Abstract
The α6β4 integrin is the receptor for the basement membrane protein laminin-5. Recent studies suggest that α6β4 integrin expression in invasive breast carcinomas may be a poor prognostic factor. Because we have not had reliable results with commercially available antibodies for the immunohistochemical detection of α6β4 integrin in archival paraffin-embedded tissues, we designed a probe to detect β4 integrin subunit mRNA in paraffin sections. In situ hybridization for β4 mRNA was performed on paraffin-embedded tissue sections of 25 invasive breast carcinomas using a hyperbiotinylated oligonucleotide DNA probe. Immunohistochemical staining was performed on corrresponding frozen tumor sections using two commercially available antibodies to the β4 integrin subunit. All cases positive for β4 protein by one or both antibodies were also positive for β4 mRNA by in situ hybridization, but three cases with β4 mRNA expression were negative by immunohistochemistry with both antibodies. These findings suggest that in situ hybridization appears to be a sensitive method for detecting β4 integrin mRNA, but it appears to identify some cases that either lack β4 protein or express variants not recognized with commercial antibodies directed to particular extracellular or cytoplasmic domains.
Integrins are glycoprotein heterodimers that serve as the principal cell surface receptors for extracellular matrix proteins.1,2,3,4 Each integrin heterodimer is composed of a single α and a single β subunit. At present, 18 α subunits and eight β subunits have been identified. The β4 subunit associates exclusively with α6. There has been particular interest recently in the α6β4 integrin, a receptor for some of the isoforms of laminin, because of its unique signaling properties and its putative role in tumor cell invasion and metastasis.5,6,7,8,9,10
A number of lines of evidence support the hypothesis that α6β4 expression plays a role in tumor invasion and metastasis. Two previous studies from Italy and Germany demonstrated a strong correlation between α6β4 expression in breast cancer and reduced patient survival.11,12 Similarly, reduceddisease-free survival for tumors with α6β4 expression was recently reported in patients with squamous cell carcinoma of the head and neck.13
The immunohistochemical evaluation of α6β4 integrin expression in patient specimens has been hampered to date by the lack of available antibodies that show reproducible immunohistochemical staining results in archival paraffin-embedded tissue sections. The few studies published so far have used frozen tissue specimens11,12,13 or fixation methods different from those routinely used on clinical specimens,14 so the number of specimens with long clinical follow-up has been limited. In this study, we designed an oligonucleotide probe for the colorimetric detection of β4 integrin subunit mRNA in formalin-fixed, paraffin-embedded sections of invasive breast carcinomas and compared β4 mRNA expression with immunohistochemical staining results on corresponding frozen tissue sections.
Materials and Methods
Cell Culture
Breast carcinoma cell lines MDA-MB-231 and MDA-MB-134 were obtained from the laboratory of Dr. Janet Price, Department of Cancer Biology at M. D. Anderson Cancer Center, where they were previously characterized by flow cytometry as α6β4 positive and α6β4 negative, respectively.15 The cell lines were cultured in Eagle’s minimum essential medium (MEM) supplemented with 10% fetal bovine serum (FBS), l-glutamine, sodium pyruvate, and nonessential amino acids and vitamins (Gibco, Grand Island, NY). The cells were maintained in monolayer culture in a humidified incubator at 37°C in an atmosphere of 5% CO2 and 95% air.
Tissues
Twenty-five frozen cases of invasive breast carcinoma that had corresponding paraffin-embedded tissue blocks available were selected from the M. D. Anderson Breast Tumor Bank. Twenty-three of the invasive breast carcinomas were of ductal type, one was ductal type with mucinous features, and one was a mixed ductal and lobular carcinoma. Seventeen of the cases were of nuclear grade 3 (high) and eight were of nuclear grade 2 (intermediate). The frozen tissues were procured from fresh surgical resection specimens and snap-frozen in OCT. Corresponding formalin-fixed, paraffin-embedded tumor blocks were retrieved from the surgical pathology files of M. D. Anderson Cancer Center.
Immunohistochemistry
Cytospin preparations were made of each cell line, and 5-μm sections were cut from the frozen tissue. The cytospins and frozen tissue sections were fixed in cold acetone for 5 minutes and air-dried. Immunohistochemical staining was performed using two different antibodies against the β4 integrin subunit: a commercially available rabbit polyclonal antibody directed against a synthetic peptide with the sequence NH2-(K)GTLSTHMDQQFFQT-amide derived from the cytoplasmic domain of β4 (1:1000, Chemicon, Temecula, CA); and a monoclonal antibody derived against a prokaryotic recombinant protein corresponding to part of the extracellular domain (clone ELF1, 1:50, Novocastra, Burlingame, CA). Immunohistochemical staining was performed using the Vector ABC kit and a standard avidin-biotin peroxidase method. Tumors were considered positive if they exhibited immunohistochemical staining in 5% or more of the tumor cells.
Probes
An oligonucleotide probe was designed to recognize a portion of the cytoplasmic domain of the β4 integrin subunit common to splice variants β4A, β4B, β4C, and β4D (Figure 1).4 The 40-mer oligonucleotide probe had a GC content of 56.1% and the following sequence:
Figure 1.
Splice variants of the β4 integrin subunit. The β4 probe recognizes a 40-nucleotide sequence in the connecting segment between the two pairs of fibronectin type III repeats in a region that does not overlap with the insertions for variants β4B and β4C.
5′-GTAGTCCCTGGGCAGTGTGGTCGAGTGTGAGTGTT-CTGAG-3′. The custom probe was purchased from Research Genetics (Huntsville, AL), where it was conjugated with a 3′ hyperbiotinylated tail. A polyd(T) 20-oligonucleotide probe was also purchased from Research Genetics.
In Situ Hybridization
Histological sections from the paraffin blocks were cut at 4 μm intervals using RNase-free conditions (all instruments, glassware, and slides washed overnight in 0.1% DEPC water, and histological sections cut using a 0.1% DEPC water bath). Tissue sections were mounted on silane-treated ProbeOn slides (Fisher Scientific, Pittsburgh, PA) and pre-heated at 65° for 45 minutes before beginning the assay. In situ hybridization was performed using the Microprobe System (Fischer Scientific, Pittsburgh, PA) and the chromagen Fast Red (Biomeda Corp., Foster City, CA) as previously described.16,17 Briefly, the glass slides were placed into the Microprobe holder, and paraffin sections were dewaxed with Autodewaxer and dehydrated using Autoalcohol (Research Genetics). This was followed by digestion with Pepsin Reagent (Fisher Scientific) for 4 minutes at 100°C. Sections were hybridized with probe at 45°C for 1 hour, then washed three times at 45°C with 0.3 M NaCl and 0.03 M sodium citrate. Incubation with chromogen was performed at 45°C for 30 minutes, followed by an additional incubation for 10 minutes. Phosphatase Enhancer Reagent (Fisher Scientific) was applied to the samples for 1 minute before both incubations with chromagen. The same procedure was performed on the cytospin preparations, with the exception of the dewaxing and dehydration steps. Tumors were considered positive if they exhibited hybridization signal in 5% or more of the tumor cells. In situ hybridization with the polyd(T) probe was performed on each specimen to verify the integrity of mRNA, and competition with a 100-fold excess of unlabeled probe was performed on representative slides to demonstrate specificity of the β4 probe.
Results
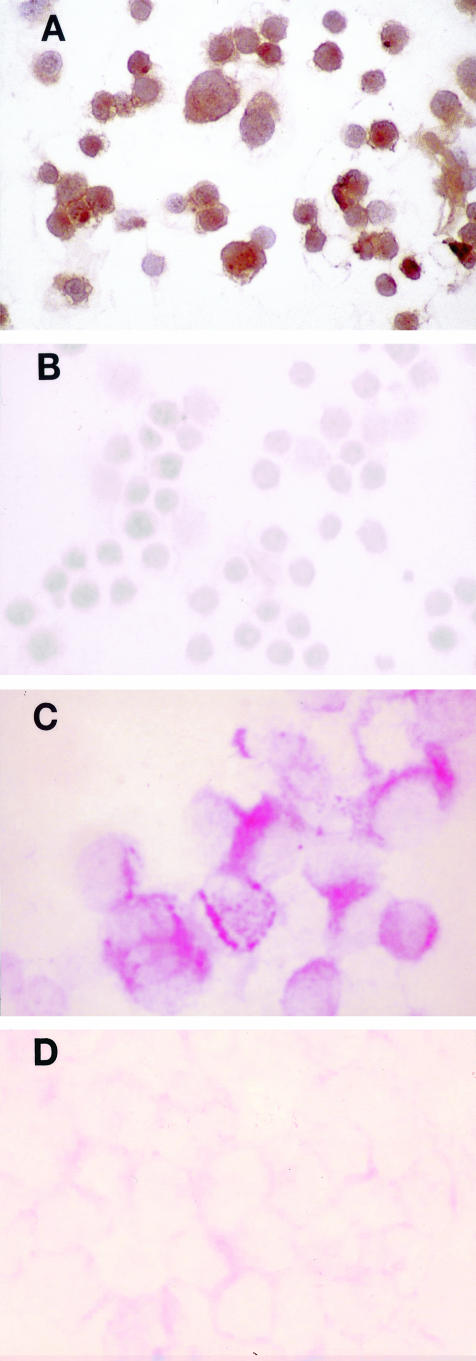
Immunohistochemical staining for β4 protein confirmed β4 expression in the breast cancer cell line MDA-MB-231 and the absence of β4 in MDA-MB-134 (Figure 2, A and B). In situ hybridization detected β4 integrin subunit mRNA in the MDA-MB-231 cells but not in the MDA-MB-134 cells (Figure 2, C and D), and competition with excess unlabeled probe confirmed specificity of the β4 probe.
Figure 2.
Immunohistochemical staining for β4 protein in breast cancer cell lines MDA-MB-231 (A) and MDA-MB-134 (B) (magnification, ×400; immunoperoxidase with DAB chromogen). In situ hybridization for β4 integrin subunit mRNA in MDA-MB-231(C) and MDA-MB-134 (D) (magnification, ×400; alkaline phosphatase with Fast Red chromogen).
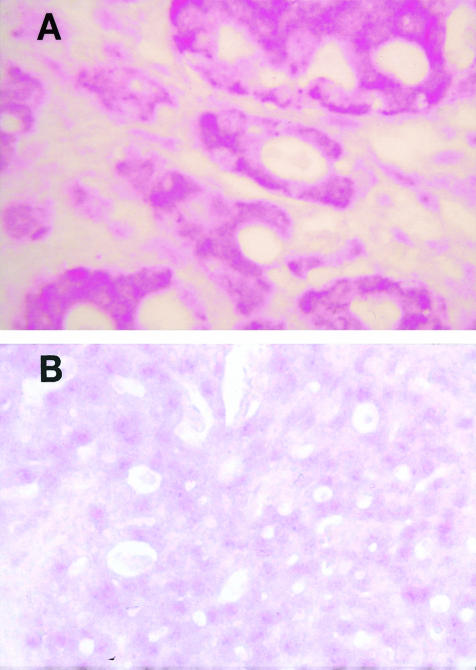
In situ hybridization detected β4 integrin subunit mRNA in 15 of the 25 formalin-fixed, paraffin-embedded breast carcinoma specimens (Table 1). Positive cases showed heterogeneous expression of β4 mRNA, with some areas of the tumors showing much greater expression than other areas. In situ hybridization produced a granular red staining reaction within the cytoplasm of tumor cells (Figure 3A). The cytoplasm of endothelial cells also showed a positive signal and served as an internal control. Competition with 100-fold excess of unlabeled probe almost completely abolished the signal, indicating specificity of the β4 probe (Figure 3B).
Table 1.
Comparison of In Situ Hybridization (ISH) and Immunohistochemical (IHC) Staining Results for β4 Integrin Subunit in Invasive Breast Carcinoma Specimens
| Case no. | Age | Histologic classification | Grade | IHC (cytoplasmic domain) | IHC (extracellular domain) | ISH (paraffin) |
|---|---|---|---|---|---|---|
| 1 | 46 | Ductal | 3 | − | + | + |
| 2 | 39 | Ductal | 3 | + | + | + |
| 3 | 89 | Ductal | 3 | + | + | + |
| 4 | 44 | Ductal | 3 | − | − | − |
| 5 | 58 | Ductal | 3 | − | + | + |
| 6 | 41 | Ductal | 2 | − | − | − |
| 7 | 48 | Ductal | 3 | − | − | − |
| 8 | 75 | Ductal | 3 | − | − | − |
| 9 | 43 | Ductal | 3 | − | − | − |
| 10 | 73 | Ductal | 3 | + | − | + |
| 11 | 84 | Mucinous | 2 | − | − | − |
| 12 | 62 | Ductal | 3 | − | − | − |
| 13 | 41 | Ductal | 3 | − | − | + |
| 14 | 39 | Ductal | 3 | − | − | − |
| 15 | 42 | Ductal | 3 | + | − | + |
| 16 | 65 | Ductal | 3 | + | + | + |
| 17 | 49 | Ductal | 2 | − | − | − |
| 18 | 49 | Ductal | 2 | + | + | + |
| 19 | 39 | Ductal | 3 | − | − | + |
| 20 | 60 | Ductal | 3 | + | + | + |
| 21 | 64 | Ductal | 2 | − | − | − |
| 22 | 59 | Ductal | 3 | + | + | + |
| 23 | 54 | Ductal | 2 | + | + | + |
| 24 | 78 | Ductal | 2 | − | − | + |
| 25 | 60 | Mixed ductal/lobular | 2 | + | + | + |
Figure 3.
In situ hybridization for β4 integrin subunit mRNA in a paraffin-embedded, formalin-fixed section of invasive ductal carcinoma (A), and competition with 100-fold excess of unlabeled probe (B) (magnification, ×200; alkaline phosphatase with Fast Red chromogen).
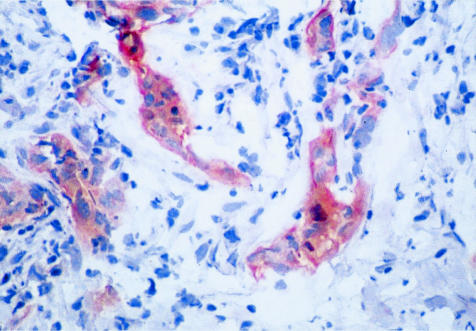
Using the polyclonal antibody directed against a cytoplasmic β4 epitope, immunohistochemical stains on the frozen sections were positive in 10 of the cases found to be positive for β4 mRNA by in situ hybridization. Tumor cells positive for β4 protein exhibited staining diffusely throughout the cytoplasm (Figure 4). With the monoclonal antibody directed against an extracellular epitope of β4, 10 cases were positive by immunohistochemical staining, eight of which were the same as those that were positive with the polyclonal antibody to the cytoplasmic domain. Neither of the antibodies detected β4 protein expression in cases that were negative for β4 mRNA by in situ hybridization (Table 1).
Figure 4.
Immunohistochemical staining for β4 protein in a frozen section of invasive ductal carcinoma (magnification, ×200; immunoperoxidase with DAB chromogen).
Discussion
The extracellular matrix comprises an extensive network of proteins that, in part, provide structural properties of tissues. In addition, the interactions between extracellular matrix proteins and their cell surface receptors provide cross-talk between the cells and their extracellular environment.18 As extracellular matrix protein receptors, integrins mediate a complex array of mechanical and biochemical signals. Most integrins contain a short cytoplasmic tail and participate in intracellular signaling by associating with various adaptor proteins. On ligand stimulation, integrins typically localize to particular sites in the cell membrane referred to as focal adhesions, and the formation of focal adhesions promotes the assembly of actin filaments.18 In contrast, the β4 integrin subunit contains a long cytoplasmic tail, interacts with keratin filaments rather than actin filaments, and participates in the formation of hemidesmosomes.19,20
Recent exciting data suggests that α6β4-mediated signal transduction plays an important role in tumor invasion and metastasis.6,7,8,9,10 The mechanisms whereby α6β4 expression may lead to increased invasive or metastatic behavior are unknown, but new information about α6β4 integrin signaling pathways is beginning to shed some light on this subject. On binding laminin-5, one of the principal ligands for α6β4, the β4 subunit becomes phosphorylated and subsequently activates downstream signaling pathways.5,7,10,21 Tumor cell invasion involves the formation of actin-containing motility structures such as lamellae and filopodia. It has been shown that α6β4 is localized in lamellae and filopodia of invasive tumor cells,22 and the formation of these structures is dependent on phosphatidylinositol 3-OH kinase (PI3K).10 Moreover, the α6β4 integrin appears to preferentially activate PI3K.9
Immunohistochemical staining for β4 protein in frozen sections of normal breast tissue show it to be expressed in the myoepithelial cell layer of normal ducts and lobules (personal observation). The myoepithelial cell layer is in contact with the extracellular basement membrane, which is known to contain laminin-5, the principal ligand for α6β4. The luminal epithelial cell layer of normal ducts, however, does not show α6β4 expression by immunohistochemistry (personal observation). Since most invasive breast cancers show a morphological and immunohistochemical phenotype more like the luminal epithelial cells than the myoepithelial cells,23 those tumors that show α6β4 expression should be regarded as having overexpression of this integrin. In this regard, α6β4-positive breast carcinomas acquire a receptor for extracellular matrix that their non-neoplastic counterparts, the luminal ductal epithelial cells, do not express. This may play an important role in allowing the tumor cells to invade the stroma and to metastasize.
The immunohistochemical evaluation of α6β4 integrin expression in patient specimens has been hampered by the lack of available antibodies that show reproducible staining results on archival paraffin-embedded tissue sections. Hanby et al14 reported successful immunohistochemical staining for β4 on tissues fixed in formalin at 4°C, but they did not see staining when tissues were fixed at room temperature (the temperature at which most archival tissues are fixed). We have made many attempts to use such antibodies against the α6 and β4 subunits on formalin-fixed, paraffin-embedded tissue sections following various antigen-retrieval methods without success to date.
As a cross-linking fixative, formalin preserves tissues and inactivates cellular enzymes by cross-linking enzymes and other proteins not only to each other but to RNA and DNA as well. When RNA is complexed in this way, it is less accessible to RNase degradation. Despite initial concerns about the degradation of RNA in fixed tissue sections, experience has shown in situ hybridization to be a powerful method for evaluating gene expression in archival tissues.24,25,26,27
The immunohistochemical staining results obtained with the antibody directed against the extracellular domain of β4 were different from those obtained with the antibody against a portion of the cytoplasmic domain, but all cases positive for β4 protein by one or both antibodies were positive for β4 mRNA by in situ hybridization on the paraffin sections. This observation suggests that the β4 protein (or a variant thereof) in some tumors is not detected with particular commercially available antibodies to β4. Three cases were positive by in situ hybridization but negative by immunohistochemistry with both antibodies. In situ hybridization, therefore, appears to be a sensitive method for detecting β4 integrin mRNA in archival tissues, but it appears to identify some cases that either lack β4 protein or express variants not recognized with commercial antibodies directed to particular extracellular or cytoplasmic domains.28,29
In the absence of reliable immunohistochemistry for detection of β4 protein in archival formalin-fixed, paraffin-embedded tissues, the use of an oligonucleotide probe to detect β4 mRNA in archival tissues could allow a large number of invasive breast carcinomas with corresponding clinical follow-up data to be evaluated. This would have many advantages over the use of frozen tissue samples for immunohistochemical analyses. A larger number of archival cases would be available for evaluation, expression of α6β4 in metastases could be compared to its expression in the corresponding primary breast tumors, and possible β4 variants not recognized by particular antibodies could be detected with the probe for β4 mRNA. Although β4 mRNA expression may not correlate with functional β4 protein expression in all cases, in situ hybridization may nevertheless be useful to determine whether β4 mRNA expression has any prognostic or predictive value in invasive breast carcinoma.
Acknowledgments
We thank Dr. Janet Price, Department of Cancer Biology at M.D. Anderson Cancer Center, for providing the breast carcinoma cell lines.
Footnotes
Supported in part by Career Development Award DAMD17–01-1–0298 from the U.S. Department of Defense (to M.Z.G.).
References
- Keely P, Parise L, Juliano R. Integrins and GTPases in tumour cell growth, motility, and invasion. Trends Cell Biol. 1998;8:101–106. doi: 10.1016/s0962-8924(97)01219-1. [DOI] [PubMed] [Google Scholar]
- Varner JA, Cheresh DA. Integrins and cancer. Curr Opin Cell Biol. 1996;8:724–730. doi: 10.1016/s0955-0674(96)80115-3. [DOI] [PubMed] [Google Scholar]
- Hughes PE, Pfaff M. Integrin affinity modulation. Trends Cell Biol. 1998;8:359–364. doi: 10.1016/s0962-8924(98)01339-7. [DOI] [PubMed] [Google Scholar]
- Melker AA de, Sonnenberg A. Integrins: alternative splicing as a mechanism to regulate ligand binding and integrin signaling events. Bioessays. 1999;21:499–509. doi: 10.1002/(SICI)1521-1878(199906)21:6<499::AID-BIES6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Chung J, Bachelder RE, Lipscomb EA, Shaw LM, Mercurio AM. Integrin α6β4 regulation of eIF-4E activity and VEGF translation: a survival mechanism for carcinoma cells. J Cell Biol. 2002;158:165–174. doi: 10.1083/jcb.200112015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitz I, Gipson IK, Mercurio AM. Traction forces mediated by α6β4 integrin: implications for basement membrane organization and tumor invasion. Mol Biol Cell. 2001;12:4030–4043. doi: 10.1091/mbc.12.12.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM. Identification of insulin receptor substrate 1 (IRS-1) and IRS-2 as signaling intermediates in the α6β4 integrin-dependent activation of phosphoinositide 3-OH kinase and promotion of invasion. Mol Cell Biol. 2001;21:5082–5093. doi: 10.1128/MCB.21.15.5082-5093.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercurio AM, Rabinovitz I, Shaw LM. The α6β4 integrin and epithelial cell migration. Curr Opin Cell Biol. 2001;13:541–545. doi: 10.1016/s0955-0674(00)00249-0. [DOI] [PubMed] [Google Scholar]
- Gambaletta D, Marchetti A, Benedetti L, Mercurio AM, Sacchi A, Falcioni R. Cooperative signaling between α(6)β(4) integrin and ErbB-2 receptor is required to promote phosphatidylinositol 3-kinase-dependent invasion. J Biol Chem. 2000;275:10604–10610. doi: 10.1074/jbc.275.14.10604. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Rabinovitz I, Wang HH-F, Toker A, Mercurio AM. Activation of phosphoinositide 3-OH kinase by the α6β4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- Friedrichs K, Ruis P, Franke F, Gille I, Terpe HJ, Imhof BA. High expression level of α6 integrin in human breast carcinoma is correlated with reduced survival. Cancer Res. 1995;15:901–906. [PubMed] [Google Scholar]
- Tagliabue E, Ghirelli C, Squicciarini P, Aiello P, Colnaghi MI, Menard S. Prognostic value of α6β4 integrin expression in breast carcinomas is affected by laminin production from tumor cells. Clin Cancer Res. 1998;4:407–410. [PubMed] [Google Scholar]
- Wolf GT, Carey TE, Schmaltz SP, McClathey KD, Poore J, Glaser L, Hayashida DJS, Hsu S. Altered antigen expression predicts outcome in squamous cell carcinoma of the head and neck. J Natl Cancer Inst. 1990;82:1566–1572. doi: 10.1093/jnci/82.19.1566. [DOI] [PubMed] [Google Scholar]
- Hanby AM, Gillett CE, Pignatelli M, Stamp GWH. β 1 and β 4 integrin expression in methacarn and formalin-fixed material from in situ ductal carcinoma of the breast. J Pathol. 1993;171:257–262. doi: 10.1002/path.1711710405. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay R, Theriault RL, Price JE. Increased levels of α6 integrins are associated with the metastatic phenotype of human breast cancer cells. Clin Exp Metastasis. 1999;17:325–332. doi: 10.1023/a:1006659230585. [DOI] [PubMed] [Google Scholar]
- Leary JJ, Brigati DJ, Ward DC. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: bio-blots. Proc Natl Acad Sci USA. 1983;80:4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radinsky R, Bucana CD, Ellis LM, Sanchez R, Cleary KR, Brigati DJ, Fidler IJ. A rapid colorimetric in situ messenger RNA hybridization technique for analysis of epidermal growth factor receptor in paraffin-embedded surgical specimens of human colon carcinomas. Cancer Res. 1993;53:937–943. [PubMed] [Google Scholar]
- Slade MJ, Coope RC, Gomm JJ, Coombes RC. The human mammary gland basement membrane is integral to the polarity of luminal epithelial cells. Exp Cell Res. 1999;247:267–278. doi: 10.1006/excr.1998.4340. [DOI] [PubMed] [Google Scholar]
- Spinardi L, Ren Y-L, Sanders R, Giancotti FG. The β4 subunit cytoplasmic domain mediates the interaction of α6β4 integrin with the cytoskeleton of hemidesmosomes. Mol Biol Cell. 1993;3:871–884. doi: 10.1091/mbc.4.9.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinardi L, Einheber S, Cullen T, Milner TA, Giancotti FG. A recombinant tail-less integrin β 4 subunit disrupts hemidesmosomes, but does not suppress α6β4-mediated cell adhesion to laminins. J Cell Biol. 1995;129:473–487. doi: 10.1083/jcb.129.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitz I, Mercurio AM. The integrin α6β4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J Cell Biol. 1997;139:1873–1884. doi: 10.1083/jcb.139.7.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, Nonni AV, Fulford L, Merrett S, Chaggar R, Eusebi V, Lakhani SR. CGH analysis of ductal carcinoma of the breast with basaloid/myoepithelial cell differentiation. Br J Cancer. 2001;85:422–427. doi: 10.1054/bjoc.2001.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitteny AF, Fouque B, Mongin C, Teoule R, Boch B. Histologic detection of mRNA with biotinylated synthetic oligonucleotide probes. J Histochem Cytochem. 1988;36:563–571. doi: 10.1177/36.6.3259249. [DOI] [PubMed] [Google Scholar]
- Farquharson M, Harvie R, McNicol AM. Detection of mRNA using a digoxigenin end-labeled oligodeoxynucleotide probe. J Clin Pathol. 1990;43:424–428. doi: 10.1136/jcp.43.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp RL, Charette LA, Rimm DL. Validation of tissue microarray technology in breast carcinoma. Lab Invest. 2000;80:1943–1949. doi: 10.1038/labinvest.3780204. [DOI] [PubMed] [Google Scholar]
- Hoos A, Urist MJ, Stojadinovic A, Mastorides S, Dudas M, Kuo D, Leung DHY, Brennan MF, Lewis JJ, Cordon-Cardo C. Validation of tissue microarrays for immunohistochemical profiling of cancer specimens using the example of human fibroblastic tumors. Am J Pathol. 2001;158:1245–1251. doi: 10.1016/S0002-9440(10)64075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AS, Lotz MM, Mercurio A. A novel structural variant of the human β4 integrin cDNA. Cell Adhes Commun. 1994;2:1–6. doi: 10.3109/15419069409014197. [DOI] [PubMed] [Google Scholar]
- van Leusden MR, Kuikman I, Sonnenberg A. The unique cytoplasmic domain of the human integrin variant β4E is produced by partial retention of intronic sequences. Biochem Biophys Res Commun. 1997;235:826–830. doi: 10.1006/bbrc.1997.6892. [DOI] [PubMed] [Google Scholar]