Abstract
Splenic marginal zone lymphoma (SMZL) is a lymphoma type of putative marginal zone B-cell origin. No specific genetic alterations have yet been demonstrated in SMZL. Clinically, SMZL is a low-grade B-cell non-Hodgkin lymphoma. However, the presence of p53 mutation, 7q22–7q32 deletion or the absence of somatic hypermutations of immunoglobulin genes has been correlated with a worse prognosis. In this study, we analyzed genome-wide gene expression of 24 cases of SMZL using the microarray technique. The AP-1 transcription factors c-jun, junD, junB, and c-fos as well as Notch2 were found to be specifically up-regulated. These data were confirmed by real-time PCR and immunohistochemical staining of tissue sections. The absence of concordant high expression of the MAP kinases, the signaling cascade leading to AP-1 up-regulation, suggests autoregulation of the AP-1 transcription factors and an important role in SMZL oncogenesis. High expression of Notch2, a transcription factor that induces marginal zone B-cell differentiation, is highly suggestive for a marginal zone B-cell origin of SMZL. In addition, SMZL with the 7q deletion showed high expression of TGF-β1 and low expression of the DNA helicase XPB, a crucial part of the nucleotide excision repair complex, possibly explaining the more aggressive clinical course of those cases.
Splenic marginal zone lymphoma (SMZL) is a distinct lymphoma type that is characterized by splenomegaly, circulating lymphoma cells and frequent bone marrow involvement.1,2 Typically, circulating lymphoma cells display villous projections in blood films. When prominent, the term SMZL with circulating villous lymphocytes is used. Peripheral lymph node involvement is usually absent. The lymphoma usually displays an indolent clinical course, but can transform to diffuse large B-cell lymphoma. The most frequent recurrent cytogenetic finding is the 7q22–7q32 deletion.3,4 Anotherfrequent, but non-specific, anomaly is trisomy 3.5 Whether t(11;14)(q13;q32) can be present in SMZL is a matter of debate.6 The pattern of somatic immunoglobulin gene mutations is heterogeneous with a predominance of cases with mutated immunoglobulin and about one-third of the cases displaying non-mutated genes.7 In addition, on-going immunoglobulin gene mutations are observed as for extranodal marginal zone lymphoma. One study reported a worse prognosis of cases with the 7q22–7q32 deletion and a correlation of the deletion with no or minimal somatic immunoglobulin gene mutations.8 Interestingly, SMZL has been shown to arise after chronic infection with hepatitis C virus or malaria in a number of cases.9,10 Defined gene mutations characterizing SMZL have not yet been shown. Of interest, though, is that both the cyclin D3 gene and the cyclin-dependent kinase 6 gene (CDK6) have been shown to be overexpressed secondary to gene rearrangements involving those genes in a number of cases.11,12 This may point to a deregulation of cell cycle control in SMZL.
Gene expression profiling yields a wealth of information on the biology of tumors. The technique allows for better classification of hematological disease, and allows the identification of biological variables that predict treatment response and prognosis.13 To investigate the biology of splenic marginal zone lymphoma, we studied genome-wide gene expression using the microarray technique in a series of 24 well-characterized cases.
Materials and Methods
Tissue Samples
All patient samples used in this study were primarily obtained for diagnosis. Only material that was left over was used for this study. Twenty-two samples of SMZL were obtained from the Department of Academic Hematology and Cytogenetics, The Royal Marsden Hospital, London. These samples consisted of frozen mononuclear cell suspensions of peripheral blood (13 cases), bone marrow (1 case), spleen (5 cases), or without recorded tissue origin (3 cases). The samples had been stored at −70°C until use. These SMZL samples were well characterized by morphological examination of blood smears and immunophenotypic analysis14 and contained a high number of tumor cells as demonstrated by flow cytometry. The bone marrow or spleen histology of the majority of cases of which tissue was available has been reviewed. Clinical data, cytogenetic analysis, immunophenotypic data as well as p53 mutation patterns were available for most of the patients. These samples have also been used in our previous study on the somatic mutation pattern of the rearranged immunoglobulin genes.7 The samples available for this study contained 105 cells, or more.
In addition to those samples, 13 frozen tissue samples were obtained from the Department of Pathology, The Norwegian Radium Hospital. The tissue samples were snap-frozen in liquid nitrogen and stored at −70°C until used. These samples comprised two cases of splenic marginal zone lymphoma (SMZL), two cases of extranodal marginal zone lymphoma of MALT type (MZL), four cases of follicular lymphoma (FL), three cases of B-cell chronic lymphocytic leukemia (CLL), and two cases of mantle cell lymphoma (MCL). All diagnoses were made according to the World Health Organization classification.15 A summary of survival data as well as previously obtained genetic data are given in Table 1.
Table 1.
Summary of Ig Mutation Status, Chromosome 7 Allelic Loss and p53 Deletions in SMZL Cases
| Case | % Identity of closest VH germline | 7 q Allelic loss | p53 Deletion | Survival |
|---|---|---|---|---|
| 1 | 100% | + | − | No follow up |
| 2 | 100% | + | Nd | Dead, 12 months |
| 3 | 100% | − | − | Alive, 72 months |
| 6 | 100% | − | − | Alive, 14 months |
| 7 | 99.1% | + | Nd | Alive, 5 months |
| 8 | 98.6% | − | − | No follow up |
| 9 | 98.3% | − | − | No follow up |
| 10 | 98.0% | + | − | Alive, 72 months |
| 11 | 96.9% | − | Nd | Dead, 60 months |
| 12 | 94.1% | − | − | No follow up |
| 13 | 95.6% | − | + | Alive, 48 months |
| 14 | 92.4% | − | − | Alive, 84 months |
| 15 | 94.4% | − | − | No follow up |
| 16 | 96.4% | − | − | Alive, 84 months |
| 17 | 97.3% | − | Nd | Alive, 144 months |
| 18 | 91.2% | − | − | Alive, 48 months |
| 19 | 93.2% | − | − | Alive, 12 months |
| 20 | 94.6% | − | Nd | Dead, 1 months |
| 21 | 90.1% | + | + | Dead, 32 months |
| 22 | 88.0% | Nd | − | No follow up |
| 23 | 90.5% | + | Nd | No follow up |
| 24 | Nd | + | Nd | Dead, 48 months |
| 26 | Nd | − | Nd | No data |
| 27 | Nd | − | Nd | No data |
Nd, not determined; +, positive for 7q allelic loss/p53 deletion; −, negative for 7q allelic loss/p53 deletion.
Reference RNA
Stratagene’s universal reference RNA (Stratagene, La Jolla, CA) was used as a common reference in all hybridizations. This RNA pool is composed of total RNA from 10 human cell lines, giving the expression profile of the majority of human genes. We generated a stock of amplified reference RNA from parallel amplifications. The stock was divided into aliquots, ready for labeling.
Total RNA Isolation
Total RNA was isolated using Trizol according to the manufacturer’s protocol (Life Technologies Ltd, Paisley, UK). RNA concentrations were determined by OD260 reading in 50 mmol/L NaOH (GeneQuant, Amersham Pharmacia Biotech AB, Uppsala, Sweden). The total RNA was diluted at 0.1 μg/μl for RNA amplification.
RNA Amplification
Because of the relative low number of cells in part of our samples, we used an mRNA amplification method to obtain sufficient quantities of RNA for expression profiling using cDNA microarrays. For homogeneity, amplification was performed for all of the samples in the study. RNA amplification was carried out as described earlier16 and is based on a slightly modified protocol according to Baugh et al.17 A detailed protocol is available at the following site: http://pubgeneserver.uio.no/SMZL/index.html. Briefly, the initial amount of total RNA used for all amplifications was 0.2 μg and all samples went through two rounds of amplification. In a 10-μl reverse transcription reaction, 0.2 μg total RNA was primed with a dT/T7-primer for synthesis of the first strand. The synthesis of the second strand was performed after RNase digestion. The purified double-stranded cDNA served as template for the first round of in vitro aRNA transcription. Subsequently, cDNA was synthesized by priming the aRNA with random hexamers. The synthesis of the second strand was initiated by annealing a dT/T7-primer to the aRNA:cDNA heteroduplex after partially digesting the aRNA. After purification, a second round of in vitro transcription was performed.
Fluorescent Labeling
Three μg of aRNA from each lymphoma sample and 2.5 μg of the reference RNA was labeled with Cy3- or Cy5-labeled dCTP (Amersham Pharmacia Biotech AB) during reverse transcription. The reaction mixture consisted of random hexamers (16 μg), 40 U RNAsin (Promega, Madison, WI), first strand buffer, 0.1 mol/L DTT, 0.5 mmol/L of dATP, dCTP, dGTP, and 0.2 mmol/L dTTP and was incubated for 5 minutes at 65°C in a water bath. The tube was then transferred to a heating block (42°C), and 4 μl of either fluorophore was added to the respective tubes followed by 400 U SuperScript II (Invitrogen, Groningen, The Netherlands). The reaction was stopped after 60 minutes by adding 5 μl 0.5 mol/L EDTA (pH 8.0). Residual RNA was hydrolyzed with 10 μl 1 mol/L NaOH and incubated at 65°C for 15 minutes. Twenty-five μl 1 mol/L Tris-HCl (pH 7.5) was added to neutralize the mixture. Labeled Cy3- and Cy5-cDNA was diluted with TE-buffer (pH 7.5) and concentrated using Microcon YM-30 columns (Amicon, Millipore Corporation, Bedford, MA), thus yielding 17 to 20 μl samples.
DNA Arrays, Hybridization, and Scanning
The DNA microarrays used in this study were printed at the core facility of the Norwegian Microarray Consortium at the Norwegian Radium Hospital (Oslo, Norway) using a Micro Grid II robotic printer (Bio Robotics, Cambridge, UK). These 13,000 human cDNA arrays were printed on amino silane-coated slides (CMT GAPS, Corning Life Sciences, Corning, NY) and represent about 11,500 genes. A list of the DNA probes with the clone ID numbers is available at http://pubgeneserver.uio.no/SMZL/index.html. The microarray hybridization volume of 40 μl consisted of 17 μl of each of the labeled probes, 3.5X SSC (pH 7.5), 0.3% SDS, 1.25X Denhardt’s solution, 4 μg yeast tRNA, and 4 μg BSA. The final mix was heated for 2 minutes at 100°C and spun down for 10 minutes at 13,000 rpm before it was applied on a microarray slide under the LifterSlip (Erie Scientific Company, Portsmouth, NH). The slide was then placed in an ArrayIT hybridization chamber (Telechem, Sunnyvale, CA) and submersed in a water bath for overnight hybridization at 65°C. Before scanning, the coverslip was removed in a solution of 2X SSC and 0.1% SDS, and the slide was washed in the following solutions for 5 minutes at room temperature; 1X SSC, 0.2X SSC, and 0.05X SSC. The slide was dried by centrifugation. Scanning was performed with a ScanARRAY 4000 scanner (Packard Biosciences, Biochip Technologies LLC, Meriden, CT), and data were acquired using GenePix Pro 3.0 software (Axon Instruments Inc., Union City, CA).
Data Analysis
The GenePix export files were processed with custom Perl scripts to facilitate import of data into Matlab (Mathworks, Natick, MA). The data analyses were carried out on normalized log-ratios from the ratio of means measurements of the GenePix export files. The log-ratios were normalized to have zero median. However, before normalization, the data were filtered, ie, poor quality measurements were removed. The spots that were removed included those spots with illegal ratios, spots that had been flagged during scanning, and spots that corresponded to poor quality cDNA probes, as determined by PCR.
Analysis of variance analyses were done with the anova1 Matlab function. In these analyses, all measurements that had been filtered were left out from the analysis. This resulted in a varying number of observations in each group for different genes. However, the varying number of observations is accounted for in the computed P values from the analysis of variance tests.
For the k-means clustering, filtered measurements were replaced with the median log-ratio before calculation. Since all arrays were normalized to have a zero median, this was equivalent to setting all missing values to zero. The k-means analyses were done with an in-house Matlab implementation of the k-means clustering algorithm. The k-means clustering was used to cluster the genes into 100 clusters, ie, k = 100. Briefly, the k-means algorithm picks k initial cluster centers and assigns the data points (log-ratio expression value vectors) to the nearest center. Then, until no data point is re-assigned to a new cluster, the algorithm moves each data point to its nearest cluster center and re-computes the cluster centers. The cluster center is calculated as the point of mass of the data points belonging to the cluster. Euclidian distance was used as the distance measure. The clustering analysis was done on a data set with a total of 64 arrays. These arrays included analysis with forward- and reverse-labeled amplified aRNA on most of the cases, some duplicate analysis on a few cases, and some normal peripheral blood hybridizations (see web supplement http://pubgeneserver.uio.no/SMZL/index.html). The figures show data from a reduced data set where measurements from the several hybridizations with aRNA from the same sample have been averaged. The averaging procedure was to replace, for each gene, measurements from several arrays with the median value for each array element. The figures were also generated in Matlab.
Quantitative Real-Time PCR
Total RNA was treated with DNase (Sigma-Aldrich, St. Louis, MO). To verify the efficiency of DNA removal, genomic albumin levels were measured by real-time PCR using forward and reverse primers that were complementary to adjacent exon regions (forward primer 5′-GCGTAGCAACCTGTTACATATTAAAGTT, reverse primer 5′-AAATGGACACTGCTG AAGATACTGA, probe FAM5′-AAGGCAATCAA-CACCCT-GAAACAA-TAMRA, sensitivity of the test: one cell equivalent of DNA) and only samples without detection of albumin were used. RNA was then reverse-transcribed to cDNA using Powerscript reverse transcriptase (BD Biosciences Clontech, Palo Alto, CA) by priming with random hexamers (PE Applied Biosystems, Foster City, CA). TaqMan probes and primers for junD, junB, c-fos, and β-glucuronidase (GUS) were designed using Primer Express 1.0 (PE Applied Biosystems) and sequence data from the NCBI database. The following primer and probes were used: c-fos: forward 5′-CAGCGAGCAACTGAGAAGCC, reverse 5′-CGCTGTGAAGCA-GAGCTGG, FAM5′-CAGCGAACGAGCAGTGACCGTGC-TAMRA; junD: forward 5′-TGACGCTGAGCCTGA GTGAG, reverse 5′TCGGGAGAGGCGAGCA, FAM5′-TGAGCGCTGCCGCCACCT, junB: forward 5′GTCACCGAGGAGCAGGAGG, reverse 5′-TCTTGTGCAGATCGTCCAGG, FAM5′-TTCGCCGACGGCTTTGTCAAAG-TAMRA, GUS: forward 5′-GAAAATATGTGGTT GGAGAGCTCATT, reverse 5′-CCGAGTGAAGATCCCCTTTTTA, FAM5′-CCAGCACTCTCGTCGGTGACTGTTCA-TAMRA. For detection of c-Jun, Notch2, MAP3K8, MAP2K3, MAPK1, and MAP2K6 mRNA expression we used assays-on-demand gene expression products (PE Applied Biosystems, Hs00277190_s1, Hs00225747_m1, Hs00177127_m1, Hs00178297_m1, Hs00177066_m1, and Hs00177150_m1). For all PCR reactions, 0.5 μl of cDNA, 300 nmol/L of specific primers, 100 nmol/L probe, and 12.5 μl of the Universal TaqMan 2X PCR mastermix (PE Applied Biosystems) were mixed to a final volume of 25 μl. For each gene, all reactions were performed in duplicates in the same TaqMan run. The thermal cycling conditions were 2 minutes at 50°C, 10 minutes at 95°C, followed by 45 cycles of 95°C for 15 seconds and 60°C for 1 minute. PCR was performed on an ABI PRISM 7700 instrument (PE Applied Biosystems). Relative mRNA concentrations were calculated using the comparative CT method (PE Applied Biosystems, User Bulletin No. 2, 1997) with β-glucuronidase (GUS) as an internal control. The average slope value for the relative standard curves for all genes tested was −3.42 ± 0.15 (mean ± SD). Ratios between the relative mRNA expression levels among patient samples and in the microarray reference sample (Stratagene) were calculated.
Immunohistochemical Staining
Immunohistochemical staining was performed on formalin-fixed, paraffin-embedded tissue of the 13 cases collected at the Norwegian Radium Hospital. Five micrometer sections were cut, de-waxed and submitted to a pH 6.1 citrate buffer antigen retrieval method according to the instructions of the manufacturer (Target Retrieval Solution, Dako, Glostrup, Denmark). The sections were incubated for 30 minutes with the following primary rabbit polyclonal antibodies, respectively: anti-c-Jun (dilution 1:20), anti-Jun B (dilution 1:20), anti-Jun D (dilution 1:100), anti-c-Fos (dilution 1:100), and anti L-plastin (dilution 1:400). All primary antibodies were obtained from Santa Cruz Biotechnology, Santa Cruz, CA, except for L-plastin (Neo Markers, Fremont, CA). Anti-rabbit peroxidase-labeled dextran-coupled antibody (Dako Envision System, Dako) was used as a second step, according to the instructions of the manufacturer. The color reaction was developed with 3,3′-diaminobenzidine tetrachloride and H2O2.
Analysis of Chromosome 7q Deletions
DNA from the SMZL cases was isolated from Trizol fractions after removal of total RNA and proteins. We used DNA from six normal blood samples as control material. Loss of heterozygosity (LOH) was determined by using a set of 10 primers pairs on chromosome 7 (1 for the short arm and 9 for the long arm, covering the chromosome region 7q22–36), as described by Mateo et al.3 The primer pairs used were: D7S503, D7S471, D7S486, D7S522, D7S480, D7S685, D7S487, D7S514, D7S530, and D7S550. Forward primers were labeled with fluorescent dyes FAM, HEX, or NED, while the reverse primers were tailed (PE Applied Biosystems). PCR reactions were performed in a final volume of 20 μl containing 20 to 50 ng DNA, 0.1 μmol/L of each primer, 2.5 mmol/L of each dNTP, 2 mmol/L MgCl2, 0.63 U AmpliTaq Gold DNA polymerase, and PCR Gold buffer at a 1X final concentration (PE Applied Biosystems). Forty cycles of PCR were performed in a DNA thermal cycler (Eppendorf), with denaturing at 94°C for 30 seconds, annealing at 53°C for 15 seconds, extension at 72°C for 30 seconds, and a final extension at 72°C for 8 minutes. PCR products were diluted in water and run on an ABI Prism 310 DNA Genetic Analyzer (PE Applied Biosystems) as described.18 Since no control normal cells were available for our cases, loci were regarded as informative only when both polymorphic alleles were detected, relying on the fact that all of the samples contained some non-neoplastic cells. Loss of heterozygosity (LOH) was then defined as a minimum of 70% reduction of the signal intensity of one of the alleles.
Results
In this study we analyzed the gene expression profile of 24 SMZL samples and compared the profile to a set of samples covering various lymphoma types. We used cDNA microarrays covering 13,000 array elements, representing approximately 11,500 genes. Due to the limited amount of RNA available from a number of samples, mRNA was amplified to generate sufficient material for expression profiling. All samples were co-hybridized with amplified RNA of a common reference. We used a dye swap strategy to eliminate differential expression due to differences in dye incorporation of the Cy3 and Cy5 fluorophores during cDNA synthesis. The average of the two hybridizations for each sample was used in downstream data analysis.
First, the expression data were explored by k-means clustering, dividing the gene expression profiles into 100 clusters (data available as a web supplement at http://pubgeneserver.uio.no/SMZL/index.html). K-means clustering analysis was performed to identify patterns of gene expression and thereby to identify groups of genes that may participate in similar pathways, important to SMZL. Based on this clustering analysis we found a set of genes specifically up-regulated in the SMZL samples compared to control lymphoma samples (Figure 1A). Subsequently, analysis of variance analysis was performed to identify significantly differentially expressed genes, either up-regulated or down-regulated, in SMZL samples versus the samples comprising other lymphoma types (data available as a web supplement at http://pubgeneserver.uio.no/SMZL/index.html). The two cases of extra-nodal marginal zone lymphoma of MALT type were excluded from the analysis of variance analysis because it is as yet unclear to what degree MALT lymphoma is similar or distinct from SMZL. Computed P values for the cluster in Figure 1A were all P < 0.05 and most were P < 0.001. The cluster of genes, illustrated in Figure 1, shows the specific expression of AP-1 and Notch2 transcription factors in SMZL. The AP-1 transcription factors included c-fos (FOS), junB (JUNB), junD (JUND), and c-jun (JUN). Analysis of variance analysis of the genes that were most significantly up-regulated in SMZL as compared to the other lymphoma types included both c-jun and junD (Table 2). The latter stresses the importance of the genes in the cluster identified by k-means statistics (Figure 1A), for the biology of SMZL. AP-1 transcription factors form heterodimers that are involved in cell proliferation and differentiation. MAP3K8, a member of the MAP kinase pathway, and important for signal transduction leading to AP-1 gene activation, is also found in this cluster of genes. Of interest, no other MAP kinases, which are well represented on the arrays, are present in this cluster, and importantly, analysis of variance analysis does not show significant expression of any of the other MAP kinases with the exception of MAP2K3. The Notch2 membrane receptor is normally expressed by B-cells, but its activation and subsequent function as a transcription factor is important for normal marginal zone B-cell differentiation. Several other genes that are or might be important for B-cell differentiation are also found in the same cluster with the AP-1 and NOTCH2 genes: IL 6, a cytokine which is important for terminal B-cell differentiation and is regulated by AP-1 transcription factors; and NR4A2 (Nur77), a transcription factor which is important for T-cell apoptosis in the thymus, but is also expressed by activated B-cells. In addition, a series of genes, of which their function is not known in B-cell maturation, are expressed in the same cluster. Some of those genes, such as COX2 (PTGS2), are regulated by the AP-1 transcription factors.
Figure 1.
Gene expression signature of SMZL. The samples studied are listed on the horizontal axis and the vertical axis displays the clustered genes. The level of expression of the different genes are depicted according to the color scale shown at the bottom where the color code represents expression values ranging from −6 to 6 on a log base 2 scale. A: Cluster 50 after k-means clustering (k = 100) along with the computed P values for each gene in the cluster, obtained after analysis of variance analysis of the comparison of SMZL cases with all other lymphoma cases in the study. A complete list of all of the clusters is available in the supplementary data. B: Selected T-cell genes. C: Selected B-cell genes. D: The average expression of AP-1 transcription factors and Notch2. E: Real-time PCR validation of AP-1 transcription factors and Notch2.
Table 2.
The 17 Most Significantly Up-Regulated Genes in SMZL with Comparison to Other Lymphoma Types
| IMAGE clone no. | p-value | Gene symbol (description) |
|---|---|---|
| 767784 | 1.4 1012 | JUND |
| 324180 | 5.0 1012 | EVI-5 |
| 323185 | 9.2 1012 | PRKR |
| 3248637 | 1.1 1011 | PTGS2 (cyclo-oxygenase 2) |
| 502634 | 1.5 1011 | ESTs |
| 125666 | 1.7 1011 | ESTs |
| 262023 | 6.7 1011 | LOC200227 |
| 358531 | 1.1 1010 | JUN |
| 757242 | 1.4 1010 | SLC30A6 |
| 416744 | 1.5 1010 | c-terminal binding protein 2 |
| 741960 | 4.0 1010 | PAEP |
| 344589 | 4.5 1010 | LCP1 (L-plastin) |
| 768370 | 5.0 1010 | ras homolog gene family, member B |
| 504179 | 5.3 1010 | LOC90139 (p53-induced protein) |
| 2398504 | 6.8 1010 | RGS2 |
| 294127 | 8.1 1010 | hypothetical protein FLJ22313 |
| 45376 | 9.8 1010 | ACAA2 |
The p-values after ANOVA analysis are shown.
Figure 1C shows the relative expression of typical B-cell genes such as CD20 (MS4A1), CD79a and CD79b, RGS1 as well as the MHC class II genes HLA-DR, and HLA-DQ. The cluster illustrates the high expression of B-cell markers observed in all samples relative to the common reference sample. In contrast, the expression of T-cell markers such as CD3, CD4, and CD5 were relatively low in the SMZL samples (Figure 1B). The high expression of genes expressed by B-lymphocytes is expected in view of the predominance of lymphoma cells in the samples as indicated by flow cytometric analysis.
Up-regulation of the AP-1 transcription factors and Notch2 in SMZL, as compared to other lymphoma types, was confirmed by quantitative RT-PCR. The mRNA expression levels of c-jun, junD, junB, c-fos, and Notch2 are given in Table 3 and graphically illustrated in Figure 1E. Compared with the microarray results in Figure 1D, we found an even higher expression level in SMZL by RT-PCR analysis than by microarray analysis, except for Notch2. However, Notch2 was still found by RT-PCR analysis to be significantly higher in SMZL than in other lymphoma groups (P = 0.012).
Table 3.
Summary of Real-Time PCR Validation of AP-1 Transcription Factors, Notch2, and Some of the MAP Kinases
| Gene symbol | SMZL mean log2-ratio (±SD) | Other lymphoma cases mean log2-ratio (±SD) | p-value |
|---|---|---|---|
| FOS | 4.5 (±2,0) | −1.9 (±0,9) | 1.6 10−11 |
| JUND | 4.7 (±1,8) | 0.8 (±0,7) | 7.7 10−8 |
| JUN | 3.6 (±1,7) | −1.1 (±0,9) | 3.1 10−10 |
| JUNB | 4.4 (±1,5) | 0.9 (±0,7) | 9.9 10−9 |
| NOTCH2 | 0.6 (±1,1) | −0.5 (±1,1) | 0.012 |
| MAP3K8 | 3.7 (±2,3) | 4.1 (±1,0) | 0.62 |
| MAP2K3 | 1.6 (±1,1) | 0.6 (±0,7) | 0.007 |
| MAPK1 | −0.5 (±1,7) | 0.4 (±0,5) | 0.09 |
| MAP2K6 | −1.7 (±5,0) | 1.6 (±0,9) | 0.04 |
Gene expression values are represented as log2-ratio. The p-values were obtained by ANOVA analysis of expression data of SMZL cases as compared to all other lymphoma cases.
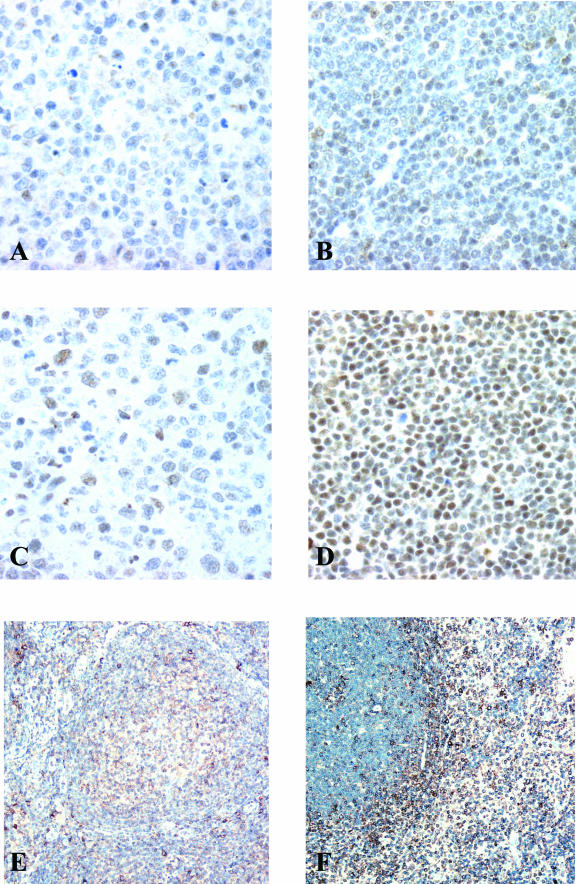
The expression of the AP-1 transcription factors in SMZL was also confirmed by immunohistochemical staining of tissue sections (Table 4 and Figure 2). Although AP-1 transcription factors are not uniquely expressed in SMZL, their expression is markedly elevated and uniform with expression in most of the lymphoma cells as compared to the other lymphoma types. Figure 2 also illustrates the high expression of L-plastin in SMZL, one of the genes that were found to be very significantly up-regulated.
Table 4.
Immunohistochemical Staining Results
| c-Jun | JunD | JunB | c-Fos | L-Plastin | |
|---|---|---|---|---|---|
| Case | |||||
| SMZL 26 | + | ++ | −/+ | −/+ | + |
| SMZL 27 | + | +/++ | −/+ | −/+ | ++ |
| MZL 1 | +/++ | −/+ | −/+ | −/+ | +/++ |
| MZL 2 | + | + | −/+ | −/+ | ++ |
| MCL 1 | −/+ | −/+ | − | − | + |
| MCL 2 | + | + | −/+ | − | + |
| B-CLL 1 | + | + | −/+ | − | + |
| B-CLL 2 | + | + | −/+ | −/+ | −/+ |
| B-CLL 3 | var −/+/++ | ++ | −/+ | −/+ | −/+ |
| FL 1 | var−/+/++ | var −/+ | var−/+/++ | − | ++ |
| FL 2 | var−/+/++ | var −/+ | var−/+/++ | − | −/+ |
| FL 3 | var−/+/++ | var −/+ | var−/+ | − | + |
−, no expression; +, clear expression; ++, strong expression; var, pronounced variable expression levels of the cells.
SMZL, splenic marginal zone lymphoma; MZL, marginal zone lymphoma; MCL, mantle cell lymphoma; FL, follicular lymphoma; B-CLL, B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma.
Figure 2.
Immunohistochemical staining for c-jun, jun-D, and L-plastin. c-jun, jun-D, and L-plastin are higher and more uniformly expressed in the nuclei of SMZL cells (B, D, and F, respectively) compared to follicular lymphoma cells (A, C, and E, respectively). L-plastin is most highly expressed in SMZL cells spreading to the red pulp (F). Magnification, ×250.
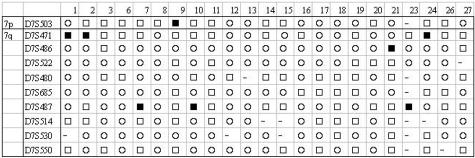
Since deletion of the chromosome 7q21–32 region is the most frequent genetic alteration in SMZL that may be related to a worse prognosis, we analyzed LOH at that region and studied the gene expression differences between cases with LOH and those without. LOH was detected in 7 of 23 SMZL cases (30%) which is within the range detected in previous reports (30 to 80%).3,4 A set of nine different microsatellite markers were used and LOH was detected by three different markers (D7S471, D7S486, and D7S487). The results of the analyses are illustrated in Figure 3. There is likely to be an underestimation of cases with LOH, since 54% of the loci were not informative. The latter is due to the lack of normal control cells for the cases. All results of the analysis of variance statistical analysis of differentially expressed genes are available as a web supplement at http://pubgeneserver.uio.no/SMZL/index.html. In total, 51 array elements showed significant differential expression at P < 0.001, whereas 614 array elements showed significant differential expression at P < 0.01. Three array elements, TGF-β1, ERCC3, and the unknown gene FLJ10153 reached a highly significant difference in expression at P < 0.001 indicating that those are important genes for the biology of SMZL cases having the 7q deletion. Of those three, TGF-β1 was up-regulated whereas the others were down-regulated in cases with the 7q deletion. No correlation between 7q allelic loss and differences in survival were found (data not shown). This may be explained by too few cases with allelic losses and a relatively short follow-up period.
Figure 3.
Graphic representation of microsatellite markers at 7q22–36 in SMZL cases. The case numbers are in the horizontal axis while the microsatellite markers are in the vertical axis. ▪ indicates allelic loss (LOH), □ indicates heterozygous loci but no allelic loss, ○ indicates non-informative loci, and - indicates no PCR product detected.
Discussion
Expression of the AP-1 Transcription Factors c-jun, junD, junB, and c-fos and Clustering with Notch2 Expression in SMZL
AP-1 Transcription Factors
The AP-1 transcription factors, especially c-jun and junD, are highly and specifically up-regulated in SMZL. The AP-1 transcription factor dimers control cell proliferation and differentiation, and are central transcription factors for mitogen-activated transduction pathways.19 These factors are rapidly and transiently expressed following stress signals, including a number of growth factor pathways. Jun and fos proteins form heterodimers, the DNA binding affinities and trans-activation capacities of which varies with their exact protein composition. The c-jun/c-fos dimer promotes the cell cycle G1 transition, whereas c-jun/JunB dimer inhibits this transition.20 Both the expression level of the various proteins of the AP-1 group, as well as their phosphorylation state, determine transcriptional regulation. c-jun promotes cell proliferation through up-regulation of cyclin D1 and repression of p53, p21, and p16. Overexpression of the AP-1 transcription factors may result in oncogenesis under experimental conditions.21 Hitherto, overexpression of c-jun and junB has been shown in Hodgkin’s disease, and amplification and overexpression of junB has been associated with primary cutaneous T-cell lymphomas.22,23 In Hodgkin’s disease, AP-1 transcription factor activation is independent of activation through the MAP kinase pathways. At least c-jun has been shown to be autoregulated in Hodgkin’s disease through binding to its own promotor. Interestingly, all MAP kinases with the exception of MAP3K8 and MAP2K3 are expressed at low levels in SMZL, suggesting that the AP-1 transcription factors are autoregulated in SMZL as in Hodgkin’s disease.
Of interest, cyclin D1 has been described to be a major target of AP-1 transcription factors, thus promoting G1 cell cycle transition.24 However, in Hodgkin’s disease, cyclin D2 seems to be a major target of both up-regulated AP-1 and NFκB transcription factors.22,23 Neither cyclin D1 nor cyclin D2 transcripts are up-regulated in splenic lymphoma, suggesting that other factors of the cell cycle machinery are targeted. It is of interest that cyclin D3 as well as its binding partner CDK6 have been reported to be overexpressed in a few cases of SMZL, where overexpression was caused by the translocation of either of the genes to immunglobulin loci.11,12 However, our gene expression data do not show that cyclin D3 or CDK6 are highly expressed in the majority of our cases of SMZL. The genes targeted by up-regulation of the AP-1 transcription factors in SMZL have yet to be identified.
Notch2
The Notch family of proteins are highly conserved transmembrane receptors that regulate cell fate determination in various types of tissues and are very important for lymphopoiesis.25 On binding of ligand, the intracytoplasmic domain of the receptor is cleaved by proteolysis and translocates to the nucleus. In the nucleus, Notch functions as a transcriptional regulator, important for cell differentiation.26 Whereas Notch1 is important for T-cell versus B-cell cell fate determination, Notch2 is necessary for the B-cell to further differentiate into a marginal zone B-cell instead of into a germinal center B-cell.27,28 Our data show that Notch2 is up-regulated in SMZL and therefore is an argument in favor of an origin of SMZL from marginal zone B-cells rather than from post-germinal center cell B-cells. Indeed, the origin from marginal zone B-cells of SMZL has hitherto been an area of controversy.2 Expression of Notch2 was previously shown in chronic lymphatic leukemia, another B-cell lymphoma that is likely of non-germinal center cell origin.29 Notch1 has been reported to repress AP-1 mediated transactivation.30 The effect of Notch2 on AP-1 mediated transactivation is not known, but high expression of both of these transcription factors in SMZL, as well as the clustering of both genes, does not suggest repression. The clustering of both genes in our analysis may suggest a synergistic activation of target genes. Of interest, Bcl6, a crucial transcriptional repressor necessary for germinal center cell development and absent from marginal zone B-cells, represses AP-1 function.31 It is therefore likely that AP-1 transcription factors and Notch2 are both important for non-germinal center cell B-cell maturation.
Other Genes Highly Up-Regulated in SMZL
Among the genes that are most significantly up-regulated in SMZL compared to other lymphoma types are LCP1 and EVI-5. LCP1, also named L-plastin or leukocyte plastin, codes for an actin-bundling protein that is necessary for the stabilization of actin filaments. L-plastin is up-regulated in leukocytes on migration. L-plastin localizes to filopodia and pseudopodia, membrane protrusions, in fibroblasts and lymphocytes.32 Of interest, many of the cases of SMZL we analyzed here have so-called circulating villous lymphocytes. The latter are a typical finding in leukemic SMZL.33 Although speculative, up-regulation of L-plastin in our cases might be due to the presence of villi on the tumor cells.
Equally of interest is the high expression of the human homologue of the viral ecotropic integration site (EVI-5). This gene, which has been cloned from a t(1;10)(q22;q21) in a case of neuroblastoma, and has also been named NB4S, may be involved in cell growth.34 The high and specific expression of this gene in SMZL prompts further research into its function and involvement in the biology of malignant disease.
TGF-β1 Is Highly Expressed and ERCC3 (XPB) Is Down-Regulated in SMZL with the 7q Deletion
The 7q22–7q32 deletion has been detected in up to 80% of SMZL.3,4 The deletion has been associated with a worse clinical outcome and with the absence of immunoglobulin somatic hypermutations.8 We analyzed the gene expression profile of SMZL with the 7q deletion and compared the results with SMZL without the deletion. Two known genes were particularly differentially expressed with high statistical significance; TGF-β1 is highly expressed in cases showing the deletion whereas XPB is expressed at much lower levels in these cases. High expression of TGF-β1 has previously been reported in extra-nodal marginal zone lymphoma of the stomach.35 TGF-β1 could potentially function as an autocrine growth factor in SMZL. TGF-β1 is known to activate the AP-1 transcription factors and its up-regulation may enhance the constitutive activation of AP-1 transcription factors resulting in increased proliferation and worse prognosis.36 The fact that junD is significantly more up-regulated in our cases with the 7q deletion lends support to this hypothesis. By which mechanisms TGF-β1, which is localized on chromosome 19, is overexpressed in SMZL with the 7q deletion is not known. Equally of interest is the low expression of XPB in SMZL with the 7q deletion. XPB is a DNA helicase and a critical member of the general transcription factor TFIIH and the nucleotide excision repair complex.37,38 XPB is equally important for p53-dependent apoptosis, in instances where the damage may be too extensive to repair.38 While disruption of the XPB gene is incompatible with life, certain mutations of the XPB gene are well known to result in defective DNA repair and increased risk for malignancy.37,38 Therefore, down-regulation of XPB expression in SMZL with 7q deletion could result either in a failure to repair potential carcinogenic mutations or in a defect of apoptosis. This may potentially explain why SMZL with 7q deletion displays a worse prognosis. Down-regulation of this gene is not readily explained by gene dosage, as the gene localizes to chromosome 2, and mechanisms leading to its down-regulation need yet to be investigated. The detection of 7q deletions in our cases lacks sensitivity because normal control cells of the patients were not available. Therefore, the list of potentially interesting genes that are differentially expressed in SMZL with the 7q deletion is likely incomplete and needs further study.
Note Added in Proof
The clone identification number (ID) is a code (5-8 digits) assigned by the I.M.A.G.E. Consortium. Information concerning the respective clones is routinely gathered from the NCBI database. Newer versions of reference sequences (RefSeq) may replace old ones, thus providing new information linked to the respective clones. The record following image clone Id 357892, previously identified as NOTCH2 (Figure 1A) was replaced after submission of this article. A query of clone Id 357892 shows that it is linked to RefSeq NM_203458 and described as a novel notch2 protein, N2N. Nucleotide comparison of RefSeq NM_203458 to RefSeq for NOTCH2, NM_024408 shows 96% homology. Hence the expression measurement obtained for clone Id 357892 may theoretically be a sum of both NOTCH2 and N2N expression. However, we want to emphasize that the primers used for RT-PCR are exclusive for the NOTCH 2 transcript, thus the major conclusions of this study remain valid.
Footnotes
Supported by grants from the Norwegian Cancer Society and the Norwegian Research Council.
G.T. and V.N. contributed equally to this study.
References
- Thieblemont C, Felman P, Callet-Bauchu E, Traverse-Glehen A, Salles G, Berger F, Coiffier B. Splenic marginal-zone lymphoma: a distinct clinical and pathological entity. Lancet Oncol. 2003;4:95–103. doi: 10.1016/s1470-2045(03)00981-1. [DOI] [PubMed] [Google Scholar]
- Maes B, Wolf-Peeters C. Marginal zone cell lymphoma: an update on recent advances. Histopathology. 2002;40:117–126. doi: 10.1046/j.1365-2559.2002.01360.x. [DOI] [PubMed] [Google Scholar]
- Mateo M, Mollejo M, Villuendas R, Algara P, Sanchez-Beato M, Martinez P, Piris MA. 7q31–32 allelic loss is a frequent finding in splenic marginal zone lymphoma. Am J Pathol. 1999;154:1583–1589. doi: 10.1016/S0002-9440(10)65411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruszka-Westwood AM, Hamoudi R, Osborne L, Matutes E, Catovsky D. Deletion mapping on the long arm of chromosome 7 in splenic lymphoma with villous lymphocytes. Genes Chromosomes Cancer. 2003;36:57–69. doi: 10.1002/gcc.10142. [DOI] [PubMed] [Google Scholar]
- Dierlamm J, Pittaluga S, Wlodarska I, Stul M, Thomas J, Boogaerts M, Michaux L, Driessen A, Mecucci C, Cassiman JJ. Marginal zone B-cell lymphomas of different sites share similar cytogenetic and morphologic features. Blood. 1996;87:299–307. [PubMed] [Google Scholar]
- Oscier DG, Matutes E, Gardiner A, Glide S, Mould S, Brito-Babapulle V, Ellis J, Catovsky D. Cytogenetic studies in splenic lymphoma with villous lymphocytes. Br J Haematol. 1993;85:487–491. doi: 10.1111/j.1365-2141.1993.tb03337.x. [DOI] [PubMed] [Google Scholar]
- Tierens A, Delabie J, Malecka A, Wang J, Gruszka-Westwood A, Catovsky D, Matutes E. Splenic marginal zone lymphoma with villous lymphocytes shows on-going immunoglobulin gene mutations. Am J Pathol. 2003;162:681–689. doi: 10.1016/S0002-9440(10)63862-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algara P, Mateo MS, Sanchez-Beato M, Mollejo M, Navas IC, Romero L, Sole F, Salido M, Florensa L, Martinez P, Campo E, Piris MA. Analysis of the IgV(H) somatic mutations in splenic marginal zone lymphoma defines a group of unmutated cases with frequent 7q deletion and adverse clinical course. Blood. 2002;99:1299–1304. doi: 10.1182/blood.v99.4.1299. [DOI] [PubMed] [Google Scholar]
- Hermine O, Lefrere F, Bronowicki JP, Mariette X, Jondeau K, Eclache-Saudreau V, Delmas B, Valensi F, Cacoub P, Brechot C, Varet B, Troussard X. Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N Engl J Med. 2002;347:89–94. doi: 10.1056/NEJMoa013376. [DOI] [PubMed] [Google Scholar]
- Bates I, Bedu-Addo G, Rutherford T, Bevan DH. Splenic lymphoma with villous lymphocytes in tropical West Africa. Lancet. 1992;340:575–577. doi: 10.1016/0140-6736(92)92108-r. [DOI] [PubMed] [Google Scholar]
- Sonoki T, Harder L, Horsman DE, Karran L, Taniguchi I, Willis TG, Gesk S, Steinemann D, Zucca E, Schlegelberger B, Sole F, Mungall AJ, Gascoyne RD, Siebert R, Dyer MJ. Cyclin D3 is a target gene of t(6;14)(p21.1;q32.3) of mature B-cell malignancies. Blood. 2001;98:2837–2844. doi: 10.1182/blood.v98.9.2837. [DOI] [PubMed] [Google Scholar]
- Brito-Babapulle V, Gruszka-Westwood AM, Platt G, Andersen CL, Elnenaei MO, Matutes E, Wotherspoon AC, Weston-Smith SG, Catovsky D. Translocation t(2;7)(p12;q21–22) with dysregulation of the CDK6 gene mapping to 7q21–22 in a non-Hodgkin’s lymphoma with leukemia. Haematologica. 2002;87:357–362. [PubMed] [Google Scholar]
- Staudt LM. Molecular diagnosis of the hematologic cancers. N Engl J Med. 2003;348:1777–1785. doi: 10.1056/NEJMra020067. [DOI] [PubMed] [Google Scholar]
- Gruszka-Westwood AM, Hamoudi RA, Matutes E, Tuset E, Catovsky D. p53 abnormalities in splenic lymphoma with villous lymphocytes. Blood. 2001;97:3552–3558. doi: 10.1182/blood.v97.11.3552. [DOI] [PubMed] [Google Scholar]
- Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. Lyon: IARC Press; World Health Organization Classification of TumoursPathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. 2001 [Google Scholar]
- Nygaard V, Loland A, Holden M, Langaas M, Rue H, Liu F, Myklebost O, Fodstad O, Hovig E, Smith-Sorensen B. Effects of mRNA amplification on gene expression ratios in cDNA experiments estimated by analysis of variance. BMC Genomics. 2003;4:11. doi: 10.1186/1471-2164-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh LR, Hill AA, Brown EL, Hunter CP. Quantitative analysis of mRNA amplification by in vitro transcription. Nucleic Acids Res. 2001;29:E29. doi: 10.1093/nar/29.5.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basirico R, Pirrotta R, Fabbiano F, Mirto S, Cascio L, Pagano M, Cammarata G, Magrin S, Santoro A. Submicroscopic deletions at 7q region are associated with recurrent chromosome abnormalities in acute leukemia. Haematologica. 2003;88:429–437. [PubMed] [Google Scholar]
- Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- van Dam H, Castellazzi M. Distinct roles of Jun: Fos and Jun: ATF dimers in oncogenesis. Oncogene. 2001;20:2453–2464. doi: 10.1038/sj.onc.1204239. [DOI] [PubMed] [Google Scholar]
- Vogt PK. Jun, the oncoprotein. Oncogene. 2001;20:2365–2377. doi: 10.1038/sj.onc.1204443. [DOI] [PubMed] [Google Scholar]
- Mao X, Orchard G, Lillington DM, Russell-Jones R, Young BD, Whittaker SJ. Amplification and overexpression of JUNB is associated with primary cutaneous T-cell lymphomas. Blood. 2003;101:1513–1519. doi: 10.1182/blood-2002-08-2434. [DOI] [PubMed] [Google Scholar]
- Mathas S, Hinz M, Anagnostopoulos I, Krappmann D, Lietz A, Jundt F, Bommert K, Mechta-Grigoriou F, Stein H, Dorken B, Scheidereit C. Aberrantly expressed c-Jun and JunB are a hallmark of Hodgkin lymphoma cells, stimulate proliferation, and synergize with NF-kappa B. EMBO J. 2002;21:4104–4113. doi: 10.1093/emboj/cdf389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakiri L, Lallemand D, Bossy-Wetzel E, Yaniv M. Cell cycle-dependent variations in c-Jun and JunB phosphorylation: a role in the control of cyclin D1 expression. EMBO J. 2000;19:2056–2068. doi: 10.1093/emboj/19.9.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pear WS, Radtke F. Notch signaling in lymphopoiesis. Semin Immunol. 2003;15:69–79. doi: 10.1016/s1044-5323(03)00003-4. [DOI] [PubMed] [Google Scholar]
- Baron M. An overview of the Notch signalling pathway. Semin Cell Dev Biol. 2003;14:113–119. doi: 10.1016/s1084-9521(02)00179-9. [DOI] [PubMed] [Google Scholar]
- Kuroda K, Han H, Tani S, Tanigaki K, Tun T, Furukawa T, Taniguchi Y, Kurooka H, Hamada Y, Toyokuni S, Honjo T. Regulation of marginal zone B cell development by MINT, a suppressor of Notch/RBP-J signaling pathway. Immunity. 2003;18:301–312. doi: 10.1016/s1074-7613(03)00029-3. [DOI] [PubMed] [Google Scholar]
- Tanigaki K, Han H, Yamamoto N, Tashiro K, Ikegawa M, Kurodo K, Suzuki A, Nakano T, Honjo T. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Immunol. 2002;3:443–450. doi: 10.1038/ni793. [DOI] [PubMed] [Google Scholar]
- Hubmann R, Schwarzmeier JD, Shehata M, Hilgarth M, Duechler M, Dettke M, Berger R. Notch2 is involved in the overexpression of CD23 in B-cell chronic lymphocytic leukemia. Blood. 2002;99:3742–3747. doi: 10.1182/blood.v99.10.3742. [DOI] [PubMed] [Google Scholar]
- Chu J, Jeffries S, Norton JE, Capobianco AJ, Bresnick EH. Repression of activator protein-1-mediated transcriptional activation by the Notch-1 intracellular domain. J Biol Chem. 2002;277:7587–7597. doi: 10.1074/jbc.M111044200. [DOI] [PubMed] [Google Scholar]
- Vasanwala FH, Kusam S, Toney LM, Dent AL. Repression of AP-1 function: a mechanism for the regulation of Blimp-1 expression and B lymphocyte differentiation by the B cell lymphoma-6 protooncogene. J Immunol. 2002;169:1922–1929. doi: 10.4049/jimmunol.169.4.1922. [DOI] [PubMed] [Google Scholar]
- Samstag Y, Eibert SM, Klemke M, Wabnitz GH. Actin cytoskeletal dynamics in T lymphocyte activation and migration. J Leukoc Biol. 2003;73:30–48. doi: 10.1189/jlb.0602272. [DOI] [PubMed] [Google Scholar]
- Catovsky D, Matutes E. Splenic lymphoma with circulating villous lymphocytes/splenic marginal-zone lymphoma. Semin Hematol. 1999;36:148–154. [PubMed] [Google Scholar]
- Roberts T, Chernova O, Cowell JK. NB4S, a member of the TBC1 domain family of genes, is truncated as a result of a constitutional t(1;10)(p22;q21) chromosome translocation in a patient with stage 4S neuroblastoma. Hum Mol Genet. 1998;7:1169–1178. doi: 10.1093/hmg/7.7.1169. [DOI] [PubMed] [Google Scholar]
- Knorr C, Amrehn C, Seeberger H, Rosenwald A, Stilgenbauer S, Ott G, Muller Hermelink HK, Greiner A. Expression of costimulatory molecules in low-grade mucosa-associated lymphoid tissue-type lymphomas in vivo. Am J Pathol. 1999;155:2019–2027. doi: 10.1016/S0002-9440(10)65521-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piek E, Roberts AB. Suppressor and oncogenic roles of transforming growth factor-beta and its signaling pathways in tumorigenesis. Adv Cancer Res. 2001;83:1–54. doi: 10.1016/s0065-230x(01)83001-3. [DOI] [PubMed] [Google Scholar]
- Bernstein C, Bernstein H, Payne CM, Garewal H. DNA repair/pro-apoptotic dual-role proteins in five major DNA repair pathways: fail-safe protection against carcinogenesis. Mutat Res. 2002;511:145–178. doi: 10.1016/s1383-5742(02)00009-1. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhu Q, Wani MA, Wani G, Chen J, Wani AA. Tumor suppressor p53-dependent recruitment of nucleotide excision repair factors XPC and TFIIH to DNA damage. DNA Repair (Amst) 2003;2:483–499. doi: 10.1016/s1568-7864(03)00002-8. [DOI] [PubMed] [Google Scholar]