Abstract
Accurate detection of central nervous system (CNS) involvement in children with newly diagnosed acute lymphoblastic leukemia (ALL) could have profound prognostic and therapeutic implications. We examined various cerebrospinal fluid (CSF) preservation methods to yield adequate DNA stability for polymerase chain reaction (PCR) analysis and developed a quantitative real-time PCR assay to detect occult CNS leukemia. Sixty CSF specimens were maintained in several storage conditions for varying amounts of time, and we found that preserving CSF in 1:1 serum-free RPMI tissue culture medium offers the best stability of DNA for PCR analysis. Sixty CSF samples (30 at diagnosis and 30 at the end of induction therapy) from 30 children with ALL were tested for CNS leukemic involvement by real-time PCR using patient-specific antigen receptor gene rearrangement primers. Six of thirty patient diagnosis samples were PCR-positive at levels ranging from 0.5 to 66% leukemic blasts in the CSF. Four of these patients had no clinical or cytomorphological evidence of CNS leukemia involvement at that time. All 30 CSF samples drawn at the end of induction therapy were PCR-negative. The data indicate that real-time PCR analysis of CSF is an excellent tool to assess occult CNS leukemia involvement in patients with ALL and can possibly be used to refine CNS status classification.
Despite the routine administration of central nervous system (CNS)-directed therapy, CNS relapse continues to afflict 3 to 5% of children with acute lymphoblastic leukemia (ALL).1,2,3 Of these relapses, more than 60% occur in patients with no clinical or cytomorphological evidence of CNS involvement at initial diagnosis.4 The significance of blasts detected by examination of cytospin preparations in patients with low CNS cell counts remains controversial.4,5,6 Unequivocal detection of leukemic cells in the cerebrospinal fluid (CSF) may help refine our classification of CNS disease and identify a subgroup of patients at higher risk of CNS relapse. Although immunocytochemistry7 and flow cytometry8,9 may be helpful in some cases, these methods require fresh specimens (within a few hours of procurement) and an adequate number of cells available for examination.
Real-time polymerase chain reaction (PCR) analysis of CSF (with clone-specific primers derived from the same patient’s bone marrow at diagnosis) may be sensitive enough to detect or confirm low levels of CNS leukemic involvement. Real-time PCR could also permit a rapid throughput of large numbers of specimens in a highly standardized format. To implement real-time PCR analysis of genomic DNA from CSF samples in multi-institutional studies, an examination of the factors affecting DNA stability in CSF is necessary. Minimizing the extent of DNA degradation would allow the use a single core molecular diagnostics facility to which CSF specimens are shipped. Our goals were to study the relative merits of various methods of preserving CSF for PCR, to develop a real-time PCR assay for quantifying CNS leukemia, and to evaluate the feasibility of detecting CNS leukemia in pediatric ALL patients in a multi-institutional study.
Patients and Methods
Patients
For the initial storage optimization and CSF DNA stability studies, CSF was collected from 60 children with varied diagnoses, including leukemia. For real-time PCR analysis of CNS leukemic involvement in ALL, 60 CSF samples were obtained at diagnosis and the end of induction therapy from 30 children enrolled on the ALL standard-risk Children’s Oncology Group protocol (COG-1991). The ALL patient CSF specimens (1 ml each) were incubated in 1:1 RPMI and shipped overnight from the local institution, except for patient 27, whose specimens were processed within 1 hour of collection. Peripheral blood (patients 1, 3, and 23) or bone marrow (remainder of patients) was obtained at the time of initial diagnosis. Eligibility criterion for CSF analysis was at least 5% blasts in the initial diagnostic peripheral blood or bone marrow, and the identification of at least one clonal antigen receptor gene rearrangement in the leukemic blasts at initial diagnosis. This study was approved by the Institutional Review Board of New York Medical College and informed consents were obtained before collecting samples.
Stability of CSF Genomic DNA and CSF Storage Optimization
Sixty CSF samples were each divided into four equal aliquots of 0.5 ml. For the first group of 20 samples, aliquot 1 was processed within 1 hour for a baseline control, aliquot 2 was incubated for 2 days before processing, and aliquots 3 and 4 were shipped to the University of Wisconsin overnight, shipped back to New York Medical College overnight, then processed 2 days (aliquot 3) or 7 days (aliquot 4) after procurement. The remaining 40 specimens were allocated the same except that aliquots 2 to 4 were stored in 25% ethanol for specimens 21 to 40, and in an equal volume of serum-free RPMI tissue culture medium for specimens 41 to 60. All incubations and shipping conditions were at room temperature.
CSF DNA Extraction
CSF samples were centrifuged for 10 minutes at 10,000 × g and cell pellets were washed once in 1 ml of 25% ethanol. We optimized the DNA extraction method using several protocols until one method (described here) provided suitable yield for PCR analysis. CSF cell pellets were air-dried and lysed in 15 μl of cell lysis buffer containing 1× PCR buffer II without MgCl2 (Perkin Elmer-Cetus, Foster City, CA), 0.1% Igepal (Sigma-Aldrich Co., St Louis, MO), 0.1% Tween 20 (Sigma-Aldrich Co.), and 17 ng/μl of proteinase K (Fisher Scientific, Springfield, NJ). The lysates were incubated for 1 hour at 56°C, and denatured for 10 minutes at 95°C. The final volumes were adjusted to 30 μl with nuclease-free water, and DNA was stored at −20°C until used. Because of low volume and DNA content, DNA quantitation by spectrophotometry was only performed when possible. DNA amount and quality were normalized by amplification of the human β-globin gene by real-time PCR as described below.
Quantitative Amplification of CSF DNA by Real-Time PCR
The number of amplifiable genome targets was assessed by real-time PCR amplification of the human β-globin gene using the LightCycler (Roche Diagnostics, Indianapolis, IN), run software version 5.32 as described in detail.10 The standard curve comprised of human genomic DNA (Roche Diagnostics) with 500, 200, 50, and 10 starting templates/reaction, which were each tested in duplicates. All patient CSF DNA samples (4 μl each) were tested in triplicates. Each DNA sample set (aliquots 1 to 4) was amplified by real-time PCR simultaneously to avoid interassay error. Water replaced DNA in the negative control. Standard and melting curve analyses and results from amplification of the CSF samples were generated at the end of the reaction using the LightCycler data analysis software version 3.5.28 (Roche Diagnostics).
Sequencing Leukemia Clone-Specific Antigen Receptor Gene Rearrangements
Peripheral blood or bone marrow aspirates were drawn at the time of diagnosis, and mononuclear cells were isolated using Histopaque 1077 (Sigma-Aldrich Co.). DNA was extracted from samples that were drawn in heparin using the High Pure kit (Roche Diagnostics), and from samples that were collected in ethylenediaminetetraacetic acid using a standard phenol/chloroform method. Clonal IgH, TCRδ, and TCRγ gene rearrangements were amplified by PCR and up to two stable monoclonal PCR targets were sequenced as previously described.11,12 Clone-specific primers were designed using Oligo Primer Analysis software version 4.0 (National Biosciences, Plymouth, MN) following stringent guidelines explained in detail.11
Detection of CNS Leukemia in ALL Patients by Real-Time PCR
Sixty CSF DNA samples (at diagnosis and the end of induction) from 30 ALL patients enrolled on COG-1991 were analyzed for CNS leukemia by real-time PCR using the LightCycler (Roche Diagnostics). Use of SYBRGreen dye master mix (Sigma Aldrich Co.) obviated the need for designing and optimizing patient-specific fluoresceinated primers and probes and enabled utilization of the same patient-specific primers used for detection of residual leukemia in remission bone marrow samples. Procedures for quantification of copy number and evaluation of intra- and interassay precision and reproducibility have been previously reported.10 Standard curves comprised of leukemia DNA diluted to 1000 (5%), 500 (2.5%), and 50 (0.25%) DNA targets per reaction, tested in duplicates, and 5 (0.025%) and 1 (0.01%) DNA targets per reaction, tested in triplicates. The diluant was human genomic DNA from nonleukemia cell lines so that each reaction contained 20,000 total genomic copies (based on the conversion of 1 μg DNA = 130,000 human genomic DNA). The blast percentage in the samples from which the leukemia DNA was derived was normalized to 100% before the DNA dilutions. Patient CSF DNA samples (4 μl each) were tested in triplicates. Negative controls were DNA from pooled normal bone marrow from 20 children who were PCR-negative for residual leukemia and water. Percentage of positive blasts in the CSF was calculated by an automated comparison of the average real-time PCR result to the standard curve, divided by the normalized β-globin value for that CSF sample. The sensitivity limit was 1 × 10−4. The entire assay required less than 1 hour of bench time for each patient.
Cytology
Patient CSF samples were classified into either CNS-1, -2, or -3, or traumatic lumbar puncture (TLP) with or without blasts according to criteria set forth by the Children’s Oncology Group. Specifically, CNS-1 was defined as fewer than 5 white blood cells (WBCs)/μl and fewer than 10 red blood cells (RBCs)/μl with no blasts detected on the cytospin. CNS-2 was defined as fewer than 5 WBCs/μl and fewer than 10 RBCs/μl, with blasts detected on the cytospin. A sample was classified as CNS-3 if any one of the following criteria was met: 5 or more WBCs/μl and fewer than 10 RBCs/μl with blasts present on the cytospin; or clinical signs of CNS disease were present, such as cranial nerve palsy, hypothalamic syndrome, spinal cord compression, or coma. A TLP was defined as the presence of 10 or more RBCs/μl in the CSF.
Data Analysis
To compare the real-time PCR results of samples at each incubation time to the samples processed immediately, the paired two-tailed Student’s t-test was used (Excel, Microsoft, Redwood, WA).
Results
Preservation of CSF DNA
Sixty CSF samples were subjected to various storage conditions and examined for DNA stability by real-time PCR amplification of the β-globin gene. Storage of CSF without additives for 2 days and without shipping resulted in no significant change in amplification of the DNA (P = 0.08) compared to the CSF aliquots processed immediately. However, storage for 2 and 7 days in addition to shipping resulted in an average of a 40% and 41% decrease in amplification, respectively (P = 0.001 for both). Storage of CSF in 25% ethanol for 2 days resulted in a nonsignificant 15% and 3% average decrease in amplifiable genomic DNA, with and without shipping, respectively, compared to the CSF aliquots processed immediately. But storage for 7 days in 25% ethanol in addition to shipping resulted in a significant 64% decrease (P = 0.001) in DNA amplification. Preservation of CSF in 1:1 RPMI resulted in no significant change in amplification even after 7 days of storage and shipping (P = 0.73), compared to the CSF aliquots processed immediately.
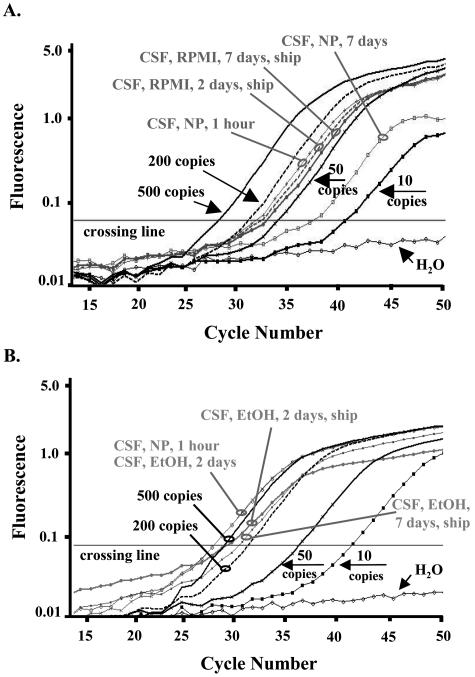
Representative data from two CSF sample sets are shown in Figure 1. Real-time PCR analysis on a sample set of CSF samples stored in 1:1 RPMI showed similar amplification efficiency of the β-globin gene up to 7 days of storage with shipping, compared to the CSF aliquot that was processed immediately. Storage of CSF without preservatives for 7 days with shipping resulted in a decrease to 25 β-globin copies compared to 100 β-globin copies from the aliquot processed immediately (Figure 1A). PCR analysis on a representative CSF sample set stored in 25% ethanol resulted in progressively decreased numbers of starting β-globin DNA targets from 630 (processed immediately and stored 2 days) to 490 (2 days and shipping), and to 285 (7 days and shipping), as shown in Figure 1B.
Figure 1.
Real-time PCR assays for determination of the most suitable storage condition for DNA stability in CSF. Samples were amplified using primers specific to the human β-globin gene. Amplification plot of cycle number versus fluorescence shows PCR results of dilutions of standard human genomic DNA (Roche) down to 10 starting template copies and DNA extracted from equal aliquots of CSF stored in 1:1 RPMI (for 2 days and 7 days with shipping) or with no medium (for 1 hour and 7 days) (A), or CSF stored in 25% ethanol for 2 days without shipping, and 2 and 7 days with shipping (B). Standards were amplified in duplicates and samples were amplified in triplicates but results are shown in singles for simplification. The cycle number in which the fluorescence level surpasses the crossing line is inversely proportional to the number of PCR DNA targets at the beginning of the reaction. NP, no preservatives; NBM, normal bone marrow; EOI, end of induction.
Real-Time PCR Quantitation of CNS Leukemia
A total of 45 clonal rearrangements, comprising 24 IgH, 16 TCRδ, and 5 TCRγ, were sequenced from material obtained at initial diagnosis from 30 ALL patients. A single clonal rearrangement was sequenced in 15 patients, and two rearrangements were sequenced in the remaining 15 patients. Table 1shows the diagnostic CNS status, CSF cytology, and real-time PCR results for each patient. Table 2shows the concordance between cytomorphology and PCR for diagnostic CSF samples. One patient had a CNS-3 classification at diagnosis (patient 4). Quantitative real-time PCR analysis of the diagnostic CSF from this patient revealed a leukemic infiltration of 66%. Four patients were CNS-2 at diagnosis and only one of them (patient 12) was positive for CNS leukemia by real-time PCR. Patient 12 did not have a traumatic tap, but did have 1 RBC/μl in the CSF, signaling a possibility of low leukemic infiltration from peripheral blood; however, the peripheral blood harbored only 2% blasts compared to the 15% blasts identified in the CSF by PCR analysis. Twenty-three patients were CNS-1, and three of these CSF samples were PCR-positive at levels ranging from 0.5% to more than 10% leukemic blasts in the CSF. Two patients (patients 11 and 15) experienced a TLP at diagnosis and CSF cytomorphological analysis was negative for blasts in both patients. Real-time PCR was negative for leukemic blasts in the CSF from patient 11, but the CSF from patient 15 harbored 47% blasts, comparable to the 55% blasts seen in the peripheral blood by cytology. None of the patients in this cohort suffered a TLP with blasts detectable on a cytospin. The 30 CSF samples drawn at the end of induction therapy all had either a CNS-1 or TLP, without blasts status, and were PCR-negative for CNS leukemia.
Table 1.
Cytology and Classification Status of CSF and Quantitative Real-Time PCR Results
| Patient | Clone type | PCR sensitivity | Diagnosis CSF
|
% blasts in PB | ||||
|---|---|---|---|---|---|---|---|---|
| PCR result | CNS status | % blasts | WBC | RBC | ||||
| 1 | IgH | 1 × 10−4 | >10% | CNS-1 | 0 | 0 | 0 | 88 |
| TCR Dδ2-Dδ3 | 5 × 10−4 | >10% | ||||||
| 2 | IgH | 1 × 10−4 | Negative | CNS-1 | 0 | 0 | 0 | 38 |
| 3 | IgH | 1 × 10−4 | Negative | CNS-1 | 0 | 0 | 0 | 6 |
| TCR Vδ2-Dδ3 | 1 × 10−4 | Negative | ||||||
| 4 | IgH | 1 × 10−4 | 66% | CNS-3 | 90 | 7 | 0 | 24 |
| 5 | TCR Vγ-Jγ | 1 × 10−4 | 0.5% | CNS-1 | 0 | 0 | 0 | 57 |
| 6 | IgH | 1 × 10−4 | Negative | CNS-1 | 0 | 2 | 0 | 0 |
| TCR Vδ2-Dδ3 | 5 × 10−4 | Negative | ||||||
| 7 | IgH L | 1 × 10−4 | Negative | CNS-1 | 0 | 0 | 0 | 0 |
| IgH U | 1 × 10−4 | Negative | ||||||
| 8 | TCR Vδ2-Dδ3 | 5 × 10−3 | Negative | CNS-2 | 7 | 1 | 1 | 30 |
| 9 | TCR Vγ-Jγ | 1 × 10−4 | Negative | CNS-1 | 0 | 1 | 1 | 17 |
| 10 | IgH | 1 × 10−4 | Negative | CNS-1 | 0 | 0 | 0 | 80 |
| TCR Vδ2-Dδ3 | 1 × 10−4 | Negative | ||||||
| 11 | TCR Vγ-Jγ | 1 × 10−4 | Negative | TLP-neg | 0 | 0 | 10 | 57 |
| 12 | TCR Vδ2-Dδ3 | 1 × 10−4 | 12% | CNS-2 | ≥1 | 1 | 1 | 2 |
| TCR Dδ2-Dδ3 | 1 × 10−4 | 15% | ||||||
| 13 | IgH | 1 × 10−4 | Negative | CNS-1 | 0 | 0 | 0 | 0 |
| 14 | IgH L | 1 × 10−4 | Negative | CNS-2 | 2 | 2 | 0 | 46 |
| IgH U | 1 × 10−4 | Negative | ||||||
| 15 | IgH | 1 × 10−4 | 47% | TLP-neg | 0 | 0 | 388 | 55 |
| TCR Vδ2-Dδ3 | 1 × 10−4 | 45% | ||||||
| 16 | TCR Vδ2-Dδ3 | 1 × 10−4 | Negative | CNS-1 | 0 | 1 | 1 | 74 |
| 17 | IgH | 1 × 10−4 | Negative | CNS-1 | 0 | 1 | 1 | 17 |
| TCR Vγ-Jγ | 1 × 10−4 | Negative | ||||||
| 18 | IgH | 1 × 10−4 | Negative | CNS-1 | 0 | 1 | 0 | 0 |
| TCR Vδ2-Dδ3 | 1 × 10−4 | Negative | ||||||
| 19 | IgH L | 1 × 10−4 | Negative | CNS-1 | 0 | 1 | 1 | 13 |
| IgH U | 5 × 10−3 | Negative | ||||||
| 20 | IgH | 1 × 10−4 | Negative | CNS-2 | 6 | 1 | 1 | 67 |
| 21 | IgH | 5 × 10−3 | Negative | CNS-1 | 0 | 0 | 1 | 0 |
| 22 | IgH | 1 × 10−4 | Negative | CNS-1 | 0 | 0 | 0 | 1 |
| TCR Vδ2-Dδ3 | 1 × 10−4 | Negative | ||||||
| 23 | TCR Vγ-Jγ | 1 × 10−4 | Negative | CNS-1 | 0 | 0 | 0 | 77 |
| 24 | IgH | 1 × 10−4 | Negative | CNS-1 | 0 | 1 | 1 | 22 |
| 25 | IgH | 1 × 10−4 | Negative | CNS-1 | 0 | 0 | 0 | 0 |
| TCR Vδ2-Dδ3 | 1 × 10−4 | Negative | ||||||
| 26 | IgH | 1 × 10−4 | Negative | CNS-1 | 0 | 1 | 2 | 83 |
| TCR Vδ2-Dδ3 | 1 × 10−4 | Negative | ||||||
| 27 | TCR Vδ2-Dδ3 | 1 × 10−4 | Negative | CNS-1 | 0 | 0 | 0 | 4 |
| 28 | IgH | 1 × 10−4 | 3.4% | CNS-1 | 0 | 0 | 0 | 0 |
| TCR Vδ2-Dδ3 | 1 × 10−4 | 4.4% | ||||||
| 29 | IgH | 1 × 10−4 | Negative | CNS-1 | 0 | 1 | 0 | 60 |
| 30 | TCR Vδ2-Dδ3 | 1 × 10−4 | Negative | CNS-1 | 0 | 0 | 0 | 0 |
WBC, white blood cell count (cells/μl); RBC, red blood cell count (cells/μl); PB, peripheral blood; U (upper); L (lower) bands on gel.
The end of induction CSF for every patient was PCR-negative and was either CNS-1 or TLP−.
Precision of Real-Time PCR Detection of CNS Leukemia
The concordance between two clonal real-time PCR targets in individual patients was examined in 15 patient samples to gain insight into the precision of the assay. Four of the patients whose CSF was PCR-positive had two PCR targets (patients 1, 12, 15, and 28). In each case, the quantitative real-time PCR results for both PCR targets closely matched. Patient 1 had a CSF leukemic cell burden of greater than 10%, detected by both IgH and TCR Dδ2-Dδ3 clone-specific primers. There was not enough DNA from this patient to quantify further. Patient 12 was positive at levels of 12% and 15% leukemic cells in the CSF, using primers specific to clonal TCR Vδ2-Dδ2 and TCR Dδ2-Dδ3, respectively. Patient 15 was positive for CNS leukemia at levels of 46% and 47% using TCR Vδ2-Dδ3 and IgH-specific primers, respectively. Patient 28 was positive for leukemia in the CSF at levels of 3.4% and 4.4% using primers specific to IgH and TCR Vδ2-Dδ3 rearrangements, respectively. The remaining 11 patients for whom two clonal rearrangements were tested by real-time PCR were negative for both PCR targets.
Specificity of Real-Time PCR Detection of CNS Leukemia
High-resolution melting curve analysis of all positive clone-specific PCR products displayed distinct profiles of the antigen receptor gene rearrangement fragments. The melting peak patterns from all six positive CSF samples (10 clonal PCR targets) were the same as the leukemic DNA, supporting the positive identity of leukemia cells in the CSF. Analysis of the water and pooled normal bone marrow DNA PCR products revealed no peaks, confirming the specificity of the reactions. Direct automated sequence analysis of the positive clone-specific PCR products was performed to confirm the identity of the leukemic clonal gene rearrangement sequence, except for patient 1, whose PCR products were not saved, and patient 15 IgH, in which the PCR product size was too small (51 bp). The PCR products from the remaining patients (seven clonal PCR targets) had the same unique DNA sequence as the leukemic clone.
Discussion
Our study shows that preserving CSF in tissue culture medium, RPMI (in a 1:1 mixture) offers the best stability for DNA. A small loss in individual cases is still manageable because the DNA samples are normalized to the amplified fixed-copy gene, β-globin. Ethanol (25%) offers good stability of the genomic DNA up to 2 days with shipping, but routine shipping of CSF in ethanol is not recommended because if the package is delayed beyond 2 days, the amount of DNA damage/loss would be significant. Storing CSF without any preservatives is the least desirable method, because even after 2 days with shipping, there is a significant loss of DNA. Although freezing CSF cell pellets or mailing CSF on ice packs may enhance the stability of DNA, these methods are not practical for multi-institutional trials and were not studied. However, if there is a delay in shipment, it would be prudent to store the specimens at 4°C and ship with ice packs to improve preservation. The experiments in this study were conducted during a hot season, between March and July, and shipping fresh specimens overnight at room temperature was sufficient for good quality DNA.
Quantitative detection of residual leukemia in the bone marrow by real-time PCR has been demonstrated in previous studies.10,13 Our present study, however, is the first to show that real-time PCR can be used to detect low levels of leukemic DNA in the CSF. Although a larger prospective study combined with patient follow-up will be necessary to determine the predictive value of outcome, this study demonstrates the ability of real-time PCR analysis to precisely detect occult CNS leukemia DNA. All 15 patients who had two characterized PCR targets in the diagnostic marrow showed concordance between both PCR targets from the CSF. Specificity of the reaction was excellent in this group of patients evidenced by the positive identity of patient-specific PCR products after sequencing. If a high standard of quality control is used, including confirmation that the negative controls do not amplify and the melting peak patterns of the positive sample matches the positive controls, then real-time PCR may have suitable clinical applications.
Real-time PCR improves our ability to detect occult CNS leukemia. In this study, 3 of the 23 CNS-1 patients at diagnosis were positive by PCR. This is not surprising given the higher sensitivity of real-time PCR (1 × 10−4). Because the majority of isolated CNS relapses occur in patients who have no clinical or cytomorphological evidence of CNS disease at diagnosis,4 the PCR-positive CNS-1 subgroup of patients may represent those at a higher risk of relapsing in the CNS. However, a plausible explanation for the discrepancy of results between PCR and cytomorphology could be related to the ordering of the vials used for both tests. In this study, the ordering of CSF tubes sent for PCR analysis was not specified. If the first-drawn CSF vial was used for PCR, peripheral blood (with blasts) contamination from slight trauma could be higher than in subsequent vials allocated for cytology and cytospin. Standardization of vial allocation for PCR studies may be necessary for clinically useful results.
PCR analysis may be used to clarify the diagnosis when the findings by cytomorphology are equivocal. Flow cytometry and TdT staining, although useful in some cases, are difficult to rely on when the cells are few. The frequency with which conventional CSF analysis yields erroneous results is not known. In this study, only one of four of the CNS-2 patients at diagnosis was PCR-positive. All three PCR-negative samples had 1 or 0 WBC/μl in the CSF. Poor sensitivity of the assay is an unlikely explanation because in all three cases the sensitivity of the assay was higher than the reported blast percentage in the CSF (Table 1). However, it is possible that poor yield during sample processing and DNA extraction, the presence of PCR inhibitors or other technical problems could have caused false-negative PCR results. A possibility that the blasts identified by morphology were normal immature lymphocytes cannot be ruled out. The discordance between PCR detection of blasts and cytomorphology may partially explain conflicting reports in the literature4,5,6 regarding the clinical significance of blasts in CSF with fewer than 5 WBCs/μl.
PCR-based analysis of CSF may help refine the detection of blasts introduced into the CSF during a TLP. Two patients in this study experienced a TLP at the start of therapy. Although both samples were reported as TLP without blasts by cytology, one was positive for CNS leukemia by PCR. We are currently analyzing a larger cohort of patients and following them during and after therapy to determine whether CNS-2 and TLP status refined by real-time PCR analysis can be a better predictor of outcome than cytomorphology alone.
We conclude that preserving CSF in RPMI maintains DNA stability up to 7 days. In addition, real-time PCR analysis of CSF is a quick and reliable tool to assess CNS involvement in patients with ALL, and could possibly be used to detect CNS involvement in other malignancies. A larger study is in progress to determine the frequency of CNS leukemia at diagnosis and during therapy by PCR, as well as to determine whether PCR analysis can refine CNS classification and aid in outcome prediction.
Table 2.
Concordance between Cytomorphology and PCR among 30 Patients with ALL at Diagnosis
| CNS-1 | CNS-2 | CNS-3 | TLP− | PCR+ | |
|---|---|---|---|---|---|
| 23 | 3 | ||||
| 4 | 1 | ||||
| 1 | 1 | ||||
| 2 | 1 | ||||
| Total | 23 | 4 | 1 | 2 | 6 |
Figure 2.
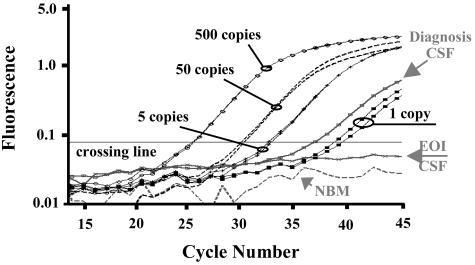
Quantitative real-time PCR analysis of CNS-leukemia involvement in pediatric ALL. Amplification plot of cycle number versus fluorescence shows real-time PCR results for patient-specific amplification of the Vδ2-Dδ2 clonal rearrangement identified at diagnosis in patient 28. Curves represent dilutions of patient diagnosis bone marrow DNA down to 1 copy PCR targets (diluted in a background of 20,000 genomic copies of non-leukemic DNA), patient diagnosis and end of induction CSF bone marrow DNA samples, and a normal bone marrow negative control DNA. Standards and patient samples were amplified in triplicates, but standards are shown in duplicates and samples are shown in singles, for simplification. The cycle number in which the fluorescence level surpasses the crossing line is inversely proportional to the number of PCR DNA targets at the beginning of the reaction. EOI, end of induction; NBM, normal bone marrow.
Footnotes
Supported by the Children’s Cancer Fund (grant to S.P.).
Work was performed at the Department of Pediatrics Hematology/Oncology, New York Medical College, Valhalla, NY.
References
- Bostrom BC, Sensel MR, Sather HN, Gaynon PS, La MK, Johnston K, Erdmann GR, Gold S, Heerema NA, Hutchinson RJ, Provisor AJ, Trigg ME. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: a report from the Children’s Cancer Group. Blood. 2003;101:3809–3817. doi: 10.1182/blood-2002-08-2454. [DOI] [PubMed] [Google Scholar]
- Schrappe M, Reiter A, Ludwig WD, Harbott J, Zimmermann M, Hiddemann W, Niemeyer C, Henze G, Feldges A, Zintl F, Kornhuber B, Ritter J, Welte K, Gadner H, Riehm H. Improved outcome in childhood acute lymphoblastic leukemia despite reduced use of anthracyclines and cranial radiotherapy: results of trial ALL-BFM 90. German-Austrian-Swiss ALL-BFM Study Group. Blood. 2000;95:3310–3322. [PubMed] [Google Scholar]
- Silverman LB, Declerck L, Gelber RD, Dalton VK, Asselin BL, Barr RD, Clavell LA, Hurwitz CA, Moghrabi A, Samson Y, Schorin MA, Lipton JM, Cohen HJ, Sallan SE. Results of Dana-Farber Cancer Institute Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1981–1995). Leukemia. 2000;14:2247–2256. doi: 10.1038/sj.leu.2401980. [DOI] [PubMed] [Google Scholar]
- Burger B, Zimmermann M, Mann G, Kuhl J, Loning L, Riehm H, Reiter A, Schrappe M. Diagnostic cerebrospinal fluid examination in children with acute lymphoblastic leukemia: significance of low leukocyte counts with blasts or traumatic lumbar puncture. J Clin Oncol. 2003;21:184–188. doi: 10.1200/JCO.2003.04.096. [DOI] [PubMed] [Google Scholar]
- Gilchrist GS, Tubergen DG, Sather HN, Coccia PF, O’Brien RT, Waskerwitz MJ, Hammond GD. Low numbers of CSF blasts at diagnosis do not predict for the development of CNS leukemia in children with intermediate-risk acute lymphoblastic leukemia: a Childrens Cancer Group report. J Clin Oncol. 1994;12:2594–2600. doi: 10.1200/JCO.1994.12.12.2594. [DOI] [PubMed] [Google Scholar]
- Mahmoud HH, Rivera GK, Hancock ML, Krance RA, Kun LE, Behm FG, Ribeiro RC, Sandlund JT, Crist WM, Pui CH. Low leukocyte counts with blast cells in cerebrospinal fluid of children with newly diagnosed acute lymphoblastic leukemia. N Engl J Med. 1993;329:314–319. doi: 10.1056/NEJM199307293290504. [DOI] [PubMed] [Google Scholar]
- Dagdemir A, Ertem U, Duru F, Kirazli S. Soluble L-selectin increases in the cerebrospinal fluid prior to meningeal involvement in children with acute lymphoblastic leukemia. Leuk Lymphoma. 1998;28:391–398. doi: 10.3109/10428199809092695. [DOI] [PubMed] [Google Scholar]
- Homans AC, Barker BE, Forman EN, Cornell CJ, Jr, Dickerman JD, Truman JT. Immunophenotypic characteristics of cerebrospinal fluid cells in children with acute lymphoblastic leukemia at diagnosis. Blood. 1990;76:1807–1811. [PubMed] [Google Scholar]
- Subira D, Castanon S, Roman A, Aceituno E, Jimenez-Garofano C, Jimenez A, Garcia R, Bernacer M. Flow cytometry and the study of central nervous disease in patients with acute leukaemia. Br J Haematol. 2001;112:381–384. doi: 10.1046/j.1365-2141.2001.02505.x. [DOI] [PubMed] [Google Scholar]
- Pine SR, Moy FH, Wiemels JL, Gill RK, Levendoglu-Tugal O, Ozkaynak MF, Sandoval C, Jayabose S. Real-time quantitative PCR: standardized detection of minimal residual disease in pediatric acute lymphoblastic leukemia. Polymerase chain reaction. J Pediatr Hematol Oncol. 2003;25:103–108. doi: 10.1097/00043426-200302000-00004. [DOI] [PubMed] [Google Scholar]
- Mayer SP, Giamelli J, Sandoval C, Roach AS, Fevzi OM, Tugal O, Rovera G, Jayabose S. Quantitation of leukemia clone-specific antigen gene rearrangements by a single-step PCR and fluorescence-based detection method. Leukemia. 1999;13:1843–1852. doi: 10.1038/sj.leu.2401530. [DOI] [PubMed] [Google Scholar]
- van der Velden VH, Wijkhuijs JM, Jacobs DC, van Wering ER, van Dongen JJ. T cell receptor gamma gene rearrangements as targets for detection of minimal residual disease in acute lymphoblastic leukemia by real-time quantitative PCR analysis. Leukemia. 2002;16:1372–1380. doi: 10.1038/sj.leu.2402515. [DOI] [PubMed] [Google Scholar]
- Pongers-Willemse MJ, Verhagen OJ, Tibbe GJ, Wijkhuijs AJ, de Haas V, Roovers E, van der Schoot CE, van Dongen JJ. Real-time quantitative PCR for the detection of minimal residual disease in acute lymphoblastic leukemia using junctional region specific TaqMan probes. Leukemia. 1998;12:2006–2014. doi: 10.1038/sj.leu.2401246. [DOI] [PubMed] [Google Scholar]