Abstract
Several diagnostic strategies have been applied to the detection of FMR1 gene repeat expansions in fragile X syndrome. Here, we report a novel polymerase chain reaction-based strategy using the Expand Long Template PCR System (Roche Diagnostics, Mannheim, Germany) and the osmolyte betaine. Repeat expansions up to ∼330 CGGs in males and up to at least ∼160 CGGs in carrier women could be easily visualized on ethidium bromide agarose gels. We also demonstrated that fluorescence analysis of polymerase chain reaction products was a reliable tool to verify the presence of premutation and full mutation alleles both in males and in females. This technique, primarily designed to detect premutation alleles, can be used as a routine first screen for expanded FMR1 alleles.
Fragile X syndrome is the most common inherited form of mental retardation. This syndrome is caused by the expansion of CGG repeats in the 5′-untranslated region of the fragile X mental retardation 1 (FMR1) gene and hypermethylation of its 5′ upstream CpG island.1 The CGG repeat element is polymorphic, varying from 6 to 44 repeats in the normal range, from 45 to 54 repeats in the gray zone, and from 55 to 200 repeats in the premutation range.2 For alleles below the gray zone, the CGG repeat is generally stable in parent-to-offspring transmissions. However, CGG elements in the premutation range become increasingly unstable with increasing repeat number, and alleles exceeding ∼59 CGG repeats can expand to a full mutation in a single generation, almost exclusively by transmission from mother to son. The FMR1 premutation is typically associated with specific clinical manifestations unique to the premutation range: premature ovarian failure has been observed in ∼20% of women,3,4,5,6 whereas the fragile X-associated tremor/ataxia syndrome has been found in at least one-third of carrier males more than 50 years old.7,8,9 Individuals affected with fragile X syndrome have FMR1 alleles with a CGG repeat number greater than 200.
At present, DNA analysis of the CGG expansion is primarily performed using Southern blot analysis, which is able to detect alleles spanning the range from normal to large full mutation alleles; however, this method lacks the resolution to accurately size alleles. An alternative approach, using polymerase chain reaction (PCR) amplification of the region spanning the CGG repeat, provides much greater resolution, although it suffers from the difficulty of amplifying CGG repeats greater than ∼100 to 150 repeats, because of the high GC content of the sequence being amplified.
Radioactive or chemiluminescent probing, or fluorescence PCR, can overcome most problems, at least in the premutation range. Several studies have already described a number of PCR techniques, which use diverse combinations of DNA polymerase, 7-deaza-dGTP, and co-solvents such as dimethyl sulfoxide (DMSO) and betaine.10,11,12,13,14,15 However, the largest allele that has been amplified to date is 250 CGG repeats,13 and PCR results with alleles of greater than ∼100 repeats are highly variable.
To address this issue, we propose an improved PCR method aimed at the identification of premutation and full mutation alleles up to 300 CGGs, using the Expand Long Template PCR system (Roche Diagnostics, Mannheim, Germany) in conjunction with the use of betaine. PCR products can be directly visualized on agarose gels after ethidium bromide staining and correctly sized on acrylamide gels. Alternatively, PCR products can be run on an automatic sequencer.
Materials and Methods
Samples
We analyzed 178 subjects with alleles in the normal and gray-zone ranges (11 to 54 CGG), 26 in the premutation range (55 to 200 CGG), and 13 in the full mutation range (>200 CGG) (Table 1). The sizes of the trinucleotide repeat in the full mutation samples had previously been determined using EcoRI and NruI digested Southern blots and the StB12.3 probe.16
Table 1.
Genotypes of the Controls and Patients Tested in the Study
| Normal-gray zone (6–54 CGGs)
|
Premutation (55–200 CGGs)
|
Full mutation (>200 CGGs)
|
|||||
|---|---|---|---|---|---|---|---|
| Males (n = 127) | Females (n = 51) | Males (n = 13) | Females (n = 13) | Males (n = 10) | Females (n = 3) | ||
| Triplets | No. | Triplets | No. | Triplets | Triplets | Triplets | Triplets |
| 11 | 1 | 19/28 | 1 | 65 | 12/98 | ∼210 | 29/∼280 |
| 18 | 1 | 19/30 | 2 | 71 | 28/84 | ∼230 | 29/∼600 |
| 19 | 3 | 20/23 | 1 | 80 | 29/64 | ∼250 | 35/>400 |
| 20 | 5 | 20/26 | 1 | 81 | 29/86 | 280–1000 | |
| 22 | 1 | 20/29 | 1 | 83 | 29/115 | ∼300 | |
| 23 | 1 | 20/30 | 4 | 86 | 29/118 | >300 | |
| 24 | 1 | 20/31 | 1 | 90 | 29/∼160 | 300–600 | |
| 26 | 1 | 20/32 | 1 | 95 | 30/65 | 300–900 | |
| 27 | 1 | 21/22 | 1 | 98 | 30/70 | 330–1000 | |
| 28 | 12 | 22/34 | 1 | 106 | 30/98 | 400–750 | |
| 29 | 30 | 22/28 | 1 | 109 | 31/70 | ||
| 30 | 41 | 23/29 | 2 | 126 | 32/81 | ||
| 31 | 8 | 23/32 | 1 | ∼165 | 33/118 | ||
| 32 | 5 | 25/29 | 1 | ||||
| 33 | 1 | 27/30 | 1 | ||||
| 34 | 1 | 28/29 | 1 | ||||
| 35 | 1 | 28/31 | 1 | ||||
| 37 | 4 | 28/32 | 1 | ||||
| 38 | 1 | 28/33 | 1 | ||||
| 39 | 3 | 28/38 | 1 | ||||
| 40 | 1 | 28/42 | 1 | ||||
| 41 | 1 | 28/51 | 1 | ||||
| 43 | 1 | 29/29 | 4 | ||||
| 44 | 1 | 29/30 | 2 | ||||
| 45 | 1 | 29/31 | 2 | ||||
| 29/44 | 1 | ||||||
| 30/30 | 7 | ||||||
| 30/31 | 1 | ||||||
| 30/32 | 1 | ||||||
| 30/33 | 3 | ||||||
| 30/39 | 1 | ||||||
| 30/41 | 1 | ||||||
| 31/32 | 1 | ||||||
Normal subjects were unselected; underlined, females homozygotes for normal alleles (21.6%).
PCR Protocol
Genomic DNA was isolated using either the Qiaquick DNA Blood kit (Qiagen, Hilden, Germany) or the Puregene DNA Blood kit (Gentra Systems, Minneapolis, MN). PCR reactions were performed using the c (fluorescent labeled) and f primers (6-FAM-agccccgcacttccaccaccagctcctcca; 5′-gctcagctccgtttcggtttcacttccggt).17 The reactions were performed with the Expand Long Template PCR System (Roche Diagnostics), using buffer 2, 500 μmol/L dNTPs, 0.33 μmol/L of each primer, and 100 ng of genomic DNA. Different betaine concentrations (B0300; Sigma-Aldrich, St. Louis, MO) were tested, ranging from 1.3 to 2.2 mol/L. Hot-start PCR was performed as indicated by the manufacturer (Roche Diagnostics). The total PCR reaction volume was 30 μl. The PCR cycling profile was as follows: denaturation at 98°C for 10 minutes; 10 cycles at 97°C for 35 seconds, 64°C for 35 seconds, 68°C for 4 minutes; 25 cycles at 97°C for 35 seconds, 64°C for 35 seconds, 68°C for 4 minutes, plus a 20-second increment for each cycle; and a final extension at 68°C for 10 minutes. The expected constant region of the PCR product was 221 bp (ie, excluding the CGG repeat itself).
Agarose and Fluorescent Analysis of the PCR Products
Five microliters of PCR product was electrophoresed at 6 V/cm for 45 minutes on a 2.0% TBE 1× agarose gel containing ethidium bromide, followed by visualization on a UV light transilluminator. Alternatively, the fragments were separated on an automatic sequencer ABI-Prism 3100-Avant (Applera, Foster City, CA) using a 36-cm capillary, the POP4 polymer, and the Genescan ROX-500 or ROX-2500 as internal standard markers (Applera). For the Prism-based approach, 1 μl of PCR product was added to 10 μl of formammide and 0.05 μl of ROX marker and heated to 95° for 2 minutes. We performed electrokinetic injection at 2 kV for 15 seconds, and samples were separated at 15 kV for 25 minutes at 60°C. Data were collected using the 3100 Avant (ver. 1.0) data collection software and elaborated using Genescan ver. 3.7 (Applera). Three independent investigators verified samples blindly.
Cloning and Sequencing
The CGG region of the FMR1 gene was amplified in 17 male patients using unlabeled c and f primers using the conditions described above. PCR products were purified using the Perfectprep Gel cleanup kit (Eppendorf, Amburg, Germany) and directly sequenced using the Big Dye Terminator cycle sequencing kit (ver. 3.1; Applera), and 1.7 mol/L betaine. Sequences were run on an automatic sequencer (ABI-Prism 3100-Avant) using a 36-cm capillary and the POP6 polymer and analyzed using the sequencing analysis software. The same FMR1 gene region was also amplified for five different premutation subjects using primers c and f, tagged at the 5′ end, respectively, with a BamHI and an EcoRI restriction site (see amplification conditions above, with 1.5 mol/L betaine). Fragments from 300 to 500 bp were gel purified using the Perfectprep Gel cleanup kit (Eppendorf), doubly digested with BamHI/EcoRI, and cloned into a pBluescriptSK vector (Stratagene, La Jolla, CA). Clones with a single insert of different sizes were sequenced using M13-21 and M13 reverse primers.
Results
Agarose Detection of CGG Repeat Expansions
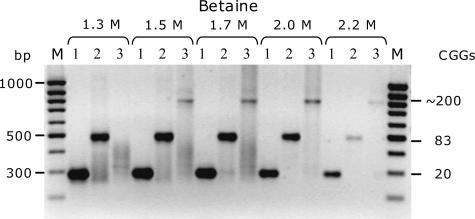
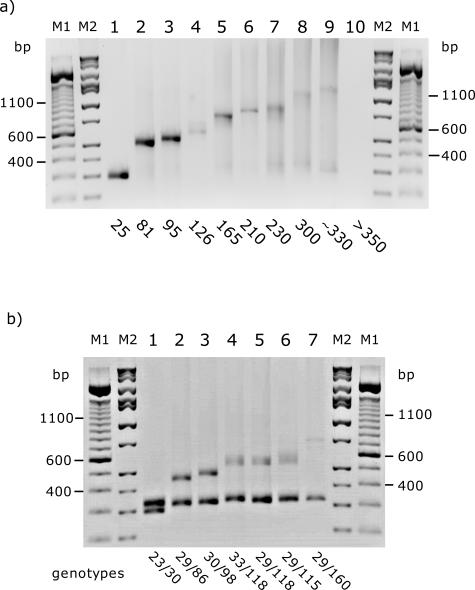
The FMR1 CGG repeat region was amplified using the Expand Long Template kit (Roche Diagnostics) and betaine; PCR products were visualized directly on agarose gels. To establish the optimal amplification conditions, we tested two males carrying alleles of 83 or 200 CGG repeats and a control male with an allele of 20 CGG repeats, with the three available buffers in the kit (buffers 1, 2, and 3) and with different concentrations of betaine (1.3, 1.5, 1.7, 2.0, and 2.2 mol/L). Optimal results were obtained with buffer 2 (2.25 mmol/L MgCl2) and betaine at concentrations of 1.7 to 2.0 mol/L (Figure 1). Although alleles larger than 250 CGG repeats became progressively fainter, they were still visible for a male with ∼330 CGGs (∼1200 bp) (Figure 2a). We did not detect any amplification product for alleles >330 CGG (Figure 2, lane 10). Carrier females with alleles of at least 160 CGG repeats (the highest premutation allele tested) yielded PCR products in which both the normal and the expanded alleles were clearly visible as two distinct bands (Figure 2b). For carrier females, the larger allele appeared proportionally weaker with increasing repeat length, but the larger alleles were still visible and discrete in the higher premutation range. The addition of 7-deaza-dGTP/dGTP to the reaction mix or the combination of 7-deaza-dGTP and betaine did not improve amplification, and in several cases, it led to a complete absence of the PCR product (data not shown).
Figure 1.
Effect of betaine concentration on the PCR amplification of the CGG repeat region of the FMR1 gene. Three male subjects, carrying 20, 83, and ∼200 CGG repeats, were tested with increasing concentrations of betaine, from 1.3 to 2.2 mol/L. M, 100-bp ladder molecular weight marker; three marker bands of 300, 500, and 1000 bp are indicated.
Figure 2.
Detection of PCR products from the amplification of the CGG region of the FMR1 gene. Five microliters of PCR product was loaded per lane: a, samples male; b, female samples. CGG genotype is indicated below each lane. M1, 100-bp ladder molecular weight marker. M2, molecular weight marker Hi-Low DNA Marker (Bionexus, Oakland, CA).
Fluorescence Detection of Premutation and Full Mutation FMR1 Alleles
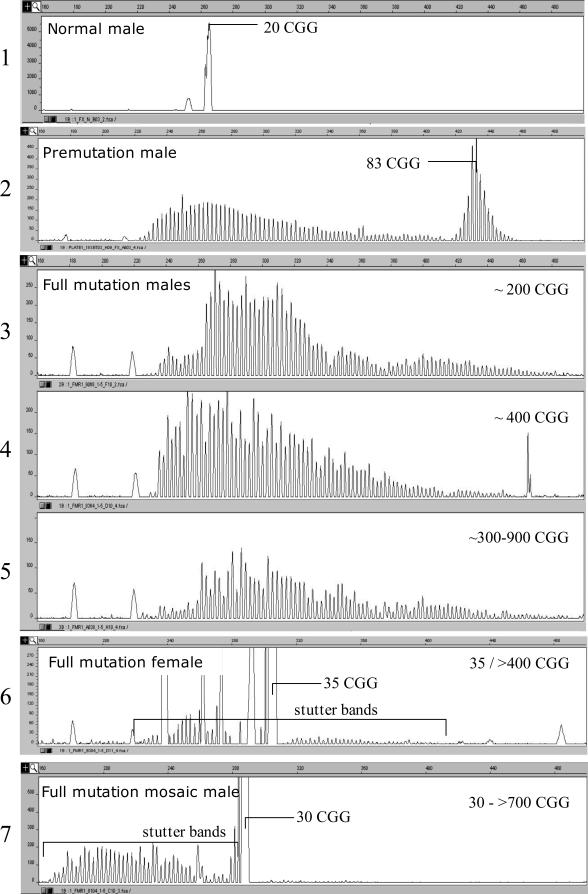
The profile of the samples in Figure 1 (20, 83, and ∼200 repeats), loaded on the automatic sequencer, presented a peculiar fluorescent pattern at lower betaine concentrations (1.7 mol/L) (Figure 3, panels 1 to 3). The two individuals, with either premutation or full mutation alleles, showed multiple low-intensity stutter spikes starting from the normal CGG repeat size range, probably generated by polymerase slippage (Figure 3, panels 2 and 3). In the normal individual and in the premutation carrier, higher peaks corresponding to the 20 and 83 CGGs, respectively, were also present. Fragments larger than 100 repeats were more difficult to visualize, likely because the peak corresponding to the expansion became proportionally weaker with increasing number of CGG repeats (data not shown). As also observed by other investigators, the ratio of the normal to expanded peak areas differed considerably between agarose and fluorescence, with the larger allele being proportionally weaker.10,18
Figure 3.
Automated fluorescence analysis of the PCR products for premutation and full mutation subjects. PCR reactions were performed using 1.7 mol/L betaine. Panels 1 and 2, premutation (male) carriers with alleles of 83 and 90 CGG repeats, respectively. Panels 3 to 5, full mutation males; descending array of peaks visible from 240 to 500 bp. Panel 6, full mutation carrier female with 35/>400 CGG repeats; a main offscale peak corresponds to the normal 35-CGG allele, and a set of low-intensity stutter bands is generated by the full mutation. Panel 7, full mutation mosaic male; the profile is similar to that of a full mutation carrier female in panel 6.
To verify whether the stutter bands on the left portion of the electropherogram (larger repeat sizes) could be used as the hallmark of an expanded allele, we tested 178 subjects in the normal and gray zone, 26 premutation carriers, and 13 subjects with full expansion alleles (Table 1), all confirmed by Southern blot analysis. One or two peaks in the normal range always characterized the pattern observed in normal controls, even if the sample was overloaded. Heterozygous normal females could be clearly detected even if the two alleles differed by a single triplet (data not shown). In premutation male carriers, a gradually descending array of peaks is present, generally accompanied by a bell-shaped array of peaks on the right portion of the curve (smaller fragment sizes) (Figure 3, panel 2). The highest peak in the bell-shaped array is consistent in size with the estimated number of expanded CGGs.
Analysis of full mutation alleles yielded only arrays of multiple peaks (starting from ∼220 bp), because the full mutation was far larger than the resolution range (see samples in Figure 3, panel 3–5). It is noteworthy that in all cases, we were able to distinguish between normal, premutation, and larger (full mutation) expanded alleles. We also performed our test on three females with full mutation alleles (see pattern in Figure 3, panel 6). A major off-scale peak, corresponding to the normal allele, is clearly visible in the left portion of the graph (35 CGGs in the figure), and an array of low-intensity stutter peaks, generated by PCR slippage, is the hallmark of the second expanded allele. We could also test two mosaic full-mutated males whose pattern was similar to that of full mutation females (Figure 3, panel 7).
Size Determination of the Normal, Gray-Zone, and Premutation Alleles
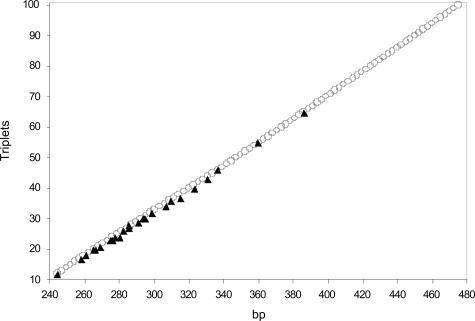
To determine the correspondence between the size of the fluorescent PCR fragments and the number of CGG repeats, we sequenced 17 male subjects and 10 clones containing the FMR1 region, with 12 to 65 CGG repeats (Table 2). Data are plotted in Figure 4 (black triangles) and show a linear correlation (R2 = 0.998) between the size in base pairs (abscissa) and the number of repeats (ordinate). The apparent constant region was 212.4 bp and increased in ∼2.7-bp increments for each CGG repeat.
Table 2.
Comparison of Sequence and Fragment Analysis Methods to Estimate the FMR1 Gene Triplet Number
| Subjects | Repeat structure | No. of sequenced triplets | Length (bp) | No. of estimated triplets* |
|---|---|---|---|---|
| B960 | (CGG)12 | 12 | 244.66 | 8 |
| C383 | (CGG)10-AGG-(CGG)9 | 20 | 265.65 | 15 |
| A241 | (CGG)10-AGG-(CGG)9 | 20 | 264.58 | 15 |
| C009 | (CGG)10-AGG-(CGG)10 | 21 | 268.60 | 16 |
| B771 | (CGG)12-AGG-(CGG)10 | 23 | 275.70 | 18 |
| B513 | (CGG)9-AGG-(CGG)14 | 24 | 276.96 | 19 |
| C244 | (CGG)10-AGG-(CGG)16 | 27 | 285.00 | 21 |
| C229 | (CGG)9-AGG-(CGG)9-AGG-(CGG)9 | 29 | 290.26 | 28 |
| B185 | (CGG)9-AGG-(CGG)9-AGG-(CGG)9 | 29 | 290.43 | 23 |
| A341 | (CGG)10-AGG-(CGG)9-AGG-(CGG)9 | 30 | 294.28 | 24 |
| B258 | (CGG)9-AGG-(CGG)9-AGG-(CGG)12 | 32 | 298.23 | 26 |
| B514 | (CGG)9-AGG-(CGG)24 | 34 | 306.43 | 28 |
| C163 | (CGG)9-AGG-(CGG)9-AGG-(CGG)6-AGG-(CGG)10 | 37 | 314.76 | 31 |
| A965 | (CGG)10-AGG-(CGG)9-AGG-(CGG)9-AGG-(CGG)9 | 40 | 322.91 | 34 |
| C044 | (CGG)9-AGG-(CGG)9-AGG-(CGG)23 | 43 | 330.38 | 36 |
| B179 | (CGG)9-AGG-(CGG)9-AGG-(CGG)26 | 46 | 336.19 | 38 |
| C789 | (CGG)9-AGG-(CGG)9-AGG-(CGG)45 | 65 | 385.94 | 55 |
| Clones | ||||
| FX-C2 | (CGG)17 | 17 | 257.48 | 12 |
| FX-D5 | (CGG)18 | 18 | 260.50 | 13 |
| FX-D1 | (CGG)23 | 23 | 274.57 | 18 |
| FX-F3 | (CGG)24 | 24 | 279.73 | 19 |
| FX-D6 | (CGG)26 | 26 | 281.90 | 20 |
| FX-G9 | (CGG)28 | 28 | 284.87 | 21 |
| FX-F10 | (CGG)10-AGG-(CGG)9-AGG-(CGG)9 | 30 | 293.62 | 24 |
| FX-A12 | (CGG)10-AGG-(CGG)9-AGG-(CGG)9 | 30 | 293.65 | 24 |
| FX-G5 | (CGG)9-AGG-CAG-(CGG)25 | 36 | 309.29 | 29 |
| FX-G10 | (CGG)9-AGG-(CGG)14-AGG-(CGG)7-AGG-(CGG)14-AGG-(CGG)7 | 55 | 359.31 | 46 |
The number of triplets is estimated using the formula: No. of triplets = (bp − 221)/3.
Figure 4.
PCR fragment length (abscissa) versus number of CGG repeats (ordinate). ▴, data from 18 sequenced male subjects and 10 clones (Table 2). ○, derived from the average size of the stutter bands generated in 17 premutation or full mutation male subjects.
We also took into consideration the regularly spaced array of spikes generated in premutation and in full mutation subjects, likely corresponding to fragment populations containing a variable number of CGG expansions. The average distance between two peaks was measured in the arrays of 17 male carriers of pre- or full mutation alleles, and the mean measure of the peaks from 12 to 100 triplets is plotted in Figure 4 (open circles). In this latter instance, there was also an approximately linear correlation between the size of the fragment and the number of CGG repeats, with an average increase of ∼2.6 bp per repeat; the difference between two peaks tended to decrease with increasing size. As revealed by the graph (Figure 4), there is good overlap between the two data sets, showing that the stutter bands may be a reliable tool to determine the size, at least up to 100 repeats. In Table 3, we provide a reference ladder to be used for estimating the number of CGG repeats from the apparent size of the fragment. The ladder was determined by fitting the sequenced samples and stutter bands. We found that the relationship, size (bp) = 2.7078 × (no. of CGG repeats) + 212.15, derived from the sequenced samples, was the best fit for normal and gray-zone alleles (up to ∼50 to 55 repeats), and that size (bp) = 2.5464 × (no.CGG repeats) + 220.5, derived from the stutter bands, was the best fit in the premutation range (up to ∼100 CGG repeats). The sizes of the alleles (55 to 100 repeats) in Table 3 are further confirmed by two samples among the largest alleles sequenced thus far (55 and 65 repeats), the size of which perfectly match with our estimates (compare Tables 2and 3). Finally, we evaluated the measurement error due to technical artifacts, analyzing 32 nonconsecutive runs of a single heterozygous female sample whose genotype was 30 of 68 repeats. The two alleles gave measured sizes of 293.7 ± 1.40 bp (mean ± SD) and 394.8 ± 2.6 bp (confidence interval 95%), corresponding to 30 and 68 (± 1) CGG repeats.
Table 3.
Reference Table to Estimate the Number of Triplets from the Size in Fluorescence and Theoretical Estimation Based on the Formula 221 + (3 × CGG)
| No. of CGGs | Size (bp)
|
No. of CGGs | Size (bp)
|
||
|---|---|---|---|---|---|
| Fluorescence* | Theoretical | Fluorescence* | Theoretical | ||
| 11 | 241.93 | 254 | 56 | 363.10 | 389 |
| 12 | 244.64 | 257 | 57 | 365.64 | 392 |
| 13 | 247.35 | 260 | 58 | 368.19 | 395 |
| 14 | 250.06 | 263 | 59 | 370.74 | 398 |
| 15 | 252.76 | 266 | 60 | 373.28 | 401 |
| 16 | 255.47 | 269 | 61 | 375.83 | 404 |
| 17 | 258.18 | 272 | 62 | 378.38 | 407 |
| 18 | 260.89 | 275 | 63 | 380.92 | 410 |
| 19 | 263.60 | 278 | 64 | 383.47 | 413 |
| 20 | 266.30 | 281 | 65 | 386.02 | 416 |
| 21 | 269.01 | 284 | 66 | 388.56 | 419 |
| 22 | 271.72 | 287 | 67 | 391.11 | 422 |
| 23 | 274.43 | 290 | 68 | 393.66 | 425 |
| 24 | 277.13 | 293 | 69 | 396.20 | 428 |
| 25 | 279.84 | 296 | 70 | 398.75 | 431 |
| 26 | 282.55 | 299 | 71 | 401.29 | 434 |
| 27 | 285.26 | 302 | 72 | 403.84 | 437 |
| 28 | 287.97 | 305 | 73 | 406.39 | 440 |
| 29 | 290.67 | 308 | 74 | 408.93 | 443 |
| 30 | 293.38 | 311 | 75 | 411.48 | 446 |
| 31 | 296.09 | 314 | 76 | 414.03 | 449 |
| 32 | 298.80 | 317 | 77 | 416.57 | 452 |
| 33 | 301.50 | 320 | 78 | 419.12 | 455 |
| 34 | 304.21 | 323 | 79 | 421.67 | 458 |
| 35 | 306.92 | 326 | 80 | 424.21 | 461 |
| 36 | 309.63 | 329 | 81 | 426.76 | 464 |
| 37 | 312.34 | 332 | 82 | 429.30 | 467 |
| 38 | 315.04 | 335 | 83 | 431.85 | 470 |
| 39 | 317.75 | 338 | 84 | 434.40 | 473 |
| 40 | 320.46 | 341 | 85 | 436.94 | 476 |
| 41 | 323.17 | 344 | 86 | 439.49 | 479 |
| 42 | 325.87 | 347 | 87 | 442.04 | 482 |
| 43 | 328.58 | 350 | 88 | 444.58 | 485 |
| 44 | 331.29 | 353 | 89 | 447.13 | 488 |
| 45 | 334.00 | 356 | 90 | 449.68 | 491 |
| 46 | 336.71 | 359 | 91 | 452.22 | 494 |
| 47 | 339.41 | 362 | 92 | 454.77 | 497 |
| 48 | 342.12 | 365 | 93 | 457.32 | 500 |
| 49 | 344.83 | 368 | 94 | 459.86 | 503 |
| 50 | 347.54 | 371 | 95 | 462.41 | 506 |
| 51 | 350.25 | 374 | 96 | 464.95 | 509 |
| 52 | 352.95 | 377 | 97 | 467.50 | 512 |
| 53 | 355.66 | 380 | 98 | 470.05 | 515 |
| 54 | 358.37 | 383 | 99 | 472.59 | 518 |
| 55 | 361.08 | 386 | 100 | 475.14 | 521 |
Data are dependent on electrophoretic conditions and must be validated with a reference sequenced sample.
Discussion
Many laboratories performing fragile X diagnostic testing use PCR as a pre-screen and proceed to Southern blot only for those samples that fail to amplify (males) or show a single normal allele (females). Several methods have been published using different combinations of 7-deaza-2′-dGTP, betaine, DMSO, and several DNA polymerase brands and mixes. The presence of 7-deaza-2′-GTP greatly reduces the detection of stained PCR products with ethidium bromide;15 PCR-based methods using this analog thus require an additional detection method such as silver staining,11 inclusion of an α-32P-labeled dNTP in the reaction, or hybridization with a radioactive or chemiluminescent probe. The use of betaine instead of DMSO to reduce secondary structures or the Expand Long Template PCR system (Roche Diagnostics) has been independently proposed in the analysis of repeat ex-pansions,12,13,14 often in conjunction with fluorescence PCR-based assays.
Here, we describe a simple and robust procedure that can be used to screen for FMR1 expansions, which can be detected directly on agarose or acrylamide gels using ethidium bromide staining. The difference in the current protocol with respect to known methods is the addition of betaine to the Expand Long Template PCR system, a combination that greatly facilitates the detection of expanded CGG repeats without the need for more laborious detection strategies (radioactive or chemiluminescent hybridization). We tested the optimal concentration of betaine and demonstrated that our system is able to amplify and visualize directly on agarose gels expanded alleles of up to ∼330 CGG repeats in males, and of at least ∼160 CGG repeats (the largest heterozygous repeat measured) in carrier females.
We also showed that fluorescence analysis of PCR products using automated sequencing methods can be a useful tool to verify the presence of pre- and full mutation alleles; in these cases the fluorescence profile shows an array of “stutter” bands (visible as a smear on the agarose) that were never observed in more than 178 controls. These series of peaks therefore appear to be specific for the presence of expanded alleles, although the lower limit of this phenomenon has not been defined. Of course, gel-based PCR and Southern blot methods are still necessary to size the number of expanded repeats above 100 CGG and to evaluate the methylation status of the expanded allele; however, the use of this fluorescence method will dramatically reduce the number of Southern blots required for screening studies.
Recently, great interest has been focused on premutation carrier detection, because premutation alleles have been found to be associated with premature ovarian failure in females and fragile X tremor/ataxia syndrome in males.7,19 Screening studies have shown that fragile X tremor/ataxia syndrome could be a frequent genetic cause of late-onset sporadic ataxia.20,21 Therefore, this method represents a useful tool especially in the case of screening of large population samples.
Our test can also determine the number of CGG repeats in the normal to premutation allele range. Sequencing the FMR1 repeat in 17 subjects and 10 clones, we demonstrated that there is a direct, approximately linear correlation between the size of the PCR fragments and the number of repeats, with an apparent constant region of 212.4 bp, instead of the real 221 bp, and an increase of 2.7 bp for each additional triplet. The last column in Table 2 reports the estimated size of the PCR product based on the formula 221 + 3n, where n is the number of CGG repeats. It should be noted that the difference between the expected and observed size increases with the expansion.
Additional data obtained on the stutter bands of premutation subjects confirm the peculiar migration rate of these PCR fragments and suggest that with increasing size, the difference between two peaks tends to diminish. We have provided (Table 3) a quick reference ladder to estimate the number of CGG repeats from the apparent size of the PCR fragment. Although it is validated by sequences only up to 65 triplets, the estimate is more reliable than the currently used formula 221 + 3n (see Tables 2and 3). Furthermore, it should be noted that an intrinsic measurement error (estimated ± 1 triplet), probably due to technical artifacts, is always present.
One possible concern with using PCR for FMR1 analysis is that affected male mosaics for normal and full mutation alleles might be missed, because the PCR would only pick up the normal allele.11 Combined with the expected full mutation positive rate of about 1 in 3500, these apparent mosaics appear less than once in 3000 “query FMR1” tests. The possibility of normal/premutation or normal/full mutation males should be given consideration, however, when testing in known fragile X families. We could test only two full mutation mosaic males, who gave a detectable pattern similar to that generated by the presence of an expansion in carrier females. The presence of such profiles both in males and females should induce further analysis to prove the carrier status. Additional patients with full mutation mosaicism need to be analyzed to define the limits of this test.
Our technique, primarily designed to detect premutation alleles, can be used as a routine first screen for expanded FMR1 alleles. In the absence of an automatic sequencer, agarose analysis can be useful to detect premutations and small expansions; apparently homozygous females and males without detectable PCR bands should be analyzed by Southern blot. Capillary electrophoresis will recognize normal heterozygous females (∼80% of females in our survey), full mutation males, and carrier females. Furthermore, it will give an evaluation of the precise triplet repeat length ± 1 CGG (up to ∼100 triplets). For diagnostic purposes, we recommend that the PCR analysis should be accompanied by Southern Blot analysis in homozygous females and as a confirmatory test also to evaluated methylation status in all carriers of premutated or full-mutated alleles.
Footnotes
Supported by the “Associazione E.E. Rulfo per la Genetica Medica,” by the “Associazione Gli Amici di Valentina,” by the Regione Piemonte CIPE36/2002, by the National Institute of Child Health and Development (grant HD40661 to P.J.H.) and by University of California Davis Health Systems Research award (F.T.).
References
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP, Eussen BE, van Ommen G-JB, Blonden LAJ, Riggins GJ, Chastain JL, Kunst CB, Galjaard H, Caskey CT, Nelson DL, Oostra BA, Warren ST. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Hagerman PJ, Hagerman RJ. Fragile X-associated tremor/ataxia syndrome (FXTAS). Ment Retard Dev Disabil Res Rev. 2004;10:25–30. doi: 10.1002/mrdd.20005. [DOI] [PubMed] [Google Scholar]
- Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, Holden JJ, Yang KT, Lee C, Hudson R, Gorwill H, Nolin SL, Glicksman A, Jenkins EC, Brown WT, Howard-Peebles PN, Becchi C, Cummings E, Fallon L, Seitz S, Black SH, Vianna-Morgante AM, Costa SS, Otto PA, Mingroni-Netto RC, Murray A, Webb J, Mac Swinney F, Dennis N, Jacobs P, Syrrou M, Georgiou I, Patsalis PC, Giovannucci Uzielli ML, Guarducci S, Lapi E, Cecconi A, Ricci U, Ricotti G, Biondi C, Scarselli B, Vieri F. Fragile X premutation is a significant risk factor for premature ovarian failure: the International Collaborative POF in Fragile X study—preliminary data. Am J Med Genet. 1999;83:322–325. [PMC free article] [PubMed] [Google Scholar]
- Murray A. Premature ovarian failure and the FMR1 gene. Semin Reprod Med. 2000;18:59–66. doi: 10.1055/s-2000-13476. [DOI] [PubMed] [Google Scholar]
- Murray A, Ennis S, Morton N. No evidence for parent of origin influencing premature ovarian failure in fragile X premutation carriers. Am J Hum Genet. 2000;67:253–254. doi: 10.1086/302963. ; author reply, 256–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SL. Premature ovarian failure among fragile X premutation carriers: parent-of-origin effect? Am J Hum Genet. 2000;67:11–13. doi: 10.1086/302985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J, Grigsby J, Gage B, Hagerman PJ. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57:127–130. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- Jacquemont S, Hagerman RJ, Leehey M, Grigsby J, Zhang L, Brunberg JA, Greco C, Des Portes V, Jardini T, Levine R, Berry-Kravis E, Brown WT, Schaeffer S, Kissel J, Tassone F, Hagerman PJ. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72:869–878. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont S, Hagerman RJ, Leehey MA, Hall DA, Levine RA, Brunberg JA, Zhang L, Jardini T, Gane LW, Harris SW, Herman K, Grigsby J, Greco CM, Berry-Kravis E, Tassone F, Hagerman PJ. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. J Am Med Assoc. 2004;291:460–469. doi: 10.1001/jama.291.4.460. [DOI] [PubMed] [Google Scholar]
- O’Connell CD, Atha DH, Jakupciak JP, Amos JA, Richie K. Standardization of PCR amplification for fragile X trinucleotide repeat measurements. Clin Genet. 2002;61:13–20. doi: 10.1034/j.1399-0004.2002.610103.x. [DOI] [PubMed] [Google Scholar]
- Haddad LA, Mingroni-Netto RC, Vianna-Morgante AM, Pena SD. A PCR-based test suitable for screening for fragile X syndrome among mentally retarded males. Hum Genet. 1996;97:808–812. doi: 10.1007/BF02346194. [DOI] [PubMed] [Google Scholar]
- Houdayer C, Lemonnier A, Gerard M, Chauve C, Tredano M, de Villemeur TB, Aymard P, Bonnefont JP, Feldmann D. Improved fluorescent PCR-based assay for sizing CGG repeats at the FRAXA locus. Clin Chem Lab Med. 1999;37:397–402. doi: 10.1515/CCLM.1999.065. [DOI] [PubMed] [Google Scholar]
- Hamdan H, Tynan JA, Fenwick RA, Leon JA. Automated detection of trinucleotide repeats in Fragile X syndrome. Mol Diagn. 1997;2:259–269. doi: 10.1054/MODI00200259. [DOI] [PubMed] [Google Scholar]
- Hecimovic S, Vlasic J, Barisic L, Markovic D, Culic V, Pavelic K. A simple and rapid analysis of triplet repeat diseases by expand long PCR. Clin Chem Lab Med. 2001;39:1259–1262. doi: 10.1515/CCLM.2001.202. [DOI] [PubMed] [Google Scholar]
- Brown WT, Nolin S, Houck G, Jr, Ding X, Glicksman A, Li SY, Stark-Houck S, Brophy P, Duncan C, Dobkin C, Jenkins E. Prenatal diagnosis and carrier screening for fragile X by PCR. Am J Med Genet. 1996;64:191–195. doi: 10.1002/(SICI)1096-8628(19960712)64:1<191::AID-AJMG34>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Rousseau F, Heitz D, Biancalana V, Blumenfeld S, Kretz C, Boue J, Tommerup N, Van Der Hagen C, DeLozier-Blanchet C, Croquette MF, Gilgenkrantz S, Jalbert P, Voelckel MA, Oberlé I, Mandel JL. Direct diagnosis by DNA analysis of the fragile X syndrome of mental retardation. N Engl J Med. 1991;325:1673–1681. doi: 10.1056/NEJM199112123252401. [DOI] [PubMed] [Google Scholar]
- Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, Verkerk AJ, Holden JJ, Fenwick RG, Jr, Warren ST, Oostra BA, Nelson DL, Caskey CT. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67:1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Dorschner MO, Barden D, Stephens K. Diagnosis of five spinocerebellar ataxia disorders by multiplex amplification and capillary electrophoresis. J Mol Diagn. 2002;4:108–113. doi: 10.1016/S1525-1578(10)60689-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Leavitt BR, Farzin F, Jacquemont S, Greco CM, Brunberg JA, Tassone F, Hessl D, Harris SW, Zhang L, Jardini T, Gane LW, Ferranti J, Ruiz L, Leehey MA, Grigsby J, Hagerman PJ. Fragile-X-associated tremor/ataxia syndrome (FXTAS) in females with the FMR1 premutation. Am J Hum Genet. 2004;74:1051–1056. doi: 10.1086/420700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussino A, Gellera C, Saluto A, Mariotti C, Arduino C, Castellotti B, Camerlingo M, de Angelis V, Orsi L, Tosca P, Migone N, Taroni F, Brusco A. FMR1 gene premutation is a frequent genetic cause of late-onset sporadic cerebellar ataxia. Neurology. 2005;64:145–147. doi: 10.1212/01.WNL.0000148723.37489.3F. [DOI] [PubMed] [Google Scholar]
- Seixas AI, Maurer MH, Lin M, Callahan C, Ahuja A, Matsuura T, Ross CA, Hisama FM, Silveira I, Margolis RL. FXTAS, SCA10, and SCA17 in American patients with movement disorders. Am J Med Genet A. 2005;136:87–89. doi: 10.1002/ajmg.a.30761. [DOI] [PubMed] [Google Scholar]