Abstract
We report the use of molecular techniques in the diagnosis of a case of culture-negative necrotizing fasciitis occurring in a 32-year-old female with no significant past medical history and who died within 36 hours of admission. Paraffin-embedded tissue sections from the popliteal fossa region obtained at autopsy showed hemorrhage, necrosis, and mild inflammation by hematoxylin and eosin staining. Tissue gram stain showed numerous gram-positive organisms arranged in clusters. The sequences of the first 500 bp of bacterial 16S rRNA gene amplified from the lesion were identical to a Lancefield group A β-hemolytic Streptococcus pyogenes. Streptococcal pyrogenic exotoxin A and B superantigen genes were detected and an emm type 1 was determined by polymerase chain reaction and sequencing from the lesion. This confirmed the etiology of the patient’s rapid deterioration with multisystem organ failure.
Case History
The patient was a 32-year-old white female with no significant past medical history other than a right groin endometrioma diagnosed 4 years previously. She initially presented to the emergency room with a 3-day history of fever, chills, nausea, vomiting, nonbloody diarrhea, myalgias, and severe right posterior leg pain in the region of the popliteal fossa. She reported taking ibuprofen and promethazine for pain and nausea, and there was no reported history of exposure to children with a possible group A streptococcal (GAS) infection.
On admission, the patient was a thin, awake, alert female with normal mentation who was in moderate distress. The physical examination revealed a blood pressure of 52/31 mmHg, temperature 36.4°C, supine heart rate of 114 beats per minute, respiratory rate of 18 breaths per minute, and an oxygen saturation of 99% on room air. Examination of the extremities showed a markedly tender right calf and popliteal region with mild erythema and mild edema. The skin was flushed.
Initial laboratory data showed a white blood cell count of 11,200/μL, hematocrit of 40%, and a platelet count of 161,000/μL. The differential count was 86% neutrophils, 3% lymphocytes, 1% monocytes, 1% eosinophils, 8% metamyelocytes, and 1% myelocytes. Prothrombin time (PT) was 41.2 seconds and partial thromboplastin time (PTT) was 73.4 seconds and liver enzymes were normal. Chemistries showed a sodium of 125 mEq/L, potassium of 4.4 mEq/L, chloride of 94 mEq/L, CO2 of 20 mmol/L, blood urea nitrogen of 47 mg/dl, creatinine of 3.4 mg/dl, and glucose of 93 mg/dl. Antistreptolysin O titer was 70 IU/ml (<125 = negative). Other antibody tests for streptococcal enzymes were not performed. Blood cultures before and after the administration of antibiotics were negative. Two separate right knee aspiration cultures preformed after administration of antibiotics were negative. X-rays of the right knee and right lower extremity ultrasound showed only subcutaneous edema, and a chest X-ray showed an accentuated interstitial pattern suggesting hydrostatic edema.
In the emergency room, the patient was started on IV fluids, vasopressors, and an antibiotic regimen consisting of cefazolin, vancomycin, and gentamicin, and on admission to the medical intensive care unit, this was changed to include clindamycin. Throughout the course of the following 24 hours the patient developed acidosis, rapidly deteriorated, and developed ventricular fibrillation at which point successful resuscitation was performed without a return in baseline mental status. An electroencephalogram showed general suppression of activity with absence of reactivity. After further discussion with the family, support was withdrawn and the patient died 36 hours after admission. Consent was obtained and a full autopsy was performed.
Materials and Methods
A postmortem tissue culture from the right popliteal fossa lesion, as well as lung and blood cultures were performed. Standard hematoxylin and eosin (H&E)-stained sections of lesional material from the popliteal fossa and other organs were prepared. Tissue gram stain was performed to evaluate for bacterial organisms in tissue sections according to previously described methods by Brown and Hopps with modifications using a 1% fast green counterstain.1
Nucleic acid was extracted from the paraffin-embedded tissues by using IsoQuick (Orca Research, Inc., Bothell, WA) according to the manufacturer’s instructions, as previously described.2 Polymerase chain reaction (PCR) amplification of the first 500 bp of the 16S rRNA gene was performed using the MicroSeq 500 16S bacterial sequencing kit according to procedures described previously.3 Bi-directional sequences of the PCR amplification product were determined and a phylogenetic analysis was performed by online analysis at the Ribosomal Database Project II site (http://rdp.cme.msu.edu/html/index.html) and the MicroSeq Database Library (Applied Biosystems, Foster City, CA).
PCR amplification experiments for speA, speB, speC, and human β-actin genes of the extracted DNA were performed by adding 5 μl of the extracted tissue DNA into 50 μl of master mix (0.5 μmol/L each primer, 2.5 U Ampli Taq Gold DNA polymerase, 2.0 mmol/L MgCl2, 350 μmol/L total dNTPs, and 25 mmol/L KCl). The mixtures were placed in a GeneAmp PCR system 9700 (Applied Biosystems). Thermal cycling parameters included an initial 10 minutes at 95°C, 50 cycles of 30 seconds at 95°C, 30 seconds at 45°C, and 45 seconds at 72°C, and a final extension at 72°C for 10 minutes. The primer sequences for speA, speB, speC, and human β-actin genes were published previously.4,5 Amplified DNA fragments were detected by an agarose gel electrophoresis. Both speA-specific (specA-347P, 5′-CTG AAC TTA AGA ACC AAG AGA TGG C-3′) and speB-specific (5′-CGC ACT AAA CCC TTC AGC TCT T-3′), 5′-biotinylated capture probes were applied to confirm the amplification products in a colorimetric microtiter plate.5 Two prototype strains, ATCC12353 and BAA-572, were included as positive controls for speA/B and speC, respectively.6,7 A PCR amplification followed by a sequencing method was used to determine the emm type as described by Beall and colleagues8 and the website at http://www.cdc.gov/ncidod/biotech/strep/protocols.htm.
Results
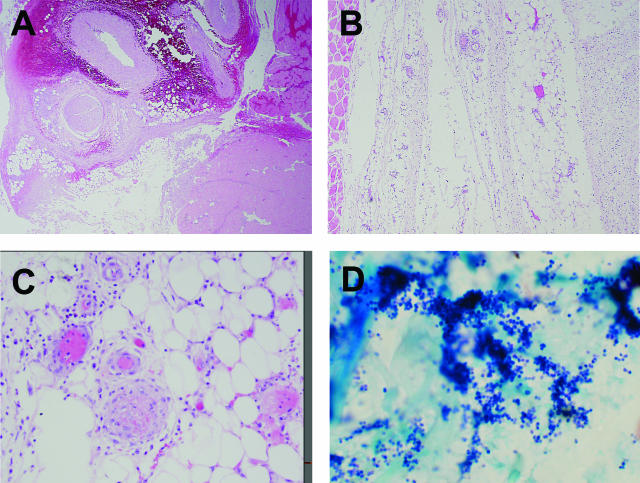
The postmortem tissue culture from the right popliteal fossa lesion, as well as lung and blood cultures were negative. The most significant finding at autopsy was the presence of focal hemorrhage, necrosis, and mild mixed inflammation with a predominance of mononuclear cells in the soft tissue of the popliteal fossa region (Figure 1; A to C). Histological sections of this region showed that the inflammation was not a prominent feature, was sparse within the interstitium and associated adipose tissue, and exhibited relative sparing of the skeletal muscle fibers (Figure 1; A to C). Mild chronic inflammation of the small arteries and arterioles with arteriole thrombosis was also present (Figure 1C). Organisms were not identified on routine H&E sections, but phenol green tissue gram stain showed numerous large collections of gram-positive cocci almost exclusively in clusters with rare gram-positive cocci in chains (Figure 1D).
Figure 1.
Photomicrographs of lesional tissue isolated from the popliteal fossa at autopsy. A: Hemorrhage and necrosis (H&E). B: The overall sparsely cellular inflammatory infiltrate with necrosis in the fascia and associated adipose tissue (H&E). C: Portion of adipose tissue with the predominantly mononuclear inflammatory infiltrate, associated vasculitis, and a thrombosed vessel (H&E). D: Gram-positive cocci in clusters (Phenol Green gram stain). Original magnifications: ×20 (A); ×40 (B); ×200 (C); ×400 (D).
In the liver, there was extensive hepatocyte necrosis and, in the kidney, there was marked acute tubular necrosis. These findings are consistent with the clinical findings of multiorgan system failure. In the lungs there were multiple areas of parenchymal hemorrhage and patchy bronchopneumonia bilaterally. In the small and large intestine, there were multiple regions of mucosal hemorrhage and necrosis. The brain showed cerebral edema, scattered ischemic pyramidal cells of the bilateral hippocampal formation, and widespread acute ischemic necrosis of Purkinje cells in the cerebellum.
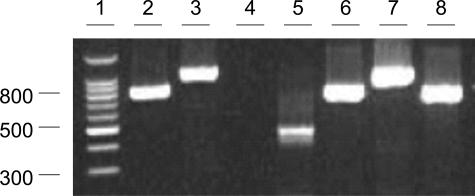
The human β-actin gene was detectable in the DNA extracted from the paraffin-embedded tissues, indicating these DNA samples were free of amplification inhibitors. PCR amplification for the 500 bp of the bacterial 16S rRNA genes was positive. Sequence of the PCR product (GenBank accession number: AY850393) showed a 100% identity to a Lancefield group A, type 1 S. pyogenes ATCC 12344 strain (Y. Mori, T. Takahashi, M. Katsumi, K. Katoh, T. Hiramune, N. Kikuchi, unpublished; GenBank accession number AB002521). An 818- and a 1106-bp DNA fragment were successfully amplified from the extracted tissue DNA when the speA- and speB-specific primer set was used (Figure 2). The fragments were confirmed speA- and speB-specific through an internal probe hybridization. In contrast, the primer set targeting the speC gene was negative. A 997-bp fragment of the emm gene corresponding to 28 to 1024 nucleotide positions was amplified by PCR. The sequence determined (GenBank accession number: AY960591) was most closely related to an emm type 1,9 diverging from the published sequence at only three nucleotide positions (99.7% identity). In summary, these findings demonstrated that the patient’s rapid deterioration with multisystem organ failure could have been the result of a SPE-A and SPE-B superantigen-producing, emm type 1 S. pyogenes infection in the popliteal fossa region.
Figure 2.
Streptococcal pyrogenic exotoxin gene detection by PCR. Three spe genes and a human β-actin gene were detected by individual PCR, respectively. Lanes 2 to 5 contained tissue DNA specimens amplified by speA-, speB-, speC-, and β-actin-specific primer sets. Lanes 6 and 7 contained a DNA specimen extracted from ATCC123536 amplified by speA- and speB-specific primer sets. Lane 8 contained a DNA specimen extracted from BAA-5727 amplified by a speC-specific primer set. Molecular sizes (lane 1) are in bp.
Discussion
Our patient initially presented with a history of acute onset of leg pain along with clinical symptoms of a flu-like syndrome with fever, chills, nausea, vomiting, diarrhea, and myalgias. The severe leg pain is characteristic of necrotizing fasciitis, and flu-like symptoms are reported in up to 20% of patients with GAS toxic shock-like syndrome.10,11 The marked hypocalcemia (3.2 to 6.2 mg/dL) seen in this patient is also a characteristic finding in GAS necrotizing fasciitis secondary to extensive adipocyte necrosis.12 Histologically, the overall sparsely cellular mononuclear cell-predominant inflammatory infiltrate of fascia and adipose tissue, hemorrhage, necrosis, and a sparing of the muscle are characteristic findings in GAS necrotizing fasciitis. The predominantly mononuclear cell infiltrate is consistent with previously published data in cases of necrotizing fasciitis showing superantigen involvement as well as bacterial production of proteases that degrade interleukin-8 and C5a that recruit polymorphonuclear leukocytes to the site of infection.13,14
GAS necrotizing fasciitis has shown a marked increase throughout the past 20 years and has a reported incidence of 3.5 to 20 cases/100,000 persons.10,12,15 The toxin-producing bacteria release superantigens that contribute to the development of fascial necrosis with relative limited involvement of the skin and underlying muscle. As previous authors have noted, necrotizing fasciitis due to any organism results in severe systemic toxicity and can be rapidly fatal secondary to refractory hypotension and multiorgan system failure.12,16,17,18,19 It most commonly occurs in the abdominal wall, extremities, and perineum. The introduction of a pathogen into the subcutaneous space can occur via disruption of the overlying skin such as a cut or abrasion or surgical incision, but most often the pathogen is bloodborne.12
Multiple factors seem to be involved in the relative severity of GAS necrotizing fasciitis and these include low antibody levels to cell-wall attached proteins, bacterial protease destruction of interleukin-8 and C5a recruitment of neutrophils, and the presence of superantigens such as SPE-A, SPE-B, SPE-C, SME-Z.13,14,20 It has also been shown that patients who have neutralizing antibodies to these superantigens are protected against developing severe disease.21,22 Recent data suggests that SME-Z may be the most important of these superantigens in eliciting the severe immunological response.23
These superantigens interact with MHC II class receptors on antigen-presenting cells and T cell receptors on T cells simultaneously to cause massive production in proinflammatory cytokines such as interleukin-1β, interleukin-2, tumor necrosis factor-α, tumor necrosis factor-β, and interferon-γ that lead to the rapid clinical deterioration with toxic shock syndrome, refractory hypotension, multiorgan system failure, and death.24 Therefore, early treatment with intravenous antibiotics including both a β-lactam agent and clindamycin as well as intravenous IgG, and aggressive surgical debridement have been shown to be important in improving patient outcomes.25,26,27,28
SPE-A and SPE-B are the most common superantigens involved in severe GAS infections seen in the United States.12 In our patient, PCR amplification and detection of a portion of the speA and speB genes were positive, illustrating the possible cause of this patient’s rapid clinical deterioration with a toxic shock-like syndrome.6,7 The presence of speA and speB also confirms the work of Norrby-Teglund and colleagues,14,22,28,29 and others that implicates superantigens as playing a central role in the pathogenesis of necrotizing GAS soft tissue infections.
In select culture-negative patients who have surgical debridement, PCR detection of streptococcal 16S rRNA and spe exotoxin DNA may be helpful in solidifying the diagnosis from paraffin-imbedded tissue. These methods have been used effectively to aid in the diagnosis of the etiological agent of culture-negative endocarditis at autopsy and could also be easily applied to paraffin-imbedded tissue blocks from routine surgical pathology specimens and would be particularly useful in patients who are repeatedly culture-negative.2,30
Acknowledgments
We thank Haijing Li, Rosemary Verrall, Shufang Meng, and Joni Williams for their excellent technical assistance.
Footnotes
This study was presented in part at the 105th General Meeting of the American Society for Microbiology, Atlanta, GA, June 5 to 9, 2005.
References
- Brown RC, Hopps HC. Staining of bacteria in tissue sections: a reliable gram stain method. Am J Clin Pathol. 1973;60:234–240. doi: 10.1093/ajcp/60.2.234. [DOI] [PubMed] [Google Scholar]
- Khulordava I, Miller G, Haas D, Li H, McKinsey J, Vanderende D, Tang YW. Identification of the bacterial etiology of culture-negative endocarditis by amplification and sequencing of a small ribosomal RNA gene. Diagn Microbiol Infect Dis. 2003;46:9–11. doi: 10.1016/s0732-8893(03)00011-7. [DOI] [PubMed] [Google Scholar]
- Tang YW, Von Graevenitz A, Waddington MG, Hopkins MK, Smith DH, Li H, Kolbert CP, Montgomery SO, Persing DH. Identification of coryneform bacterial isolates by ribosomal DNA sequence analysis. J Clin Microbiol. 2000;38:1676–1678. doi: 10.1128/jcm.38.4.1676-1678.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black CM, Talkington DF, Messmer TO, Facklam RR, Hornes E, Olsvik O. Detection of streptococcal pyrogenic exotoxin genes by a nested polymerase chain reaction. Mol Cell Probes. 1993;7:255–259. doi: 10.1006/mcpr.1993.1038. [DOI] [PubMed] [Google Scholar]
- Li H, Dummer JS, Estes WR, Meng S, Wright PF, Tang YW. Measurement of human cytomegalovirus loads by quantitative real-time PCR for monitoring clinical intervention in transplant recipients. J Clin Microbiol. 2003;41:187–191. doi: 10.1128/JCM.41.1.187-191.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks CR, Ferretti JJ. Nucleotide sequence of the type A streptococcal exotoxin (erythrogenic toxin) gene from Streptococcus pyogenes bacteriophage T12. Infect Immun. 1986;52:144–150. doi: 10.1128/iai.52.1.144-150.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoot JC, Barbian KD, Van Gompel JJ, Smoot LM, Chaussee MS, Sylva GL, Sturdevant DE, Ricklefs SM, Porcella SF, Parkins LD, Beres SB, Campbell DS, Smith TM, Zhang Q, Kapur V, Daly JA, Veasy LG, Musser JM. Genome sequence and comparative microarray analysis of serotype M18 group A Streptococcus strains associated with acute rheumatic fever outbreaks. Proc Natl Acad Sci USA. 2002;99:4668–4673. doi: 10.1073/pnas.062526099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall B, Facklam R, Thompson T. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J Clin Microbiol. 1996;34:953–958. doi: 10.1128/jcm.34.4.953-958.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti JJ, McShan WM, Ajdic D, Savic DJ, Savic G, Lyon K, Primeaux C, Sezate S, Suvorov AN, Kenton S, Lai HS, Lin SP, Qian Y, Jia HG, Najar FZ, Ren Q, Zhu H, Song L, White J, Yuan X, Clifton SW, Roe BA, McLaughlin R. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc Natl Acad Sci USA. 2001;98:4658–4663. doi: 10.1073/pnas.071559398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelsom J, Halstensen A, Haga T, Hoiby EA. Necrotizing fasciitis due to group A streptococci in western Norway: incidence and clinical features. Lancet. 1994;344:1111–1115. doi: 10.1016/s0140-6736(94)90629-7. [DOI] [PubMed] [Google Scholar]
- McHenry CR, Piotrowski JJ, Petrinic D, Malangoni MA. Determinants of mortality for necrotizing soft-tissue infections. Ann Surg. 1995;221:558–565. doi: 10.1097/00000658-199505000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RJ, Dafoe DC, Raffin TA. Necrotizing fasciitis. Chest. 1996;110:219–229. doi: 10.1378/chest.110.1.219. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Grass C, Dan-Goor M, Maly A, Eran Y, Kwinn LA, Nizet V, Ravins M, Jaffe J, Peyser A, Moses AE, Hanski E. Effect of bacterial pheromone peptide on host chemokine degradation in group A streptococcal necrotising soft-tissue infections. Lancet. 2004;363:696–703. doi: 10.1016/S0140-6736(04)15643-2. [DOI] [PubMed] [Google Scholar]
- Norrby-Teglund A, Thulin P, Gan BS, Kotb M, McGeer A, Andersson J, Low DE. Evidence for super-antigen involvement in severe group A streptococcal tissue infections. J Infect Dis. 2001;184:853–860. doi: 10.1086/323443. [DOI] [PubMed] [Google Scholar]
- O’Brien KL, Beall B, Barrett NL, Cieslak PR, Reingold A, Farley MM, Danila R, Zell ER, Facklam R, Schwartz B, Schuchat A. Epidemiology of invasive group A streptococcus disease in the United States, 1995–1999. Clin Infect Dis. 2002;35:268–276. doi: 10.1086/341409. [DOI] [PubMed] [Google Scholar]
- Stevens DL. Streptococcal toxic-shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment. Emerg Infect Dis. 1995;1:69–78. doi: 10.3201/eid0103.950301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens DL. Invasive group A streptococcus infections. Clin Infect Dis. 1992;14:2–13. doi: 10.1093/clinids/14.1.2. [DOI] [PubMed] [Google Scholar]
- Schwartz B, Facklam RR, Breiman RF. Changing epidemiology of group A streptococcal infection in the USA. Lancet. 1990;336:1167–1171. doi: 10.1016/0140-6736(90)92777-f. [DOI] [PubMed] [Google Scholar]
- Johnson DR, Stevens DL, Kaplan EL. Epidemiologic analysis of group A streptococcal serotypes associated with severe systemic infections, rheumatic fever, or uncomplicated pharyngitis. J Infect Dis. 1992;166:374–382. doi: 10.1093/infdis/166.2.374. [DOI] [PubMed] [Google Scholar]
- Akesson P, Rasmussen M, Mascini E, von Pawel-Rammingen U, Janulczyk R, Collin M, Olsen A, Mattsson E, Olsson ML, Bjorck L, Christensson B. Low antibody levels against cell wall-attached proteins of Streptococcus pyogenes predispose for severe invasive disease. J Infect Dis. 2004;189:797–804. doi: 10.1086/381982. [DOI] [PubMed] [Google Scholar]
- Mascini EM, Jansze M, Schellekens JFP, Musser JM, Faber JAJ, Verhoef-Verhage LAE, Schouls L, van Leeuwen WJ, Verhoef J, van Dijk H. Invasive group A streptococcal disease in The Netherlands: evidence for a protective role of anti-exotoxin A antibodies. J Infect Dis. 2000;181:631–638. doi: 10.1086/315222. [DOI] [PubMed] [Google Scholar]
- Norrby-Teglund A, Kaul R, Low DE, McGeer A, Andersson J, Andersson U, Kotb M. Evidence for the presence of streptococcal-superantigen-neutralizing antibodies in normal polyspecific immunoglobulin G. Infect Immun. 1996;64:5395–5398. doi: 10.1128/iai.64.12.5395-5398.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unnikrishnan M, Altmann DM, Proft T, Wahid F, Cohen J, Fraser JD, Sriskandan S. The bacterial superantigen streptococcal mitogenic exotoxin Z is the major immunoactive agent of Streptococcus pyogenes. J Immunol. 2002;169:2561–2569. doi: 10.4049/jimmunol.169.5.2561. [DOI] [PubMed] [Google Scholar]
- Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am. 2003;8:1454–1460. [PubMed] [Google Scholar]
- Fink S, Chaudhuri TK, Davis HH. Necrotizing fasciitis and malpractice claims. South Med J. 1999;92:770–774. doi: 10.1097/00007611-199908000-00004. [DOI] [PubMed] [Google Scholar]
- Lille ST, Sato TT, Engrav LH, Foy H, Jurkovich GJ. Necrotizing soft tissue infections: obstacles in diagnosis. J Am Coll Surg. 1996;182:7–11. [PubMed] [Google Scholar]
- Zimbelman J, Palmer A, Todd J. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J. 1999;18:1096–1100. doi: 10.1097/00006454-199912000-00014. [DOI] [PubMed] [Google Scholar]
- Norrby-Teglund A, Norrby SR, Low DE. The treatment of severe group A streptococcal infections. Curr Infect Dis Rep. 2003;5:28–37. doi: 10.1007/s11908-003-0062-2. [DOI] [PubMed] [Google Scholar]
- Malak K, Norrby-Teglund A, McGeer A, El-Sherbini H, Dorak MT, Khurshid A, Green K, Peeples J, Wade J, Thomson G, Schwartz B, Low DE. An immunogenetic and molecular basis for differences in outcomes of invasive group A streptococcal infections. Nat Med. 2002;8:1398–1404. doi: 10.1038/nm1202-800. [DOI] [PubMed] [Google Scholar]
- Jobbagy Z, Fabian CB, Memoli VA, Schwartzman JD. A novel Streptococcus organism identified in a case of fulminant endocarditis using 16S rDNA sequencing. J Mol Diagn. 2004;6:145–148. doi: 10.1016/S1525-1578(10)60503-X. [DOI] [PMC free article] [PubMed] [Google Scholar]