Abstract
The risk of developing second primary cancers is increased in patients with breast cancer. The lung is one of the major target organs, and therefore a differential diagnosis between primary and metastatic cancers is required for the treatment of lung tumors in patients with a history of breast cancer. However, biopsy specimens frequently result in small, fragmented tissues containing only a few, degenerated cancer cells. We attempted to find a useful marker for differential diagnosis, using the online SAGE database. We selected three molecules, small breast epithelial mucin (SBEM), prostate epithelium-specific Ets transcription factor (PDEF), and mammaglobin (MGB1), as potential markers for breast cancer. SBEM and PDEF proved of no use for practical differential diagnosis because they are expressed in the normal bronchus. In contrast, expression of MGB1 was detected in all 22 primary breast cancers, but not in 22 normal lung tissues. Furthermore, all 12 metastatic breast cancers examined demonstrated positive MGB1 transcripts, whereas one of 48 primary lung adenocarcinomas expressed MGB1. This suggests that MGB1 can serve as a differential molecular marker. In practice, prospective examination, using the nine cases with a history of breast cancer, confirmed the usefulness of MGB1 in differential diagnosis.
The lung is a major target of hematogeneous metastases from a variety of cancers. Thus, a diagnosis differentiating between primary and metastatic cancers is always required in clinical practice. In our institute, on average 250 lung biopsies are performed every year, and about two thirds of the tumors are diagnosed as malignant. Metastatic cancers make up 10% to 20% of these. Although this incidence may not be very high, a differential diagnosis of the metastatic cancer is important to determine the therapeutic strategy. For example, in the case of a small solitary lung tumor without any lymphadenopathy, the patient may be treated with chemotherapy or may undergo partial resection of the lung when the lung tumor is diagnosed as a metastatic breast cancer. On the other hand, standard lobectomy may be the treatment of choice when the diagnosis is of a primary non-small cell lung cancer.
The risk of developing second primary cancers is increased in patients with breast cancer, and the lung is one of the major sites involved.1,2,3 Some articles have described an increased risk of primary lung cancers in association with radiation therapy following mastectomy.4,5 Furthermore, the long latent period before identification of metastasis makes a differential diagnosis challenging. Indeed, a latent period of more than 10 years is not rare in patients with breast cancer. Histologically, a differential diagnosis between metastatic breast cancer and primary lung adenocarcinoma is difficult. Cytoplasm with secretory feature and stromal fibrosis were frequently observed in both adenocarcinomas. Moreover, metastatic breast cancer can grow along with the alveolar septa, in a similar manner to bronchioloalveolar carcinomas.6 Difficulty is also caused by the need to carry out a differential diagnosis on biopsy specimens. Often, lung biopsies produce small amounts of fragmented tissue, which contain only a few degenerated cancer cells. Therefore, the differential diagnosis has to draw on auxiliary analysis, such as immunohistochemistry.
There are a limited number of immunohistochemical markers to identify breast cancers. Gross cystic disease fluid protein-15 (GCDFP-15) is one such marker; it is positive in only a few normal breast epithelia, but frequently expressed in breast carcinomas showing apocrine features. Estrogen receptor (ER) is also commonly used for differential diagnosis. The combined application of GCDFP-15 and ER are very helpful for differential diagnosis, but each marker is not complete. About 50% to 60% of breast cancers express one or both, but the remaining tumors are negative for both markers. Indeed, Perry et al7 reported that GCDFP-15 and ER are specific, but insensitive, for breast origin through the differential diagnosis of 68 metastatic adenocarcinomas to the brain. Currently, extensive human genome data have been accumulated, and the data contain much information that can be directly used in clinical practice. In the present study, we searched for molecules of potential breast-specific expression using the online database of serial analysis of gene expression (SAGE) from the National Center for Biotechnology Information (NCBI). Our results demonstrated that one of the molecules examined was specifically expressed in breast cancers, indicating that the molecule can serve as a differential marker.
Materials and Methods
Patients
Using a database of the Department of Pathology and Molecular Diagnostics of Aichi Cancer Center Hospital (Nagoya, Japan), we first analyzed the incidence of lung biopsies that required a differential diagnosis of primary or metastatic lung cancer. For RT-PCR studies, 70 primary lung cancers, 51 metastatic lung cancers, and 22 normal lung tissue samples, as well as 22 invasive breast cancers were analyzed. All these tissues were obtained immediately after surgery, snap-frozen, and stored at −80°C until use. In addition, for prospective analysis, nine touch-imprint slides were prepared from fine-needle biopsy specimens.
Reverse Transcription and PCR (RT-PCR) Analysis
Total RNA was extracted using a standard acid guanidinium thiocyanate-phenol-chloroform method,8 and was digested with DNase I, followed by conversion to cDNA with Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA) and random hexamers. After confirmation of RNA integrity and negative contamination of genomic DNA by RT-PCR for β-actin (275 and 369 bp products for cDNA and genomic DNA, respectively), cDNA was subjected to PCR analysis. Gene-specific amplification was performed using Amplitaq Gold (Applied Biosystems, Foster City, CA). The nucleotide sequences of MGB1, SBEM and PDEF were obtained from GenBank (NM 002411, NM 058173, and NM 012391, respectively) and primers were designed to span an exon junction as follows: MGB1, 5′-ACCATGAAGTTGCTGATGGTC-3′ and 5′-AAACACCTCAACATTGCTCAGA-3′; SBEM, 5′-GTATCCAGCTACTGGTCCTGCT-3′ and 5′-CAATTGCAGAAGACTCAAGCTG-3′; PDEF, 5′-CGGTCATTGACAGCCAAG-3′ and 5′-AGGAGCCACTTCTGCACATT-3′.
Products were analyzed by electrophoresis on 2.5% high-resolution gels (NuSieve GTG agarose, BioWhittaker Molecular Applications, Rockland, ME). Some products were directly sequenced using an ABI 310 Genetic Analyzer and BigDye Primer Cycle Sequencing Kits (ABI, Foster City, CA), to confirm the amplified sequences. To determine the precise location of the mRNA expression of the gene of interest, parts of the tissues were isolated with a laser-captured microdissection system (Arcturus, Mountain View, CA). Extraction of RNA and RT-PCR were carried out as described above.
Results
Incidence of Lung Biopsies Requiring Differential Diagnosis
For the years 2000–2002, 834 lung biopsies were submitted to our department; 564 were diagnosed as malignancies or suspicious malignancies. A differential diagnosis between primary and metastatic cancers was required for 174 (21%) of the specimens submitted, because these patients had histories of cancer before the identification of lung tumors. The primary sites of the previous cancers included lung (26%), head and neck (22%), breast (13%), stomach (13%), colon (7%), and others (19%). We have reported on the differential diagnosis of metachronous9 and synchronous lung cancers,10 and, therefore, this study focused on the differential diagnosis of primary lung cancer and metastatic breast cancer.
Searching for Sensitive Markers of Metastatic Breast Cancer
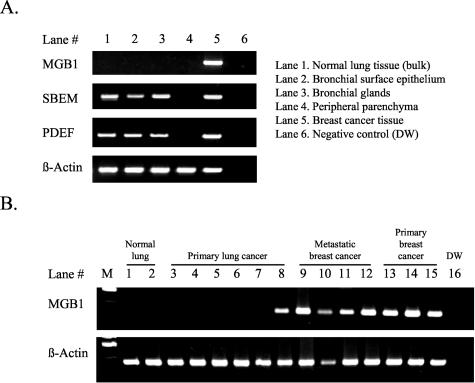
To obtain markers that are sensitive and specific to breast cancers, we searched the online SAGE database of the NCBI (http://www.ncbi.nlm.nih.gov/SAGE/). Three candidates, mammaglobin (MGB1, Hs.46452), small breast epithelial mucin (SBEM; Hs.348419), and prostate epithelium-specific Ets transcription factor (PDEF; Hs.79414), were selected. Although only a few articles have described these molecules, they support breast-specific expression.11,12,13,14,15 Detailed results from the SAGE database and the literature are summarized in Table 1. We next examined whether expression of these molecules could be used as practical distinguishing markers between primary lung cancers and metastatic breast cancers. In the 22 primary breast cancers, MGB1 was expressed in all 22, SBEM in 20, and PDEF in 20. In contrast to the results from the SAGE database, SBEM and PDEF transcripts were detected in 21 and 20 of 22 normal lung tissues, respectively, whereas MGB1 was not expressed in any (Table 1). SBEM has also been reported to be expressed in salivary glands,14 which histologically resemble bronchial glands. We therefore determined the precise location of expressions in isolated bronchial glands, bronchial surface epithelium, and peripheral lung tissue, using a laser-capture microdissection device. SBEM expression was detected both in bronchial glands and bronchial surface epithelium, but not in parenchyma without bronchioles (Figure 1). The same experiment was carried out on PDEF, which was also expressed in bronchial cells.
Table 1.
Expression of MGB1, SBEM, and PDEF in Breast Cancers and in the Normal Lung
| MGB1 | SBEM | PDEF | |
|---|---|---|---|
| SAGE database* | |||
| Normal tissues | 9:2 | 376:0 | 4:0 |
| Cancer tissues | 17:4 | 32:0 | 126:0 |
| Reported description | |||
| Tissue-specific expression | Yes | Yes | Yes |
| Expressing organ(s) | Breast† | Breast and salivary glands | Breast and prostate |
| Expression in breast cancers | Yes | Yes | Yes |
| References | 11, 16 | 14 | 12, 13 |
| RT-PCR | |||
| Primary breast cancer (n = 22)‡ | 22 | 20 | 20 |
| Normal lung (n = 22) | 0 | 21 | 20 |
Ratio of total breast counts to lung counts; libraries used were GSM692, 677, 691, 760, and 780 for normal breast tissue; GSM 670, 671, 672, 673, and 694 for breast cancer tissue, and GSM762 for normal lung tissue.
One article reported low-level of expression in gynecological malignancies, using nested RT-PCR.
All are invasive ductal carcinoma.
Figure 1.
Expression analysis of potential breast-specific molecules (A) reveals that bulk tissue of normal lung (lane 1) expressed SBEM and PDEF, but not MGB1. Detailed examination using laser capture microdissection (lanes 2 to 4) demonstrated that bronchial surface epithelium and bronchial gland cells, but not the peripheral lung, were the source of the expression. In tumors (B), MGB1 expression was specific to breast cancers (B, lanes 9 to 15), with the notable exception of small-cell lung carcinomas (B, lane 8). DW indicates distilled water.
Expression of MGB1 in Primary and Metastatic Cancers of the Lung
The lack of expression of MGB1 in the normal lung prompted us to examine whether MGB1 could be used in differential diagnosis between primary lung cancers and metastatic breast cancers. All of the 14 metastatic breast cancers of the lung were confirmed to express MGB1, whereas only seven of 70 primary lung cancers (10%) were positive (Table 2). No MGB1 expression could be detected in the 47 cases of adenocarcinoma of the lung except for one, which interestingly metastasized to the breast 1 year after the lung operation. Detailed immunohistochemical analysis of this case revealed that both lung and breast tumors were positive for thyroid transcription factor-1 (TTF-1) and surfactant apoprotein A, and negative for estrogen and progesterone receptors. The morphological features were that of an ordinary lung adenocarcinoma. Five of the remaining six tumors with unexpected MGB1 expression were three small cell lung cancers, two large cell neuroendocrine carcinomas and a squamous cell carcinoma expressing neuroendocrine markers (CD56 and synaptophysin) in parts. Conversely, for high-grade neuroendocrine tumors of the lung, half the small cell lung cancers and large cell neuroendocrine carcinomas were positive for MGB1 expression.
Table 2.
Expression of MGB1 in Breast and Lung Tumors
| n | Positive cases | Frequency | |
|---|---|---|---|
| Cancers metastatic to lung (total)
|
51
|
16
|
31%
|
| Metastatic breast cancer | 12 | 12 | 100% |
| Metastatic colon cancer | 15 | 0 | 0% |
| Metastatic sarcoma | 12 | 0 | 0% |
| Metastatic salivary gland cancer | 4 | 3 | 75% |
| Metastatic cancer, others
|
8
|
1*
|
13%
|
| Primary lung cancers (total)
|
70
|
7
|
11%
|
| Adenocarcinoma | 48 | 1† | 2% |
| Squamous cell carcinoma | 12 | 1 | 8% |
| High-grade neuroendocrine tumor | 10 | 5‡ | 50% |
| Low-grade neuroendocrine tumor§ | 3 | 0 | 0% |
A metastatic endometroid cancer.
A primary lung adenocarcinoma that metastasized to the breast.
Positive cases were 2 of 4 large cell neuroendocrine carcinomas and 3 of 6 small cell carcinomas.
All these were carcinoid tumors.
We also examined the expression status of MGB1 in various other metastatic cancers. All 15 colon cancers and 12 sarcomas were negative for MGB1 expression, whereas three of four salivary gland carcinomas (adenoid cystic carcinomas) and one each of metastatic esophageal and endometrial cancers showed MGB1 expression. All of the PCR products of MGB1 in the non-breast metastatic cancer cases were confirmed by direct sequencing of the products. The other metastatic cancers, including one each of thyroid, tongue, gastric, pancreas and uterine cervix cancers, were negative for MGB1 expression. Because MGB1 transcripts were detected in the metastatic tumors, 17 primary esophageal cancers, and 10 primary salivary tumors were examined: the esophageal cancers were all negative for MGB1, and six of the 10 salivary gland tumors expressed MGB1.
Practical Application of MGB1 in Differential Diagnosis
These results indicated that MGB1 could serve as a marker of breast cancers, and thus we prospectively evaluated MGB1 expression in touch-imprint specimens of fine-needle biopsies from nine lung tumors with a breast cancer history. In six of the nine, MGB1 expression was detected, and detailed immunohistochemical results of these cases were summarized in Table 3. Among these, a representative case (Case 2) is presented below.
Table 3.
Summary of Prospective Analysis, Using Biopsy Specimens
| Case | Years after breast cancer | Lung tumor | MGB1 | ER | TTF-1 | Surfactant | Evaluation |
|---|---|---|---|---|---|---|---|
| 1 | 2 | Solitary, lymphadenopathy | + | − | − | − | Metastatic breast cancer |
| 2 | 13 | Solitary, lymphadenopathy | − | − | + | + | Primary lung cancer |
| 3 | 20 | Solitary, 20 mm | + | + | − | − | Metastatic breast cancer |
| 4 | 11 | Solitary, 18 mm | + | + | − | − | Metastatic breast cancer |
| 5 | 0 | Multiple* | − | − | + | + | Primary lung cancer |
| 6 | 12 | Solitary, 37 mm | + | − | − | − | Metastatic breast cancer |
| 7 | 2 | Solitary, 25 mm | + | − | − | − | Metastatic breast cancer |
| 8 | 2 | Solitary, 20 mm | − | − | + | − | Primary lung cancer |
| 9 | 12 | Solitary, 15 mm | + | + | − | − | Metastatic breast cancer |
Simultaneous presentation of multiple lung nodules and a breast tumor.
Case 2
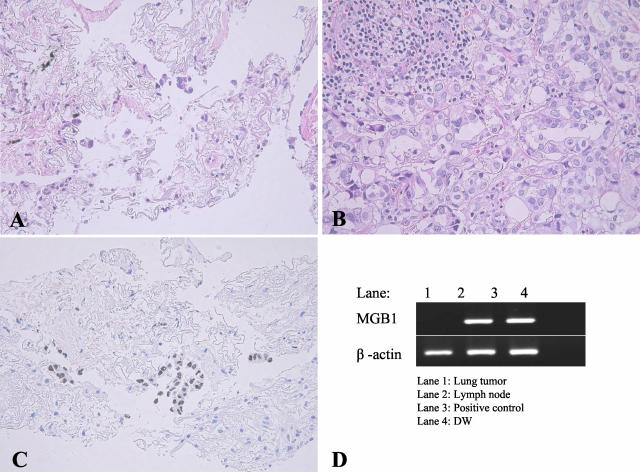
A small lung nodule and right cervical lymphadenopathy were found in an 80-year-old woman, and both lesions were biopsied. She had undergone mastectomy for breast cancer 13 years before the biopsy. In the fine-needle lung biopsy, atypical carcinoma cells were identified (Figure 2). However, the tumor cells were so few that a diagnosis differentiating between metastatic breast cancer and primary lung cancer was difficult. The lymph node biopsied was replaced by an infiltration of metastatic cancer, which histologically resembled breast cancer cells. Immunohistochemical and transcript profiles were as follows: TTF-1−, surfactant apoprotein A− and ER+ in the breast cancer; TTF-1+, surfactant apoprotein A+, ER− and MGB1− in the lung tumor, and TTF-1−, surfactant apoprotein A−, ER+, MGB1+ in metastatic cancer cells of the lymph node. This suggested that the lung tumor was a primary lung adenocarcinoma, and the tumor cells in the lymph node were metastases from the breast cancer.
Figure 2.
Practical application of MGB1 to an 80-year-old woman, who had a history of breast cancer 13 years previously. A lung tumor (A) and a lymph node of her left neck (B) were biopsied. In the lung tumor specimen, a few degenerated atypical cells are seen in the background fibrosis. Although the atypical cells are suggestive of an adenocarcinoma, it is impossible to determine whether the adenocarcinoma is primary or metastatic simply from HE sections (A). Positive staining of TTF-1 (C) and the absence of a transcript for MGB1 (D) suggests that this lung tumor is a primary pulmonary adenocarcinoma. By contrast, the metastatic cancer in the lymph node specimen is negative for TTF-1 and positive for MGB1 (D), suggesting metastatic breast cancer.
Discussion
We describe here a novel approach to the differential diagnosis of lung tumors in patients with a history of breast cancer. Immunohistochemical analysis is easier and more practical than nucleic acid-based assays; however, accumulated human genome data can be used for the nucleic acid-based assays. In this study, three candidate molecules, MGB1, SBEM, and PDEF, were selected from the public database as highly expressed in breast cancers but not expressed or very low in normal lung. Only a few articles have described these molecules, but they support breast-specific expression.11,12,13,14,15 However, both SBEM and PDEF could be detected in normal lung. Such a discrepancy might result from our highly sensitive RT-PCR analysis, which we used to obviate the possibility of pseudo-tissue-specific expression due to a low level of expression. Indeed, expression of SBEM and PDEF were detected in the bronchus, which was a minor component in the lung parenchyma.
In contrast, MGB1 exhibited a very restricted expression pattern in the breast and salivary gland. Gruenewald et al11 reported that MGB1 is also expressed in normal and cancerous tissues of the ovary, endometrium, and uterine cervix, as well as in normal breast and breast cancer tissues. Indeed, a metastatic endometrial cancer in our series expressed this molecule. MGB1 is a member of the uteroglobulin gene family, and is characterized as being a small secretory protein with glycosylation sites. It is regulated by steroid hormones, including estrogen and androgen.16 It is of note that all members of this gene family show tissue-specific expression: Clara cell antigen (secretoglobin, family 1A, member 1) in the lung and kidney;17 prostatein-like lipophilin A (secretoglobin, family 1D, member 1) in the prostate and tears;18 and uteroglobin-related protein 1 (secretoglobin, family 3A, member 2) in the lung.19 Furthermore, all but uteroglobin-related protein 1 are localized in tandem in chromosomal region 11q12.2. In addition, their physiological functions remain unclear.
The present study demonstrated that MGB1 is a sensitive and specific marker to identify metastatic breast cancers in the differential diagnosis. However, there was a notable exception of unexpected MGB1 expression in small cell carcinomas. Interestingly, small cell carcinomas unexpectedly expressed TTF-1, which regulates functional pulmonary molecules, such as surfactant apoprotein, and is expressed commonly in terminal airway unit cells and their cancers. Recent research suggests that stem cells can express a broad range of genes.20,21 Morphologically, small cell carcinomas appear as very primitive or undifferentiated cells, and thus the unexpected co-expression of MGB1 and TTF-1 may have some association with the multi-lineage gene expression of stem cells. Indeed, ectopic expression of c-Kit, stem cell factor,22,23 and some cancer testis antigens24 are more common in small cell lung carcinomas than in non-small cell lung cancers.
In contrast to morphological analysis, RNA just for RT-PCR is relatively tolerant of degeneration, and RT-PCR analysis of MGB1 can be applied to a small number of tumor cells, even in a biopsy. Application of the assay to paraffin-embedded tissues may be more practical. However, a preliminary study using paraffin-embedded tissues resulted in successful detection for MGB1 amplification in only half of the positive controls studied (data not shown). RT-PCR of shorter sequences and RT-PCR followed by RNA amplification may lead to more consistent detection. Alternative material for the assay is a touch-imprint specimen of the biopsy, as described. These specimens provide high quality and sufficient amounts of RNA to perform RT-PCR. Moreover, tumor cells were significantly enriched by the procedure.25 A similar approach using SAGE and cDNA-microarray databases may allow the identification of molecules specific for other organs or cancers that are applicable for tumors of unknown origin.
In interpreting the results of MGB1 expression, attention should be paid to two factors. First, high-grade neuroendocrine cancers may show positive transcripts for MGB1. In this case, TTF-1, which is commonly used for the identification of pulmonary adenocarcinomas, may also be positive. When tumor cells show poorly differentiated or undifferentiated morphology, and/or atypical gene expression, such as the MGB1+, TTF-1+ phenotype, the possibility of high-grade neuroendocrine tumors should be excluded. Second, metastatic salivary gland cancers and gynecologic malignancies are possible tumors that may express MGB1. Like lung cancers, ovarian cancers and endometrial cancers preferentially occur as second primary neoplasms.3 However, gynecological tumors rarely metastasize to the lung as solitary tumors without peritoneal involvement.
Although a validation study based on a larger series of various cancers is needed, the strategy described in this study provides a useful tool to develop methods for the differential diagnosis of primary and metastatic tumors. It appears to be more important to develop novel methods for the differential diagnosis of squamous cell carcinomas in those patients with head and neck cancers, because differential diagnosis is required second most frequently for these cases. Squamous cell carcinomas of the head and neck often arise in a multi-focal fashion, which is explained by the field cancerization theory, and squamous cell carcinomas of the head and neck and of the lung are morphologically indistinguishable. It is therefore of urgent need to develop markers distinguishing squamous cell carcinomas arising from the two organs.
Acknowledgments
We thank K. Hayashi for excellent technical assistance and M. Mabuchi and H. Ishida for management of paraffin blocks and preparations of slides.
Footnotes
This study is supported partly by Grant-in-Aid for Encouragement of Young Scientists (B) and by Grant-in-Aid for Scientific Research (C, 14571294) from the Ministry of Education, Science, Sports and Culture, Japan.
References
- Levi F, Te VC, Randimbison L, La Vecchia C. Cancer risk in women with previous breast cancer. Ann Oncol. 2003;14:71–73. doi: 10.1093/annonc/mdg028. [DOI] [PubMed] [Google Scholar]
- Prochazka M, Granath F, Ekbom A, Shields PG, Hall P. Lung cancer risks in women with previous breast cancer. Eur J Cancer. 2002;38:1520–1525. doi: 10.1016/s0959-8049(02)00089-8. [DOI] [PubMed] [Google Scholar]
- Volk N, Pompe-Kirn V. Second primary cancers in breast cancer patients in Slovenia. Cancer Causes Control. 1997;8:764–770. doi: 10.1023/a:1018487506546. [DOI] [PubMed] [Google Scholar]
- Neugut AI, Robinson E, Lee WC, Murray T, Karwoski K, Kutcher GJ. Lung cancer after radiation therapy for breast cancer. Cancer. 1993;71:3054–3057. doi: 10.1002/1097-0142(19930515)71:10<3054::aid-cncr2820711027>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Inskip PD, Stovall M, Flannery JT. Lung cancer risk and radiation dose among women treated for breast cancer. J Natl Cancer Inst. 1994;86:983–988. doi: 10.1093/jnci/86.13.983. [DOI] [PubMed] [Google Scholar]
- Dail DH. Uncommon tumors. Dail DH, Hammer SP, Colby TV, editors. New York: Springer-Verlag; Pulmonary PathologyTumors. 1995:182–184. [Google Scholar]
- Perry A, Parisi JE, Kurtin PJ. Metastatic adenocarcinoma to the brain: an immunohistochemical approach. Hum Pathol. 1997;28:938–943. doi: 10.1016/s0046-8177(97)90009-5. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Mitsudomi T, Yatabe Y, Koshikawa T, Hatooka S, Shinoda M, Suyama M, Sugiura T, Ogawa M, Takahashi T. Mutations of the P53 tumor suppressor gene as clonal marker for multiple primary lung cancers. J Thorac Cardiovasc Surg. 1997;114:354–360. doi: 10.1016/S0022-5223(97)70180-6. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Yatabe Y, Koshikawa T, Haruki N, Hatooka S, Shinoda M, Suyama M, Ogawa M, Hamajima N, Ueda R, Takahashi T, Mitsudomi T. High frequency of clonally related tumors in cases of multiple synchronous lung cancers as revealed by molecular diagnosis. Clin Cancer Res. 2000;6:3994–3999. [PubMed] [Google Scholar]
- Grunewald K, Haun M, Fiegl M, Urbanek M, Muller-Holzner E, Massoner A, Riha K, Propst A, Marth C, Gastl G. Mammaglobin expression in gynecologic malignancies and malignant effusions detected by nested reverse transcriptase-polymerase chain reaction. Lab Invest. 2002;82:1147–1153. doi: 10.1097/01.lab.0000027840.16064.8a. [DOI] [PubMed] [Google Scholar]
- Ghadersohi A, Sood AK. Prostate epithelium-derived Ets transcription factor mRNA is overexpressed in human breast tumors and is a candidate breast tumor marker and a breast tumor antigen. Clin Cancer Res. 2001;7:2731–2738. [PubMed] [Google Scholar]
- Oettgen P, Finger E, Sun Z, Akbarali Y, Thamrongsak U, Boltax J, Grall F, Dube A, Weiss A, Brown L, Quinn G, Kas K, Endress G, Kunsch C, Libermann TA. PDEF, a novel prostate epithelium-specific ets transcription factor, interacts with the androgen receptor and activates prostate-specific antigen gene expression. J Biol Chem. 2000;275:1216–1225. doi: 10.1074/jbc.275.2.1216. [DOI] [PubMed] [Google Scholar]
- Miksicek RJ, Myal Y, Watson PH, Walker C, Murphy LC, Leygue E. Identification of a novel breast- and salivary gland-specific, mucin-like gene strongly expressed in normal and tumor human mammary epithelium. Cancer Res. 2002;62:2736–2740. [PubMed] [Google Scholar]
- Watson MA, Fleming TP. Mammaglobin, a mammary-specific member of the uteroglobin gene family, is overexpressed in human breast cancer. Cancer Res. 1996;56:860–865. [PubMed] [Google Scholar]
- Watson MA, Darrow C, Zimonjic DB, Popescu NC, Fleming TP. Structure and transcriptional regulation of the human mammaglobin gene, a breast cancer associated member of the uteroglobin gene family localized to chromosome 11q13. Oncogene. 1998;16:817–824. doi: 10.1038/sj.onc.1201597. [DOI] [PubMed] [Google Scholar]
- Singh G, Katyal SL. Clara cell proteins. Ann NY Acad Sci. 2000;923:43–58. doi: 10.1111/j.1749-6632.2000.tb05518.x. [DOI] [PubMed] [Google Scholar]
- Lehrer RI, Xu G, Abduragimov A, Dinh NN, Qu XD, Martin D, Glasgow BJ. Lipophilin, a novel heterodimeric protein of human tears. FEBS Lett. 1998;432:163–167. doi: 10.1016/s0014-5793(98)00852-7. [DOI] [PubMed] [Google Scholar]
- Niimi T, Keck-Waggoner CL, Popescu NC, Zhou Y, Levitt RC, Kimura S. UGRP1, a uteroglobin/Clara cell secretory protein-related protein, is a novel lung-enriched downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor. Mol Endocrinol. 2001;15:2021–2036. doi: 10.1210/mend.15.11.0728. [DOI] [PubMed] [Google Scholar]
- Akashi K, He X, Chen J, Iwasaki H, Niu C, Steenhard B, Zhang J, Haug J, Li L. Transcriptional accessibility for genes of multiple tissues and hematopoietic lineages is hierarchically controlled during early hematopoiesis. Blood. 2003;101:383–389. doi: 10.1182/blood-2002-06-1780. [DOI] [PubMed] [Google Scholar]
- Shamblott MJ, Axelman J, Littlefield JW, Blumenthal PD, Huggins GR, Cui Y, Cheng L, Gearhart JD. Human embryonic germ cell derivatives express a broad range of developmentally distinct markers and proliferate extensively in vitro. Proc Natl Acad Sci USA. 2001;98:113–118. doi: 10.1073/pnas.021537998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi K, Takahashi T, Sekido Y, Ueda R, Hida T, Ariyoshi Y, Takagi H. Coexpression of the stem cell factor and the c-kit genes in small-cell lung cancer. Oncogene. 1991;6:2291–2296. [PubMed] [Google Scholar]
- Sekido Y, Obata Y, Ueda R, Hida T, Suyama M, Shimokata K, Ariyoshi Y, Takahashi T. Preferential expression of c-kit protooncogene transcripts in small cell lung cancer. Cancer Res. 1991;51:2416–2419. [PubMed] [Google Scholar]
- Sugita M, Geraci M, Gao B, Powell RL, Hirsch FR, Johnson G, Lapadat R, Gabrielson E, Bremnes R, Bunn PA, Franklin WA. Combined use of oligonucleotide and tissue microarrays identifies cancer/testis antigens as biomarkers in lung carcinoma. Cancer Res. 2002;62:3971–3979. [PubMed] [Google Scholar]
- Maitra A, Wistuba II, Virmani AK, Sakaguchi M, Park I, Stucky A, Milchgrub S, Gibbons D, Minna JD, Gazdar AF. Enrichment of epithelial cells for molecular studies. Nat Med. 1999;5:459–463. doi: 10.1038/7458. [DOI] [PubMed] [Google Scholar]