Abstract
T-cell receptor gamma (TRG) gene rearrangement status is useful for the differential diagnosis of T-cell lesions. The BIOMED-2 protocol that uses two sets of Jγ and four sets of Vγ primers in a multiplex, two-tube reaction followed by capillary gel electrophoresis is emerging as a standard assay for this application. Here, we report a computer-aided method to evaluate the significance of a peak in this TRG clonality assay. A best-fit normal distribution (ND) curve and the χ2 error for each peak are used to determine whether a peak is significantly taller than the background (cutoff for Vγ1–8 is 1). Eighty clinical samples that have been previously analyzed by a GC-clamped primer polymerase chain reaction/denaturing gradient gel electrophoresis assay were reanalyzed with the BIOMED-2 assay and scored by the ND method and four previously published methods: relative peak height (RPH), relative peak ratio (RPR), height ratio (HR), and peak height ratio (Rn). A greater than 90% concordance rate was observed between RPH and ND analysis, whereas RPR, Rn, and HR had a lower threshold to call a peak positive. The advantage of the ND method is that it is more objective, reproducible, and can be automated.
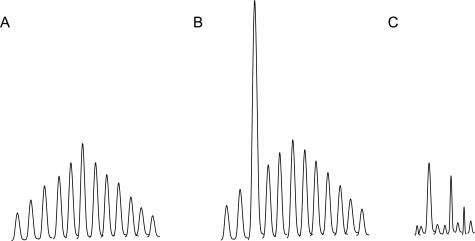
Polymerase chain reaction (PCR) amplification of gene segments for the rearranged antigen receptors [T-cell receptor (TCR) β (TRB)] and γ (TRG) chains and immunoglobulin (Ig) heavy (IGH) and κ light chains (IGK) by fluorescently labeled primers, followed by capillary gel electrophoresis (CE)1,2 has increasingly been used as an ancillary diagnostic test to assess T- and B-cell clonality.3,4 The test is based on the realization that during the assembly of the TCR and Ig genes, the length and the sequence content of the rearranged genes are highly polymorphic such that each B cell and T cell has its unique set of antigen receptor genes. If a reactive tissue is sampled, the distribution of the sizes of the investigated segment is expected to follow a normal (Gaussian) distribution (Figure 1A). If a clonal population is present, a fragment of a distinct size can be expected to predominate in the product mixture (Figure 1B). There is a plethora of published reports with different primer designs and experimental protocols exploiting this principle of clonality analysis.5,6,7,8,9 Currently, two commercially available kits are most widely used by diagnostic laboratories in commercial facilities and academic medical centers. One5,10 was developed by BIOMED-2, a European consortium, and the second was developed and marketed by InVivoScribe Technologies LLC (San Diego, CA) using proprietary primer sequences.11 For B cells, both kits use three separate PCR reactions with consensus primers to the three framework regions of the IGH gene (FRI, FRII, and FRIII). For T cells, both have four distinct primers for the V segments (Vγ1–8, Vγ9, Vγ10, and Vγ11) and two primers for the J segments (Jγl.l, Jγ1.3, and Jγ2.1; and Jγ2.3) of the TRG gene that are fluorescently labeled with different fluorophores.
Figure 1.
Common peak distributions seen in capillary gel electrophoresis of TRG clonality assays. A: Reactive, polyclonal pattern; (B) clonal peak in a polyclonal background. C: Scattered peaks without a definitive polyclonal background.
For the IGH test, the interpretation of the CE result is usually more straightforward. The reactive samples have a range of product peaks at three-base intervals showing a normal distribution. When a clonal population is present, one or two distinct peaks are generally observed in addition to the reactive background. Because the consensus primers used for the three framework regions cover a large repertoire of the VDJ segments, the background amplification is more robust, and the same rearrangements can often be amplified in more than one reaction, providing easily obtainable interobserver concordance (data not shown). TRG reactions, on the other hand, frequently present interpretative challenges in clinical specimens because more than one prominent peak of varying heights is common in TRG amplifications, either with or without an accompanying recognizable polyclonal background (Figure 1C). In those specimens, it is difficult to reach interobserver consensus without objective criteria for interpretation. Four methods have been previously proposed to aid in the determination of the significance of a peak by calculating the ratio of the height of the peak in question to that of the polyclonal background.8,12,13,14 These methods work well for experienced observers when a background amplification is clearly present but are less useful in cases where background amplifications are not robust. Three of these methods rely on determination of the background height, which can only be deciphered subjectively by the observer; thus reproducible measurements are more difficult to perform in practice. This is especially true in the subsets of reactions where the V segment usage is underrepresented, producing a small background.
Here, we report a new method to evaluate the significance of a peak in a TRG clonality assay. The data are exported from the capillary gel instrument software (Applied Biosystems 3100xl GeneMapper v3.7; Foster City, CA) and fitted to a normal distribution curve by computer algorithms. Individual peaks are evaluated for their closeness to the fitted curve. Specimens where all data points can be successfully fitted to a normal distribution curve are considered to be reactive or nonclonal, and specimens with peaks having χ2 errors significantly deviated from the curve are interpreted as clonal. This method is objective as the curve fitting and calculation steps are fully computerized. It also takes into consideration the size of the individual peaks and theoretically can improve the sensitivity of tests when the clonal peak is at the edge of the expected size range for a given primer set compared with relative peak height (RPH). We performed titration experiments and used a training set of cases to select a cutoff value to separate clonal and reactive specimens. Using the cutoff value, we evaluated and compared the TRG gene rearrangement results of 80 cases of T-cell lesions analyzed by GC-clamped primers and denaturing gradient gel electrophoresis (DGGE)3 with those by fluorescently labeled BIOMED-2 primers10 (InVivoScribe Technologies, LLC) and CE. The latter results were scored by five methods: RPH,12 relative peak ratio (RPR),13 height ratio (HR),8 peak height ratio (Rn),5 and our new method, normal distribution (ND). A 90% concordance rate is observed between RPH and ND analysis. The RPR, Rn, and HR methods have a lower threshold for peak calling and tend to overcall when compared with results obtained by DGGE and the RPH or ND methods. The advantage of the new method is that it is automated, more objective, and reproducible.
Materials and Methods
Samples and Cell Lines
Twenty-nine polyclonal samples (22 reactive tissue samples and seven specimens of peripheral blood lymphocytes) and 51 samples with clonal TRG gene rearrangements identified by DGGE and/or Southern blots were used to validate the new method (see also Table 1). The histological diagnosis of these 51 samples include 11 cases of peripheral T-cell lymphoma, 21 cases of mycosis fungoides, 14 cases of atypical lymphoid infiltrate, one case of anaplastic large cell lymphoma, one case of T-cell acute lymphoblastic leukemia, and three cases of T-cell large granular lymphocytic leukemia. Twenty-five cases were amplified from frozen tissue, nine cases from blood/bone marrow, and 56 cases from paraffin-embedded tissue. These clinical samples, which were sequentially accessioned in our lab over a 6-month period in 2004 (July to December), were previously tested by either Southern blot and/or DGGE. PEER and Jurkat T cell line DNA and human polyclonal control DNA are supplied with the TCRγ Gene Clonality Assay Kit (InVivoScribe Technologies, LLC).
Table 1.
Features of Cases Used in the Evaluation of the New Method
| Histologic diagnosis | |
| Peripheral T-cell lymphoma, NOS | 11 |
| Mycosis fungoides | 21 |
| Atypical lymphoid infiltrate | 14 |
| Anaplastic large cell lymphoma | 1 |
| Acute T-cell lymphoblastic lymphoma | 1 |
| Large granular lymphocyte leukemia, T cell phenotype | 3 |
| Reactive, nonneoplastic | 29 |
| Tissue type (excluding blood/marrow) | |
| Frozen | 25 |
| Paraffin-embedded | 56 |
| Tissue source | |
| Blood/bone marrow | 9 |
| Skin | 49 |
| Lymph node | 18 |
| Others | 14 |
| Vγ usages (by DGGE)* | |
| Vγ1–8 | 44 |
| Vγ9 | 7 |
| Vγ10 | 12 |
NOS, nitric-oxide synthase.
One case can have more than one rearranged allele detected.
DNA Extraction and Dilution
DNA was extracted from fresh/frozen tissue with PureGene DNA Isolation kits (Gentra Systems, Minneapolis, MN) according to the manufacturer’s instruction. Paraffin-embedded tissue was cleared of paraffin by xylene, followed by 100% ethanol rinse. The deparaffinized tissue was then processed with QIAGEN QIAamp DNA micro kits (QIAGEN 56304; QIAGEN, Valencia, CA). Serial twofold dilutions of tumor DNA into polyclonal human DNA were prepared with PEER and Jurkat DNA.
DGGE and Southern Blot Analysis
Amplification of TRG gene by GC clamped primers followed by DGGE was performed according to methods described by Greiner et al.3 When high-molecular weight DNA was available, the DNA was also digested with two or three restriction enzyme combinations and separated on 0.8% agarose gel and transferred to a nylon membrane. Jγ probes for TRG were radiolabeled with [32P]dCTP, hybridized to the membrane overnight, washed, and exposed to X-ray film according to standard Southern blot procedures.
Polymerase Chain Reaction and Capillary Gel Electrophoresis
The T-cell clonality assay was performed according to manufacturer’s instruction with reagents purchased from InVivoScribe Technologies LLC (TCRG Gene Clonality Assay for ABI Fluorescence Detection, InVivoScribe no. l-207-0021). The kit uses the primer sequences published by the BIOMED-25 and had all reagents needed except for sample DNA and Taq polymerase (AmpliTAQ Gold; Applied Biosystems). Two multiplex PCR reactions were performed for each specimen. Tube A contained primers that target the Vγ1–8 + Vγ10 sequences and Jγ1.1/2.1 and Jγ1.3/2.3. Tube B contained primers that target the Vγ9 + Vγ11 sequences and all Jγ1.1/2.1 and Jγ1.3/2.3. JγP primer was not used in this kit. A Control Size Ladder master mix was used in a third reaction. The PCR reactions were conducted in either a PE 9600 thermocycler (Perkin-Elmer, Foster City, CA) or an ABI 2700 thermocycler (Applied Biosystems) with 0.5 μg of DNA used in each reaction. The PCR program was composed of heating initially to 95°C for 7 minutes, followed by 35 cycles of 95°C for 45 seconds, 60°C for 45 seconds, 72°C for 90 seconds, plus a final extension of 72°C for 10 minutes. After PCR, 10 μl of HiDi formamide/Genescan 400HD ROX size standard mixture was mixed with 1 μl of PCR product, transferred to a 96-well plate, heated to 95°C for 3 minutes, snap-chilled for 5 minutes, and loaded onto a 3130xl Genetic Analyzer (ABI, Foster City, CA) for capillary gel electrophoresis.
Data Analysis
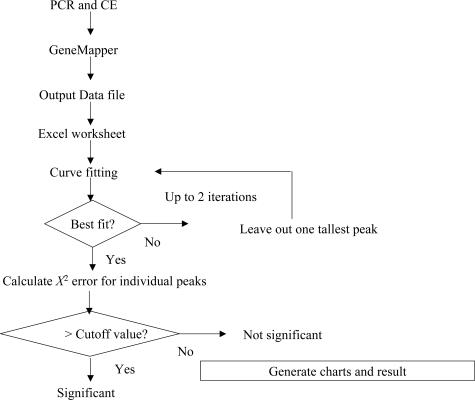
The raw data for each sample run were loaded into ABI GeneMapper v3.7 software for peak calling and measurement of peak size and area under the curve. All of the data on peak size, height, and area were exported as a comma-delimited file and imported into Microsoft Excel (Redmond, WA) for analysis. First, the expected size range data provided by BIOMED-2 were used to generate a population of idealized ND of peaks for each case. The height of each peak was then calculated for every given product size (expected, E). The observed (O) peak height at each size was then fitted into this idealized distribution by repeated iterations until the sum of χ2 error [Σ(O − E)2/E] for all of the peaks reached a minimum (χ2 goodness-of-fit test).15 If no χ2 error was greater than 1, then the null hypothesis of a normal distribution for the dataset was accepted, and the specimen was declared polyclonal. If χ2 error of any of the peaks was greater than 1, then that data point was temporarily removed, and the process of curve fitting was repeated. This step mimics the situation where a clonal population is present in a mixture of reactive T cells, and if the clonal T cells are removed from the specimen, the remaining T cells can be expected to give rise to a normal distribution. This process may be repeated once more because neoplastic T cells may have two rearranged alleles. At the end of the process, the result of the analysis was shown in a maximum of eight separate charts, one for each V and J primer combination. A summary was also provided to highlight peaks with large χ2 errors. A computer program in Microsoft Visual Basic for Application (VBA) has been written and implemented as a macro that can be invoked by clicking an icon on the standard toolbar. This program is available on request.
To compare the existing methods, the data were also examined to produce a value based on the RPH,12 RPR,13 HR,8 and Rn14 methods. In brief, the data were inspected by one of us (D.H.) to determine the maximal height of the polyclonal background (h0). The height of the peak of interest (h1) was read from the GeneMapper output. In cases where a reliable h0 could not be determined (discernable background distribution is not always present), we excluded such cases from this study. In the BIOMED-2 assay, the reactions were separate, and the product lengths were different. In the original publications, these methods were designed to analyze pooled PCR products from several individual V-J amplifications. We adapted these methods to each individual BIOMED-2 reaction. RPH was calculated as (h1 − h0)/h0, and RPR was calculated as h1/h0. HR is the same as RPR. Rn is the ratio between h1 and the average of the two immediate flanking peaks.
Results
Development of an Algorithm to Detect the Presence of a Clonal T-Cell Population
Our initial aim was to try to fit the CE data into a computer-generated, idealized ND curve. This alone worked fairly well for cases that clearly fell into a normal distribution by visual inspection (cases with a pattern as in Figure 1A) but did not work well for cases that were clonal (Figure 1B) or were without a discernable polyclonal background (Figure 1C). In those situations, attempts to fit all of the data into a curve appeared to skew the best-fit curve toward the higher peaks. This is because most curve-fitting algorithms tend to be more weighted toward the higher peaks; as a result, the taller peaks are fitted onto a curve while the smaller peaks are ignored by the algorithm. Recognizing this fact, we next tried to remove the tallest outlier from the dataset before curve fitting and to see whether that would yield a more realistic normal distribution representing the “reactive” portion of the data. Because both rearranged alleles in a clonal T cell may be amplifiable by the same Vγ-Jγ primer set, we also factored in the situations where two outliers may need to be removed to identify the best-fit curve. After a best-fit curve was obtained with this modified dataset, all of the peaks in the original dataset could then be evaluated to determine whether each peak belongs on the curve by χ2 analysis. One or two peaks too tall to fit into the curve were considered as evidence of the presence of a clonal population.
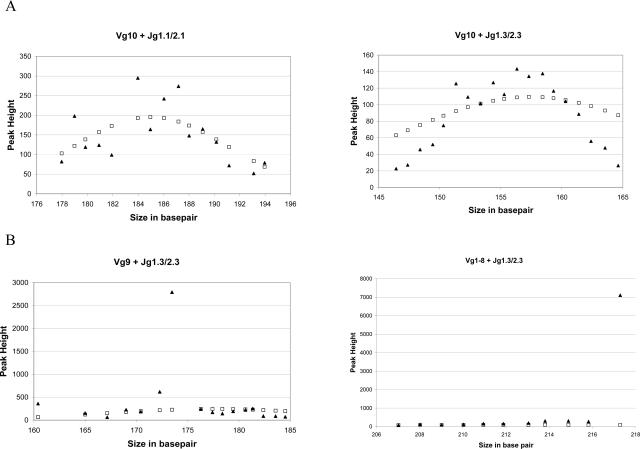
We tested this algorithm first by using DNA isolated from a reactive sample (human tonsil) in a standard BIOMED-2 TRG assay. As shown in Figure 2A, the peaks from polyclonal samples were fitted into an idealized ND curve created by the mean and SD of the known size range of each of the TRG primer combinations. This was true with all eight possible primer combinations in the BIOMED-2 test (only Vγ10 reactions are shown as examples). Next, we tested two clonal T-cell samples, one with a known Vγ1–8 and one with a known Vγ9 rearrangement. As expected, when all of the data points from the PCR amplifications were used, the curve that minimized the χ2 error had a total error too big to be accepted as a fit. When we removed the outlier peak and repeated the curve fitting, a best-fit curve that included most of the reactive peaks was successfully found for both cases (Figure 2B). Using the best-fit curve, we evaluated all of the peaks, and the clonal peaks in both cases gave χ2 errors more than 100-fold greater than the rest of the peaks. These initial data supported our notion that an objective method that quantitatively measures the extent of deviation of a peak from a normal distribution curve can be adapted to analyze the results from a TRG assay.
Figure 2.
Representative best-fit curves (open square, idealized normal distribution; filled triangle, original data). A: Data from reactive specimens can be fitted into an idealized normal distribution curve. B: Clonal specimens have one or more peaks that do not belong to a normal distribution.
Having refined our algorithm, we next determined whether cutoff values for χ2 error can be selected with acceptable sensitivity and specificity for evaluation of clinical samples. We first selected 10 clonal and 10 polyclonal clinical samples and used the data as a training set to identify a reasonable cutoff value. To facilitate this, we developed a computer program that automates the process of curve fitting and identification of the peaks with large χ2 errors. The program is written with VBA and runs on Microsoft Excel 2000 and above. It takes exported delimited files from GeneMapper 3.7 and outputs the result as individual charts for each TRG primer reaction and a summary table. The input files can be processed individually or as a batch. A flow diagram of this program is illustrated in Figure 3. Using this program, we analyzed the dataset of 20 samples and examined the χ2 errors of all of the peaks. Without exceptions, the 10 polyclonal samples gave no peak with a χ2 error greater than 0.08. The large peaks of the 10 clonal samples, on the other hand, had χ2 error values ranging from 1.05 to over 100. Based on this data, we tentatively set the cutoff for χ2 error at 1. Errors less than 0.1 are considered to be insignificant and those between 1 and 0.1 to be equivocal; although in this training set, we did not see any peaks with errors that fell within this range.
Figure 3.
Data flowchart in normal distribution analysis.
Different Cutoff Values for χ2 Error May Be Necessary for Different Vγ-Jγ Primer Combinations
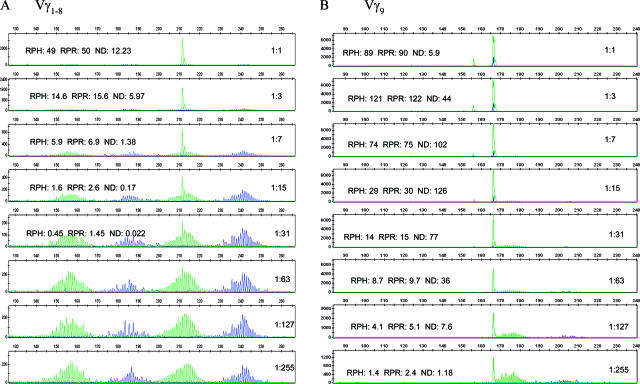
Next, we PCR-amplified and analyzed T cell line (PEER) DNA serially diluted into tonsil DNA from a ratio of 1:1 down to 1:255 (50% down to 0.5% tumor) to refine the cutoff value of our method. For Vγ 1–8 reactions (Figure 4A), the χ2 error ranged from a high of 12.23 at 50% tumor to a low of 0.022 at 3% tumor. Surveying the χ2 errors of other nonclonal peaks in the reaction showed that more than 95% have a χ2 error of less than 0.05. At 1:7 dilution, which is equivalent to about 15% tumor cells, the χ2 error was 1.38. Based on this data, we selected a χ2 error of 1 as a cutoff to give us a specificity of 100% in our training set and a sensitivity of about 10% tumor cells, which is roughly twice as many cells as identified by the lower limit of detection of Southern blot assays. The results for Vγ9 and Vγ10 primer sets were distinctly different from those of the Vγ1–8. With the Vγ9 primer set, a 1:255 dilution yielded a χ2 error of 1, ie, as little as 0.5% clonal cells (Figure 4B). This is probably due to the underrepresentation of T cells in a mixed cell population that use Vγ9 in TRG rearrangement compared with Vγ1–8. We found similar results for Vγ10 reactions (data not shown). Thus, a uniform χ2 error cutoff for all V regions will not identify similar percentages of clonal cells.
Figure 4.
Serial twofold dilutions of cell line DNA containing a Vγ1 (A) and Vγ9 (B) rearrangement (PEER) into polyclonal human genomic DNA (tonsil) were made from 1:1 to 1:255 (equivalent to 50% tumor down to less than 0.5% tumor). The DNA was amplified with the InvivoScribe BIOMED-2 T-cell clonality assay kit, separated by capillary gel electrophoresis, and analyzed with the GeneMapper software v3.7. The χ2 errors (ND) are calculated by the normal distribution program described in this article. RPR and RPH are determined by the methods as described by Greiner et al13 and Lee et al.12
Validation of the New Method with 80 Clinical Samples
To further validate our algorithm and computer program, we took sequentially accessioned clinical samples over a 6-month period in 2004 (July to December) that were previously tested by the Southern blot and/or DGGE (see Materials and Methods) technique and amplified them by the BIOMED-2 kit. Of these, 80 samples were successfully amplified (Table 1) and were analyzed by four previously published methods, RPH,12 RPR,13 HR,8 and Rn,14 or our new method ND. The results of the five methods were compared with the results previously determined by Southern blot or DGGE. As shown in Table 2, there was excellent agreement between the RPH and the ND methods. RPR, HR, and Rn had lower threshold for calling a peak significant, and more cases were called positive with these methods than with RPH, ND, and DGGE/Southern. With Vγ9 and Vγ10 reactions, where lacking a well-formed background distribution of PCR products is common, there were more discrepancies among the methods. When compared with DGGE/Southern blot results, the nine false-positive peaks were all in the Vγ9 and Vγ10 reactions (six in Vγ9 and three in Vγ10).
Table 2.
Comparison of Five Methods of Interpretation of TRG Clonality Assay
| DGGE/Southern
|
|||
|---|---|---|---|
| POS | NEG | ||
| RPH ≥ 3 | POS | 48 | 8 |
| NEG | 3 | 21 | |
| RPR ≥ 2 | POS | 50 | 19 |
| HR ≥ 2 | NEG | 1 | 10 |
| ND ≥ 1 | POS | 49 | 9 |
| NEG | 2 | 20 | |
| Rn ≥ 3 | POS | 48 | 17 |
| NEG | 3 | 12 | |
DGGE/Southern, denaturing gradient gel electrophoresis and/or Southern blots; RPH, relative peak height, (h1 − h0)/h0 (where h1 is the height of peak of interest and h0 the highest peak height of the polyclonal background peaks); RPR, relative peak ratio, h1/h0; HR, height ratio is the same as the RPR; Rn, peak height ratio, h1/ha (where ha is the average of two peaks immediately adjacent to peak of interest); ND, normalized χ2 error (O − E)2/E from the best-fit curve; NEG, negative; POS, positive.
Discussion
For molecular diagnostic testing to be more clinically relevant, there needs to be standardization of methods and criteria for interpretation.16 Two recent developments in the area of T- and B-cell clonality testing by PCR have moved us closer to reaching these goals. The first development is the availability of the consensus primers put forth by BIOMED-2, the European Consortium.5,10,17 The second is the use of capillary gel electrophoresis, which has significantly improved the resolution and quantitation of PCR products.11,18,19,20 Although the use of common primers effectively eliminates one variable in comparing results among different laboratories, interpretation of a CE result is actually more challenging than anticipated, and this is especially true for paucicellular specimens analyzed by PCR for TRG. Instead of having PCR products distributed throughout the expected size range, giving rise to a normal distribution, isolated and discontinuous peaks of random heights are frequently seen.12,21,22 Because the currently available guidelines for interpretation of CE results all rely on the identification of a “background” distribution, these guidelines are difficult to apply to in many cases. The problem is somewhat exacerbated by the possibility that molecular diagnosticians may be influenced by the clinical context when analyzing test results and may “overcall” discontinuous peaks.12 To overcome this potential obstacle to standardization, we aimed to develop an objective method applying established statistical methods such that the significance of individual peaks can be determined solely on the heights and distribution of these peaks. Because the method is fully automated, it is reproducible and operator independent. It can be easily adapted to most laboratories performing the same test, and it only needs a regular PC running a widely available spreadsheet program.
In our method, a χ2 error is calculated for each peak, and it is proportional to the degree of deviation of this peak from the best-fit curve. Although the height of each peak should ideally be proportional to the size of the population it represents, in reality, it is also influenced by other factors such as PCR efficiency of the actual sequence. A cutoff value therefore has to be determined empirically using tumor dilutions and clinical specimens with known TRG rearrangement status. We chose a cutoff value of 1 after empirical analysis of 20 samples in a training set. This cutoff value clearly stratified clonal verus polyclonal samples for Vγ1–8 reactions and seemed to correspond to a lower limit sensitivity of detection between 7 and 15% tumor DNA (Figure 4). Lowering the cutoff value will probably increase the sensitivity but at the expense of specificity. For Vγ10 and Vγ9 reactions, we recommend raising this cutoff value since as little as 0.5% tumor will produce a peak with a χ2 error of more than one with these primer pairs. Because only subsets of T cells that used their Vγ10 and Vγ9 contribute to these reactions, the percentage of tumor cells in these reactions is proportionally increased. There is limited data on the relative frequency of Vγ10 and Vγ9 T cells in different types of normal tissues, body fluids, or peripheral blood. The published results range from a low of 5% to a high of 40% for Vγ9-rearranged T cells.23,24,25,26 Our results suggest the relative frequency of Vγ10 and Vγ9 T cells to be in the 5 to 7% range in a variety of tissues tested. It has been well documented that many reactive skin lesions can have T-cell proliferations with a limited T-cell receptor repertoire.27,28 The chance of having a small clone comprising 1% of the total T-cell population may be significantly high, and using the same cutoff value for these two reactions as for Vγ1–8 is likely to increase the false positive rate. Our suggestion at this point is to increase the cutoff value at least 10- to 20-fold for Vγ9 and Vγ10 to avoid overcalling positives in these reactions. Collection and analysis of more clinical samples is required for further refinement of the precise cutoff values for these reactions. It is also possible that different cutoff values may need to be developed for different specimens (skin, blood, body fluids versus solid tissues). As for Vγ11 reactions, we only detected two Vγ11 clonal peaks after reviewing over 400 clinical samples tested in our laboratory over a 2-year period, suggesting that Vγ11 is infrequently rearranged in neoplastic cells. Although we did not perform dilution study for Vγ11 and do not have sufficient data at this point, the cutoff value for the Vγ11 reaction is likely to be similar to those for Vγ9 and Vγ10.
When we performed the comparison study on the 80 samples, we found that it was much easier to analyze the data with our automated computer program compared with the manual RPH and RPR methods. Many cases did not have a well-formed background distribution of PCR products, making it difficult to reproducibly assign a value to the maximal height of the polyclonal background (h0) required for these methods; in fact, we chose to have only one of us calculate the RPH and RPR because our initial attempt with multiple interpreters led to poor interobserver correlation. The RPH method produced results that correlate very well with both the DGGE and the ND methods, indicating that it is a good, reliable method when used by experienced observers. The peak height ratio (Rn) method, which uses the average of the two immediately adjacent peaks as the denominator, does not rely on identification of a polyclonal background, is also objective, can be automated, and has excellent reproducibility among different observers. It performs very well when the background polyclonal peaks are well formed. In cases where discontinuous peaks are present without a well-formed background distribution of PCR products, it has slightly higher false-positive rate because the immediate adjacent peaks are sometimes very low. This is especially true in the Vγ9 and Vγ10 reactions, where it is not uncommon to observe poorly formed background distribution in clinical specimens.
The normal (Gaussian) size distribution of the TRG PCR products reflects the relative frequency of the size of the rearranged V-N-J gene segments. In both RPH and RPR methods, the background peak height (h0) value is taken from the highest peak in the background, regardless of its location relative to the normal distribution. When a suspicious “clonal” peak migrates near the center of the background, it is reasonable to assign its significance by comparing it with h0. However, when a suspicious peak migrates near the edge of the normal distribution where the background peaks are low, it may not meet the cutoff criteria using the RPH or RPR methods, leading to undercalling a clonal population in some cases. This is another area where the ND method may theoretically offer an advantage over other published methods. Because the χ2 error value is inversely proportional to the expected value (the height of the background peak) at that location, a peak near the edge of the background distribution does not need to be as tall as the one at the center of the background to have χ2 error value exceeding the cutoff value. This can theoretically increase the sensitivity of those cases with clonal peaks occurring near the edge of the expected size ranges.
Because the curve-fitting process relies on approximation of raw data to an idealized normal distribution, the quality of the fit will improve when more data points are available. In cases where only one or two PCR product peaks are present, it is not always possible for the program to properly fit the data into a curve. In these situations, either the sample is truly made of a clonal process where only the one (or two) dominant allele is amplified or the sample is T cell-poor (either the specimen is paucicellular or the specimen is cellular but contains few T cells) and the PCR reactions mimic single-cell PCR conditions. Interpretation of this type of result will require knowledge of the nature and size of the specimen and quality of the DNA. In our experience with clinical specimens, we found that the height of the peak is helpful in evaluation of these peaks. It is rare for a paucicellular specimen to produce peaks over 800 fluorescence units in height, whereas if a peak is over the cutoff value but is only 300 fluorescent units in height, then it should be interpreted with caution.
Our intention is to provide an objective, quantitative, and reproducible method to aid in the determination of the significance of a peak in TRG capillary gel electrophoresis clonality assays. The use of algorithm-driven curve fitting and calculation of χ2 error of a peak achieves these goals. Although the cutoff value is determined empirically, theoretically, if there are more than 10 data points in the dataset, the probability that the peak is part of a normal distribution is less than 0.02. The method also allows for differential interpretation of the significance of peak heights in the more common Vγ1–8 rearrangements versus the less common Vγ9 and Vγ10 rearrangements. Armed with objective data, molecular diagnosticians can correlate the results with other available clinical, laboratory, and histopathological information and exercise their best judgment in the medical interpretation. We will provide our computer program to other laboratories who request it to advance the standardization of TRG clonality tests. Molecular diagnosticians can then focus on using the data to better define clinically relevant criteria for the diagnosis of T-cell lymphoproliferative disorders.
References
- Miller JE, Wilson SS, Jaye DL, Kronenberg M. An automated semiquantitative B and T cell clonality assay. Mol Diagn. 1999;4:101–117. doi: 10.1016/s1084-8592(99)80035-6. [DOI] [PubMed] [Google Scholar]
- Simon M, Kind P, Kaudewitz P, Krokowski M, Graf A, Prinz J, Puchta U, Medeiros LJ, Sander CA. Automated high-resolution polymerase chain reaction fragment analysis: a method for detecting T-cell receptor gamma-chain gene rearrangements in lymphoproliferative diseases. Am J Pathol. 1998;152:29–33. [PMC free article] [PubMed] [Google Scholar]
- Greiner TC, Raffeld M, Lutz C, Dick F, Jaffe E. Analysis of T cell receptor γ gene rearrangements by denaturing gradient gel electrophoresis of GC-clamped polymerase chain reaction products. Am J Pathol. 1995;146:46–55. [PMC free article] [PubMed] [Google Scholar]
- Theodorou I, Bigorgne C, Delfau MH, Lahet C, Cochet G, Vidaud M, Raphael M, Gaulard P, Farcet JP. VJ rearrangements of the TCR gamma locus in peripheral T-cell lymphomas: analysis by polymerase chain reaction and denaturing gradient gel electrophoresis. J Pathol. 1996;178:303–310. doi: 10.1002/(SICI)1096-9896(199603)178:3<303::AID-PATH475>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- van Dongen JJ, Langerak AW, Bruggemann M, Evans PA, Hummel M, Lavender FL, Delabesse E, Davi F, Schuuring E, Garcia-Sanz R, van Krieken JH, Droese J, Gonzalez D, Bastard C, White HE, Spaargaren M, Gonzalez M, Parreira A, Smith JL, Morgan GJ, Kneba M, Macintyre EA. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98–3936. Leukemia. 2003;17:2257–2231. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- Hodges E, Williams AP, Harris S, Smith JL. T-cell receptor molecular diagnosis of T-cell lymphoma. Methods Mol Med. 2005;15:197–215. doi: 10.1385/1-59259-936-2:197. [DOI] [PubMed] [Google Scholar]
- Beaubier NT, Hart AP, Bartolo C, Willman CL, Viswanatha DS. Comparison of capillary electrophoresis and polyacrylamide gel electrophoresis for the evaluation of T and B cell clonality by polymerase chain reaction. Diagn Mol Pathol. 2000;9:121–131. doi: 10.1097/00019606-200009000-00001. [DOI] [PubMed] [Google Scholar]
- Sprouse JT, Werling R, Hanke D, Lakey C, McDonnel L, Wood BL, Sabath DE. T-cell clonality determination using polymerase chain reaction (PCR) amplification of the T-cell receptor gamma-chain gene and capillary electrophoresis of fluorescently labeled PCR products. Am J Clin Pathol. 2000;113:838–850. doi: 10.1309/02M7-5JCC-YRTK-MGDR. [DOI] [PubMed] [Google Scholar]
- Krafft AE, Taubenberger JK, Sheng ZM, Bijwaard KE, Abbondanzo SL, Aguilera NS, Lichy JH. Enhanced sensitivity with a novel TCRgamma PCR assay for clonality studies in 569 formalin-fixed, paraffin-embedded (FFPE) cases. Mol Diagn. 1999;4:119–133. doi: 10.1016/s1084-8592(99)80036-8. [DOI] [PubMed] [Google Scholar]
- Sandberg Y, Heule F, Lam K, Lugtenburg PJ, Wolvers-Tettero IL, van Dongen JJ, Langerak AW. Molecular immunoglobulin/T-cell receptor clonality analysis in cutaneous lymphoproliferations. Experience with the BIOMED-2 standardized polymerase chain reaction protocol. Haematologica. 2003;88:659–670. [PubMed] [Google Scholar]
- Oda RP, Wick MJ, Rueckert LM, Lust JA, Landers JP. Evaluation of capillary electrophoresis in polymer solutions with laser-induced fluorescence detection for the automated detection of T-cell gene rearrangements in lymphoproliferative disorders. Electrophoresis. 1996;17:1491–1498. doi: 10.1002/elps.1150170914. [DOI] [PubMed] [Google Scholar]
- Lee SC, Berg KD, Racke FK, Griffin CA, Eshleman JR. Pseudo-spikes are common in histologically benign lymphoid tissues. J Mol Diagn. 2000;2:145–152. doi: 10.1016/S1525-1578(10)60630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner TC, Rubocki RJ. Effectiveness of capillary electrophoresis using fluorescent-labeled primers in detecting T-cell receptor gamma gene rearrangements. J Mol Diagn. 2002;4:137–143. doi: 10.1016/s1525-1578(10)60694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo V, Lessin SR, Wilson RB, Rennert H, Tozer C, Benoit B, Leonard DG. Detection of clonal T-cell receptor gamma gene rearrangements using fluorescent-based PCR and automated high-resolution capillary electrophoresis. Mol Diagn. 2001;6:169–179. doi: 10.1054/modi.2001.27056. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Iowa City: Iowa State University Press,; Statistical Methods. (8th ed.) 1989 [Google Scholar]
- Neumaier M, Braun A, Wagener C. Fundamentals of quality assessment of molecular amplification methods in clinical diagnostics. International Federation of Clin Chem Scientific Division Committee on Molecular Biology Techniques. Clin Chem. 1998;44:12–26. [PubMed] [Google Scholar]
- Sandberg Y, van Gastel-Mol EJ, Verhaaf B, Lam KH, van Dongen JJ, Langerak AW. BIOMED-2 multiplex immunoglobulin/T-cell receptor polymerase chain reaction protocols can reliably replace Southern blot analysis in routine clonality diagnostics. J Mol Diagn. 2005;7:495–503. doi: 10.1016/S1525-1578(10)60580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega F, Medeiros LJ, Jones D, Abruzzo LV, Lai R, Manning J, Dunmire V, Luthra R. A novel four-color PCR assay to assess T-cell receptor gamma gene rearrangements in lymphoproliferative lesions. Am J Clin Pathol. 2001;116:17–24. doi: 10.1309/5WFQ-N12E-DT05-UX1T. [DOI] [PubMed] [Google Scholar]
- Lukowsky A. Clonality analysis by T-cell receptor gamma PCR and high-resolution electrophoresis in the diagnosis of cutaneous T-cell lymphoma (CTCL). Methods Mol Biol. 2003;218:303–20. doi: 10.1385/1-59259-356-9:303. [DOI] [PubMed] [Google Scholar]
- Sprouse JT, Werling R, Hanke D, Lakey C, McDonnel L, Wood BL, Sabath DE. T-cell clonality determination using polymerase chain reaction (PCR) amplification of the T-cell receptor gamma-chain gene and capillary electrophoresis of fluorescently labeled PCR products. Am J Clin Pathol. 2000;13:838–50. doi: 10.1309/02M7-5JCC-YRTK-MGDR. [DOI] [PubMed] [Google Scholar]
- Lukowsky A, Richter S, Dijkstal K, Sterry W, Muche JM. A T-cell receptor gamma polymerase chain reaction assay using capillary electrophoresis for the diagnosis of cutaneous T-cell lymphomas. Diagn Mol Pathol. 2002;1:59–66. doi: 10.1097/00019606-200206000-00001. [DOI] [PubMed] [Google Scholar]
- Lawnicki LC, Rubocki RJ, Chan WC, Lytle DM, Greiner TC. The distribution of gene segments in T-cell receptor gamma gene rearrangements demonstrates the need for multiple primer sets. J Mol Diagn. 2003;5:82–87. doi: 10.1016/s1525-1578(10)60456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara J, Benedict SH, Yumura K, Ha-Kawa K, Gelfand EW. Rearrangement of variable region T cell receptor gamma genes in acute lymphoblastic leukemia. V gamma gene usage differs in mature and immature T cells. J Clin Invest. 1989;83:1277–1283. doi: 10.1172/JCI114012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migone N, Casorati G, Di Celle PF, Lusso P, Foa R, Lefranc MP. Nonrandom TRG gamma variable gene rearrangement in normal human T cells and T cell leukemias. Eur J Immunol. 1988;18:173–178. doi: 10.1002/eji.1830180126. [DOI] [PubMed] [Google Scholar]
- Moisan JP, Bonneville M, Bouyge I, Moreau JF, Soulillou JP, Lefranc MP. Characterization of the T-cell receptor gamma (TRG) gene rearrangements in alloreactive T-cell clones. Hum Immun. 1989;24:95–110. doi: 10.1016/0198-8859(89)90050-5. [DOI] [PubMed] [Google Scholar]
- Kohsaka H, Chen PP, Taniguchi A, Ollier WE, Carson DA. Regulation of the mature human T cell receptor gamma repertoire by biased V-J gene rearrangement. J Clin Invest. 1993;91:171–178. doi: 10.1172/JCI116167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady SP, Magro CM, Diaz-Cano SJ, Wolfe HJ. Analysis of clonality of atypical cutaneous lymphoid infiltrates associated with drug therapy by PCR/DGGE. Hum Pathol. 1999;30:130–136. doi: 10.1016/s0046-8177(99)90266-6. [DOI] [PubMed] [Google Scholar]
- Dippel E, Assaf C, Hummel M, Schrag HJ, Stein H, Goerdt S, Orfanos CE. Clonal T-cell receptor gamma-chain gene rearrangement by PCR-based GeneScan analysis in advanced cutaneous T-cell lymphoma: a critical evaluation. J Pathol. 1999;188:146–154. doi: 10.1002/(SICI)1096-9896(199906)188:2<146::AID-PATH334>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]