Abstract
Mutations in the BRAF oncogene at amino acid 600 have been reported in 40 to 70% of human metastatic melanoma tissues, and the critical role of BRAF in the biology of melanoma has been established. Sampling the blood compartment to detect the mutational status of a solid tumor represents a highly innovative advance in cancer medicine, and such an approach could have advantages over tissue-based techniques. We report the development of a fluorescence-based polymerase chain reaction (PCR) assay to detect mutant BRAF alleles in plasma. A mutant-specific PCR assay was optimized to specifically amplify the mutant BRAF allele without amplifying the wild-type allele. Experiments mixing DNA from a BRAF mutant melanoma cell line with wild-type human placental DNA in varying proportions were performed to determine the threshold of this assay and to compare it with routine DNA sequencing. The assay was then applied to tissue and plasma specimens from patients with metastatic melanoma. The assay detected 0.1 ng of mutant DNA mixed in 100 ng of wild-type DNA and was 500-fold more sensitive than DNA sequencing. The assay detected mutant BRAF alleles in plasma samples from 14 of 26 (54%) metastatic melanoma patients. These data demonstrate the feasibility of blood-based testing for BRAF mutations in metastatic melanoma patients.
Mutations in the BRAF oncogene are the most frequently reported molecular alterations in melanoma. Several groups, including ours, have identified these mutations in primary and metastatic melanoma tumors and melanoma cell lines at rates ranging from 31 to 75%.1,2,3,4,5,6,7,8 BRAF, a serine-threonine kinase, activates the mitogen-activated protein kinase cascade, a pathway critical in tumor cell proliferation.9,10,11 The codon 600 mutation (formerly 5994), which accounts for >90% of BRAF mutations identified in melanoma, possesses constitutive kinase activity and can transform primary mouse embryonic fibroblasts.2,12,13,14 In addition, two studies have demonstrated that knockdown of mutant BRAF V600E expression in cultured human melanoma cell lines inhibits cell growth and invasion and promotes apoptosis.15,16 Thus, the BRAF oncogene is an attractive target of new treatments for metastatic melanoma.
For clinical trials of treatments with well-defined molecular targets, patient selection based on the mutational status of their metastatic tumor allows for substantially fewer patients to be enrolled to achieve appropriate statistical power.17 This is based on the fact that agents that target specific molecular alterations in tumor cells have shown the highest response rates in tumors that carry these alterations.18,19,20,21 Some ongoing clinical trials of BRAF inhibitors plan to include tumor mutational typing in their analyses. The final results of these trials are pending.22,23,24 As more potent and specific BRAF inhibitors become available, mutational typing is expected to acquire greater clinical relevance to determine the most suitable patients for these therapies.
Although the benefits of molecularly targeted therapies are clear,18,19,20,21 patient selection based on the mutational status of their tumor can be quite restrictive. A substantial number of patients who might benefit from the treatment will be ineligible because of the lack of an accessible tissue sample. In addition, determining the presence of mutations is limited by the ability of the assay to detect the mutation. In melanoma, samples often have large amounts of contaminating normal tissue. Thus, a highly sensitive and less invasive method of determining patients’ mutational status is desirable.
In this study, we developed a technique to test patient plasma samples for BRAF mutations. Using a mutant-specific amplification strategy with fluorescent detection, we show that plasma samples from patients with metastatic melanoma contain ample DNA for analysis, and we demonstrate that this assay may be a useful tool for determining the BRAF mutational status of a patient’s tumor, especially when an appropriate tissue sample is not available for analysis.
Materials and Methods
Patients and Sample Characteristics
The study cohort consisted of 26 metastatic melanoma patients with stage IV disease (age range, 35 to 92; mean, 63; 20 males, six females; tumor biopsy sites included 21 skin or subcutaneous metastases, three lymph node, one brain, and one local recurrence) accrued from either New York University School of Medicine (16 patients) or Memorial Sloan-Kettering Cancer Center (10 patients). The study was approved by the institutional review boards of both institutions, and all patients signed informed consent at time of enrollment. All patients had recurrent stage IV disease at time of blood draw. Peripheral blood samples were collected in two ethylenediamine tetraacetic acid tubes, placed immediately on ice, and transported to the laboratory where samples were centrifuged to separate cells and plasma. The plasma supernatant was transferred to a clean tube and stored at −80°C before use. For DNA extraction, plasma samples were thawed at room temperature, and 1-ml aliquots were placed into 1.5-ml microcentrifuge tubes. Samples were centrifuged at 16,000 rpm in an Eppendorf model 5415R microcentrifuge (Eppendorf-5 Prime, Inc., Boulder, CO) for 30 minutes. The supernatant was discarded, and the pellet was resuspended in 200 μl of Tris-ethylenediamine tetraacetic acid buffer. This material was used to isolate DNA using the QIAmp DNA Blood Mini kit (QIAgen, Valencia, CA).
For analysis of tumor samples, hematoxylin and eosin-stained slides were reviewed to ensure enough viable tumor. Unstained cut sections mounted on slides were macrodissected to remove contaminating normal cells when possible, and tissue sections were scraped into a 1.5-ml microcentrifuge tube. Subsequent steps followed the protocol for DNA isolation from paraffin slides in the QIAmp Mini Blood DNA kit. Peaks with an amplitude of 50 or more [default analysis parameters of the Genescan software (Applied Biosystems, Foster City, CA)] were considered positive. All blood and tissue samples were run in triplicate; a positive result required at least two positive replicates. The human melanoma cell line SK-MEL 29, mutant for BRAF, and human placental DNA, wild type for BRAF, were included in every run as positive and negative controls, respectively.
Mutation Detection
Mutant-specific polymerase chain reaction (MS-PCR) was accomplished using two primers: forward, 5′-GGTGATTTTGGTCTAGCTACATA-3′; and reverse, 5′-HEX-GGCCAAAAATTTAATCAGTGGA-3′.
The forward, mutant-specific primer was based on a design by Xu et al25 with minor modifications. This design used two bases at the 3′ end of the primer that do not anneal to the wild-type sequence. In the presence of the mutant sequence, the terminal 3′ base will anneal, allowing amplification to proceed. The reverse primer described by Davies et al2 was labeled with the HEX fluorochrome to permit detection using an ABI 310 Genetic Analyzer (Applied Biosystems). For amplification of the entire BRAF exon 15, we used the primers of Davies et al.2
DNA templates were extracted and purified from the human melanoma cell line SK-MEL 29 and human plasma samples using the QIAamp Mini DNA kit (QIAgen). Human placental DNA (Sigma-Aldrich, St. Louis, MO) was used as a negative control DNA template. Amplification was accomplished using 49 cycles of PCR at an annealing temperature of 62°C. For initial optimization experiments, an annealing temperature gradient was tested using an Eppendorf Mastercycler PCR machine. Subsequent amplifications were performed using a Perkin Elmer 9700 machine (Applied Biosystems). The amplified products were analyzed using ABI 310 Genetic Analyzer and Genescan 3.1 software (Applied Biosystems). Conventional DNA sequencing was accomplished as described by Gorden et al.3 Sequences were analyzed using SeqScape software (Applied Biosystems).
Results
Annealing Temperature Analysis
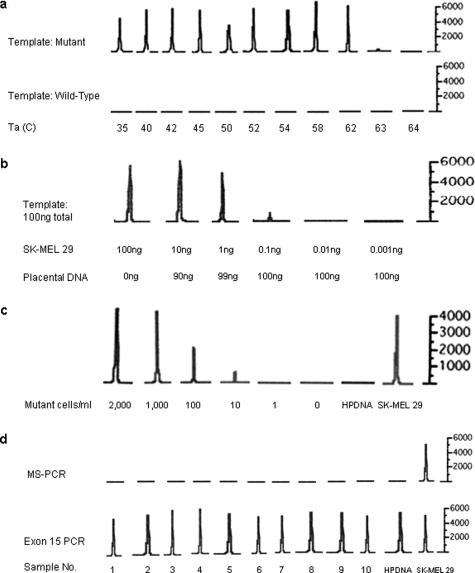
A gradient of annealing temperatures from 35 to 64°C was tested using DNA from the melanoma cell line SK-MEL 29, which carries the BRAF V600E mutation.3 Amplified products could be detected at annealing temperatures ranging from 35 to 62°C, with markedly reduced or absent amplification using annealing temperatures greater than 62°C. The negative control human placental DNA (wild-type BRAF) failed to amplify at any annealing temperature, indicating the highly specific nature of this PCR reaction (Figure 1a).
Figure 1.
Sensitivity and specificity of the MS-PCR assay. a: Annealing temperature gradient. Annealing temperatures between 35 and 64°C were tested. The amplified products were analyzed using an ABI 310 Genetic Analyzer, and the resulting electropherograms are presented. In the top row, amplification of templates containing the BRAF mutation is markedly reduced or absent at annealing temperatures of 63°C or higher. At all temperatures, the reaction failed to amplify the wild-type control (bottom row). The mutant template consisted of DNA purified from the BRAF mutant melanoma cell line SK-MEL 29; the wild-type template consisted of human placental DNA. The y axis is relative fluorescence units. b: Mixing experiment. The specified quantities of DNA from the BRAF mutant cell line SK-MEL 29 were mixed with human placental DNA (wild-type BRAF) to a sum of 100 ng of DNA. The mixture was used as a template for the MS-PCR. The limit of detection for mutant BRAF alleles using this assay was 0.1 ng of cell line DNA. c: Dilution experiment. Normal blood samples (5 ml each) were spiked with decreasing numbers of SK-MEL 29 cells, mutant for BRAF. Peripheral blood mononuclear cells were isolated, and DNA was extracted. The lowest concentration for which a peak is present is 10 cells/ml. Mutant cells were undetectable at concentrations below this and in the negative control human placental DNA (HPDNA). d: Negative controls. Ten peripheral blood samples from normal volunteers were analyzed by MS-PCR. None of the samples had detectable mutant BRAF alleles (top panel). In the bottom panel, the entire BRAF exon 15 was amplified (as expected) from all volunteer samples and from human placental (HPDNA) and SK-MEL 29 control DNA samples.
Analysis of Sensitivity Using Cell Line DNA
Two approaches were used to characterize the sensitivity of the assay and further define its specificity. In the first, decreasing amounts of DNA from the BRAF mutant cell line SK-MEL 29 were mixed with human placental DNA. Each mixture contained a total of 100 ng of DNA, and these mixtures were used as templates for the MS-PCR assay. The assay was able to detect mutant BRAF alleles for samples containing as little as 0.1 ng of mutant DNA per 100 ng of DNA (Figure 1b). Below this concentration, there was no amplification of the mutant allele. In the second approach, whole blood from a normal healthy volunteer was spiked with decreasing numbers of cells of the BRAF mutant tumor cell line SK-MEL 29. The limit of detection of the assay was 50 melanoma cells mixed in 5 ml of whole blood (10 cells/ml) (Figure 1c). In both of these experiments, there was no allelic amplification at very low concentrations of mutant BRAF, despite the presence of abundant wild-type DNA, confirming the specificity of this assay. In addition, testing of peripheral blood cell samples from 10 normal controls via MS-PCR demonstrated no detectable PCR product (Figure 1d).
Comparison of MS-PCR with Direct Sequencing Using Purified DNA
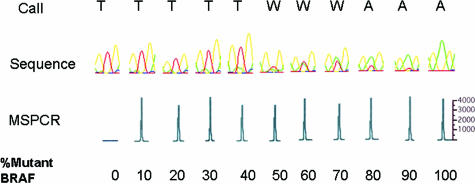
To compare the sensitivities of the MS-PCR assay and direct sequencing techniques in detecting the BRAF hotspot codon 600 mutation, we applied both techniques to samples composed of a mixture of mutant and wild-type DNA (Figure 2). Direct sequencing with automated base calling could not definitively detect mutations when the sample was comprised of less than 50% of melanoma DNA. Manual inspection of the electropherogram could only detect mutant peaks when at least 30% mutant DNA was present. The MS-PCR could detect mutant alleles in the 10% mixture, and given the results of the sensitivity analysis shown in Figure 1, it can detect mutations at much lower proportions.
Figure 2.
Sensitivity of MS-PCR versus sequencing. The sensitivity of the MS-PCR technique is compared with DNA sequencing by testing purified DNA containing 0 to 100% mutant BRAF DNA. In the two top rows, SeqScape software (Applied Biosystems) was used to identify mutant peaks (A) in place of wild type (T) (W denotes the presence of both peaks). BRAF mutations are detected when at least 50% of the sample DNA is derived from mutant tumor cells. At concentrations of 40% or less mutant DNA, conventional sequencing is unable to confidently detect the mutation. In the third row, MS-PCR is able to detect mutations with as little as 10% mutant BRAF DNA. The y axis is relative fluorescence units.
MS-PCR Can Detect Mutant BRAF Alleles in Patient Samples
Plasma Samples
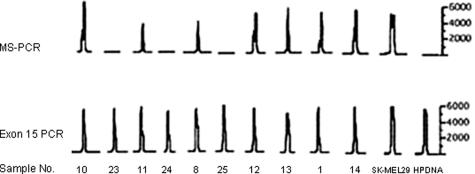
To test the clinical utility of the MS-PCR assay in melanoma patients, plasma samples from 26 patients with metastatic melanoma were tested for BRAF mutations. To control for DNA quality, all samples also underwent PCR amplification of the entire BRAF exon 15. The electropherograms for 10 patients (Figure 3) and MS-PCR results for all patients screened (Table 1) are provided. Fourteen of 26 (54%) patients had detectable mutant BRAF alleles in their plasma samples. All patients had detectable amplification of the entire BRAF exon 15, indicating adequate DNA quality in each sample. Thus, the assay is able to detect mutant BRAF alleles in plasma samples of patients in the targeted population.
Figure 3.
Detection of mutant BRAF alleles in plasma samples from patients with metastatic melanoma. Representative electropherogram results from 10 patients with metastatic melanoma. MS-PCR results are shown in the top panel; control amplification of the entire BRAF exon 15 is shown in the bottom panel. In this group, 7 of 10 (70%) patients had detectable BRAF mutations in their plasma samples. The placental DNA samples were used as controls and were negative for the mutant BRAF and positive for BRAF exon 15, as expected. The y axes are relative fluorescence units.
Table 1.
MS-PCR Results of Metastatic Melanoma Patients
| Patient | Plasma MS-PCR | Tissue MS-PCR |
|---|---|---|
| 1 | Mutant | Mutant |
| 2 | Mutant | Mutant |
| 3 | Mutant | Mutant |
| 4 | Mutant | Mutant |
| 5 | Mutant | Mutant |
| 6 | Mutant | Mutant |
| 7 | Mutant | Mutant |
| 8 | Mutant | Wild type |
| 9 | Mutant | Wild type |
| 10 | Mutant | N/A |
| 11 | Mutant | N/A |
| 12 | Mutant | N/A |
| 13 | Mutant | N/A |
| 14 | Mutant | N/A |
| 15 | Wild type | Wild type |
| 16 | Wild type | Wild type |
| 17 | Wild type | Wild type |
| 18 | Wild type | Mutant |
| 19 | Wild type | Mutant |
| 20 | Wild type | Mutant |
| 21 | Wild type | Mutant |
| 22 | Wild type | Mutant |
| 23 | Wild type | N/A |
| 24 | Wild type | N/A |
| 25 | Wild type | N/A |
| 26 | Wild type | N/A |
N/A, not available.
Tissue Samples
Tumor tissue was available from metastatic lesions of 17 of 26 patients. Of the 17 samples, 12 (71%) had detectable mutant BRAF alleles. Comparison of the plasma and tissue results for these 17 patients revealed concordance of results in 10 of 17 (59%) patients: seven patients had detectable BRAF mutations in both plasma and tumor tissue, and three had wild-type BRAF alleles in both their plasma and tumor tissues. Seven of 17 patients had discordance between the plasma and tissue results: two had mutations detected only in their plasma, and five had mutations detected only in their tumor tissue (Table 1).
Discussion
Given that patients with metastatic cancers have higher concentrations of DNA in plasma than healthy individuals (see review26), it would be clinically valuable to develop assays that use the accessibility of tumor DNA from plasma samples. In this study, we developed a fluorescence-based mutant-specific PCR assay that can detect BRAF mutations in plasma samples from patients with metastatic melanoma. The method has several technical features that make it an attractive assay for future study. It requires only a small amount of DNA, is more sensitive than direct sequencing techniques, and is highly specific for the V600E mutation.
A similar technique reported by Miller et al27 compared the sensitivity of allele-specific PCR using agarose gel electrophoresis with DNA sequencing. Allele-specific PCR could detect the mutation in as low as a 1% concentration of mutant DNA. Sequencing required at least 5% mutant DNA for visual detection of the mutant allele on the electropherogram. Comparison of BRAF mutation frequencies in melanocytic lesions using these two methods revealed higher detected mutation rates with the allele-specific PCR, as would be expected with a more sensitive assay. We have confirmed this finding by comparing sequencing with our MS-PCR and fluorescent detection system. In addition, the technique we describe can detect BRAF mutations in blood samples, greatly expanding on previous work and broadening the potential uses for BRAF mutation testing.
Our detected rate of BRAF mutations [14 of 26 (54%)] in the plasma of patients with metastatic melanoma corresponds well with previously described rates in tumor tissue from this patient population.2,3,4,28 In addition, MS-PCR of 17 tissue samples from these patients revealed concordant results in 10 of 17 (59%) patients (seven mutant in blood and tissue, and three wild type in blood and tissue). Among the discordant results, two patients had tumors negative for mutant BRAF but plasma that was positive. Interestingly, one patient had a positron emission tomography/computed tomography scan performed at the time of the subcutaneous tumor biopsy analyzed in this study, revealing multifocal disease with metastases to the lung, peritoneum, and muscle. Thus, it is possible that these other metastatic sites contained the mutation, and it was from one of these sources that the blood-borne mutant BRAF allele originated. In support of this hypothesis, we have shown in a previous study that there is heterogeneity in BRAF mutation status between metastases in the same patient.29 On the other hand, the mutant BRAF alleles in the bloodstream may have been derived from a portion of the biopsied tumor that was not used for the tissue-based PCR. A recent study examining short-term human melanoma cultures revealed a single culture in which both BRAF and NRAS mutations could be detected (a rare finding); however, these mutations were mutually exclusive at the single-cell level.30 These results provide additional direct evidence that some melanomas may be heterogeneous with respect to BRAF mutations. In the two cases presented here, it is possible that the MS-PCR results of the plasma are a more accurate representation of the patients’ tumor(s) than the single biopsy specimen available for analysis. The other five patients with discordant results had tumors that were positive for mutant BRAF, but plasma samples were negative. One explanation for this discordance is that shedding of mutant BRAF in the bloodstream may be intermittent. Curry et al31 showed in their study of circulating melanoma cells that the sensitivity of detecting circulating tumor markers increased with serial blood draws. In this regard, we are embarking on a study with a larger sample size that will incorporate serial blood draws. In general, we may find that repeated sampling of the blood compartment has the potential to be the easiest and most useful method to detect tumor-associated BRAF mutations in melanoma and other tumor types in which substantial frequencies of BRAF mutations have been detected (eg, thyroid, colon, and ovarian).12,25,32,33
Although there is not enough clinical data available today to determine whether therapeutic response to currently available BRAF-inhibiting agents correlates with BRAF mutational status, preclinical testing of these drugs reveals that BRAF V600E is more sensitive to these agents than wild-type BRAF.34,35 In addition, given preclinical data showing that BRAF mutant cells are extremely sensitive to mitogen-activated protein kinase kinase inhibition compared with wild-type and RAS mutant cells, it is possible that patient stratification based on BRAF mutation status may ultimately be useful for molecularly targeted therapies aimed at multiple kinases in the mitogen-activated protein kinase pathway.36
There are important limitations to this assay. Because not all melanomas harbor BRAF mutations, and this assay was specifically designed to distinguish BRAF mutant from BRAF wild-type tumors, it cannot be used as a tool for staging and monitoring all melanoma patients. In addition, not all patients with BRAF mutant tumors will necessarily have BRAF mutant DNA in their circulation. Thus, the potential clinical utility of this assay at this point is as an adjunctive study to tissue-based testing. On the other hand, it could serve as a stand-alone assay in patients with tumors that are unable to be biopsied. Finally, we recognize that although the assay detects the V600E mutation and is predicted to detect the much less common V600K mutation, it is not expected to detect the V600R mutation. This mutation is quite rare; it is estimated to occur in only 3 to 6% of melanomas with BRAF mutations. Thus, the current assay remains a potentially powerful clinical tool3,37 and can be modified to detect the more rare mutation if needed.
Our laboratory is currently pursuing several avenues of study to expand on this work. We are developing a quantitative PCR assay based on this qualitative technique, which may expand the potential utility of the assay and may be useful for staging and monitoring of patients with BRAF mutations. We are also working to determine whether the presence of circulating mutant BRAF alleles correlates with patients’ responses to BRAF inhibitors and clinical outcome. Last, we are also investigating whether testing DNA isolated from peripheral blood cells may be advantageous when compared with plasma-based assays for the detection of BRAF mutations. We are hopeful that with further refinements, the discordance between blood- and tissue-based results will decrease and that in the future, we will be able to add blood-based testing to the techniques used to type patients for targeted therapies. As such, our lab is working to explore these routes to improve blood-based testing of patients with melanoma.
Acknowledgments
We thank the patients for participating in this study. We are grateful to Daniel Freedberg for laboratory assistance and to Caroline Chang and Joanna Spira for help with the clinical data.
Footnotes
Supported by National Institutes of Health grant R21 CA109388 (to D.P. and I.O.) and grant T32 AR07190-31 (to M.Y.), and in part by the use of facilities at the Manhattan Veterans Affairs Medical Center.
References
- Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, Moses TY, Hostetter G, Wagner U, Kakareka J, Salem G, Pohida T, Heenan P, Duray P, Kallioniemi O, Hayward NK, Trent JM, Meltzer PS. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Gorden A, Osman I, Gai W, He D, Huang W, Davidson A, Houghton AN, Busam K, Polsky D. Analysis of BRAF and N-RAS mutations in metastatic melanoma tissues. Cancer Res. 2003;63:3955–3957. [PubMed] [Google Scholar]
- Kumar R, Angelini S, Czene K, Sauroja I, Hahka-Kemppinen M, Pyrhonen S, Hemminki K. BRAF mutations in metastatic melanoma: a possible association with clinical outcome. Clin Cancer Res. 2003;9:3362–3368. [PubMed] [Google Scholar]
- Shinozaki M, Fujimoto A, Morton DL, Hoon DS. Incidence of BRAF oncogene mutation and clinical relevance for primary cutaneous melanomas. Clin Cancer Res. 2004;10:1753–1757. doi: 10.1158/1078-0432.ccr-1169-3. [DOI] [PubMed] [Google Scholar]
- Dong J, Phelps RG, Qiao R, Yao S, Benard O, Ronai Z, Aaronson SA. BRAF oncogenic mutations correlate with progression rather than initiation of human melanoma. Cancer Res. 2003;63:3883–3885. [PubMed] [Google Scholar]
- Uribe P, Wistuba II, Gonzalez S. BRAF mutation: a frequent event in benign, atypical, and malignant melanocytic lesions of the skin. Am J Dermatopathol. 2003;25:365–370. doi: 10.1097/00000372-200310000-00001. [DOI] [PubMed] [Google Scholar]
- Maldonado JL, Fridlyand J, Patel H, Jain AN, Busam K, Kageshita T, Ono T, Albertson DG, Pinkel D, Bastian BC. Determinants of BRAF mutations in primary melanomas. J Natl Cancer Inst. 2003;95:1878–1890. doi: 10.1093/jnci/djg123. [DOI] [PubMed] [Google Scholar]
- Govindarajan B, Bai X, Cohen C, Zhong H, Kilroy S, Louis G, Moses M, Arbiser JL. Malignant transformation of melanocytes to melanoma by constitutive activation of mitogen-activated protein kinase kinase (MAPKK) signaling. J Biol Chem. 2003;278:9790–9795. doi: 10.1074/jbc.M212929200. [DOI] [PubMed] [Google Scholar]
- Cohen C, Zavala-Pompa A, Sequeira JH, Shoji M, Sexton DG, Cotsonis G, Cerimele F, Govindarajan B, Macaron N, Arbiser JL. Mitogen-activated protein kinase activation is an early event in melanoma progression. Clin Cancer Res. 2002;8:3728–3733. [PubMed] [Google Scholar]
- Satyamoorthy K, Li G, Gerrero MR, Brose MS, Volpe P, Weber BL, Van Belle P, Elder DE, Herlyn M. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res. 2003;63:756–759. [PubMed] [Google Scholar]
- Rajagopalan H, Bardelli A, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Tumorigenesis: rAF/RAS oncogenes and mismatch-repair status. Nature. 2002;418:934. doi: 10.1038/418934a. [DOI] [PubMed] [Google Scholar]
- Brose MS, Volpe P, Feldman M, Kumar M, Rishi I, Gerrero R, Einhorn E, Herlyn M, Minna J, Nicholson A, Roth JA, Albelda SM, Davies H, Cox C, Brignell G, Stephens P, Futreal PA, Wooster R, Stratton MR, Weber BL. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62:6997–7000. [PubMed] [Google Scholar]
- Mercer K, Giblett S, Green S, Lloyd D, Darocha Dias S, Plumb M, Marais R, Pritchard C. Expression of endogenous oncogenic V600EB-raf induces proliferation and developmental defects in mice and transformation of primary fibroblasts. Cancer Res. 2005;65:11493–11500. doi: 10.1158/0008-5472.CAN-05-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani SR, Jacobetz MA, Robertson GP, Herlyn M, Tuveson DA. Suppression of BRAF(V599E) in human melanoma abrogates transformation. Cancer Res. 2003;63:5198–5202. [PubMed] [Google Scholar]
- Sumimoto H, Miyagishi M, Miyoshi H, Yamagata S, Shimizu A, Taira K, Kawakami Y. Inhibition of growth and invasive ability of melanoma by inactivation of mutated BRAF with lentivirus-mediated RNA interference. Oncogene. 2004;23:6031–6039. doi: 10.1038/sj.onc.1207812. [DOI] [PubMed] [Google Scholar]
- Simon R, Maitournam A. Evaluating the efficiency of targeted designs for randomized clinical trials. Clin Cancer Res. 2004;10:6759–6763. doi: 10.1158/1078-0432.CCR-04-0496. [DOI] [PubMed] [Google Scholar]
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- Tsao MS, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S, Squire J, Lorimer I, Zhang T, Liu N, Daneshmand M, Marrano P, da Cunha Santos G, Lagarde A, Richardson F, Seymour L, Whitehead M, Ding K, Pater J, Shepherd FA. Erlotinib in lung cancer: molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C, Niederwieser D, Resta D, Capdeville R, Zoellner U, Talpaz M, Druker B, Goldman J, O’Brien SG, Russell N, Fischer T, Ottmann O, Cony-Makhoul P, Facon T, Stone R, Miller C, Tallman M, Brown R, Schuster M, Loughran T, Gratwohl A, Mandelli F, Saglio G, Lazzarino M, Russo D, Baccarani M, Morra E. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346:645–652. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- Hardin EPA, Liebes L, Osman I, Hamilton A, Soon J, Polsky D, Friedman K, Wright J, Muggia F. A phase II trial of BAY 43-9006 in metastatic melanoma with molecularly targeted BRAF status. J Clin Oncol. 2006;24:8046. [Google Scholar]
- Ahmad T, Marais R, Pyle L, James M, Schwartz B, Gore M, Eisen T. BAY 43–9006 in patients with advanced melanoma: The Royal Marsden experience (Meeting Abstract). J Clin Oncol. 2004;22(14S):7506. [Google Scholar]
- Flaherty KT, Redlinger M, Schuchter LM, Lathia CD, Weber BL, O’Dwyer PJ. Phase I/II, pharmacokinetic and pharmacodynamic trial of BAY 43-9006 alone in patients with metastatic melanoma (Meeting Abstract). J Clin Oncol. 2005;23(16 S):3037. [Google Scholar]
- Xu X, Quiros RM, Gattuso P, Ain KB, Prinz RA. High prevalence of BRAF gene mutation in papillary thyroid carcinomas and thyroid tumor cell lines. Cancer Res. 2003;63:4561–4567. [PubMed] [Google Scholar]
- Ziegler A, Zangemeister-Wittke U, Stahel RA. Circulating DNA: a new diagnostic gold mine? Cancer Treat Rev. 2002;28:255–271. doi: 10.1016/s0305-7372(02)00077-4. [DOI] [PubMed] [Google Scholar]
- Miller CJ, Cheung M, Sharma A, Clarke L, Helm K, Mauger D, Robertson GP. Method of mutation analysis may contribute to discrepancies in reports of (V599E)BRAF mutation frequencies in melanocytic neoplasms. J Invest Dermatol. 2004;123:990–992. doi: 10.1111/j.0022-202X.2004.23468.x. [DOI] [PubMed] [Google Scholar]
- Omholt K, Platz A, Kanter L, Ringborg U, Hansson J. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483–6488. [PubMed] [Google Scholar]
- Chang DZ, Panageas KS, Osman I, Polsky D, Busam K, Chapman PB. Clinical significance of BRAF mutations in metastatic melanoma. J Transl Med. 2004;2:46. doi: 10.1186/1479-5876-2-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensi M, Nicolini G, Petti C, Bersani I, Lozupone F, Molla A, Vegetti C, Nonaka D, Mortarini R, Parmiani G, Fais S, Anichini A. Mutually exclusive NRAS(Q61R) and BRAF(V600E) mutations at the single-cell level in the same human melanoma. Oncogene. 2006;25:3357–3364. doi: 10.1038/sj.onc.1209379. [DOI] [PubMed] [Google Scholar]
- Curry BJ, Myers K, Hersey P. Utility of tests for circulating melanoma cells in identifying patients who develop recurrent melanoma. Recent Results Cancer Res. 2001;158:211–230. doi: 10.1007/978-3-642-59537-0_22. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Deng G, Matsuzaki K, Kakar S, Kim GE, Miura S, Sleisenger MH, Kim YS. BRAF mutation, CpG island methylator phenotype and microsatellite instability occur more frequently and concordantly in mucinous than non-mucinous colorectal cancer. Int J Cancer. 2006;118:2765–2771. doi: 10.1002/ijc.21701. [DOI] [PubMed] [Google Scholar]
- Singer G, Oldt R, III, Cohen Y, Wang BG, Sidransky D, Kurman RJ, Shih IeM. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95:484–486. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- da Rocha Dias S, Friedlos F, Light Y, Springer C, Workman P, Marais R. Activated B-RAF is an Hsp90 client protein that is targeted by the anticancer drug 17-allylamino-17-demethoxygeldanamycin. Cancer Res. 2005;65:10686–10691. doi: 10.1158/0008-5472.CAN-05-2632. [DOI] [PubMed] [Google Scholar]
- Karasarides M, Chiloeches A, Hayward R, Niculescu-Duvaz D, Scanlon I, Friedlos F, Ogilvie L, Hedley D, Martin J, Marshall CJ, Springer CJ, Marais R. B-RAF is a therapeutic target in melanoma. Oncogene. 2004;23:6292–6298. doi: 10.1038/sj.onc.1207785. [DOI] [PubMed] [Google Scholar]
- Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, Ye Q, Lobo JM, She Y, Osman I, Golub TR, Sebolt-Leopold J, Sellers WR, Rosen N. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas NE, Alexander A, Edmiston SN, Parrish E, Millikan RC, Berwick M, Groben P, Ollila DW, Mattingly D, Conway K. Tandem BRAF mutations in primary invasive melanomas. J Invest Dermatol. 2004;122:1245–1250. doi: 10.1111/j.0022-202X.2004.22523.x. [DOI] [PubMed] [Google Scholar]