Abstract
We studied the feasibility of using real-time quantitative PCR to determine HER-2 DNA amplification and mRNA expression in microdissected formalin-fixed, paraffin-embedded breast tumors and compared this with standard immunohistochemistry (IHC) and fluorescent in situ hybridization (FISH) methods. Study cases (27 carcinomas and 3 ductal breast carcinoma in situ (DCIS) cases) showed varying Her-2 expression as determined by IHC (HercepTest). In carcinomas, there was a good correlation between HER-2 DNA amplification and strong HER-2 protein expression detected by FISH and IHC, respectively. A single DCIS case was amplified in FISH, but not in IHC. Both HER-2 gene amplification and expression could be quantified in microdissected paraffin-embedded tumors using real-time PCR, DNA and RNA being successfully detected in 146 of 150 (97%) and 141 of 150 (94%) samples, respectively. PCR analysis for HER-2 DNA amplification using the LightCycler HER2/neu DNA Quantification kit (Roche Molecular Biochemicals, Mannheim, Germany) correlated fairly well with IHC and FISH. All IHC HER-2 3+ tumors were amplified according to the kit, as was the FISH-amplified DCIS case. DNA-PCR identified five additional tumors as being amplified. Interestingly, all these scored 2+ with the HercepTest, but were negative using FISH. We believe that real-time quantitative PCR analysis of HER-2 DNA amplification following microdissection represents a useful supplementary or perhaps even an alternative technique for establishing HER-2 status in paraffin-embedded tumors.
Human epidermal growth factor receptor-2 (HER-2) is a proto-oncogene located at 17q21 that encodes a 185-kd transmembrane glycoprotein with tyrosine kinase activity. HER-2 (also known as c-erbB-2 or neu) is a member of the epidermal growth factor receptor family and is now recognized as a key oncogene in several malignancies. Amplification and/or strong expression of HER-2 can be seen in 20% to 30% of invasive breast carcinomas. It is regarded as a marker of adverse clinical outcome and as a predictive marker for reduced response to certain chemotherapy and hormonal treatments.1 Even more importantly, positive HER-2 status predicts for response to therapy with trastuzumab (Herceptin), a humanized monoclonal antibody directed against the external domain of the HER-2 protein which has been shown to be effective in prolonging survival in patients with receptor-positive metastatic breast carcinoma.2 Thus, there is a clear need for reliable and robust methods for accurately determining HER-2 status in tumors. Ideally, such methods should also be simple, quantitative, and widely applicable (eg, capable of being performed on formalin-fixed, paraffin-embedded (FFPE) archival biopsies or on small and/or precancerous specimens). However, while the clinical benefit of determining HER-2 status in breast carcinomas is now accepted, there is no consensus on the best diagnostic assay to use for this purpose.
HER-2 status can be analyzed at the DNA-, the mRNA-, or the protein level. Various techniques are available for assessing these different target molecules, each with benefits and drawbacks.3 For practical reasons, immunohistochemistry (IHC) using an anti-HER-2 antibody is by far the most frequently used method for assessing HER-2 status. IHC is available as a standard technique in pathology laboratories, and can be easily used on archival FFPE tissues. Furthermore, it is tumor cell-specific, the observer being able to distinguish signals from neoplastic and non-neoplastic cells. As a result, reports published on the clinical relevance of HER-2 expression have almost all used IHC. However, major disadvantages of HER-2 IHC assessment are that it is at best a semi-quantitative method with considerable inter-observer variations reported in some studies.4 Furthermore, the many available HER-2 antibodies show variable sensitivities and specificities5 that, together with differences in tumor fixation, antigen retrieval method, and IHC technique, contribute to considerable assay imprecision. While this imprecision is improved by the use of standardized IHC tests (such as the HercepTest), there is widespread agreement that IHC HER-2 scores at the 2+ level need to be confirmed with another technique.6,7
Fluorescence in situ hybridization (FISH) is a common alternative method for assessing HER-2 status. It is a sensitive and apparently highly specific technique for measuring HER-2 gene amplification at the single-cell level,8 a change that appears to be closely correlated with strong protein expression9 and thus of clinical relevance. In contrast to IHC, FISH can give a more objective and quantitative measure of HER-2 abnormalities, and is widely regarded as the gold standard for assaying HER-2 status. However, the technique is more labor-intensive and expensive, and it requires both more specialized equipment and expertise compared with IHC.
Blotting techniques (Southern, Northern, and Western) can be used to measure HER-2 molecules, but they are technically difficult, require large amounts of fresh tissue, and are impractical for routine screening purposes. In addition, these techniques are not tumor cell-specific. Thus, the HER-2 status will be modified by the dilutional effect caused by the often large numbers of non-neoplastic cells (inflammatory, stromal, and normal) found in all tumors, resulting in an underestimation of gene amplification or expression. This problem can be reduced if analysates are enriched for target cells of interest, eg, by histological microdissection. Using laser-assisted microdissection (LAM), homogeneous tumor cell samples can be procured rapidly and precisely from both fresh/frozen or from archival FFPE tissues.10,11,12 This allows the detection of tumor-specific genetic alterations in otherwise heterogeneous samples, and enables a more correct detection and quantification of tumor DNA and mRNA.
Polymerase chain reaction (PCR)-based assays can determine changes in both HER-2 gene number and expression. By using real-time quantitative PCR, the target can be both amplified and detected simultaneously, making the assay a potentially sensitive, specific, reliable, and cost-effective way of measuring HER-2 changes. Routine methods of histological fixation and processing either destroy or damage RNA, or cause such extensive tissue cross-linking as to make it inaccessible to analysis.13 However, the high sensitivity of real-time quantitative PCR means that even minute amounts of DNA or RNA may be detectable in FFPE tissues14, opening up the possibility of performing retrospective clinical and molecular studies on the large specimen archives stored in pathology institutes.
In this study, we focused on the feasibility of using real-time quantitative PCR analysis of DNA and mRNA from laser-microdissected FFPE archival breast cancer tissues to determine HER-2 status, and compared these results with those obtained using IHC and FISH. We show that HER-2 amplification and mRNA expression can be reliably detected by real-time quantitative PCR in archival microdissected FFPE tissues. In particular, our results suggest that PCR analysis of HER-2 DNA amplification in microdissected cells using the LightCycler HER2/neu DNA Quantification kit (Roche Molecular Biochemicals, Mannheim, Germany) may be used as an alternative to the traditional tumor cell-specific assays of HER-2 status.
Materials and Methods
Tissue Samples
Thirty cases of breast tumor were selected from the archives of the pathology institutes, Aarhus University Hospital depending on their HER-2 protein status assessed by the HercepTest. Paraffin-embedded material from 27 representative primary ductal breast carcinomas (3+, n = 8; 2+, n = 8; 1+, n = 8; 0, n = 3) and three cases of ductal breast carcinoma in situ (DCIS) were used. All tissues were routine diagnostic, surgical specimens that had been fixed, processed, and stored using standard histological protocols. The oldest paraffin blocks were 48 months old. Serial sections for microdissection (5 μm) and FISH (2 μm) were cut simultaneously.
Immunohistochemistry
We used the semi-quantitative immunocytochemical HercepTest (DakoCytomation, Copenhagen, Denmark) to assess HER-2 protein status, according to the manufacturer’s instructions. In brief, deparaffinized sections (4 μm) mounted on poly-L-lysine coated slides were heated in diluted Epitope Retrieval Solution (10 mmol/L citrate buffer) for 45 minutes using a water bath at 95°C, followed by cooling at room temperature for 20 minutes. After rinsing and blocking endogenous peroxidase, the sections were covered with primary polyclonal antibody (rabbit anti-human HER2 protein) in a humid chamber for 30 minutes. Slides were washed and then incubated in Visualization Reagent (dextran polymer conjugated with horseradish peroxidase and goat anti-rabbit immunoglobulins, EnVision, DakoCytomation) for 30 minutes at room temperature, and washed again before color development in 3,3′-diaminobenzidine substrate-chromogen (DAB) solution. The sections were counter-stained in Mayer’s hematoxylin. To validate the staining procedure, we simultaneously processed negative and positive controls provided with the kit consisting of sections of FFPE breast carcinoma cell lines representing various levels of Her-2 expression. The same technician performed the staining procedure for all cases. We scored tumor cells for Her-2 in accordance with the manufacturer’s instructions: no visible staining or membrane staining in less than 10% of cells, 0; faint, incomplete membrane staining detected in more than 10% of cells, 1+; weak to moderate complete membrane staining in more than 10% of cells, 2+; strong and complete membrane staining in more than 10% of malignant cells, 3+. Scoring was performed twice (blinded) by the same experienced pathologist.
Fluorescence In Situ Hybridization
Paraffin sections were hybridized using the Vysis PathVysion HER-2/neu DNA probe kit (Abbott Laboratories, Abbott Park, IL) in accordance with the manufacturer’s protocol. For each case, two sections (2 μm) were mounted on coated-glass slides (Superfrost Plus, Menzel, Germany). One section was stained with hematoxylin and eosin and was used as a microscopic control for locating dense tumor cell populations suitable for FISH analysis. The second section was used for FISH. The slide was deparaffinized, dehydrated in graded ethanols, denatured in 70% formamide, 2X standard saline citrate (SSC; 20X SSC = 3 mol/L NaCl2, 0.3 mol/L Na citrate, pH 5.3) at 70°C. Hybridization was carried out overnight in a humid chamber at 37°C in the presence of SpectrumOrange-labeled HER-2 DNA probe and SpectrumGreen-labeled centromere 17 reference probe. After washing in 2X SSC/0.3% NP-40, the nuclei were counter-stained with DAPI (0.2 μmol/L 4.6-diamino-2-phenyldole). Slides were examined using a fluorescence microscope at ×100 magnification. At least 120 non-overlapping nuclei were counted in each patient, the counting area blindly selected by the same technician within the rather large marked area on the control H&E stain. These areas were carefully chosen to include large homogeneous tumor regions, with as few intervening inflammatory or stromal non-neoplastic cells as possible. In the tumors showing invasive carcinoma, areas with DCIS were excluded. The signals for Her-2/neu and centromere 17 were recorded and the ratio calculated for each slide by the same technician, without knowledge of the IHC HER-2 status of the patients. In accordance with the manufacturer’s protocol, we did not score nuclei with no signals, with signals of only one color, or with signals in overlapping areas. Nuclei with insufficient counter-stain to determine the nuclear border were not counted. We regarded a ratio ≥2.2 as being truly amplified and a ratio <1.8 as not being amplified. Cases falling in the cut-off area between 1.8 to 2.2 were repeated.
Laser Microbeam Microdissection (LMM)
Sections were cut under RNase-free conditions and, mounted for laser-assisted microdissection using a modification of the MOMeNT technique.15 Microdissection of FFPE tissues was carried out using a high-resolution UV-laser system (μ-Cut, Molecular Machines & Industries, Glattbrug, Switzerland) as previously described.12 In brief, the sections were deparaffinized in xylene, rehydrated in graded ethanols, stained with hematoxylin, rinsed in tap water, and finally immersed in 99% ethanol. After drying the sections, the areas of interest were identified. To increase the quality of morphology, we used Cytotec (Schuco International, London, UK) as an optical medium.12 For each analysis, five homogeneous tumor areas were laser-cut (∼300 cells each) and then captured using a sterile disposable needle. We dissected neighboring tumor areas to minimize the influence of intratumoral heterogeneity on the results. A naked area of the membrane was cut as a negative control. The tissue-fragments were put in an Eppendorf tube containing 200 μl RNA digestion buffer (20 mmol/L Tris, pH 7.5, 20 mmol/L EDTA, 1% SDS in sterile water) and 5 μl proteinase K (20 mg/ml, Roche Molecular Biochemicals), for RNA extraction, incubated overnight at 60°C, inactivated at 95°C for 10 minutes, and stored at −80°C.16 This procedure was repeated for DNA extraction using the same membrane-mounted slides. The cut fragments were transferred to an Eppendorf tube with 30 μl DNA extraction buffer containing 50 mmol/L Tris, pH. 8.1, 1 mmol/L EDTA, pH.8.0, and 0.5% Tween 20 and 0.24% proteinase K (20 mg/ml), incubated overnight at 55°C, and inactivated for 10 minutes at 95°C.17 The samples were kept at −20°C.
DNA Extraction, Total RNA Extraction, and Reverse Transcription
DNA extraction samples were used without any precipitation steps. Total RNA was extracted using the TRIzol-reagent according to the manufacturer (Life Technologies, Gaithersburg, MD), followed by precipitation with an equal volume of isopropanol, 0.1 volume 3 mol/L sodium acetate (pH 5.2) and 1 μl glycogen (20 mg/ml, Roche). The pellet was washed in 70% ethanol, dried and resuspended in 10 μl RNase-free water. Total RNA from the human bladder cell line HCV29 cell line18 was isolated with the Qiagen Total RNA Isolation kit (Qiagen Inc., Valencia, CA) and used for generating the calibration curve. Reverse transcription (RT) took place in a final reaction volume of 20 μl containing 50 units MuLV reverse transcriptase (Applied Biosystems, Foster City, CA), 10X PCR buffer, 5 pmol specific antisense primer (also used in PCR) for either Her-2/neu or β-actin, 4 U RNase inhibitor (Roche), 4 mmol/L of each dNTPs, 6.25 mmol/L MgCl2, and 2 μl template. The RT step was performed on a Perkin Elmer 9700 Thermocycler at 42°C for 30 minutes followed by 94°C for 5 minutes.
Real-Time Quantitative RT-PCR Analysis of HER-2 Gene Expression
Analysis of HER-2/neu and of housekeeping gene β-actin expression was carried out using the LightCycler system (Roche Molecular Biochemicals), following the manufacturer’s instructions. The theory and principles of real-time quantitative PCR are described in detail elsewhere.19 We used intron-spanning PCR amplicons and hydrolysis probes.16,20 A standard curve for gene expression of Her-2 and β-actin was constructed using total RNA from a human urothelial cell line HCV 29, as described in detail previously.14 In brief, 10-fold dilutions of RNA in water were made to give 10 different calibrators, each with a known amount of HCV 29 RNA. For both products a standard curve was generated and included in each PCR run. PCR amplification for analyzing gene expression was performed in a 20-μl reaction volume. A reaction mixture (Lightcycler DNA Master Hybridization Probes mixture, Roche Molecular Biochemicals) containing reaction buffer, nucleotides, and Taq polymerase was used for the PCR according to the supplier’s instructions. Probe, sense primer, antisense primer, 3.2 μl H2O and 8.0 μl cDNA were added to this reaction mixture (see Table 1 for sequence and concentration of primers and probes). Quantification was initiated by incubation for 1 minute at 94°C, followed by 40 cycles with the following profile: specific annealing temperature for 2 seconds, extension at 72°C for 10 seconds and denaturation at 94°C, immediately followed by the next amplification cycle. We calculated the relative quantification using the ratio between HER-2/neu and β-actin mRNA, to eliminate the constant differences between genes and gene transcripts, that might be generated during the process (eg, differences due to variations in tissue preparation, template extraction, mRNA integrity, and PCR17). To ensure that no DNA contamination had occurred, we included 20 samples without reverse transcriptase.
Table 1.
Sequences of Primers and Hydrolysis Probes Employed for Real-Time Quantitative RT-PCR
| Oligonucleotide | Sequence | Product size and reference (in parenthesis) |
|---|---|---|
| HER-2/neu FP(5 pmol) | 5′-cca gga cct gct gaa ctg gt-3′ | 72 bp (16) |
| HER-2/neu RP(5 pmol) | 5′-tgt acg agc cgc aca tcc-3′* | |
| HER-2/neu probe(1 pmol) | FAM-cag att gcc aag ggg atg agc tac ctg-TAMRA | |
| β-actin FP(10 pmol) | 5′-cca cac tgt gcc cat cta cg-3′ | 99 bp (20) |
| β-actin RP(10 pmol) | 5′-agg atc ttc atg agg tag tca gtc ag-3′* | |
| β-actin probe(1 pmol) | FAM-atg ccc tcc ccc atg cca tcc tgc gt-TAMRA |
The reverse primers were used in both reverse transcription and in PCR.
Abbreviations: FP, forward primer; RP, reverse primer; FAM, 6-carboxyfluorescein; TAMRA, 6-carboxytetramethylrhodamin.
Real-Time Quantitative PCR Analysis of HER-2 Gene Amplification
The real-time PCR protocol was established in accordance with the manufacturer’s instructions. Test DNA was used directly without any further purification or precipitation. The relative quantification of the HER-2/neu gene and a reference gene was performed using the LightCycler HER2/neu DNA Quantification kit (Roche Molecular Biochemicals). A 112-bp fragment of the HER-2/neu gene and a 133-bp fragment of the reference gene gastrin were amplified during the PCR reaction. Simultaneous quantification of the HER-2/neu gene and the reference gene was accomplished by using two different LightCycler hybridization probes (Red-705 and Red-640, respectively), enabling dual color detection in the same capillary. Hybridization probes, enzyme, and reference DNA were mixed together with 5 μl of template DNA, resulting in a final volume of 20 μl. Activation of the enzyme (hot-start) and denaturation of the template DNA was performed at 95°C for 10 minutes. This was followed by denaturation at 95°C for 10 seconds, annealing at 58°C for 10 seconds, and elongation at 72°C for another 10 seconds. This profile was repeated 45 times, after which the specimens were cooled to 40°C. The kit contains a calibrator DNA, to allow for reliable and reproducible data to be produced, and to make generation of a calibration curve possible. The HER-2 and gastrin copy numbers are determined using the ratio of Ct values of the samples compared to the ratio target/reference generated in the calibration curve. We included three additional samples with known amplification status as positive controls: one without amplification, and two with moderate and strong amplification, respectively, enabling us to calculate the inter-assay variation (coefficient of variation; CV). According to the manufacturers, a twofold increased ratio (≥2) should be regarded as positive for HER-2/neu DNA amplification.
Statistical Analysis
Statistical analysis was performed using SPSS Version 11, setting the statistical significance at P < 0.05. The data obtained from real-time quantitative RT-PCR were analyzed with a linear mixed model for repeated measurements, where group is the fixed effect and patient is the random effect. This statistical model furthermore allows for analysis of total variation contributed by variations between the patients in the same group (var1) and the variation secondary to tumor heterogeneity and/or measurement inaccuracies (var2). The same statistical model was used to analyze data obtained with the DNA quantification kit. CV was calculated for inter-assay variation on the data obtained by the DNA quantification kit. Results were compared using scatter plots.
Results
Immunohistochemical Analysis for HER-2/neu Using the HercepTest
HER-2 protein status was assessed with the HercepTest. Eight cases scored 3+, eight cases 2+, eight cases 1+, and 3 cases 0. All three DCIS cases scored 1+. A repeat test (blinded) gave identical results.
FISH Analysis of HER-2 Using PathVysion DNA Probe Kit
All cases were suitable for FISH analysis. Nine of 30 tumors (30%) were amplified for the HER-2/neu gene. Eight were carcinomas, all of which also showed strong expression by the HercepTest. One case (case no. 29) of DCIS was also amplified. The remaining 19 carcinomas and two DCIS cases were not amplified at the DNA level (Table 2). In the amplified cases, HER-2 signals were mostly bright and clustered, although two carcinomas showed innumerable signals. In some cases, signals were difficult to interpret, and hybridization had to be extended over 2 nights. Five of the 8 tumors, which were amplified in both IHC and FISH assays (62.5%), contained signals for more than two cep-17 regions in at least 25% of the tumor cells. In comparison, more than two signals for cep-17 were found in all 8 IHC 2+ cases, in 7 of 8 IHC 1+ cases, and in 2 of 3 IHC 0 cases. In the DCIS lesions, only the single FISH-amplified case, had more than two signals for cep-17 in some tumor nuclei. In general, neighboring tumor cells in most cases displayed distinct heterogeneity, with regard to both Her-2 and cep-17 signals.
Table 2.
Comparison of IHC, FISH, and Real-Time PCR Analyses of HER-2 in Formalin-Fixed, Paraffin-Embedded Breast Tumors
| Group* | Case no. | Hercep1&2† 0, 1+, 2+, 3+ | FISH HER-2/CEP 17‡ | Ratio mRNA§ HER-2/ β-actin N = 5 | Ratio DNA¶ HER-2/gastrin N = 5 |
|---|---|---|---|---|---|
| A | 1 | 3 | >7.0 | 149.88 [107.55; 179.38] | 23.22 [10.58; 30.48] |
| 2 | 3 | 6.0 | 85.50 [23.01; 130.19] | 19.59 [6.6; 34.25] | |
| 3 | 3 | 3.1 | No result∥ | 2.33 [0.62; 34.44] | |
| 4 | 3 | >7.5 | 182.92 [105.93; 262.1] | 67.45 [41.22; 77.66] | |
| 5 | 3 | 6.0 | 222.12 [183.96; 262.1] | 43.79 [7.3; 94.38] | |
| 6 | 3 | 6.6 | 24.46 [3.52; 43.97] | 17.98 [6.67; 25.63] | |
| 7 | 3 | 4.2 | 29.59 [10.53; 43.67] | 4.12 [1.98; 6.48] | |
| 8 | 3 | 4.0 | 65.86 [33.78; 120.2] | 7.48 [4.73; 11.18] | |
| B | 9 | 2 | 1.1 | 17.06 [9.71; 35.88] | 2.89 [1.8; 3.42] |
| 10 | 2 | 1.1 | 4.82 [3.35; 7.98] | 2.46 [1.51; 2.69] | |
| 11 | 2 | 1.1 | 58.93 [31.39; 98.55] | 2.08 [1.85; 2.38] | |
| 12 | 2 | 1.2 | 6.59 [5.42; 10.05] | 1.88 [1.38; 2.15] | |
| 13 | 2 | 0.9 | 61.61 [42.65; 82.67] | 2.13 [1.44; 2.57] | |
| 14 | 2 | 1.0 | 7.67 [4.52; 11.29] | 1.91 [0.86; 3.01] | |
| 15 | 2 | 1.0 | 4.82 [0.92; 9.69] | 2.6 [1.87; 3.93] | |
| 16 | 2 | 1.0 | 2.85 [1.93; 5.1] | 1.98 [1.24; 2.4] | |
| C | 17 | 1 | 0.8 | 18.05 [8.54; 36.41] | 0.93 [0.67; 1.14] |
| 18 | 1 | 1.1 | 43.85 [34.81; 51.71] | 0.64 [0.03; 1.24] | |
| 19 | 1 | 1.1 | 8.62 [3.46; 19.41] | 0.34 [0.09; 0.93] | |
| 20 | 1 | 0.9 | 26.23 [10.74; 63.1] | 0.37 [0.05; 0.73] | |
| 21 | 1 | 1.1 | 17.45 [10.64; 35.95] | 1.58 [1.26; 1.74] | |
| 22 | 1 | 1.1 | 9.89 [6.38; 13.27] | 1.45 [0.16; 2.57] | |
| 23 | 1 | 1.3 | 13.93 [9.55; 21.75] | 1.12 [0.28; 1.58] | |
| 24 | 1 | 0.9 | 10.61 [6.1; 13.89] | 1.24 [0.54; 3.15] | |
| D | 25 | 0 | 1.0 | 51.53 [7.77; 130.58] | 1.79 [0.86; 2.46] |
| 26 | 0 | 1.0 | 0.46 [0.32; 0.76] | 1.98 [1.19; 2.52] | |
| 27 | 0 | 1.0 | 35.51 [4.25; 104.4] | 1.53 [0.86; 1.9] | |
| E | 28 | 1 | 1.1 | 33.33 [13.99; 41.3] | 0.96 [0.34; 1.47] |
| 29 | 1 | 2.4 | 7.15 [2.95; 10.58] | 2.15 [1.99; 2.33] | |
| 30 | 1 | 1.4 | 29.92 [7.18; 95.76] | 1.86 [0.93; 2.71] |
Groups A–D = Invasive ductal carcinomas (HercepTest 3+, 2+, 1+ positive; 0, negative). Group E = DCIS.
Duplicate blinded HER-2 protein expression assay (HercepTest).
CEP 17 = probe for chromosome 17 centromere region.
Relative HER-2/neu mRNA expression (Her-2/neu/β-actin) in absolute values. Values are given as means, with min and max values.
Relative HER-2/neu gene amplification in accordance with the LightCycler HER2/neu DNA Quantification kit (Roche). Values are given as means, with min and max values.
Insufficient mRNA for analyzing both HER-2 and β-actin.
A–D = Invasive ductal carcinomas.
E = DCIS cases.
Abbreviations: DCIS, ductal carcinoma in situ; IHC, immunohistochemistry; FFPE, formalin-fixed, paraffin-embedded; FISH, fluorescence in situ hybridization.
Real-Time Quantitative RT-PCR Analysis of HER-2 mRNA Expression
When establishing the assay, we compared total RNA extraction using Qiagen RNA kit (Qiagen), Ambion Paraffin kit (Ambion, Inc., Austin, TX) and TRIzol-reagent (Invitrogen, Life Technologies, Carlsbad, CA). These extraction methods gave similar results (data not shown). Furthermore, DNAase treatment of the samples did not significantly affect the yield. The RT step was performed with specific antisense primers for either HER-2 or β-actin, resulting in a 1000-fold increase in sensitivity, compared to random hexamer primer (data not shown). We used intron-spanning primers that amplified short sequences (eg, 99 bp for β-actin and 72 bp for HER-2). This gave sufficient sensitivity to allow successful amplification, even from the minute amounts of potentially damaged RNA available in the FFPE archival material used in this assay. An initial comparison of different reverse transcriptase enzymes (Superscript (Invitrogen, Life Technologies), Sensiscript (Qiagen), and MuLV (Applied Biosystems)) and a trial of priming at specific annealing temperature in the reverse transcriptase step, did not show any one method to be superior (data not shown). Omitting the enzyme in the RT step resulted in no amplification of products.
HER-2 mRNA could be analyzed by real-time quantitative RT-PCR in all 30 cases (Table 2), although in one carcinoma (no. 3) there was insufficient cDNA available for subsequent analysis of β-actin. In all cases, at least three of the five microdissected samples from each paraffin section gave satisfactory results for HER-2 mRNA; in the majority of cases, all five samples were informative. In total, 141 of 150 samples (94%) gave informative results.
Based on the IHC and histological findings, test cases were assigned to five groups: group A, 3+ amplification; group B, 2+ amplification; group C, 1+ amplification; group D, no amplification; group E, DCIS cases only. RT-PCR data were analyzed in relation to these groups (Table 2). We performed statistical analysis for repeated measurements on the ratio between HER-2 and β-actin, as well as on the data obtained from HER-2 and β-actin alone.
After normalization of the HER-2 results to the reference gene, we found a highly significant difference between group A and the remaining groups (P = 0.0001; Table 3). The total variation was composed partly by the variation between patients in the same group (Table 3; var1 = 73%) and partly by the variation that resulted from tumor heterogeneity and/or measurement inaccuracies (Table 3; var2 = 27%). Similar results were found for HER-2 alone and after normalization to the number of cells in each experiment (n ∼24; Table 3). The same test was performed on the data obtained from β-actin alone, and showed no systematic difference between the groups (P = 0.530). In this case, the values for var1 and var2 were 81% and 19%, respectively. A similar result was found after normalization to the number of cells. Overall, the data obtained exhibited a distinct spread of values for the five samples per case in many of the patients (Table 2). Moreover, there was a distinct overlap between data in the different groups (Figure 1).
Table 3.
Real-Time Quantitative HER-2 mRNA Expression and DNA Amplification in Breast Tumors
| Analysis | Group | P-value | Var1* | Var2* | CV† | |
|---|---|---|---|---|---|---|
| mRNA | Ratio HER-2/β-actin | A against B–E | 0.0001 | 73% | 27% | n.d. |
| HER-2 alone | A against B–E | 0.0001 | 68% | 32% | ||
| HER-2/cell | A against B–E | 0.0001 | 68% | 32% | ||
| β-actin alone | A against B–E | 0.530 | 81% | 19% | ||
| β-actin/cell | A against B–E | 0.530 | 81% | 19% | ||
| DNA | Ratio HER-2/gastrin | A against B–E | 0.0001 | 54% | 46% | 12% |
Var1 and var2 are variance components describing the proportion of total variation contributed by variations between the patients within the same group and the variation secondary to tumor heterogeneity and/or measurement inaccuracies.
CV describes inter-assay variation.
Abbreviations: Var, variance; CV, coefficient of variation; n.d., not done.
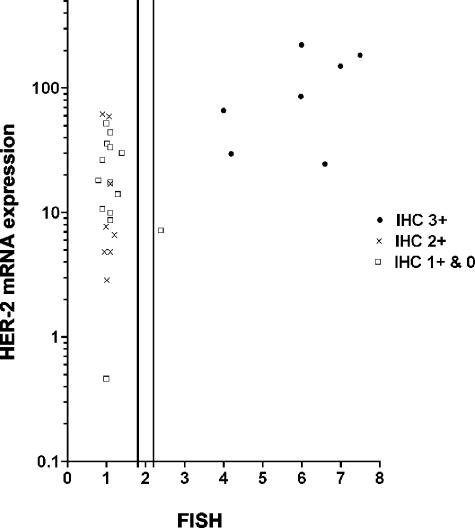
Figure 1.
Comparison of IHC, FISH and real-time RT-PCR analysis of HER-2 status in 29 breast tumors. Data are displayed as a function of IHC, FISH, and RT-PCR values. The RT-PCR values are given as a ratio between HER-2 and β-actin. The y-axis is displayed as a logarithmic scale. The 2 vertical lines on the x-axis show the lower and upper cut-off values for “borderline” amplification in FISH (1.8 and 2.2, respectively). For cases with FISH values in this area, analysis was repeated.
Real-Time Quantitative PCR Analysis of HER-2 Gene Amplification
The LightCycler HER2/neu DNA Quantification kit (Roche Molecular Biochemicals) was simple to perform and gave satisfactory results in most microdissected paraffin sections from all cases. A total of 146 of 150 samples (97.3%) were informative. The ratio between HER-2 and the reference gene gastrin showed a significant difference between group A and the remaining groups (P = 0.0001), with var1 and var2 being 54% and 46%, respectively. The inter-assay variation (CV) was 12%, leaving tumor heterogeneity to account for 34% of the total variance. The data obtained for each patient with five separate measurements showed minor variation in non-amplified tumors, whereas there was considerable spread in tumors exhibiting distinct gene amplification (Table 2 and Figure 2).
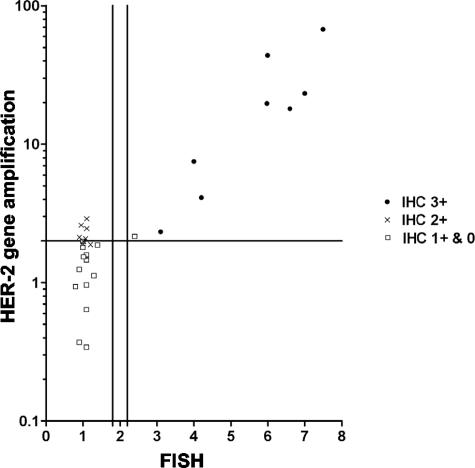
Figure 2.
Comparison of IHC, FISH and real-time PCR analysis on HER-2 status in 30 breast tumors. Data are displayed as a function of IHC, FISH and LightCycler HER2/neu DNA quantification kit values. The y-axis is displayed as a logarithmic scale. The horizontal line on the y-axis represents the cut-off value for the kit (≥2.0). The two vertical lines on the x-axis show the lower and upper cut-off values for “borderline” amplification in FISH (1.8 and 2.2, respectively). For cases with FISH values in this area, analysis was repeated. Some of the data points are partly overlapping.
Comparison of HER-2 Status Assessed by IHC and FISH
Analysis of HER-2 status in the carcinomas using IHC and FISH gave results consistent with those that could be expected on the basis of previously published data. Thus, all eight carcinomas with amplification determined by FISH, also showed strong expression of HER-2 protein at the 3+ level. One DCIS case (case no. 29) was found to be amplified using FISH, but scored only 1+ in the HercepTest.
Comparison of IHC, FISH, and Real-Time Quantitative PCR Assays of HER-2 mRNA Expression
Most cases showing amplification in FISH and strong protein expression in IHC (3+), had distinctly high expression of HER-2 mRNA measured by real-time quantitative RT-PCR (Figure 1). Cases with only moderate or lesser levels of HER-2 protein expression, showed mild to moderate expression of HER-2 mRNA. There was, however, a distinct overlap between these cases and the cases with strong protein expression (Figure 1).
Comparison of IHC, FISH, and LightCycler HER2/neu DNA Quantification Kit Assays of HER-2 Status
Cases amplified in FISH and with strong expression of HER-2 protein (IHC 3+) were all found to be amplified using the LightCycler kit (Tables 4and 5, Figure 2). In addition, the single FISH-positive, IHC-negative DCIS case, was also found to be amplified by DNA-PCR (Tables 4and 5). With the exception of this case, all other IHC 0 and 1+ tumors were not amplified by PCR. Five of the IHC 2+ cases were amplified in PCR, but were negative with FISH (Table 4 and Figure 2). The degree of polysomy 17 found by FISH in these five cases was not significantly different to that found in the other IHC 2+ cases.
Table 4.
Comparison of IHC and Real-Time LightCycler PCR Analysis of HER-2 Status in 30 Breast Tumors
| IHC 3+ | IHC 2+ | IHC negative* | Total | |
|---|---|---|---|---|
| HER-2 gene amplification† | 8 | 5 | 1 | 14 |
| HER-2 gene non-amplification‡ | 0 | 3 | 13 | 16 |
| Total | 8 | 8 | 14 | 30 |
IHC cases scoring 0 and 1.
HER-2 ratio ≥ 2.0.
HER-2 ratio < 2.0.
Table 5.
Comparison of FISH and Real-Time LightCycler PCR Analysis of HER-2 Status in 30 Breast Tumors
| FISH amplified | FISH not amplified | Total | |
|---|---|---|---|
| HER-2 gene amplification* | 9 | 5 | 14 |
| HER-2 gene non-amplification† | 0 | 16 | 16 |
| Total | 9 | 21 | 30 |
HER-2 ratio ≥ 2.0.
HER-2 ratio < 2.0.
Discussion
Recent advances in our understanding of the genetic alterations underlying the development of breast carcinoma have opened the way for new treatments of the disease based on the identification of molecularly distinct patient subgroups. In an era of patient-specific therapy, the clinical importance of demonstrating HER-2/neu amplification and/or high gene expression is compelling. What is less clear, is the optimal method for evaluating HER-2 status in the routine clinical setting. In the present study, we show that by combining the precision and high sensitivity of real-time PCR analysis with the morphological specificity of histological microdissection, it is possible to quantify both HER-2 gene amplification and gene expression in routine FFPE archive tissues. Despite the inevitable degradation of nucleic acids that occurs with formalin-fixation and paraffin-embedding, our real-time PCR protocol proved to be both robust and reliable, giving informative results in the great majority of microdissected paraffin tissue samples studied. Thus, detection of RNA and DNA were successful in 141 (94%) and 146 (97.3%) of 150 samples, respectively. Of particular interest, assessment of HER-2 amplification in microdissected tumor cells using the commercial real-time LightCycler HER2/neu DNA quantification kit correlated fairly well with the results of the more established assays of FISH and IHC, suggesting that this technique may be a feasible alternative for assessment of HER-2 status in a clinical setting.
IHC assays of HER-2 protein expression are currently the most widely used methods for assessing HER-2 status. The main advantages of these techniques, are that they are rapid and simple, they can be performed in most pathology laboratories, and that (when compared with other assays) they are relatively inexpensive. Furthermore, strong IHC-determined HER-2 protein expression is linked to clinical outcome and treatment response. However, IHC assay reliability has been questioned, because variations in tissue fixation, handling and processing, as well as differences in antibody clone used,21 objective scoring criteria, controls and inter-observer variability in interpretation can all influence the precision of the results. These causes of IHC assay variability help explain the widely differing rates of HER-2 expression in breast carcinomas reported previously.
The technical difficulties associated with IHC, have prompted the development of FISH assays for quantifying HER-2 gene amplification, particularly in cases found to be borderline by HER-2 IHC.22,23 FISH has the advantage of being a quantitative method that correlates well with the results of IHC assays,9,24 and it is generally regarded as the gold standard technique for evaluating HER-2 status. Major drawbacks are that FISH is technically difficult to carry out, laborious to set up, time consuming to perform, and costly compared to IHC. Moreover, this technique is currently only available in a minority of pathology laboratories.
A key advantage shared by IHC and FISH techniques for HER-2 evaluation is that they are based on microscopic analysis. In principle, this allows for changes in gene copy number and gene product expression to be assessed specifically in the tumor cell population. While this is an integral part of IHC assays such as the HercepTest, it is in practice only a theoretical advantage in most FISH analysis, since simultaneous morphological assessment of tissues is difficult in most routinely applied fluorescence-based in situ hybridization protocols. More precise morphological assessment is possible using chromogenic in situ hybridization (CISH).25,26 This technique uses non-fluorescence-labeled probes in a paraffin section in situ hybridization protocol. This allows HER-2 status to be assessed in breast carcinomas on a cell-to-cell basis using a bright-field microscope. However, the sensitivity, precision, clinical correlates and usefulness of this technique remain to be established. A possible source of error with CISH, is that current protocols are based on a single-color detection of one probe only, masking possible HER-2 pseudo-amplification caused by chromosome 17 polysomy.
The inability to relate molecular changes in test tissues to specific histological components (eg, tumor cells) is a major disadvantage of blotting and PCR techniques based on analysis of extracted nucleic acids. Earlier published PCR-based methods to detect HER-2 amplification have been essentially semi-quantitative, based on fixed-cycle PCR with analysis by gel electrophoresis.27 However, the development of real-time PCR methods makes it possible to perform more precise quantitative analysis of gene amplification. These methods are easy and rapid to perform, can be used to test multiple samples, have a low risk of contamination (no post-amplification manipulation of amplicons), and can be automated, making them potentially useful methods for screening tumors for HER-2 amplification in a routine clinical setting. However, dilution of tumor genomic material with nucleic acids from non-neoplastic tissue components is an important potential source of imprecision in such quantitative assays in many cancers, not least in breast carcinoma in which non-malignant cells may account for more than 50% of the tumor.28 In the setting of HER-2 analysis, this will tend to increase the number of false-negative tumors, an effect that is undesirable and possible unacceptable in a screening procedure. In keeping with this, a recent study has shown, that low-level HER-2 gene amplification was no longer detectable by real-time PCR if the breast carcinoma cells compromised less than 30% of the tumor specimen.29
To reduce the problem of tumor tissue heterogeneity, we performed histological microdissection before PCR analysis. LAM has emerged recently as a key methodology for this purpose, allowing rapid and precise collection of homogeneous tumor cells from complex tissues suitable for a variety of downstream molecular applications.12 In this study, LAM proved to be a reliable technique that, once learned, was quick and as easy to perform as FISH. Tumor cell specimens obtained in this way gave satisfactory results with both real-time quantitative RT-PCR and PCR in almost all samples, even though the actual number of cells in individual analyses was very small (∼24 in RT-PCR and ∼50 in DNA-PCR). Formalin-fixation and paraffin-embedding is highly destructive of RNA, with the result that mRNA levels may be decreased by up to 99% in such tissues, with residual RNA being extensively degraded.13,14 As a result, it has been considered essentially impossible to perform quantitative RT-PCR on archival paraffin block material. Despite this, several groups have recently published studies, in which the small amounts of degraded RNA in FFPE tissues can be successfully amplified and detected using highly sensitive real-time RT-PCR techniques.14,16,20,29 Our study contributes further to these findings. We were able to show a statistically significant difference between the different tumor groups with regard to both the ratio between HER-2 and β-actin mRNA, and HER-2 mRNA alone. In contrast, no significant differences were found between groups in levels of mRNA from the housekeeping gene β-actin. We found considerable variation in HER-2 mRNA levels comparing different patients, a higher degree of variation than was due to differences in the HER-2 data, as compared with β-actin. The rather large spread of mRNA levels within individual patients comparing the five parallel analyses, which contributed to the total variation, also calls for attention (Table 2). We believe this to be an expression of both tumor heterogeneity and of technical inaccuracies. Although we found statistically significant differences in HER-2 mRNA levels in our test tumors, there was a clear overlap of gene expression levels in the different groups. This suggests that HER-2 gene expression data from FFPE tissue studies should be used with caution, especially in the clinical setting. As discussed by Bustin,30 there are several other potentially important problems to consider with regard to the evaluation of real-time quantitative RT-PCR. The use of housekeeping genes is difficult in in vivo studies, as the mRNA level of these genes may vary considerable, and in LAM specimens it is not possible to quantitate total RNA. However, we found that β-actin mRNA expression was similar in all groups, while there were statistically significant differences in HER-2 mRNA levels comparing different groups with regard to HER-2 normalized to β-actin, HER-2 alone, and HER-2 normalized to cell number, suggesting that it was appropriate to normalize HER-2 levels to this housekeeping gene.
Compared to our real-time quantitative PCR analysis of HER-2 gene expression, evaluation of gene amplification using the LightCycler HER2/neu DNA Quantification kit (Roche Molecular Biochemicals) showed an even better agreement with both IHC and FISH data. Although DNA undergoes substantial changes during formalin-fixation, it is more stable than RNA. While DNA also undergoes degeneration in FFPE tissues, the short amplicon size used in the DNA quantification kit allows for reliable amplification of the fragmented DNA available in our microdissected samples. Compared to the data for HER-2 gene expression, we found less variation in levels of gene amplification in the different groups (Table 2). We believe that this is a result of DNA being relatively less degraded than RNA in FFPE material. The data also reveals a degree of tumor heterogeneity (Table 3; 34% (var2-CV)) in spite our attempts to minimize this phenomenon by dissecting adjacent tumor areas. We suggest that in similar future studies, at least three repeated measurements should be conducted on tumor tissue from each patient. Furthermore, the total variance was contributed less by the differences between patients in the same group (Table 3; var1 = 54%). The results from FISH analysis show that this should come as no surprise. Twenty-three of thirty cases (76.7%) showed polysomy of cep-17 and an even higher number (27 of 30) showed heterogeneity of HER-2 signals.
Using the LightCycler HER2/neu DNA Quantification kit, we found 14 tumors in which the mean HER-2 score was above the cut-off value of ≥2. Eight of these cases strongly overexpressed HER-2 protein by the HercepTest. Interestingly, five of the remaining six tumors scored +2 in the IHC assay. These cases were not FISH amplified. We have considered whether these may in fact be showing true amplification at the DNA level, although clearly the numbers included in our study are too small to allow any firm conclusions. Furthermore, it might be more appropriate for the cut-off value of the LightCycler kit (≥2 indicates Her-2 gene amplification) to be given as a range of values (analogous to the FISH assay), instead of a single value. Indeed, it should be stressed that the mean value for some of the cases came very close to the cut-off value. However, the precision of FISH analysis is also dependent on the degree of tumor heterogeneity. While we were able to use LAM to help enrich our PCR samples for tumor cells, it should be remembered that tissues examined by FISH may contain a variable number of non-malignant cells within the counting area. Using routine FISH protocols with DAPI counter-stain, fine morphological analysis is not possible, giving a potential source of error. Thus, although FISH is regarded as the gold standard to be used when assessing other methods for HER-2 analysis, it is not necessarily true that this technique will always give the most precise results. In light of this, our results raise the possibility that real-time PCR quantification of HER-2 gene amplification in microdissected breast carcinoma tissue may be as good, or even better than established techniques for determining HER-2 status.
While the advantages of IHC analysis make it likely that this technique will continue to be used as the most widespread method for primary screening of HER-2 status in breast carcinomas, microdissection with real-time PCR may be a useful supplement or even alternative to FISH for quantitative confirmation of HER-2 gene amplification. At first sight, the logistics of performing microdissection and real-time PCR might seem to be against their more widespread use. However, while the equipment and expertise for performing these techniques are not found in most routine laboratories, they are becoming increasingly available. Thus, they are already used in specialized laboratories, including many HER-2 reference pathology laboratories. FISH is also a methodology that requires special equipment and expertise that are not available in many routine laboratories. Furthermore, FISH is still quite a laborious and slow assay to use. In most routine protocols, FISH takes at least 2 days to perform. In our set up, procurement and preparation of microdissected specimens (including sectioning, deparaffinization, staining, microdissection, and protein-digestion) takes 1 to 2 days. If necessary, deparaffinization times can be further reduced. Thus, in a routine clinical setting we believe it would be possible to perform HER-2 analysis on microdissected samples within 1 to 2 days in most cases. While the microdissection is likely to be a rate-limiting step in high-throughput HER-2 testing, this is to a great extent counterbalanced by the suitability of PCR for batch analysis. For example, the LightCycler HER2/neu DNA Quantification kit analyzes 32 samples (ie, 10 tumors in triplicate) in a single run, thus making it possible to perform high-throughput analyses.
Clearly, before considering the use of microdissection with real-time quantitative PCR for HER-2 analysis, larger studies will be required to confirm our findings, and to correlate the results of the assay with clinical outcome of the patients. However, we believe that this technique may have a useful role to play in the assessment of HER-2 gene amplification in both research and clinical settings.
Acknowledgments
We thank Alice Nielsen, Inge Ray Pedersen, Esther Thomsen, and Alice Villemoes for technical assistance and Asger Roer Pedersen for statistical guidance.
Footnotes
Supported by grants from the Clinical Research Unit of the Danish Cancer Society, the Fritz, Georg and Marie Cecile Glud’s Foundation, the Johan and Lise Boserup Foundation, the Hans and Nora Buchard’s Foundation, the Astrid Thaysen Foundation, the Lily Benthine Lund Foundation of 1/6–78, and the Danish Medical Research Council.
References
- Revillion F, Bonneterre J, Peyrat JP. ERBB2 oncogene in human breast cancer and its clinical significance. Eur J Cancer. 1998;34:791–808. doi: 10.1016/s0959-8049(97)10157-5. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- Dowsett M, Cooke T, Ellis I, Gullick WJ, Gusterson B, Mallon E, Walker R. Assessment of HER2 status in breast cancer: why, when, and how? Eur J Cancer. 2000;36:170–176. doi: 10.1016/s0959-8049(99)00264-6. [DOI] [PubMed] [Google Scholar]
- Thomson TA, Hayes MM, Spinelli JJ, Hilland E, Sawrenko C, Phillips D, Dupuis B, Parker RL. HER-2/neu in breast cancer: inter-observer variability and performance of immunohistochemistry with four antibodies compared with fluorescent in situ hybridization. Mod Pathol. 2001;14:1079–1086. doi: 10.1038/modpathol.3880440. [DOI] [PubMed] [Google Scholar]
- Press MF, Hung G, Godolphin W, Slamon DJ. Sensitivity of HER-2/neu antibodies in archival tissue samples: potential source of error in immunohistochemical studies of oncogene expression. Cancer Res. 1994;54:2771–2777. [PubMed] [Google Scholar]
- Bartlett J, Mallon E, Cooke T. The clinical evaluation of HER-2 status: which test to use? J Pathol. 2003;199:411–417. doi: 10.1002/path.1354. [DOI] [PubMed] [Google Scholar]
- Dowsett M, Bartlett J, Ellis IO, Salter J, Hills M, Mallon E, Watters AD, Cooke T, Paish C, Wencyk PM, Pinder SE. Correlation between immunohistochemistry (HercepTest) and fluorescence in situ hybridization (FISH) for HER-2 in 426 breast carcinomas from 37 centres. J Pathol. 2003;199:418–423. doi: 10.1002/path.1313. [DOI] [PubMed] [Google Scholar]
- Kjeldsen E, Kølvraa S. FISH techniques, FISH probes and their applications in medicine and biology: an overview. Rautenstrauss B, Liehr T, editors. Berlin: Springer; FISH Technology. 2002:3–50. [Google Scholar]
- Jacobs TW, Gown AM, Yaziji H, Barnes MJ, Schnitt SJ. Comparison of fluorescence in situ hybridization and immunohistochemistry for the evaluation of HER-2/neu in breast cancer. J Clin Oncol. 1999;17:1974–1982. doi: 10.1200/JCO.1999.17.7.1974. [DOI] [PubMed] [Google Scholar]
- Emmert-Buck MR, Bonner RF, Smith PD, Chuaqui RF, Zhuang Z, Goldstein SR, Weiss RA, Liotta LA. Laser capture microdissection. Science. 1996;274:998–1001. doi: 10.1126/science.274.5289.998. [DOI] [PubMed] [Google Scholar]
- Schutze K, Clement-Sengewald A. Catch and move: cut or fuse. Nature. 1994;368:667–669. doi: 10.1038/368667a0. [DOI] [PubMed] [Google Scholar]
- Gjerdrum LM, Lielpetere I, Rasmussen LM, Bendix K, Hamilton-Dutoit S. Laser-assisted microdissection of membrane-mounted paraffin sections for polymerase chain reaction analysis: identification of cell populations using immunohistochemistry and in situ hybridization. J Mol Diagn. 2001;3:105–110. doi: 10.1016/S1525-1578(10)60659-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda N, Ohnishi T, Kawamoto S, Monden M, Okubo K. Analysis of chemical modification of RNA from formalin-fixed samples and optimization of molecular biology applications for such samples. Nucleic Acids Res. 1999;27:4436–4443. doi: 10.1093/nar/27.22.4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamsen HN, Steiniche T, Nexo E, Hamilton-Dutoit SJ, Sorensen BS. Towards quantitative mRNA analysis in paraffin-embedded tissues using real-time reverse transcriptase-polymerase chain reaction: a methodological study on lymph nodes from melanoma patients. J Mol Diagn. 2003;5:34–41. doi: 10.1016/S1525-1578(10)60449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm M, Wieland I, Schutze K, Rubben H. Microbeam MOMeNT: non-contact laser microdissection of membrane-mounted native tissue. Am J Pathol. 1997;151:63–67. [PMC free article] [PubMed] [Google Scholar]
- Specht K, Richter T, Muller U, Walch A, Werner M, Hofler H. Quantitative gene expression analysis in microdissected archival formalin-fixed and paraffin-embedded tumor tissue. Am J Pathol. 2001;158:419–429. doi: 10.1016/S0002-9440(10)63985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann U, Kreipe H. Real-time PCR analysis of DNA and RNA extracted from formalin-fixed and paraffin-embedded biopsies. Methods. 2001;25:409–418. doi: 10.1006/meth.2001.1263. [DOI] [PubMed] [Google Scholar]
- Christensen B, Kieler J, Vilien M, Don P, Wang CY, Wolf H. A classification of human urothelial cells propagated in vitro. Anticancer Res. 1984;4:319–337. [PubMed] [Google Scholar]
- Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- Godfrey TE, Kim SH, Chavira M, Ruff DW, Warren RS, Gray JW, Jensen RH. Quantitative mRNA expression analysis from formalin-fixed, paraffin-embedded tissues using 5′ nuclease quantitative reverse transcription-polymerase chain reaction. J Mol Diagn. 2000;2:84–91. doi: 10.1016/S1525-1578(10)60621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampaul R, Pinder S, Gullick W, Robertson J, Ellis I. HER-2 in breast cancer-methods of detection, clinical significance, and future prospects for treatment. Crit Rev Oncol Hematol. 2002;43:231–244. doi: 10.1016/s1040-8428(01)00207-4. [DOI] [PubMed] [Google Scholar]
- Wang S, Saboorian MH, Frenkel E, Hynan L, Gokaslan ST, Ashfaq R. Laboratory assessment of the status of Her-2/neu protein and oncogene in breast cancer specimens: comparison of immunohistochemistry assay with fluorescence in situ hybridisation assays. J Clin Pathol. 2000;53:374–381. doi: 10.1136/jcp.53.5.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier J, Vincent-Salomon A, Nicolas A, Beuzeboc P, Mouret E, Zafrani B, Sastre-Garau X. Strong correlation between results of fluorescent in situ hybridization and immunohistochemistry for the assessment of the ERBB2 (HER-2/neu) gene status in breast carcinoma. Mod Pathol. 2000;13:1238–1243. doi: 10.1038/modpathol.3880228. [DOI] [PubMed] [Google Scholar]
- Tsuda H, Akiyama F, Terasaki H, Hasegawa T, Kurosumi M, Shimadzu M, Yamamori S, Sakamoto G. Detection of HER-2/neu (c-erb B-2) DNA amplification in primary breast carcinoma: inter-observer reproducibility and correlation with immunohistochemical HER-2 overexpression. Cancer. 2001;92:2965–2974. doi: 10.1002/1097-0142(20011215)92:12<2965::aid-cncr10156>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Tanner M, Gancberg D, Di Leo A, Larsimont D, Rouas G, Piccart MJ, Isola J. Chromogenic in situ hybridization: a practical alternative for fluorescence in situ hybridization to detect HER-2/neu oncogene amplification in archival breast cancer samples. Am J Pathol. 2000;157:1467–1472. doi: 10.1016/S0002-9440(10)64785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandachi N, Dietze O, Hauser-Kronberger C. Chromogenic in situ hybridization: a novel approach to a practical and sensitive method for the detection of HER2 oncogene in archival human breast carcinoma. Lab Invest. 2002;82:1007–1014. doi: 10.1097/01.lab.0000024360.48464.a4. [DOI] [PubMed] [Google Scholar]
- de Cremoux P, Martin EC, Vincent-Salomon A, Dieras V, Barbaroux C, Liva S, Pouillart P, Sastre-Garau X, Magdelenat H. Quantitative PCR analysis of c-erb B-2 (HER2/neu) gene amplification and comparison with p185(HER2/neu) protein expression in breast cancer drill biopsies. Int J Cancer. 1999;83:157–161. doi: 10.1002/(sici)1097-0215(19991008)83:2<157::aid-ijc2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Lehmann U, Glockner S, Kleeberger W, von Wasielewski HF, Kreipe H. Detection of gene amplification in archival breast cancer specimens by laser-assisted microdissection and quantitative real-time polymerase chain reaction. Am J Pathol. 2000;156:1855–1864. doi: 10.1016/S0002-9440(10)65059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol. 2002;29:23–39. doi: 10.1677/jme.0.0290023. [DOI] [PubMed] [Google Scholar]