGL2 protein levels in after-ripened sly1-2 seeds. However, since after-ripened sly1-2 reached near-maximum radicle emergence (71.2%; Figure 4) after 4 d at 22°C, it does not appear that decreased RGL2 protein levels are required prior to radicle emergence in the sly1-2 or sly1-10 genotype. The sly1-t2 allele had the strongest seed germination phenotype, showing 0% germination at the conclusion of the experiment. Compared with wild-type Ws, the sly1-t2 allele also showed increased RGL2 protein accumulation. After the seeds were transferred at 22°C, the RGL2 protein persisted throughout the time course.
Next we examined the expression of another DELLA protein, RGA, during seed imbibition. In a double mutant study examining four of the five DELLA genes, mutations in RGA rescued the germination of ga1-3 seeds better than any DELLA mutation but rgl2 (Cao et al., 2005). When we examined RGA protein accumulation during seed germination, RGA protein showed a very similar expression pattern to RGL2 (see Supplemental Figure 5C online). The RGA protein accumulated at high levels after 6 d of stratification and did not disappear after transfer to 22°C in ga1-3 and sly1 mutant seeds. Somewhat higher protein accumulation was found in after-ripened sly1-2 than in dormant sly1-2 after 4 d at 22°C (see Supplemental Figure 5D online). These results indicate that at least two major DELLA proteins involved in seed germination do not disappear during sly1 seed germination.
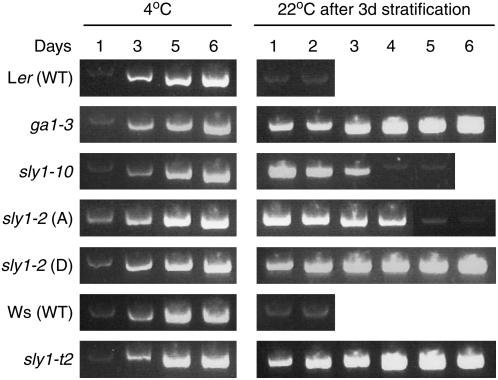
sly1 Mutations Cause Increased RGL2 mRNA Accumulation
The increased level of RGL2 protein in sly1 mutants could be either due to a requirement for SLY1 in triggering the disappearance of RGL2 protein or mRNA. It was expected that sly1 mutants act by stabilizing the RGL2 protein since SLY1 is the F-box subunit of an SCF E3 ubiquitin ligase required for DELLA protein disappearance (McGinnis et al., 2003; Dill et al., 2004; Fu et al., 2004). To determine if this is true, the effect of sly1 mutants on the accumulation of RGL2 mRNA was determined by RT-PCR analysis of the same seed lots and imbibition time points used to examine RGL2 protein expression in Figure 5B (Figure 6). Control RT-PCR reactions using the UBQ11 primers are shown in Supplemental Figure 6A online. As previously shown, the RGL2 mRNA levels gradually increased during imbibition of wild-type and ga1-3 mutant seeds over 6 d at 4°C (Lee et al., 2002). A similar pattern of expression was seen during cold imbibition of sly1-10, sly1-t2, and dormant and after-ripened sly1-2 seeds. Next, RGL2 mRNA expression was examined following transfer to 22°C. RGL2 mRNA levels decreased within 1 d (24 h) of transfer to 22°C in Ler and Ws seeds, the time point corresponding to 72.5 and 81.6% radicle emergence, respectively. RGL2 mRNA levels never decreased during imbibition of ga1-3 seeds that failed to germinate.
Figure 6.
RGL2 mRNA Accumulation during a Time Course of Seed Imbibition for Wild-Type, ga1-3, and sly1 Mutant Seeds.
RT-PCR analysis of RGL2 mRNA expression in seeds imbibing using the same time courses in Figure 6 of stratified (4°C for 1, 3, 5, and 6 d) seeds and seeds imbibing at 22°C after 3 d of stratification at 4°C. Seed samples used were identical to Figure 6 and include Ler (28 months old), ga1-3 (25 months old), sly1-10 (27 months old, #3), after-ripened (A) sly1-2 (26 months old, #21), dormant (D) sly1-2 (0.5 months old, #35), Ws (0.5 months old), and sly1-t2 (0.5 months old, #12).
In sly1 mutants, RGL2 mRNA levels appeared to decrease after radicle emergence. RGL2 mRNA levels decreased after 4 d of imbibition at 22°C in sly1-10 and after 5 d of imbibition in after-ripened sly1-2 seeds. These time points corresponded to 90.3 and 81.6% radicle emergence, respectively. Like ga1-3, the RGL2 mRNA levels remained high through 6 d of imbibition of dormant sly1-2 and sly1-t2 seeds showing 9.4 and 0% final radicle emergence, respectively (Figure 4). The decline in RGL2 mRNA levels during this time course occurred prior to the decrease in RGL2 protein levels (Figure 5B). In sly1-10, RGL2 mRNA levels decreased after 4 d of imbibition at 22°C, while RGL2 protein levels remained high at least through 5 d of imbibition. In after-ripened sly1-2 seeds, RGL2 mRNA levels decreased after 5 d of imbibition at 22°C, whereas RGL2 protein levels remained high through 6 d of imbibition. This suggests that the high levels of RGL2 protein accumulation following seed germination of sly1-10 and sly1-2 seeds are due at least in part to loss of SCFSLY1-stimulated proteolysis of RGL2 protein.
GA Partially Rescues sly1 Germination without Causing Reduced RGL2 Protein Levels
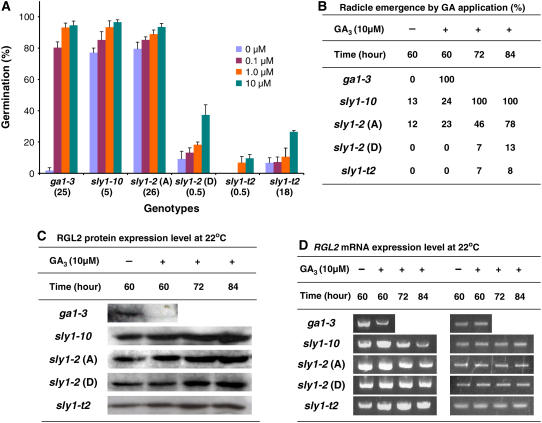
The current model is that GA stimulates germination via degradation of DELLA proteins, including RGL2, RGA, and GAI (Dill et al., 2004; Fu et al., 2004; Tyler et al., 2004). The strongest evidence of this is that RGL2 protein disappears within 5 h of adding GA to imbibing ga1-3 seeds but not sly1 mutant seeds. This result raised the question of whether GA treatment can stimulate sly1 mutant seed germination and the disappearance of RGL2 protein over a time course of imbibition. Previous work showed that GA treatment did not cause an increase in sly1-2 hypocotyl elongation (Steber and McCourt, 2001). As shown in Figure 7A, GA treatment increased the percentage of germination of all sly1 alleles tested, even dormant sly1-2 and sly1-t2. Improvement of the germination was dependent on the concentration of GA applied, and the best recovery was found at 10 μM GA3 (Figure 7A). Next, we examined the RGL2 protein levels after GA treatment of the same seed lots used for the germination experiments. Seeds were plated either in the absence or presence of 10 μM GA3 and protein extracts prepared after the indicated incubation at 22°C after 3 d of stratification at 4°C. As expected, RGL2 protein disappeared after 60 h of incubation on GA in ga1-3 but not in the sly1 mutants (Figures 7B and 7C). Further time points showed that RGL2 protein levels did not decrease in sly1 mutants after 84 h of incubation on GA. When RGL2 mRNA levels were examined by RT-PCR analysis, the apparent level of RGL2 mRNA remained constant in dormant sly1-2 and sly1-t2 and decreased slightly after 84 h of incubation in the presence of GA in after-ripened sly1-2 and sly1-10 (Figure 7D). Overall, these results suggest that GA treatment improves seed germination without reducing RGL2 protein levels in sly1-10, sly1-2, and sly1-t2 backgrounds and suggests the existence of an RGL2-independent pathway for stimulating seed germination.
Figure 7.
GA Partially Rescued sly1 Germination without Reducing RGL2 Protein Levels.
(A) Percentage of germination on indicated GA3 concentration in ga1-3 biosynthesis mutant (25 months old), sly1-10 (5 months old, #13), after-ripened (A) sly1-2 (26 months old, #21), dormant (D) sly1-2 (0.5 months old, #35), and sly1-t2 (0.5 months old, #12; 18 months old, #2). Germination was calculated based on cotyledon appearance. Age of the stock is shown below the line (months). Error bars show se (n = 30 to 60).
(B) Percentage of radicle emergence determined during time-course experiment examining RGL2 protein accumulation in the presence of 10 μM GA3 using the same seed lots used in (A) except for 0.5-month-old sly1-t2. Radicle emergence was determined at 60, 72, and 84 h at 22°C following 3 d of 4°C.
(C) RGL2 protein expression in the absence and presence of 10 μM GA3. Protein was extracted from imbibing seeds at indicated time points at 22°C following 3 d at 4°C.
(D) RGL2 mRNA accumulation in the absence and presence of GA3 during the same time-course experiment in (C). Left panel, RGL2 mRNA; right panel, ACTIN2 mRNA.
GA-Regulated Genes Show Altered Expression in sly1 Seeds
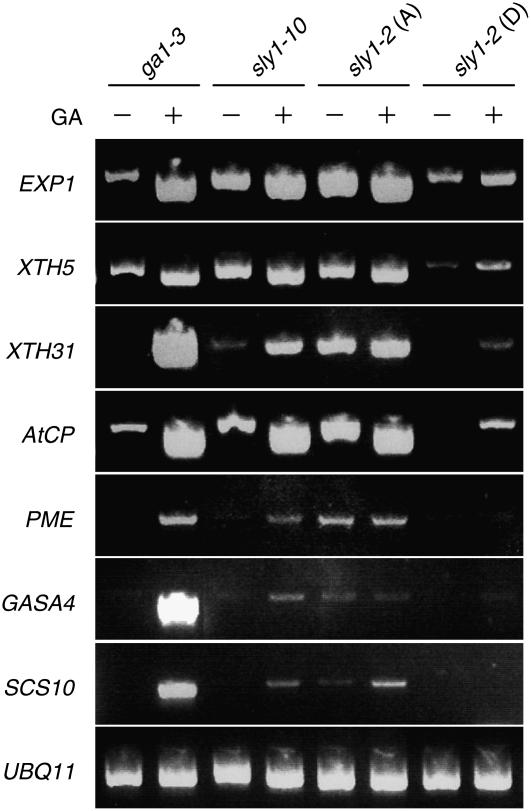
The fact that sly1 mutant seeds can germinate in the presence of high levels of RGL2 protein suggested that RGL2 protein disappearance may not be required to derepress GA-dependent gene expression in the sly1 mutant background. To investigate this notion, the mRNA levels of GA-regulated genes were examined during the imbibition of sly1 seeds and ga1-3 after 60 h of incubation at 4°C in water followed by 24 h of imbibition at 22°C on MS-agar and then 12 h of imbibition at 22°C in the presence and absence of GA3. At this time, none of the seeds had germinated. We examined seven transcripts showing GA induction in ga1-3 based on previous reports (Herzog et al., 1995; Ogawa et al., 2003; Yamauchi et al., 2004; Cao et al., 2006). The mRNA accumulation in these seven genes was upregulated after GA was applied to ga1-3 seeds (Figure 8). Dormant sly1-2 seeds show reduced accumulation of GA-induced genes in the presence and sometimes in the absence of GA compared with ga1-3. This inability to induce GA-regulated genes is consistent with a GA-insensitive phenotype. Interestingly, the expression of many GA-induced genes is increased in after-ripened sly1-2 seeds compared with dormant seeds both in the presence and absence of exogenous GA. After-ripened seeds of the weaker sly1-10 allele did not appear to have as strong a GA-insensitive phenotype as sly1-2 since some GA-regulated transcripts showed GA-induced expression. In after-ripened seeds, four transcripts, α-EXPANSIN1 (EXP1), XYLOGLUCAN ENDOTRANSGLYCOSYLASE5 (XTH5), XTH31, and CYSTEIN PROTEINASE (CP), showed increased expression in the absence of GA in after-ripened sly1-2 and sly1-10 compared with ga1-3. Three transcripts, PECTIN METHYLESTERASE (PME), GIBBERELLIC ACID STIMULATED ARABIDOPSIS4 (GASA4), and SERINE CARBOXYPEPTIDASE S10 FAMILY PROTEIN (SCS10), showed higher expression in after-ripened sly1-2 than in ga1-3 in the absence of GA and showed lower accumulation in the presence of GA. These patterns of expression are consistent with the notion that the RGL2 protein accumulating in after-ripened sly1 seeds is not fully active as a repressor of GA-regulated gene expression.
Figure 8.
Effect of sly1 Mutations on Accumulation of GA-Regulated Transcripts in Imbibing Seeds.
mRNA accumulation of seven GA-induced genes was determined by RT-PCR. mRNA was isolated from ungerminated imbibing seeds.
DISCUSSION
Mutations in SLY1 Result in Allele-Dependent Defects in Seed Germination
The data presented here provide clear evidence that sly1 mutations cause increased seed dormancy, delayed after-ripening, and decreased germination potential (Figures 2 to 5). Even sly1 mutant seed lots showing 100% seed germination germinate more slowly and have increased sensitivity to inhibition of germination by ABA, PEG 8000, and PAC (Strader et al., 2004; Figure 4; see Supplemental Figures 2 and 3 online). In addition, alleles that caused loss of more of the SLY1 protein sequence also caused a more severe seed germination defect (Figures 2 to 4; see Supplemental Figures 2 and 3 online). For example, the sly1-2 mutation resulting in loss of the C-terminal 40 amino acids showed stronger seed dormancy and required more time to after-ripen than the sly1-10 mutation, resulting in loss of the last eight amino acids of the protein (Figures 1 and 2). These results also are consistent with the fact that sly1-2 shows more severe dwarfism and reduced fertility compared with sly1-10 (McGinnis et al., 2003; Dill et al., 2004; see Supplemental Table 1 online). The new sly1-t2 allele exhibited the strongest seed dormancy of the three alleles, suggesting that sly1-t2 may be a null allele. However, a direct comparison is not possible because different ecotypes show differences in seed dormancy (Debeaujon and Koornneef, 2000). Tetrazolium staining and after-ripening experiments showed that dormant sly1-2 seeds are viable. However, sly1-2 seeds stored for ∼5 years show reduced viability, whereas Ler wild-type seeds of the same age show no decrease in germination or viability (Figure 3). The sly1-2 and sly1-10 seeds showed variability in the degree of seed coat–imposed dormancy (Figure 2). This variability is most likely due to environmental effects, possibly through differences in accumulation and sensitivity to other plant hormones, such as brassinosteroid, ethylene, and ABA (Koornneef and Karssen, 1994; Steber and McCourt, 2001; Okamoto et al., 2006). Both the sly1-2 and sly1-10 mutations result in increased sensitivity to inhibition of seed germination by ABA (Strader et al., 2004). Thus, it is possible that the variability in the sly1 germination phenotype is due to differences in ABA levels or signaling. It is interesting to note that transcript levels of GA-induced genes were lower in imbibing dormant sly1-2 seeds than in after-ripened sly1-2 seeds (Figure 8). It is possible that dormancy somehow represses GA-induced genes in sly1-2 seeds. This suggests that SLY1 contributes to, but is not essential for, the derepression of these genes during after-ripening.
The fact that sly1 mutants show increased sensitivity to PEG 8000 inhibition of seed germination compared with the wild type does suggest that sly1 mutations cause altered permeability to water. While light and transmission electron microscopy revealed no obvious morphological differences in the seed coat structure of wild-type and sly1 seeds (K.M. McGinnis and C.M. Steber, unpublished data), we cannot exclude the possibility that changes in the seed coat are involved. Many of the GA-induced genes that show decreased expression in dormant sly1-2 seeds, such as EXP1, XTH5, XTH31, and PME, are involved in cell wall loosening and may help to release the testa or endosperm barriers during seed germination (Ogawa et al., 2003; Yamauchi et al., 2004). Thus, it is possible that the sly1 mutations cause increased seed dormancy in part through effects on seed coat loosening.
Mechanisms Underlying the sly1 Mutant Seed Germination Phenotype
Analysis of RGL2 mRNA accumulation in sly1 mutants showed that the SLY1 gene is required for GA-regulated disappearance of RGL2 mRNA. Previous studies demonstrated that (1) RGL2 mRNA levels increase during cold imbibition of wild-type and ga1-3 seeds, (2) RGL2 mRNA levels decrease in wild-type seeds imbibed at 23°C during radicle emergence, (3) RGL2 mRNA levels persist at high levels in ga1-3 seeds imbibing at 23°C, and (4) GA treatment of ga1-3 seeds stimulates germination and triggers reduced accumulation of RGL2 mRNA (Lee et al., 2002; Bassel et al., 2004). Bassel et al. (2004) showed that RGL2 mRNA did not fully disappear prior to radicle emergence, indicating that RGL2 mRNA disappearance is not required for seed germination. This work further suggested that RGL2 activity might be controlled by multiple mechanisms. Here, we demonstrated that RGL2 mRNA overaccumulated in both dormant and after-ripened sly1 seeds imbibed at 22°C in a manner similar to that seen in ga1-3 seeds (Figure 6). Unlike ga1-3 seeds, however, sly1 seeds showed no significant decrease in RGL2 mRNA levels after 60 h of GA treatment (Figure 7), suggesting that SLY1 is required for GA-stimulated disappearance of RGL2 mRNA. RGL2 mRNA accumulation did decrease after germination of sly1-10 and after-ripened sly1-2 seeds (Figures 6 and 7). This suggests that RGL2 mRNA accumulation is subject to SLY1-independent downregulation after seed germination. RGL2 mRNA decreased markedly when wild-type Ler reached 72.5% radicle emergence (day 1), whereas RGL2 transcript levels remained quite high when sly1-10 (73.7%) and sly1-2 (71.2%) showed comparable radicle emergence at 3 and 4 d, respectively (Figures 4 and 6). Thus, RGL2 mRNA appears to be downregulated during seedling establishment in sly1 mutants.
Previous studies postulated that GA stimulates seed germination by causing the SCFSLY1 complex to trigger the proteolysis of the RGL2 protein via ubiquitination (Tyler et al., 2004). This model predicted that the degree of seed dormancy should directly correlate with the level of RGL2 protein in the seeds. The sly1 mutants that showed variable seed dormancy and that required a long period of after-ripening provided an excellent tool for examining this prediction. This study showed that sly1 mutants accumulate high levels of RGL2 and RGA protein during imbibition regardless of the sly1 allele or the dormancy status of the seed (Figure 5B; see Supplemental Figure 5 online). What this result does show is that the degree of sly1 dormancy is due to factors other than the absolute level of RGL2 protein accumulation. Since mutations in RGL2 rescue the increased dormancy and reduced germination capacity of dormant sly1 mutant seeds, it appears that these phenotypes are due to RGL2 overaccumulation. The increased germination of sly1 seeds due to after-ripening may be due to inactivation of RGL2 or to competition with parallel pathways. Based on previous work, we considered the possibility that other E3 ubiquitin ligases, such as the SLY1 homolog SNEEZY and the PHOR1 U-box E3 ubiquitin ligase, could replace SLY1 in GA signaling and lead to SLY1-independent fluctuations in RGL2 levels during seed germination (Monte et al., 2003; Fu et al., 2004; Strader et al., 2004). No such fluctuations were seen, suggesting that SCFSLY1 is the major E3 ubiquitin ligase transmitting the GA signal under the imbibition conditions used. RGL2 protein does not disappear in GA-treated sly1 mutant seeds, but GA,treatment does somewhat stimulate seed germination (Figure 7). This suggests that GA may stimulate sly1 mutant seed germination via an RGL2-independent pathway, either through a gene that stimulates seed germination or a gene that controls RGL2 activity (Figure 9A). This notion that there is an RGL2-independent mechanism for GA regulation of seed germination is consistent with a recent microarray study showing that there are GA-regulated genes with DELLA-independent expression (Cao et al., 2006).
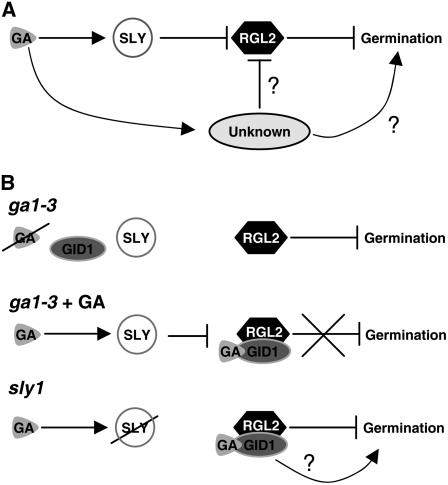
Figure 9.
Model for Control of Seed Germination by SLY1 and RGL2.
(A) There is an alternative pathway controlling seed germination that is stimulated by GA. This pathway may bypass the sly1 mutation either by inhibiting RGL2 activity without causing decreased RGL2 protein levels possibly by posttranslational modification or by activating seed germination independently of SLY1/RGL2.
(B) In the ga1-3 mutant (no GA), RGL2 does not interact with the GA receptor GID1. In this case, RGL2 functions purely as a negative regulator. Addition of GA rescues ga1-3 seed germination by causing RGL2 to be destroyed following interaction with GA-GID1 and SCFSLY1. In the sly1 mutant (GA present), RGL2 cannot be destroyed because SLY1 is defective. In this case, RGL2 acts as a repressor of GA responses when not bound to GID1, and GA-GID1-RGL2 may be inactive as a repressor or act as a positive regulator of seed germination.
Mechanisms Controlling RGL2 Function
The fact that loss-of-function mutations in DELLA genes rescue the defects of the ga1-3 GA biosynthesis mutant forms the genetic basis for the model that DELLA proteins act as negative regulators of GA signaling and that GA acts via negative regulation of DELLA proteins (Dill and Sun, 2001; King et al., 2001; Cheng et al., 2004). Previous work has also shown that DELLA proteins disappear in response to GA and that this disappearance requires the SCFSLY1 E3 ubiquitin ligase (Silverstone et al., 2001; McGinnis et al., 2003; Dill et al., 2004; Fu et al., 2004; Tyler et al., 2004). Prior to this study, it was known that GA treatment of ga1-3 seeds causes RGL2 protein to disappear prior to the disappearance of RGL2 mRNA (Tyler et al., 2004). These data together with the evidence that RGL2 persists at high levels after GA treatment of sly1 mutants are evidence that the RGL2 DELLA protein is also subject to GA-stimulated destruction via the SCFSLY1 and ubiquitin-proteasome pathway (Figure 5).
This article and others have revealed that RGL2 function in seed germination must be regulated at multiple levels, including transcript and protein accumulation (Lee et al., 2002; Bassel et al., 2004; Tyler et al., 2004; Cao et al., 2005; Hussain et al., 2005; Figures 5 to 7). This study suggests that RGL2 functions as a repressor of seed germination in dormant sly1 seeds and that the RGL2 repressor is deactivated by after-ripening either due to effects of dry storage itself or due to posttranslational regulation. Transcript levels of GA-induced genes are higher in imbibing after-ripened sly1 seeds than in ga1-3 seeds in the absence of GA (Figure 8). These data suggest that the RGL2 protein accumulating in after-ripened sly1 mutant seeds is either less active as a repressor of GA-induced genes or is competing with a transcriptional activator of GA-induced genes (Figure 9A). One possibility is that RGL2 activity is controlled by posttranslational regulation. It appears that RGL2 is phosphorylated and that phosphorylation stabilizes the protein (Hussain et al., 2005). Possibly a kinase either activates or inactivates the RGL2 repressor (Figure 9A). It is also known that the SPINDLY O-Glc-NAc transferase acts as a negative regulator of GA signaling during seed germination (Jacobsen and Olszewski, 1993). It is possible that O-Glc-NAc modification is needed for RGL2 to act as a repressor and that the RGL2 protein accumulating in sly1 mutants is incompletely modified. Accumulation of unmodified RGL2 in sly1 mutants may be due to overaccumulation of the protein or to indirect effects of the sly1 mutation. Alternatively, RGL2 activity may be regulated by protein–protein interaction. For example, RGL2 alone may act as a negative regulator of GA signaling, while RGL2 bound to the GA receptor GID1 may act as a positive regulator of GA signaling (Figure 9B). Positive regulation of GA signaling by GA-GID1-RGL2 in sly1 mutants may compete with negative regulation by RGL2, leading to an intermediate germination phenotype in spite of the fact that RGL2 is present at high levels. This may also account for the fact that the sly1 phenotypes in plant height and in fertility are less severe than those of ga1-3 (Steber et al., 1998).
METHODS
Plant Materials and Growth Conditions
Seeds of wild-type Arabidopsis thaliana (accessions Ler and/or Ws), sly1-10, sly1-2, sly1-DS, and sly1-t2 were cultivated according to McGinnis et al. (2003). The sly1-t2 line was isolated by searching the Sussman Basta lines by PCR using the T-DNA left border primer JL202 (5′-CATTTTATAATAACGCTGCGGACATCTAC-3′) and SLY1-specific primer sly1T-DNAr (5′-ACTTTCCGATTCACGATTGGTCCAGGTGA-3′) to obtain a 2.0-kb product (L. Strader and C.M. Steber, unpublished data; Krysan et al., 1999). Homozygous mutant plants were identified by PCR using the primers above and the SLY1-specific primers 2-62f (5′-AAGGCATCTGAGAAACCC-3′) (2.1-kb product) and sly1T-DNAr that do not amplify the large T-DNA insertion in sly1-t2. The sly1-DS line was obtained from ABRC (stock No. CS101918; Sundaresan et al., 1995). The sly1-t2 rgl2-13 double mutant was made by crossing the sly1-t2 allele in the Ws ecotype to rgl2-13 in the Col ecotype. For comparison in germination experiments, we also crossed sly1-t2 once to Col wild type. Failure to germinate was seen in all independent isolates of sly1-t2 recovered from the cross to Col (data not shown).
Germination Experiments
For germination experiments, 30 to 60 seeds were sterilized with 10% bleach for 15 to 20 min and plated per repetition on 0.5× MS salts (Sigma-Aldrich)/0.8% agar (MS-agar). Percentage of germination was determined following 3 d of incubation at 4°C followed by 7 d of incubation under constant fluorescent light at 22°C. The average germination rate was calculated using three independent experiments. After harvest, seeds were stored in open tubes for 2 weeks at room temperature and then stored at room temperature in closed tubes in a dessication cabinet. For viability tests, seeds were soaked in 0.5% 2,3,5-triphenyltetrazolium choloride (Sigma-Aldrich) solution at 37°C in darkness for 10 h. Fully red-stained seeds were counted as viable (Debeaujon et al., 2000). For preparation of the PEG 8000–supplemented media, a sterile 40% stock solution of PEG 8000 was prepared and then mixed into the MS-agar media to give 4, 6, and 8% PEG 8000 plates. Seeds from homozygous sly1-10, after-ripened sly1-2, and wild-type Ler were plated on this PEG-containing media. After 3 d of incubation at 4°C, they were transferred to 22°C under constant light, and the germination rate was determined over 7 d. For experiments in which GA was applied, seeds from homozygous ga1-3, sly1-10, after-ripened sly1-2, dormant sly1-2, sly1-t2, and the wild type (Ler and Ws) of similar age were used. Seeds were sown on MS-agar plates containing 0, 0.1, 1.0, and 10 μM of GA3. After 3 d of incubation at 4°C, they were transferred to 22°C under constant light, and germination rate was determined at 60, 72, and 84 h. For PAC experiments, homozygous ga1-3, sly1-10, after-ripened sly1-2, and the wild type (Ler) were used. First, these seeds were incubated in 100 μM PAC solution at 4°C for 3 d, washed five times with distilled water, and sown on MS-agar plates containing 0, 0.1, 1.0, and 10 μM of GA3. The MS-agar plates were then incubated at 22°C under constant light, and germination rate was determined after 7 d.
RT-PCR Analysis of mRNA Expression
RT-PCR analysis was used to analyze the accumulation of SLY1 mRNA expression in different mutant alleles. Total RNA was isolated from flower buds using an RNA easy kit (Qiagen), and genomic DNA contamination was removed using the DNA-Free RNA kit (ZYMO Research). cDNA was generated from 5 μg total RNA using a first-strand cDNA synthesis kit (GE Healthcare). cDNA was diluted and then used as a template for PCR amplification with the SLY1-2f (5′-CTCCGTCTGATGGTTTGCTT-3′) and SLY1-2r (5′-CCTCTCAGAGAATTTCTCACGC-3′) primers to obtain a 147-bp product and with the 2-63f and sly1-r primers to obtain a 213-bp product. SLY1-2f and SLY1-2r primers detect mRNA in sly1-10, sly1-2, and the wild type but not in sly1-t2 because they flank the T-DNA insertion. 2-63f and sly-r primers detect transcript in sly1-t2, sly1-2, and the wild type but not in sly1-10 because these primers flank the DNA rearrangement in this allele. ACTIN2 was amplified as a loading control using actin2-F (5′-CTGGATTCTGGTGATGGTGTGTC-3′) and actin2-R (5′-TCTTTGCTCATACGGTCAGCG-3′) primers (An et al., 1996). PCR was performed with Takara Ex Taq polymerase (Takara Shuzo) for 35 cycles of denaturation for 30 s at 94°C, annealing for 30 s at 59°C, and extension for 1 min at 72°C, followed by final extension for 5 min at 72°C. PCR reaction for ACTIN2 amplification was performed for 23 cycles.
RGL2 mRNA accumulation was analyzed by RT-PCR using total RNA extracted from whole seeds imbibed as indicated in Figure 7 using a phenol-sodium dodecyl sulfate extraction method (Sambrook et al., 1989). Genomic DNA contamination was removed, and cDNA strand synthesis was performed as described above. To determine the transcript level of RGL2, PCR was performed on cDNA using RGL2int-F (5′-CACCAGGAGAATCTCAACTTAG-3′) and RGL2orf3R (5′-GGCGAGTTTCCACGCCGAGG-3′) primers. The ubiquitin gene was amplified as a constitutive control as described by Tyler et al. (2004). Primer specificity was confirmed using the rgl2-13 mutant (see Supplemental Figure 6B online). To determine the transcript level of the seven GA-regulated genes, PCR was performed on cDNA using the specific primers (see Supplemental Table 2 online). cDNA synthesis was performed on mRNA extracted from seeds imbibed first for 60 h at 4°C in water followed by 24 h at 22°C on MS-agar plates, and then seeds were incubated for 12 h at 22°C on MS-agar plates in the presence and absence of 10 μM GA3. PCR reactions were performed with Mango-Taq DNA polymerase (Bioline) for 30 cycles of denaturation for 1 min at 94°C, annealing for 1 min at 60°C, and extension for 1 min at 72°C, followed by final extension for 5 min at 72°C.
Protein Gel Blot Analysis
Total protein from whole seeds was extracted in extraction buffer (phosphate buffer with 1× protease inhibitor cocktail [Roche]), and 60 μg protein was separated on an 8% SDS-PAGE and transferred onto a polyvinylidene difluoride membrane. The protein concentration was determined using Bradford reagent (Bio-Rad protein assay; Bio-Rad Laboratories). Time points for RGL2 protein extraction were taken at 24-h intervals during imbibition as indicated in Figures 5 and 7. Protein detection was performed using an enhanced chemiluminescence system (ECL; GE Healthcare) according to the manufacturer's protocol. The polyclonal RGL2 antibody (Hussain et al., 2005) was used as primary antibody (1:20,000). The anti-rabbit IgG-horseradish peroxidase (GE Healthcare) was used as a secondary antibody (1:200,000). The specificity of the RGL2 antibody was confirmed by determining that the 60.5-kD RGL2 band was absent in the sly1-2 rgl2-13 double mutant after 24 h of imbibition at 22°C (see Supplemental Figures 4A and 4B online). To detect RGA protein, polyclonal SLR1 antibody (Itoh et al., 2002) was used as primary antibody (1:15,000). The same secondary antibody was used as described above. The specificity of the SLR1 antibody was confirmed by determining that the 64.0-kD RGA band was absent in the ga1-3 rgat-2 and sly1-2 rga24 double mutants after 24 h of imbibition at 22°C (see Supplemental Figures 5A and 5B online). Time points for RGA protein extraction were taken at 24-h intervals during imbibition as indicated (see Supplemental Figure 5D online).
Accession Numbers
Arabidopsis Genome Initiative locus identifiers for the genes mentioned in this article are as follows: SLY1 (At4g24210), RGL2 (At3g03450), EXP1 (At1g69530), XTH5 or EXGT-A4 (At5g13870), XTH31 (At3g44990), At CP1 (At4g36880), PME (At3g10720), GASA4 (At5g15230), and SCS10 (At2g27920).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table 1. Phenotypes of Different sly1 Mutant Alleles.
Supplemental Table 2. Primer Sequences of GA-Regulated Genes for RT-PCR.
Supplemental Figure 1. Time-Course After-ripening Experiment in Dormant sly1-2.
Supplemental Figure 2. sly1 Mutant Seed Germination Is More Sensitive to PEG 8000.
Supplemental Figure 3. sly1 Mutant Seed Germination Is More Sensitive to PAC.
Supplemental Figure 4. RGL2 Protein Detection by Protein Gel Blot Analysis.
Supplemental Figure 5. RGA Protein Detection by Protein Gel Blot Analysis.
Supplemental Figure 6. Ubiquitin Control RT-PCR Reaction and Confirmation of Amplification in RGL2 in Different Arabidopsis Ecotypes.
Supplementary Material
Acknowledgments
We thank J. Peng and A. Hussain for kindly providing the RGL2 antibody and ga1-3 rga-t2 double mutant. We are grateful to M. Matsuoka and T.-p. Sun for providing the SLR antibody and rgl2-13 mutant allele, respectively. We thank H. Nonogaki and members of the Steber lab for helpful suggestions and comments on the manuscript. This work was supported by a Japanese Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad to T.A. and by USDA–Agricultural Research Service funds and USDA National Research Initiative 2002-01351 to C.M.S.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Camille M. Steber (csteber@wsu.edu).
Online version contains Web-only data.
References
- Alonso-Blanco, C., Bentsink, L., Hanhart, C.J., Blankestijn-de Vries, H., and Koornneef, M. (2003). Analysis of natural allelic variation at seed dormancy loci of Arabidopsis thaliana. Genetics 164 711–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, Y.Q., McDowell, J.M., Huang, S., McKinney, E.C., Chambliss, S., and Meagher, R.B. (1996). Strong, constitutive expression of the Arabidopsis ACT2/ACT8 actin subclass in vegetative tissues. Plant J. 10 107–121. [DOI] [PubMed] [Google Scholar]
- Bassel, G.W., Zielinska, E., Mullen, R.T., and Bewley, J.D. (2004). Down-regulation of DELLA genes is not essential for germination in tomato, soybean, and Arabidopsis seeds. Plant Physiol. 136 2782–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley, J.D. (1997). Seed germination and dormancy. Plant Cell 9 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley, J.D., and Black, M. (1994). Seeds: Physiology of Development and Germination. (New York: Plenum Press).
- Cao, D., Cheng, H., Wu, W., Meng Soo, H., and Peng, J. (2006). Gibberellin mobilizes distinct DELLA-dependent transcriptomes to regulate seed germination and floral development in Arabidopsis. Plant Physiol. 142 509–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, D., Hussain, A., Cheng, H., and Peng, J. (2005). Loss of function of four DELLA genes leads to light- and gibberellin-independent seed germination in Arabidopsis. Planta 223 105–113. [DOI] [PubMed] [Google Scholar]
- Cheng, H., Qin, L., Lee, S., Fu, X., Richards, D.E., Cao, D., Luo, D., Harberd, N.P., and Peng, J. (2004). Gibberellin regulates Arabidopsis floral development via suppression of DELLA protein function. Development 131 1055–1064. [DOI] [PubMed] [Google Scholar]
- Debeaujon, I., and Koornneef, M. (2000). Gibberellin requirement for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiol. 122 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeaujon, I., Leon-Kloosterziel, K.M., and Koornneef, M. (2000). Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol. 122 403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill, A., and Sun, T. (2001). Synergistic derepression of gibberellin signaling by removing RGA and GAI function in Arabidopsis thaliana. Genetics 159 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill, A., Thomas, S.G., Hu, J., Steber, C.M., and Sun, T.P. (2004). The Arabidopsis F-box protein SLEEPY1 targets gibberellin signaling repressors for gibberellin-induced degradation. Plant Cell 16 1392–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein, R.R., and Rock, C.D. (September 30, 2002). Abscisic acid biosynthesis and response. In The Arabidopsis Book, C.R. Somerville and E.M. Meyerowitz, eds (Rockville, MD: American Society of Plant Biologists), doi/10.1199/tab.0058, http://www.aspb.org/publications/arabidopsis/. [DOI] [PMC free article] [PubMed]
- Footitt, S., Slocombe, S.P., Larner, V., Kurup, S., Wu, Y., Larson, T., Graham, I., Baker, A., and Holdsworth, M. (2002). Control of germination and lipid mobilization by COMATOSE, the Arabidopsis homologue of human ALDP. EMBO J. 21 2912–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, X., Richards, D.E., Fleck, B., Xie, D., Burton, N., and Harberd, N.P. (2004). The Arabidopsis mutant sleepy1gar2-1 protein promotes plant growth by increasing the affinity of the SCFSLY1 E3 ubiquitin ligase for DELLA protein substrates. Plant Cell 16 1406–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths, J., Murase, K., Rieu, I., Zentella, R., Zhang, Z.L., Powers, S.J., Gong, F., Phillips, A.L., Hedden, P., Sun, T.P., and Thomas, S.G. (2006). Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 18 3399–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartweck, L.M., and Olszewski, N.E. (2006). Rice GIBBERELLIN INSENSITIVE DWARF1 is a gibberellin receptor that illuminates and raises questions about GA signaling. Plant Cell 18 278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog, M., Dorne, A.M., and Grellet, F. (1995). GASA, a gibberellin-regulated gene family from Arabidopsis thaliana related to the tomato GAST1 gene. Plant Mol. Biol. 27 743–752. [DOI] [PubMed] [Google Scholar]
- Hussain, A., Cao, D., Cheng, H., Wen, Z., and Peng, J. (2005). Identification of the conserved serine/threonine residues important for gibberellin sensitivity of Arabidopsis RGL2 protein. Plant J. 44 88–99. [DOI] [PubMed] [Google Scholar]
- Itoh, H., Ueguchi-Tanaka, M., Sato, Y., Ashikari, M., and Matsuoka, M. (2002). The gibberellin signaling pathway is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. Plant Cell 14 57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen, S.E., and Olszewski, N.E. (1993). Mutations at the SPINDLY locus of Arabidopsis alter gibberellin signal transduction. Plant Cell 5 887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith, K., Kraml, M., Dengler, N.G., and McCourt, P. (1994). fusca3: A heterochronic mutation affecting late embryo development in Arabidopsis. Plant Cell 6 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, K.E., Moritz, T., and Harberd, N.P. (2001). Gibberellins are not required for normal stem growth in Arabidopsis thaliana in the absence of GAI and RGA. Genetics 159 767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef, M., and Karssen, C.M. (1994). Seed dormancy and germination. In Arabidopsis, C.R. Somerville and E.M. Meyerowitz, eds (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press), pp. 313–334.
- Krysan, P.J., Young, J.C., and Sussman, M.R. (1999). T-DNA as an insertional mutagen in Arabidopsis. Plant Cell 11 2283–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushiro, T., Okamoto, M., Nakabayashi, K., Yamagishi, K., Kitamura, S., Asami, T., Hirai, N., Koshiba, T., Kamiya, Y., and Nambara, E. (2004). The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: Key enzymes in ABA catabolism. EMBO J. 23 1647–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S., Cheng, H., King, K.E., Wang, W., He, Y., Hussain, A., Lo, J., Harberd, N.P., and Peng, J. (2002). Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes Dev. 16 646–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis, K.M., Thomas, S.G., Soule, J.D., Strader, L.C., Zale, J.M., Sun, T.P., and Steber, C.M. (2003). The Arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase. Plant Cell 15 1120–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte, E., Amador, V., Russo, E., Martínez-García, J., and Prat, S. (2003). PHOR1: A U-Box GA signaling component with a role in proteasome degradation? J. Plant Growth Regul. 22 152–162. [Google Scholar]
- Nakajima, M., et al. (2006). Identification and characterization of Arabidopsis gibberellin receptors. Plant J. 46 880–889. [DOI] [PubMed] [Google Scholar]
- Nambara, E., Keith, K., McCourt, P., and Naito, S. (1995). A regulatory role for the ABI3 gene in the establishment of embryo maturation in Arabidopsis thaliana. Development 121 629–636. [Google Scholar]
- Ogawa, M., Hanada, A., Yamauchi, Y., Kuwahara, A., Kamiya, Y., and Yamaguchi, S. (2003). Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 15 1591–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto, M., Kuwahara, A., Seo, M., Kushiro, T., Asami, T., Hirai, N., Kamiya, Y., Koshiba, T., and Nambara, E. (2006). CYP707A1 and CYP707A2, which encode abscisic acid 8′-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol. 141 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski, N., Sun, T.P., and Gubler, F. (2002). Gibberellin signaling: Biosynthesis, catabolism, and response pathways. Plant Cell 14 (suppl.): S61–S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms, J.J.J., Leon-Kloosterziel, K.M., Bartels, D., Koornneef, M., and Karssen, C.M. (1993). Acquisition of desiccation tolerance and longevity in seeds of Arabidopsis thaliana. Plant Physiol. 102 1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papi, M., Sabatini, S., Altamura, M.M., Hennig, L., Schafer, E., Costantino, P., and Vittorioso, P. (2002). Inactivation of the phloem-specific Dof zinc finger gene DAG1 affects response to light and integrity of the testa of Arabidopsis seeds. Plant Physiol. 128 411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papi, M., Sabatini, S., Bouchez, D., Camilleri, C., Costantino, P., and Vittorioso, P. (2000). Identification and disruption of an Arabidopsis zinc finger gene controlling seed germination. Genes Dev. 14 28–33. [PMC free article] [PubMed] [Google Scholar]
- Parcy, F., and Giraudat, J. (1997). Interactions between the ABI1 and the ectopically expressed ABI3 genes in controlling abscisic acid responses in Arabidopsis vegetative tissues. Plant J. 11 693–702. [DOI] [PubMed] [Google Scholar]
- Penfield, S., Li, Y., Gilday, A.D., Graham, S., and Graham, I.A. (2006). Arabidopsis ABA INSENSITIVE4 regulates lipid mobilization in the embryo and reveals repression of seed germination by the endosperm. Plant Cell 18 1887–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, J., et al. (1999). ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 400 256–261. [DOI] [PubMed] [Google Scholar]
- Pysh, L.D., Wysocka-Diller, J.W., Camilleri, C., Bouchez, D., and Benfey, P.N. (1999). The GRAS gene family in Arabidopsis: Sequence characterization and basic expression analysis of the SCARECROW-LIKE genes. Plant J. 18 111–119. [DOI] [PubMed] [Google Scholar]
- Russell, L., Larner, V., Kurup, S., Bougourd, S., and Holdsworth, M. (2000). The Arabidopsis COMATOSE locus regulates germination potential. Development 127 3759–3767. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Silverstone, A.L., Jung, H.S., Dill, A., Kawaide, H., Kamiya, Y., and Sun, T.P. (2001). Repressing a repressor: Gibberellin-induced rapid reduction of the RGA protein in Arabidopsis. Plant Cell 13 1555–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steber, C.M., Cooney, S.E., and McCourt, P. (1998). Isolation of the GA-response mutant sly1 as a suppressor of ABI1-1 in Arabidopsis thaliana. Genetics 149 509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steber, C.M., and McCourt, P. (2001). A role for brassinosteroids in germination in Arabidopsis. Plant Physiol. 125 763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader, L.C., Ritchie, S., Soule, J.D., McGinnis, K.M., and Steber, C.M. (2004). Recessive-interfering mutations in the gibberellin signaling gene SLEEPY1 are rescued by overexpression of its homologue, SNEEZY. Proc. Natl. Acad. Sci. USA 101 12771–12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan, V., Springer, P., Volpe, T., Haward, S., Jones, J.D., Dean, C., Ma, H., and Martienssen, R. (1995). Patterns of gene action in plant development revealed by enhancer trap and gene trap transposable elements. Genes Dev. 9 1797–1810. [DOI] [PubMed] [Google Scholar]
- Thomas, S.G., Rieu, I., and Steber, C.M. (2005). Gibberellin metabolism and signaling. Vitam. Horm. 72 289–338. [DOI] [PubMed] [Google Scholar]
- Tyler, L., Thomas, S.G., Hu, J., Dill, A., Alonso, J.M., Ecker, J.R., and Sun, T.P. (2004). DELLA proteins and gibberellin-regulated seed germination and floral development in Arabidopsis. Plant Physiol. 135 1008–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueguchi-Tanaka, M., Ashikari, M., Nakajima, M., Itoh, H., Katoh, E., Kobayashi, M., Chow, T.Y., Hsing, Y.I.C., Kitano, H., Yamaguchi, I., and Matsuoka, M. (2005). GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 437 693–698. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, S., Kamiya, Y., and Sun, T. (2001). Distinct cell-specific expression patterns of early and late gibberellin biosynthetic genes during Arabidopsis seed germination. Plant J. 28 443–453. [DOI] [PubMed] [Google Scholar]
- Yamauchi, Y., Ogawa, M., Kuwahara, A., Hanada, A., Kamiya, Y., and Yamaguchi, S. (2004). Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. Plant Cell 16 367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.