Abstract
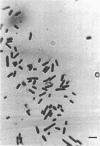
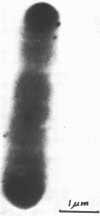
Several dissimilatory, sulfate-reducing bacteria were isolated from the rumen fluid of sheep fed purified diets containing sulfate. One isolate, strain D, was selected for characterization. This organism is a nonsporeforming, obligately anaerobic, mesophilic, nonmotile, gram-negative, straight rod. Cell-free extracts show absorption maxima for cytochrome c3 and desulfoviridin, characteristic of Desulfovibrio. Carbohydrates, as a sole carbon source, will support growth. Lactate supports growth in the presence of sulfate, not in its absence, whereas glucose or pyruvate support growth either in the presence or absence of sulfate. The isolate has a deoxyribonucleic acid base composition of 61.2% guanine plus cytosine, which is similar to that of several other species of Desulfovibrio; however, it differs from previously described species in morphology, motility, and carbon source utilization. Cell-free extracts of this bacterium exhibit adenosine 5′-triphosphate-sulfurylase, adenosine-5′-phosphosulfate-reductase, and hydrogenase activity. After incubation of cell-free extracts with adenine 5′-triphosphate and 35SO42-, adenosine-5′-phosphosulfate rather than 3′-phosphoadenosine-5′-phosphosulfate was shown to be labeled, indicating that the pathway of sulfate reduction in this organism is similar to that of other dissimilatory sulfate reducers. This is the first report of a Desulfovibrio sp. isolated from the rumen.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagi J. M., Campbell L. L. STUDIES ON THERMOPHILIC SULFATE-REDUCING BACTERIA III. : Adenosine Triphosphate-sulfurylase of Clostridium nigrificans and Desulfovibrio desulfuricans. J Bacteriol. 1962 Dec;84(6):1194–1201. doi: 10.1128/jb.84.6.1194-1201.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLENDEN D. C., GOLDBERG H. S. SILVER IMPREGNATION STAIN FOR LEPTOSPIRA AND FLAGELLA. J Bacteriol. 1965 Mar;89:899–900. doi: 10.1128/jb.89.3.899-900.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULLER C. S., AKAGI J. M. HYDROGENASE OF COLEMAN'S SULFATE-REDUCING BACTERIUM. J Bacteriol. 1964 Aug;88:440–443. doi: 10.1128/jb.88.2.440-443.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLEMAN G. S. A sulphate-reducing bacterium from the sheep rumen. J Gen Microbiol. 1960 Apr;22:423–436. doi: 10.1099/00221287-22-2-423. [DOI] [PubMed] [Google Scholar]
- Campbell L. L., Postgate J. R. Classification of the spore-forming sulfate-reducing bacteria. Bacteriol Rev. 1965 Sep;29(3):359–363. doi: 10.1128/br.29.3.359-363.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisingh J., Gomez G. G., Matrone G. Interactions of copper, molybdenum, and sulfate in ruminant nutrition. Fed Proc. 1973 Aug;32(8):1921–1924. [PubMed] [Google Scholar]
- Huisingh J., Matrone G. Copper-molybdenum interactions with the sulfate-reducing system in rumen microorganisms. Proc Soc Exp Biol Med. 1972 Feb;139(2):518–521. doi: 10.3181/00379727-139-36177. [DOI] [PubMed] [Google Scholar]
- Jones H. E. Sulfate-reducing bacterium with unusual morphology and pigment content. J Bacteriol. 1971 May;106(2):339–346. doi: 10.1128/jb.106.2.339-346.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS D. The reduction of sulphate in the rumen of the sheep. Biochem J. 1954 Mar;56(3):391–399. doi: 10.1042/bj0560391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MATRONE G., RAMSEY H. A., WISE G. H. Effect of volatile fatty acids, sodium and potassium bicarbonate in purified diets for ruminants. Proc Soc Exp Biol Med. 1959 Jan;100(1):8–11. doi: 10.3181/00379727-100-24504. [DOI] [PubMed] [Google Scholar]
- Mara D. D., Williams D. J. The evaluation of media used to enumerate sulphate reducing bacteria. J Appl Bacteriol. 1970 Sep;33(3):543–552. doi: 10.1111/j.1365-2672.1970.tb02232.x. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr, DEACON T. E., DAVIDSON J. T. STUDIES ON ADENOSINE 5'-PHOSPHOSULFATE REDUCTASE FROM DESULFOVIBRIO DESULFURICANS AND THIOBACILLUS THIOPARUS. I. THE ASSAY AND PURIFICATION. Biochim Biophys Acta. 1965 Mar 22;96:429–446. doi: 10.1016/0005-2787(65)90561-7. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr Symposium on metabolism of inorganic compounds. V. Comparative metabolism of inorganic sulfur compounds in microorganisms. Bacteriol Rev. 1962 Mar;26:67–94. doi: 10.1128/br.26.1.67-94.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTGATE J. R., CAMPBELL L. L. IDENTIFICATION OF COLEMAN'S SULFATE-REDUCING BACTERIUM AS A MESOPHILIC RELATIVE OF CLOSTRIDIUM NIGRIFICANS. J Bacteriol. 1963 Aug;86:274–279. doi: 10.1128/jb.86.2.274-279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTGATE J. A diagnostic reaction of Desulphovibrio desulphuricans. Nature. 1959 Feb 14;183(4659):481–482. doi: 10.1038/183481b0. [DOI] [PubMed] [Google Scholar]
- Peck H. D. THE ATP-DEPENDENT REDUCTION OF SULFATE WITH HYDROGEN IN EXTRACTS OF DESULFOVIBRIO DESULFURICANS. Proc Natl Acad Sci U S A. 1959 May;45(5):701–708. doi: 10.1073/pnas.45.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postgate J. R., Campbell L. L. Classification of Desulfovibrio species, the nonsporulating sulfate-reducing bacteria. Bacteriol Rev. 1966 Dec;30(4):732–738. doi: 10.1128/br.30.4.732-738.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS P. W., LIPMANN F. Isolation and identification of active sulfate. J Biol Chem. 1957 Dec;229(2):837–851. [PubMed] [Google Scholar]
- Saunders G. F., Campbell L. L., Postgate J. R. Base composition of deoxyribonucleic acid of sulfate-reducing bacteria deduced from buoyant density measurements in cesium chloride. J Bacteriol. 1964 May;87(5):1073–1078. doi: 10.1128/jb.87.5.1073-1078.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie J. W., Segel I. H. ATP-sulfurylase from Penicillium chrysogenum. I. Purification and characterization. Prep Biochem. 1971;1(2):91–117. doi: 10.1080/00327487108081932. [DOI] [PubMed] [Google Scholar]
- WILSON L. G., BANDURSKI R. S. Enzymatic reactions involving sulfate, sulfite, selenate, and molybdate. J Biol Chem. 1958 Oct;233(4):975–981. [PubMed] [Google Scholar]
- Whanger P. D., Matrone G. Effect of dietary sulfur upon the fatty acid production in the rumen. Biochim Biophys Acta. 1965 Jun 1;98(3):454–461. doi: 10.1016/0005-2760(65)90141-4. [DOI] [PubMed] [Google Scholar]