Abstract
We developed a real-time, quantitative, reverse transcription PCR assay for cyclin D1 (CCND1) expression to aid in the diagnosis of mantle cell lymphoma (MCL). The diagnosis of MCL can be problematic, and existing CCND1 expression assays show a lack of specificity, with elevated expression also detected in other lymphoproliferative disorders. We postulated that evaluating CCND1 expression relative to CCND3 expression by quantitative PCR could offer an improved specificity over an evaluation of CCND1 alone. This method quantitates both CCND1 and CCND3, each normalized to a housekeeping gene (GADPH), using the 5′-exonuclease technique. We analyzed 107 clinical specimens: MCL (17), chronic lymphocytic leukemias (CLL) (10), other non-MCL hematolymphoid disorders (41), non-malignant tissues with an epithelial component (7) and other normal samples (32). This method correctly identified 16 of 17 MCLs, and there were no false positives among any of the other diagnostic groups tested including CLL. CLL presents the major diagnostic dilemma at this institution when diagnosing MCL. Sensitivity studies showed that this method could detect an elevated CCND1/CCND3 ratio when the tumor infiltrate is at least 10% of the cells. We compared the specificity of CCND1 expression alone against the CCND1/CCND3 ratio to demonstrate the increased specificity for the latter. We conclude that the CCND1/CCND3 ratio is a sensitive and specific test for the diagnosis of MCL.
Mantle cell lymphoma (MCL) comprises approximately 6% of all non-Hodgkin’s lymphomas (NHL) and is derived from naïve, pre-germinal center B cells.1 MCL is characterized cytogenetically by the t(11;14), although the t(11;14) is also occasionally present in other hematolymphoid neoplasms including other NHLs, multiple myeloma (MM), atypical chronic lymphocytic leukemias (CLL), and splenic lymphoma with villous lymphocytes (SLVL).2,3,4 The translocation brings the cyclin D1 (CCND1) gene at 11q23 under the control of the immunoglobulin heavy chain gene on chromosome 14, resulting in an overexpression of CCND1.2 CCND1 plays an important role in regulating the transition from G1 to S phase during the cell cycle.5 CCND1 binds to and activates a cyclin-dependent kinase. The activated complex then binds to and inactivates the retinoblastoma protein through phosphorylation, removing its inhibition of transcription factor E2F, which then leads to the transcription of DNA synthesis genes. An increase in CCND1 thereby shortens the time a cell spends in the resting G phase, and accelerates the transition into the S phase.6
The diagnosis of MCL has important implications for prognosis and treatment because the clinical course is aggressive and MCL often responds poorly to conventional B-NHL therapies.2 The morphological appearance can vary broadly, and MCL may in some instances be difficult to distinguish from other lymphomas such as follicular lymphoma (FL), marginal zone lymphoma (MZL), and variant forms of CLL. Distinguishing MCL from CLL presents a common diagnostic dilemma because they can sometimes overlap both morphologically and immunophenotypically. The typical MCL immunophenotype is CD20 positive, CD5 positive, and CD23 negative, whereas CLL is usually positive for the latter.2 However, MCL shows an aberrant phenotype in approximately 7% of cases.7 While morphology and immunophenotyping are often sufficient, additional studies are sometimes necessary to make a definitive diagnosis.
The CCND1 protein (also known as BCL1 or PRAD1) can by detected by immunohistochemical (IHC) methods in approximately 70% of cases,8 although the staining and interpretation can be difficult, especially in bone marrow preparations.
By standard cytogenetic analysis, the t(11;14) is reported to be detectable in only 60 to 80% of cases for technical reasons.9 When routinely available for clinical use, it is virtually always detectable by fluorescence in situ hybridization (FISH).9 PCR for t(11;14) has complications because the breakpoints occur across 120 kb, although approximately 50% occur within a span called the major translocation cluster (MTC). Consequently, the PCR detection rate for t(11;14) is approximately 40 to 60%.2 Southern blot methods can detect 70 to 80% if multiple probes are used.9 However, this method requires large amounts of sample and is technically laborious and challenging in a clinical setting. Similarly, the labor-intensive and technically difficult Northern blot assays for CCND1 mRNA are not suitable for clinical use.
An approach used by others has been to assay for CCND1 mRNA by reverse transcription PCR (RT-PCR), since normal B cells express very low levels of CCND1.10 Again, there are caveats. Increased CCND1 levels have been reported in a minority of cases of other B-NHLs, CLLs, MMs, acute myeloid leukemias (AML), and hairy cell leukemias (HCL), sometimes in the absence of t(11;14).11,12,13 Furthermore, epithelial cells inherently express high levels of CCND1.14 Such cells may be found in significant numbers in extranodal sites being evaluated for NHL.
It is not surprising, therefore, that non-quantitative RT-PCR methods do not adequately distinguish MCL from other B-cell lymphoproliferations.15 Semi-quantitative methods and real-time quantitative methods have shown improvement in specificity. Bijwaard16 and Suzuki12 each describe a real-time quantitative method, with normalization of the results to a housekeeping gene. These appear to separate the MCLs in most cases, however neither evaluated any CLL cases. Nor do they resolve the issue of discerning MCL in a specimen containing an epithelial component, although Bijwaard16 does mention this caveat. Specht4 and Thomazy9 resolve the latter using microdissection of the specimen before RT-PCR.
We postulated that a simpler approach would be to quantitate the ratio of CCND1 to cyclin D3 (CCND3). There are previous reports by others that support this approach. Ott et al17 used immunohistochemistry to demonstrate that while CCND1 was overexpressed in MCL, it was accompanied by reduced or absent CCND3 expression. Suzuki et al18 demonstrated by Northern blotting that in lymphoid malignancies showing an overexpression of CCND1 or cyclin D2 (CCND2), the CCND3 expression was reduced. Furthermore, Uchimura et al19 used a semi-quantitative assay for cyclins D1, D2, and D3, where they showed that a strong CCND1 signal with a weaker CCND3 signal is highly specific for the t(11;14)malignancies. Therefore, we adapted Uchimura’s19 assay as a real-time quantitative RT-PCR assay. We measured CCND1, CCND3, and a housekeeping gene (glyceraldehyde dehydrogenase phosphate or GADPH) on all specimens. The CCND1 and CCND3 are each normalized to GADPH, and the results are reported as a ratio of CCND1 to CCND3.
Materials and Methods
Patients and Samples
Quantitative analyses were performed on 107 clinical samples. The specimen types were archived frozen tissue, peripheral blood, and bone marrow aspirates. The diagnostic groups are shown in Table 1. Thirty-two anonymized specimens of blood from patients not known to harbor any hematological or lymphoproliferative disorders comprised the normal group. For lymphoma samples, the diagnosis was confirmed by blinded evaluation. The diagnosis of mantle cell lymphoma was made by using the morphological and immunophenotypic criteria of the World Health Organization classification.20,21
Table 1.
Diagnostic Groups of Specimens
| Diagnostic group | Number of cases | |
|---|---|---|
| MCL | 17 | |
| CLL | 10 | |
| Normal peripheral blood | 32 | |
| Hematolymphoid disorders, excluding MCL and CLL | 41 | |
| ALL (1) | MM (9) | |
| AML (9) | MPD (5) | |
| HCL (3) | Neutropenia (2) | |
| Hyper-eosinophilia (1) | Other B-NHL (3) | |
| LPL (2) | PLL (2) | |
| MDS (3) | Reactive tonsil (1) | |
| Non-malignant tissues with an epithelial component | 7 | |
| Intestine (1) | Stomach (1) | |
| Lung (1) | Submaxillary (1) | |
| Parotid (1) | Thymus (2) | |
ALL, acute lymphocytic leukemia; AML, acute myelogenous leukemia; CLL, chronic lymphocytic leukemia; HCL, hairy cell leukemia; LPL, lymphoplasmacytic lymphoma; MCL, mantle cell lymphoma; MDS, myelodysplastic syndrome; MM, multiple myeloma; MPD, myeloproliferative disease; NHL, non-Hodgkin’s lymphoma; PLL, prolymphocytic leukemia.
Quantitative RT-PCR
For peripheral blood and bone marrow samples, mononuclear cells (MNCs) were first isolated by density gradient centrifugation as per the manufacturer’s instructions (Ficoll-Paque from Amersham Biosciences, Piscataway, NJ). RNA was isolated from the MNCs by a modified guanidium-thiocyanate method as per the manufacturer’s instructions (TRIzol, Invitrogen, Carlsbad, CA). Frozen tissues were processed in the same manner, with the exception of the Ficoll separation step. One to 10 micrograms of RNA, depending on the specimen quantity, was then treated to remove any DNA contamination (DNA-free Kit, Ambion, Inc., Austin, TX).
The reverse transcription reaction contained the following in a 40 μl volume: 400 units MMLV enzyme, 10 mmol/L dithiothreitol (DTT), 1X MMLV buffer (all from Invitrogen), 80 units RNasin (Promega, Madison, WI), 0.5 μg random hexamers (Amersham Biosciences), 0.5 mmol/L dNTPs (Amersham Biosciences) and DNase-treated total RNA. The minimum absolute RNA quantity used for the reverse transcription was one microgram, and the maximum was 10 micrograms. The reaction incubated at 25°C for 10 minutes, 37°C for 2 hours, and 70°C for 15 minutes. Control and reference RNA’s were reverse-transcribed alongside patient RNA for each run.
The quantitative real-time PCR was performed in the ABI 7700 with cycling as follows: an initial cycle for 10 minutes at 95°C, followed by 45 bi-phasic cycles of 15 seconds at 95°C and 1 minute at 60°C.
Each 96-well plate was divided into thirds, one for each target (CCND1, CCND3, and GADPH). Each third (32 wells) included the following: positive control, negative control, reference (or calibrator), no template control (water in lieu of cDNA template) and patient samples (all run in duplicate) as well as also no-RT controls for each patient sample run singly. The no-RT control is DNase-treated sample RNA that has not undergone the reverse transcription step. There was sufficient space for eight patient samples on each plate.
The CCND1 and CCND3 primers are as per Uchimura,19 and the probes for each were designed using Primer Express software (Applied Biosystems, Foster City, CA). The GADPH primers and probe were adapted from sequences used in the TaqMan GADPH Control Reagents Kit (Applied Biosystems), however we used FAM as the reporting fluorophore, rather than VIC. Primers and probes were manufactured by Operon Technologies (Alameda, CA). Note that the CCND1 and CCND3 reactions use the same forward primer (for homologous CCND regions), while the reverse primer and the probe is specific for either the D1 or D3 types. Each probe includes FAM (6-carboxy-fluorescein. emission 518 nm) at the 5′-end as the reporter and TAMRA (6-carboxy-tetramethyl-rhodamine, emission 582 nm) at the 3′-end as the quencher. Primer and probe sequences are listed in Table 2.
Table 2.
Primer and Probe Sequences
| Name | Sequence |
|---|---|
| CCND-F | 5′-CTGGCCATGAACTACCTGGA-3′ |
| CCND1-R | 5′-GTCACACTTGATCACTCTGG-3′ |
| CCND1-probe | 5′-FAM-AGAAGCGTGTGAGGCGGTAGTAGGA-TAMRA-3′ |
| CCND3-R | 5′-CCAGGAAATCATGTGCAATC-3′ |
| CCND3-probe | 5′-FAM-AGCAGCCAGGTCCCACTT-TAMRA-3′ |
| GADPH-F | 5′-GAAGGTGAAGGTCGGAGTC-3′ |
| GADPH-R | 5′-GAAGATGGTGATGGGATTTC-3′ |
| GADPH-probe | 5′-FAM-CAAGCTTCCCGTTCTCAGCC-TAMRA-3′ |
The reaction mixture for CCND1 contains the following in a final volume of 25 μl: 2.5U TaqGold, 1X TaqGold Buffer II, and 11 mmol/L MgCl2 (all from Applied Biosystems), 200 μmol/L dNTPs (Amersham Biosciences), 10 pmoles each of CCND-F primer, CCND1-R primer, and CCND1 probe, and 3 μl of cDNA.
The reaction mixture for CCND3 contains the following in a final volume of 25 μl: 2.5U TaqGold, 1X TaqGold Buffer II, and 11 mmol/L MgCl2 (all from Applied Biosystems), 200 μmol/L dNTPs (Amersham Biosciences), 10 pmoles each of CCND-F primer, CCND3-R primer, and CCND3 probe, and 3 μl of cDNA.
The reaction mixture for GADPH contains the following in a final volume of 25 μl: 2.5U TaqGold, 1X TaqGold Buffer II, and 11 mmol/L MgCl2 (all from Applied Biosystems), 250 μmol/L dNTPs (Amersham Biosciences), 50 pmoles each of GADPH-F primer and GADPH-R primer, 40 pmoles of GADPH probe, and 3 μl of cDNA.
Data Analysis
Results were calculated by the “Comparative Ct Method of Quantitation” (ΔΔDCt) as per Applied Biosystems 7700 Sequence Detection System User Bulletin No. 2 (ABI Document No. 4303859, can be downloaded from the web as a pdf from: www.wzw.tum.de/gene-quantification/pe-rel-quan.pdf). In this method no standard curve is used, instead all results are calculated relative to a reference standard, called a “calibrator.” Each of the three targets is analyzed separately, then CCND1 and CCND3 are each normalized to GADPH. The normalization is necessary to account for variabilities in RNA quantity and quality, and variabilities in reverse transcription efficiency among samples. In this study we required that the GADPH Ct be less than 30 to be considered an interpretable specimen. In our experience using GADPH in real time assays, higher Cts for the GADPH indicate insufficient template quantity, quality, and/or the presence of inhibitors. Only one sample (GADPH Ct of 36) was excluded because it failed to meet this criterion.
The ΔΔCt is then calculated separately for CCND1 and CCND3 for each sample, using the cell line Meg01 as the calibrator. Meg01 (American Type Culture Collection, Manassas, VA) was chosen as the reference calibrator material because it appears to express approximately equivalent amounts of CCND1 and CCND319 and, as a cell line, it is potentially a consistent and renewable source of calibrator template. We monitored the stability of the Meg01 cell line throughout this study, as described in the Results section below.
The ΔΔCt for CCND1 and the ΔΔCt for CCND3 are then reported as a simple ratio (ΔΔCt CCND1 value divided by the ΔΔCt CCND3 value). The relative PCR amplification efficiencies for GADPH, CCND1, and CCND3 were empirically evaluated, also as per User Bulletin No. 2 and were determined to meet the manufacturer’s criteria for equivalency.
Sensitivity
To assess the sensitivity of the assay, we performed the quantitative RT-PCR described above on a series of cell line dilutions. Cell suspensions of a t(11;14)-positive myeloma cell line (TIB-196, American Type Culture Collection, Manassas, VA) and a t(11;14)-negative cell line (OCI-Ly8, Stanford, CA) were prepared such that each contained 5 × 105 cells per microliter. The undiluted TIB-196 cell line was designated as 100%. The subsequent dilutions were named 50%, 25%, 10%, 5%, 1%, 0.5%, and 0.25%, which represented the percentage of TIB-196 cells in each, using the negative OCI-Ly8 cells as the diluent. After the cells were properly diluted, RNA was extracted as described above. The RNA was then Dnase treated, and reverse transcription and quantitative real-time PCR were performed as described above.
Results
The a CCND1/CCND3 (D1/D3) assay results for the 107 specimens in this study are tabulated in Table 3 and displayed graphically in Figure 1. Sixteen of 17 MCL cases (94.1%) were positive by this method, defined as a D1/D3 ratio of 2 (2.0). The cut-off value of 2.0 was determined by analyzing which value yielded the best specificity results.
Table 3.
Results Summary by Diagnostic Group
| Diagnostic group* | Number of cases | Mean result (CCND1/CCND3) | Range of results |
|---|---|---|---|
| MCL | n = 17 | 8.190 | 0.030 –22.500 |
| CLL | n = 10 | 0.117 | 0.014 –0.270 |
| Other hematolymphoid disorders, excluding MCL and CLL | n = 41 | 0.170 | 0.001 –1.66 |
| Non-malignant tissues containing a significant epithelial component | n = 7 | 0.194 | 0.001 –1.260 |
| Normal peripheral blood | n = 32 | 0.047 | 0.006 –0.351 |
See Table 1 for composition of the groups. The Kruskal-Wallis ANOVA test demonstrates that there is a statistically significant difference between the MCL group and the other groups (p <0.0001).
Figure 1.
CCND1/CCND3 results summary. Mean values are shown at the hash marks alongside the data points. Abbreviations: N, normal; Abn, hematolymphoid disorders other than MCL or CLL (see Table 1 for case composition of this group); Epi, non-malignant tissues containing a significant epithelial component (see Table 1 for case composition of this group); CLL, chronic lymphocytic leukemia; MCL, mantle cell lymphoma.
The MCL mean value was 8.19 (range 0.03 to 22.50). Also, all MCL cases were positive for CCND1 by immunohistochemistry; however the single case with a negative D1/D3 ratio (0.030) showed an atypical immunohistochemical staining pattern. It had only scattered CCND1 positivity, with approximately 1 to 5% of non-endothelial (presumably lymphoid) cells staining. The majority of malignant-appearing cells did not stain (Figure 2).
Figure 2.
Immunoperoxidase stain for BCL1 in lymph node tissue at ×400 magnification. A: MCL case showing a typical nuclear staining pattern. B: The single MCL case with a negative D1/D3 ratio (0.030), showing an atypical staining pattern with only scattered CCND1 positivity.
Among the 41 non-mantle-cell, hematolymphoid disorder cases, the mean D1/D3 ratio was 0.170 (range 0.001 to 1.660); all were negative. The CLL group also showed only negative results among all 10 cases, with a mean of 0.177 (range 0.014 to 0.270). Similarly, the normal control group of 32 cases was negative, with a mean of 0.047 (range 0.006 to 0.351). Among the 7 non-malignant epithelial tissues, the mean was 0.194 (range 0.001 to 1.260). In summary, a total of 90 non-mantle cell tissues were studied, and 90 of 90 (100%) were negative by this assay, with a mean of 0.120 (range 0.001 to 1.680). The specificity of the D1/D3 assay is summarized in Table 4.
Table 4.
Chi-Square Showing Specificity of Results
| Positive | Negative | |
|---|---|---|
| Diagnosis: MCL | 16/17 (94.1%) | 1/17 (5.9%) |
| Diagnosis: Non-MCL | 0/90 (0%) | 90/90 (100%) |
Statistical analysis using the Kruskal-Wallis analysis of variance test demonstrates that there is a statistically significant difference between the MCL group and the other groups (P < 0.0001).
Assays of cell-line dilutions showed a sensitivity of 10%, the lowest dilution of MCL cells in non-MCL lymphocytes that gave a D1/D3 ratio of greater than 2.0. In other words, a tumor infiltrate comprising at least 10% of the cells analyzed is required to detect an abnormal result.
The Meg01 cell line showed stable relative expression of CCND1 and CCND3 over the three passages required to complete this study (a total of 24 test plates). For all test plates, the Meg01 mean ΔCt D1-D3 was 8.70, with a coefficient of variance of 10.0%. This was evenly spread throughout the runs, with no shifts when changing lots of Meg01.
Discussion
We have developed a real-time quantitative PCR using the 5′ exonuclease method of fluorescence detection on the ABI 7700 Sequence Detector System. We adapted the semi-quantitative method of Uchimura et al19 to measure the expression of CCND1 and CCND3, both normalized to the housekeeping gene GADPH. We assayed a total of 107 specimens for this D1/D3 ratio, which included 17 MCL cases. By our D1/D3 ratio method, we were able to correctly identify 16 of 17 MCLs, with no false positives in other tissues or hematolymphoid malignancies.
The single aberrantly low D1/D3 result in MCL was an axillary lymph node. Rosenwald et al22 reported gene expression microarray data that showed nine cases of MCL without increased CCND1 expression, and some of these showed increased CCND2 or CCND3 expression. Indeed, the ΔΔCt value for CCND3 in this case is 113.4, while the other MCL cases are very low in comparison, ranging from 0.51 to 9.09.
An important issue in CCND1 assay development is specificity. Of particular concern to us was the ability of the D1/D3 ratio method to distinguish MCL from CLL. At our institution, it is common to make a diagnosis of CLL from peripheral blood and/or bone marrow, and in some cases it is difficult to definitively rule out MCL. We performed the D1/D3 assay on 10 cases of CLL and this method was 100% specific at ruling out a diagnosis of MCL, with no false positives among the CLL cases.
Another concern was whether or not contaminating epithelial cells might falsely elevate the D1/D3 ratio. Epithelia are known to express high levels of CCND114 and extra-nodal presentations are not uncommon in MCL.2 Indeed, in CCND1 expression studies that included extra-nodal specimens, elevated CCND1 were sometimes seen in non-MCL NHL cases. These reports warn of potential false positive results in CCND1 expression levels in such cases.17
CCND3 levels in epithelia have not been reported to our knowledge. However, CCND3 is nearly ubiquitously expressed, along with either CCND1 or CCND2 in a given tissue type.19,23 Therefore, we postulated that perhaps the ratio of CCND1/CCND3 might more reliably diagnose a MCL in tissues containing epithelia. To investigate this, we tested 7 non-malignant tissues containing a significant epithelial component that would be potential extranodal sites for MCL. The sites were thymus (2), lung (1), intestine (1), stomach (1), submaxillary tissue (1), and parotid (1). These tissues showed a broad range of epithelial tissue, ranging from ≤10% in the thymus specimens to >90% in the submaxillary specimen. Interestingly, an evaluation of the parotid specimen by CCND1 alone, without including CCND3, would give an apparent (false) positive result. This specimen contained 50% epithelial tissue. However, all of the epithelial specimens, including the parotid, were negative by CCND1/CCND3 ratio.
Table 5 shows the results for these specimens. All specimens yielded negative CCND1/CCND3 ratios (less than 2.0). Therefore, it appears that by this methodology, the presence of epithelial cells will not lead to false positive diagnoses of MCL in extra-nodal presentations.
Table 5.
CCND1/CCND3 Results of the Epithelia-Containing Tissue Specimens
| CCND1/CCND3 result | |
|---|---|
| All MCL cases | >2.00 × 100 |
| Thymus A | 9.87 × 10−4 |
| Thymus B | 9.39 × 10−3 |
| Lung | 1.57 × 10−2 |
| Parotid | 1.26 × 100 |
| Stomach | 4.24 × 10−2 |
| Intestine | 2.39 × 10−2 |
| Submaxillary gland | 6.87 × 10−3 |
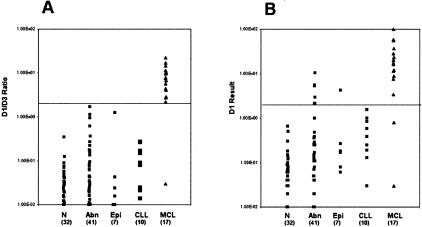
We also compared the D1/D3 ratio to the results obtained for CCND1 expression alone. The CCND1 expression is normalized to the housekeeping gene GADPH and are therefore comparable to other real-time methods reported in the literature.12,17 As can be seen in Figure 3A, the D1/D3 ratio approach improves the separation and specificity for MCL over CCND1 expression alone (Figure 3B). While there are no false positives using the D1/D3 ratio, 7 of the 90 non-MCL cases would be falsely positive if CCND1 expression alone were evaluated. The 7 cases are reactive tonsil (1), parotid tissue (1), hairy cell leukemia (2), prolymphocytic leukemia (2), and marginal zone lymphoma (1). Furthermore, there is a case of MCL that becomes falsely negative. Therefore, using an expression analysis of D1 only, there would be 7 of 90 false positives (7.8%) and 2 of 17 false negatives (11.8%). But by using the D1/D3 ratio, these values improve such that there are 0 of 90 false positives and 1 of 17 false negatives (5.9%).
Figure 3.
Comparison of the specificity of the CCND1/CCND3 ratio (A) with CCND1 expression alone (B). All results are normalized to GADPH. N, normal; Abn, hematolymphoid disrders other than MCL or CLL (see Table 1 for case composition of this group); Epi, non-malignant tissues containing a significant epithelial component (see Table 1 for case composition of this group); CLL, chronic lymphocytic leukemia; MCL, mantle cell lymphoma.
Our study contained too few MCL cases to derive any statistically significant prognostic correlations. However, Rosenwald et al22 reported data showing that the level of CCND1 expression correlates with overall survival. Additional studies are needed to demonstrate whether the D1/D3 value may have independent prognostic implications.
We did not perform testing on paraffin-embedded tissue in this study, because the described primer set yields amplicons of approximately 250 bp. In our experience, this amplicon size is larger than can routinely be obtained by isolating RNA from paraffin-embedded specimens. However, our approach of measuring a CCND1/CCND3 ratio could be tested by designing a primer set that yields smaller amplicons.
In conclusion, we describe a real-time quantitative PCR for cyclin D1 expression, reported as a ratio to cyclin D3. Our CCND1/CCND3 method reliably distinguishes MCL from other malignant and non-malignant samples. The CCND1/CCND3 ratio improves the specificity over an evaluation of CCND1 expression alone in the cases tested here. We conclude that the CCND1/CCND3 ratio offers a sensitive and specific test to aid in the diagnosis of MCL.
Footnotes
Supported by National Institutes of Health grants 2PO1CA49605 and 2PO1CA34233.
References
- Shaffer AL, Rosenwald A, Staudt LM. Lymphoid malignancies: the dark side of B-cell differentiation. Nat Rev Immunol. 2002;2:920–932. doi: 10.1038/nri953. [DOI] [PubMed] [Google Scholar]
- Weisenburger DD, Armitage JO. Mantle cell lymphoma: an entity comes of age. Blood. 1996;87:4483–4494. [PubMed] [Google Scholar]
- Jadayel D, Matutes E, Dyer MJ, Brito-Babapulle V, Khohkar MT, Oscier D, Catovsky D. Splenic lymphoma with villous lymphocytes: analysis of BCL-1 rearrangements and expression of the cyclin D1 gene. Blood. 1994;83:3664–3671. [PubMed] [Google Scholar]
- Specht K, Kremer M, Muller U, Dirnhofer S, Rosemann M, Hofler H, Quintanilla-Martinez L, Fend F. Identification of cyclin D1 mRNA overexpression in B-cell neoplasias by real-time reverse transcription-PCR of microdissected paraffin sections. Clin Cancer Res. 2002;8:2902–2911. [PubMed] [Google Scholar]
- Lukas J, Jadayel D, Bartkova J, Nacheva E, Dyer MJ, Strauss M, Bartek J. BCL-1/cyclin D1 oncoprotein oscillates and subverts the G1 phase control in B-cell neoplasms carrying the t(11;14) translocation. Oncogene. 1994;9:2159–2167. [PubMed] [Google Scholar]
- Pines J. Mammalian cell cycle control. Peters G, Vousden KH, editors. New York: Oxford University Press; Oncogenes and Tumour Suppressors. 1997:191–200. [Google Scholar]
- Argatoff LH, Connors JM, Klasa RJ, Horsman DE, Gascoyne RD. Mantle cell lymphoma: a clinicopathologic study of 80 cases. Blood. 1997;89:2067–2078. [PubMed] [Google Scholar]
- Yatabe Y, Suzuki R, Tobinai K, Matsuno Y, Ichinohasama R, Okamoto M, Yamaguchi M, Tamaru J, Uike N, Hashimoto Y, Morishima Y, Suchi T, Seto M, Nakamura S. Significance of cyclin D1 overexpression for the diagnosis of mantle cell lymphoma: a clinicopathologic comparison of cyclin D1-positive MCL and cyclin D1-negative MCL-like B-cell lymphoma. Blood. 2000;95:2253–2261. [PubMed] [Google Scholar]
- Thomazy VA, Luthra R, Uthman MO, Davies PJ, Medeiros LJ. Determination of cyclin D1 and CD20 mRNA levels by real-time quantitative RT-PCR from archival tissue sections of mantle cell ymphoma and other non-Hodgkin’s lymphomas. J Mol Diagn. 2002;4:201–208. doi: 10.1016/S1525-1578(10)60704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch F, Jares P, Campo E, Lopez-Guillermo A, Piris MA, Villamor N, Tassies D, Jaffe ES, Montserrat E, Rozman C. PRAD-1/cyclin D1 gene overexpression in chronic lymphoproliferative disorders: a highly specific marker of mantle cell lymphoma. Blood. 1994;84:2726–2732. [PubMed] [Google Scholar]
- Campo E. Genetic and molecular genetic studies in the diagnosis of B-cell lymphomas I: mantle cell lymphoma, follicular lymphoma, and Burkitt’s lymphoma. Hum Pathol. 2003;34:330–335. doi: 10.1053/hupa.2003.97. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Takemura K, Tsutsumi M, Nakamura S, Hamajima N, Seto M. Detection of cyclin D1 overexpression by real-time reverse-transcriptase-mediated quantitative polymerase chain reaction for the diagnosis of mantle cell lymphoma. Am J Pathol. 2001;159:425–429. doi: 10.1016/S0002-9440(10)61713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch F, Campo E, Jares P, Pittaluga S, Munoz J, Nayach I, Piris MA, Dewolf-Peeters C, Jaffe ES, Rozman C, Montserrat E, Cardesa A. Increased expression of the PRAD-1/CCND1 gene in hairy cell leukaemia. Br J Haematol. 1995;91:1025–1030. doi: 10.1111/j.1365-2141.1995.tb05429.x. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Lukas J, Strauss M, Bartek J. Cell cycle-related variation and tissue-restricted expression of human cyclin D1 protein. J Pathol. 1994;172:237–245. doi: 10.1002/path.1711720303. [DOI] [PubMed] [Google Scholar]
- Aguilera NS, Bijwaard KE, Duncan B, Krafft AE, Chu WS, Abbondanzo SL, Lichy JH, Taubenberger JK. Differential expression of cyclin D1 in mantle cell lymphoma and other non-Hodgkin’s lymphomas. Am J Pathol. 1998;153:1969–1976. doi: 10.1016/s0002-9440(10)65710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijwaard KE, Aguilera NS, Monczak Y, Trudel M, Taubenberger JK, Lichy JH. Quantitative real-time reverse transcription-PCR assay for cyclin D1 expression: utility in the diagnosis of mantle cell lymphoma. Clin Chem. 2001;47:195–201. [PubMed] [Google Scholar]
- Ott MM, Bartkova J, Bartek J, Durr A, Fischer L, Ott G, Muller-Hermelink HK, Kreipe H. Cyclin D1 expression in mantle cell lymphoma is accompanied by down-regulation of cyclin D3 and is not related to the proliferative activity. Blood. 1997;90:3154–3159. [PubMed] [Google Scholar]
- Suzuki R, Kuroda H, Komatsu H, Hosokawa Y, Kagami Y, Ogura M, Nakamura S, Kodera Y, Morishima Y, Ueda R, Seto M. Selective usage of D-type cyclins in lymphoid malignancies. Leukemia. 1999;13:1335–1342. doi: 10.1038/sj.leu.2401485. [DOI] [PubMed] [Google Scholar]
- Uchimaru K, Taniguchi T, Yoshikawa M, Asano S, Arnold A, Fujita T, Motokura T. Detection of cyclin D1 (bcl-1, PRAD1) overexpression by a simple competitive reverse transcription-polymerase chain reaction assay in t(11;14)(q13;q32)-bearing B-cell malignancies and/or mantle cell lymphoma. Blood. 1997;89:965–974. [PubMed] [Google Scholar]
- Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC, Grogan TM, Isaacson PG, Knowles DM, Mason DY, Muller-Hermelink H-K, Pileri SA, Piris MA, Ralfkiaer E, Warnke RA. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD. The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting, Airlie House, VA, November, 1997. Ann Oncol. 1999;10:1419–1432. doi: 10.1023/a:1008375931236. [DOI] [PubMed] [Google Scholar]
- Rosenwald A, Wright G, Wiestner A, Chan WC, Connors JM, Campo E, Gascoyne RD, Grogan TM, Muller-Hermelink HK, Smeland EB, Chiorazzi M, Giltnane JM, Hurt EM, Zhao H, Averett L, Henrickson S, Yang L, Powell J, Wilson WH, Jaffe ES, Simon R, Klausner RD, Montserrat E, Bosch F, Greiner TC, Weisenburger DD, Sanger WG, Dave BJ, Lynch JC, Vose J, Armitage JO, Fisher RI, Miller TP, LeBlanc M, Ott G, Kvaloy S, Holte H, Delabie J, Staudt LM. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell. 2003;3:185–197. doi: 10.1016/s1535-6108(03)00028-x. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Lukas J, Strauss M, Bartek J. Cyclin D3: requirement for G1/S transition and high abundance in quiescent tissues suggest a dual role in proliferation and differentiation. Oncogene. 1998;17:1027–1037. doi: 10.1038/sj.onc.1202016. [DOI] [PubMed] [Google Scholar]