Abstract
Mutations in the cardiac ryanodine type 2 receptor (RyR2) gene are associated with catecholaminergic polymorphic ventricular tachycardia. We hypothesized that these mutations could be detected at autopsy in cases of exercise-triggered sudden death. Fourteen sudden death patients, eight males and six females, were studied at autopsy based on apparent sudden cardiac death, without significant anatomical abnormalities. The coding regions of arrhythmia genes were amplified by polymerase chain reaction and directly sequenced. Three novel RyR2 mutations, R414C, F2331S, and R2401L, were identified in three unrelated patients (two males and one female; mean age at death, 12 ± 2 years), all performing strenuous activity at the time of death or collapse. These mutations were located in highly conserved regions where arrhythmia-linked RyR2 mutations clustered. Although G269S in the KVLQT1 gene was detected in a female with known family history of syncope and sudden cardiac death, no other mutations were found in any of the 14 cases, and no other mutations was found in 200 controls. The absence of structural cardiac disease in physical activity-induced sudden death and the finding of three novel RyR2 mutations suggest that mutation screening in such cases should include RyR2.
Ventricular arrhythmias, the leading cause of morbidity and mortality, cause more than 300,000 sudden cardiac deaths each year in the United States. Approximately 5 to 12% of these deaths occur in apparently healthy individuals without known cardiac or noncardiac causes.1 The molecular mechanisms underlying ventricular arrhythmias in these apparently normal hearts remained completely unknown until the arrhythmia susceptibility genes were identified.2,3 Mutations in these genes can lead to defective cardiac ion channels, causing ventricular arrhythmias. Genes for KVLQT1, KCNE1, KCNE2, and HERG encode cardiac potassium ion channels; their mutations are associated with sudden cardiac death in long QT syndrome.4 SCN5A encodes the α subunit of the voltage-gated cardiac sodium channel. Its mutations are linked to either long QT or Brugada syndrome.4,5,6 RyR2 has recently gained attention because its mutations have been shown to cause arrhythmogenic disorders including catecholaminergic polymorphic ventricular tachycardia (CPVT) and arrhythmogenic right ventricular dysplasia type 2.7,8,9,10
CPVT was first described in 1975, and later in 1995, as an arrhythmogenic disease of unknown origin.11,12 It is characterized with exercise-induced ventricular arrhythmia, syncope, or early sudden death, but not at rest. Patients with CPVT have morphologically normal hearts.9,13 The disorder locus was mapped to chromosome 1q42-q43 in affected families, the same region as RyR2 gene.14,15 Therefore, Priori and colleagues9 speculated that RyR2 mutations might be responsible for CPVT. They identified four RyR2 mutations on chromosome 1q42-q43 in individuals with CPVT. Since then, more RyR2 mutations have been detected and linked to the autosomal-dominant form of CPVT.7,8,9,10,13 The missense mutation of calsequestrin 2 causes the autosomal-recessive form of the disorder.16 So far, only one missense mutation of calsequestrin 2 has been identified in association with CPVT.16
CPVT has a high mortality rate, 30 to 50% by the age of 30 years, but the disease can be effectively treated to prevent syncope and sudden death.17 Therefore, early diagnosis is critical. In this study, we screened mutations in cardiac ion channel genes in 14 sporadic individuals lacking known cardiac disorder at autopsy. We identified three novel RyR2 mutations and one KVLQT1 mutation. This was the first time these RyR2 mutations were found at autopsy, complementing a recent report.18
Materials and Methods
Case Description
The 14 patients at autopsy included 8 males and 6 females (Table 1). The mean age at death was 17.4 ± 13 years, ranging from 1 to 43 years. At the time of collapse, nine patients were engaged in vigorous physical activity, three were in mild activity, and the activity was unknown in two. The 43-year-old female was the only subject with known family history of syncope and sudden cardiac death induced by emotional or physical activity. The autopsy and postmortem heart examination showed anatomically normal hearts in all cases.
Table 1.
Description of the Patients at Autopsy
| Case no. | Age | Gender | Activity at time of death | Mutation |
|---|---|---|---|---|
| 1 | 8 | Male | Climbing | F2331S mutation in RYR2 |
| 2 | 12 | Male | Running | R2401L mutation in RYR2 |
| 3 | 16 | Female | Swimming | R414C in RYR2 |
| 4 | 43 | Female | Shoveling snow | G269S mutation in KVLQT1 |
| 5 | 5 | Male | Drowning in shallow pond | None found |
| 6 | 24 | Female | Drowning in shallow water | None found |
| 7 | 18 | Male | Drowning in shallow water | None found |
| 8 | 10 | Female | Rest | HERG-K897T* |
| 10 | 14 | Male | Sitting at school | None |
| 11 | 20 | Female | Witnessed collapse standing | None |
| 12 | 1 | Female (sibling with possible LQT syndrome) | Breastfeeding | None |
| 13 | 20 | Male | Benchpressing | None |
| 14 | 21 | Male | Running | None |
In exon 11 of the LQT2 gene (HERG), an A-to-C transversion was found, resulting in R414C, considered a polymorphism. Additionally, a heterozygous mutation was found 28 bp upstream of the transcription initiation site of the KCNE2 gene in the patient, changing TTATTA to TTA/GTTA; this mutation is of uncertain significance.
Case 1
An 8-year-old white male became unresponsive while climbing a rock wall. He was initially resuscitated and transferred to a hospital where he expired ∼3 hours later. He had prior seizure episodes and was treated with gabapentin. Previous cardiology work-up did not show any evidence of long QT syndrome. At autopsy, the heart weighed 140 g (predicted normal value 125 g, upper limit 180 g as a function of body weight). The left ventricular cavity was 25 mm in diameter (normal, ≤40 mm), the left ventricular free wall was 8 mm thick (normal, ≤11 mm thick), the ventricular septum was 8 mm thick (normal, ≤11 mm thick), and the right ventricle was 2 mm thick (normal, ≤5 mm thick), without scars or abnormal fat infiltrates. Histological sections showed unremarkable myocardium. Coronary arteries were normal. A detailed conduction system study did not show significant abnormalities.
Case 2
A 12-year-old white male had a history of exercise-induced ventricular tachycardia and sinus node dysfunction. He suffered a syncopal/arrhythmic episode while running and could not be resuscitated. A previous catheterization study showed normal hemodynamics and normal right ventricular appearance. At autopsy, the heart weighed 210 g (apex had been removed and submitted separately). The left ventricular cavity was 25 mm in diameter, the left ventricular free wall was 11 mm thick, the ventricular septum was 10 mm thick, and the right ventricle was 5 mm thick, without gross scars or abnormal fat infiltrates. Histological sections showed unremarkable myocardium. Coronary arteries were normal. A detailed conduction system study did not show significant abnormalities.
Case 3
A 16-year-old white female had a history of attention deficit hyperactivity disorder and two episodes of syncope 1 year before death. An evaluation with electroencephalogram, computed tomography scan, and 24-hour Holter monitor were negative. Her QT interval was 0.438. She died suddenly while swimming competitively. At autopsy, her heart weight was 340 g. The left ventricular free wall thickness was 0.9 mm, interventricular septum thickness was 1.0 mm, and right ventricle thickness was 0.3 mm. Histological evaluation was unremarkable. The coronary arteries were normal in origin and course. A detailed histological examination of the conduction system was unremarkable.
Case 4
A 43-year-old white female collapsed while shoveling snow. Her sister collapsed and could not be resuscitated after being informed of the death of her sibling. Two other siblings were subsequently found to have electrocardiographic changes suggestive of long QT syndrome. At autopsy, the heart weighed 310 g with normal cardiac chamber dimensions. The left ventricular cavity was 25 mm in diameter, left ventricular free wall was 13 mm thick, ventricular septum was 11 mm thick, and right ventricle was 3 mm thick, without gross scars or abnormal fat infiltrates. Histological sections showed mild subendocardial and perivascular interstitial fibrosis in the left ventricle, with a single focus of perivascular lymphocytic infiltrate in the posterior left ventricle, with rare basophilic degeneration of myocytes. Coronary arteries were normal, with normal ostia, right dominance, and no significant atherosclerosis. A conduction system study was unremarkable.
Cases 5 to 14
At the time of collapses, three died from drowning, one each from bench press in the gym, running, snowball fighting, eating, and standing up, and in two the activity was unknown. The 10 cases included 4 females and 6 males. The age at death ranged from 1 to 24 years.
Genetic Study
Genomic DNA samples were extracted from frozen tissues by standard procedures. RyR2 is the largest cardiac ion channel gene identified, with 105 exons encoding 4967 amino acid residues.8 The mutation screening of the RyR2 gene had been performed on all 105 exons by other researchers; however, all of the mutations identified in this gene cluster occurred in three highly conserved regions known as the critical functional and regulatory domains of the channel.6,8,9,13,19
It was reasonable to study these conserved regions instead of the whole gene (on the advice of Drs. Kimmo Kontula (University of Helsinki) and Paivi Laitinen (Oulu University Hospital)). Therefore, we performed the mutation screening of RyR2 only in three hot spots, including exons of 8 to 15; 44 to 47, 49; and 83 to 105. Intronic primers amplifying the coding area were used for polymerase chain reaction amplifications (primer sequences were kindly provided by Drs. Kontula and Latinen). Polymerase chain reaction products were analyzed by agarose gel electrophoresis and were all directly sequenced on an ABI377 genetic analyzer (Applied Biosystems, Foster city, CA). Once we identified the suspected mutation, we compared it to the DNA sequences in the same area in 200 or 400 human controls to exclude DNA polymorphism. All coding areas of KVLQT1, HERG, SCN5A, KCNE1, and KCNE2 were amplified by the methods reported by others.2 The polymerase chain reaction products in this study were all sequenced on an ABI377 genetic analyzer.
Results
Case 1
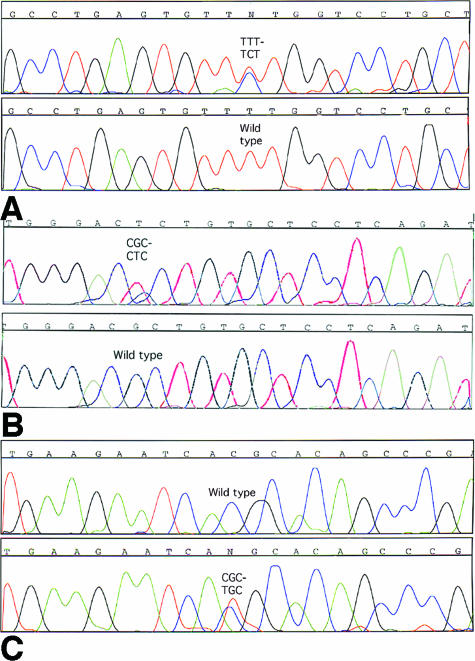
A T-to-C transition at nucleotide 7113 (7113T→C) in exon 46 of RyR2 was identified in the 8-year-old male who collapsed while climbing a rock wall. It resulted in a Phe2331-to-Ser (F2331S) missense mutation (Figure 1A). The wild-type, F2331, was detected in all 200 human controls (400 alleles) and is highly conserved among members of RyR2, even in fish RyR1(Figure 2A). F2331S is a novel RyR2 mutation.
Figure 1.
A: A C-to-T substitution at nucleotide position 7113 in exon 46 of RYR2 gene results in a Phe2331-to-Ser (F2331S) in the 8-year-old male. Residue F2331 is highly conserved in different species of three types of RyR2. B: A G-to-T transversion in exon 47 of RyR2 gene was identified in the 12-year-old male who died while running. It resulted in an Arg2401-to-Leu (R2401L) missense mutation. C: DNA sequence analysis reveals a T-to-C substitution in exon 14, converting arginine to cysteine at amino acid 414 in the 16-year-old female who died while swimming competitively.
Figure 2.
Protein sequence alignments of the victims’ protein segments with the corresponding segments in some of their homologues. The sequences were selected using a BLAST (NCBI BLAST) search of the victims’ RyR2 protein sequences translated from the victims’ RyR2 DNA sequences. Each top sequence is the mutated RyR2 protein segment from the victim, and the bar with boldface indicates the mutated position. A: F2331S mutation identified in the 8-year-old boy (F2331S mutation RyR2). Residue F2331 is highly conserved in different species of three types of RyRs. B: The R2401L mutation and its alignments. R2401 is highly conserved in all RyRs, even in fish RyR1. C: R414C missense mutation detected in the 16-year-old female. R414 is conserved in human, mouse, or rabbit RyR2 but not in horse RyR1 or in human or chicken RyR3 (data not shown). Protein sequence identifiers are listed on the right sides of protein sequences.
Case 2
The 7323G→T transversion in exon 47 of RyR2 was identified in the 12-year-old male who died suddenly while running. It resulted in an Arg2401-to-Leu (R2401L) missense mutation (Figure 1B). R2401L was absent in 200 controls (400 alleles), it was not a DNA polymorphism. Its wild type, R2401, is highly conserved in different species in RyR genes, including fish RyR1(Figure 2B).
Case 3
DNA sequence analysis revealed a T-to-C substitution at nucleotide 1361 in exon 14, converting arginine414 to cysteine (R414C) in the 16-year-old female who died while swimming competitively (Figure 1C). R414 is highly conserved in human, rabbit, and mouse RYR2(Figure 2C), but it is not conserved in human or chicken RyR3 (data not shown). R414C was not detected in 400 human controls (800 alleles).
Case 4
A G-to-A transition at 128767G→A in exon 6 resulted in Gly269-to-Ser (G269S) in the S5 transmembrane region of KVLQT1. This gene encodes potassium channel α subunits. It has six transmembrane-spanning domains, S1 to S6. The potassium channel pore signature sequence is located between S5 and S6. Most of the arrhythmia-associated missense mutations in KVLQT1 have been identified in membrane-spanning regions as well as the pore region. No other mutation was found in the above four cases.
Cases 5 to 14
Genetic screening on other cases showed negative results, and no other mutation was found in all cases. In case 8, we found an A-to-C transversion resulting in R414C in exon 11 of the LQT2 gene (HERG), which is considered a polymorphism. Additionally, a heterozygous mutation was found 28 bp upstream of the transcription initiation site of KCNE2 in the same patient, changing TTATTA to TTA/GTTA; this mutation is of uncertain significance.
Discussion
In this study, we screened for mutations in cardiac ion channel genes in 14 patients with structurally normal hearts at autopsy, and we identified three novel RyR2 missense mutations and a KVLQT1 mutation. The KVLQT1 mutation has been previously described,4,20 is therefore not novel, and is not the major focus of the current study. Briefly, there are two major family studies on KVLQT1, both identifying the G269S mutation found in the current study.4,20 Chen and colleagues20 found that 10 mutations of KCNQ1 including G269S were identified in 10 of 55 (18%) families with the LQTS phenotype. The majority (70%) of mutations identified in this study were located in the pore region and transmembrane domains S5 and S6, immediately adjacent to the pore, and 30% were in the C-terminus.20
Mammalian tissues express three isoforms of RyRs, each encoded by specific genes known as RyR1, RyR2, and RyR3. RyR2 protein is highly homologous to RyR1, and it is the predominant isoform in the heart. RyR2 is the major Ca2+ release channel required for excitation-contraction coupling in cardiac muscle.21,22,23 The voltage-gated L-type calcium channels are activated by the cardiomyocyte membrane depolarization in phase zero of action potential. The Ca2+ influx through these activated channels activates RyR2 to initiate the rapid release of Ca2+ from the sarcoplasmic reticulum into the cytosol via the process known as Ca2+-induced Ca2+ release.24,25 The RyR2 macromolecular complex is a tetramer that includes four 565-kd RyR2 polypeptides, each of which binds to a 12-kd calstabin 2 protein (also known as FKBP12.6 in cardiac muscle). The calcium release channel also contains cAMP-dependent protein kinase A, phosphatases PP1 and PP2A, the anchoring protein mAKAP, calsequestrin 2, and the intrinsic membrane proteins junction and triadin.26,27 The functional receptor has transmembrane domains in the C-terminals of RyR2 monomers, forming a pore region with a diameter of 1 to 2 nm. The rest of the molecule is the large N-terminal region protruding into the cytosol. This region contains domains for cooperative and regulative interactions among RyR2 and its channel modulators.26,28
CPVT RyR2 mutations cluster in three highly conserved regions that are homologous to three mutational hot spots in the skeletal muscle type 1 RyR receptor underlying malignant hyperthermia and central core disease.29,30 The three RyR2 mutations we identified are all located in these hot spots in the N-terminal region of RyR2. R414C was detected in the 16-year-old drowning female. Residue R414 is conserved in human and rabbit RyR2, but it is not conserved in human or chicken RyR3 or in horse RyR1. F2331S and R2401L, identified separately in the 8-year-old and the 12-year-old males, respectively, are located in the binding domain of RyR2 to its gating protein calstabin 2. F2331 and R2401 are highly conserved in different species of all RyRs. Interestingly, a slightly different mutation at the same residue, R2401H, in RyR2 was recently identified in a 20-year-old male with CPVT.31 It provides clinical evidence that residue R2401 is critical for RyR2 normal function. R2401H is the only CPVT-linked mutation reported in the calstabin-binding region in which the mutation carrier escaped from early sudden cardiac death.
To date, seven other RyR2 mutations clustered in this domain have been detected and associated with arrhythmia or early sudden cardiac death. P2328S was detected by Laitinen and colleagues8 in a large family without structural heart disease but with a history of ventricular tachycardia and early sudden death in response to vigorous exercise. P2328S is located only three residues from F2331S, the mutation identified in our laboratory. Priori and colleagues9 identified R2474S in an 8-year-old male with exercise-induced ventricular tachycardia and repeated syncopal episodes. His identical twin had repeated syncopal events and died at 7 years of age. Autopsy failed to show any structural heart abnormality. S2246L was detected in another 8-year-old male with CPVT, who had recurrent exercise-induced syncopal events since the age of 3 years.9 E2311D was found in association with polymorphic ventricular tachycardia in a child.13 Tiso and colleagues10 identified two more RyR2 mutations in this region (N2386I and T2504 mol/L) in large families affected with arrhythmogenic right ventricular dysplasia type 2. Another RyR2 mutation, Y2392C, was linked to CPVT and sudden death.10 Taken together, 10 mutations of RyR2 in this highly conserved region have been identified in patients with severe forms of CPVT- or arrhythmogenic right ventricular dysplasia type 2-linked ventricular arrhythmias or early sudden death7,8,9,10,31 as well as in patients in this report.
Calstabin 2 plays a critical role in RyR2 channel gating in cardiac muscle. Brillantes and colleagues,32 Kaftan and colleagues,33 Marx and colleagues,34 and Wehrens and colleagues35 have found that this immunophilin stabilizes a closed state of the channel, preventing aberrant activation of the channel during the resting phase of the cardiac cycle. In addition, calstabin 2 is required for coupled gating between RyRs. Marx and colleagues36 showed that protein kinase A phosphorylation of RyR2 dissociates calstabin 2 and regulates the channel open probability, a reflection of ion channel activation. Wehrens and colleagues35 expressed CPVT RyR2 mutations, located in the RyR2 binding domain to calstabin 2, in vesicles prepared from human embryonic cell lines transfected with mutant RyR2. At simulated resting conditions, these CPVT-associated RyR2 mutations presented normal single channel properties. Under conditions simulating exercise, RyR2 with S2246L or R2474S had increased RyR2 channel activity by reducing the affinity of RyR2 for calstabin 2. Calstabin 2-deficient mice (calstabin 2−/−) remained undistinguishable from calstabin 2+/+ mice at rest, but calstabin 2−/− mice consistently exhibited exercise-induced fatal arrhythmias that were remarkably similar to the clinical phenotype as individuals with these mutant RyR2.36 Marks19 suggested that the depletion of the channel-stabilizing protein calstabin 2 and defective RyR2 channel may have caused a leaky RyR2 channel. The author further used a derivative of 1,4-benzothazepine (JTV519) that increased the affinity of calstabin 2 for RyR2.19 It stabilized the closed state of RyR2 and prevented the Ca2+ leak. Its application in calstabin 2+/− mice prevented ventricular arrhythmias and sudden death. It may provide a new therapeutic strategy for CPVT patients.37
George and colleagues38 clearly showed for the first time that mutant RyR2 with S2246L did not alter the beating frequency or intracellular Ca2+ handling in resting cardiac cells, but mediated significantly augmented Ca2+ release and caused longer relaxation time after exposure to RyR agonists of β-adrenergic stimulation. It provided further information for us to understand how RyR2 mutations could cause arrhythmia and sudden death. However, they did not find any change in the affinity of mutant RyR2 to calstabin 2. The precise mechanisms by which RyR2 mutations cause the augmented Ca2+ release remains to be defined.
Study Limitations
DNA samples from the victims’ relatives are not available for family study at present time. We do not know if these are inherited or de novo mutations. We plan to perform the structure-function study in the future to show the effects of the mutations on cardiomyocytes.
In summary, the clinical phenotype of three victims with RyR2 mutations suggests an exertional arrhythmia, which is characteristic of RyR2 mutation-linked CPVT. We identified three novel RyR2 mutations among 14 autopsy cases. It suggested that some CPVT patients were sporadic and undiagnosed. Our results reveal that RyR2 mutation contributes to unexpected sudden death in the absence of morphological abnormality.
References
- Kannel WB, Cupples LA, D’Agostino RB. Sudden death risk in overt coronary heart disease: the Framingham Study. Am Heart J. 1987;113:799–804. doi: 10.1016/0002-8703(87)90722-8. [DOI] [PubMed] [Google Scholar]
- Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001;104:569–580. doi: 10.1016/s0092-8674(01)00243-4. [DOI] [PubMed] [Google Scholar]
- Marban E. Cardiac channelopathies. Nature. 2002;415:213–218. doi: 10.1038/415213a. [DOI] [PubMed] [Google Scholar]
- Splawski I, Shen J, Timothy KW, Lehmann MH, Priori S, Robinson JL, Moss AJ, Schwartz PJ, Towbin JA, Vincent GM, Keating MT. Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation. 2000;102:1178–1185. doi: 10.1161/01.cir.102.10.1178. [DOI] [PubMed] [Google Scholar]
- Bezzina C, Veldkamp MW, van Den Berg MP, Postma AV, Rook MB, Viersma JW, van Langen IM, Tan-Sindhunata G, Bink-Boelkens MT, van Der Hout AH, Mannens MM, Wilde AA. A single Na(+) channel mutation causing both long-QT and Brugada syndromes. Circ Res. 1999;85:1206–1213. doi: 10.1161/01.res.85.12.1206. [DOI] [PubMed] [Google Scholar]
- Chen Q, Kirsch GE, Zhang D, Brugada R, Brugada J, Brugada P, Potenza D, Moya A, Borggrefe M, Breithardt G, Ortiz-Lopez R, Wang Z, Antzelevitch C, O’Brien RE, Schulze-Bahr E, Keating MT, Towbin JA, Wang Q. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998;392:293–296. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- Bauce B, Rampazzo A, Basso C, Bagattin A, Daliento L, Tiso N, Turrini P, Thiene G, Danieli GA, Nava A. Screening for ryanodine receptor type 2 mutations in families with effort-induced polymorphic ventricular arrhythmias and sudden death: early diagnosis of asymptomatic carriers. J Am Coll Cardiol. 2002;40:341–349. doi: 10.1016/s0735-1097(02)01946-0. [DOI] [PubMed] [Google Scholar]
- Laitinen PJ, Brown KM, Piippo K, Swan H, Devaney JM, Brahmbhatt B, Donarum EA, Marino M, Tiso N, Viitasalo M, Toivonen L, Stephan DA, Kontula K. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation. 2001;103:485–490. doi: 10.1161/01.cir.103.4.485. [DOI] [PubMed] [Google Scholar]
- Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino V, Danieli GA. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- Tiso N, Stephan DA, Nava A, Bagattin A, Devaney JM, Stanchi F, Larderet G, Brahmbhatt B, Brown K, Bauce B, Muriago M, Basso C, Thiene G, Danieli GA, Rampazzo A. Identification of mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2). Hum Mol Genet. 2001;10:189–194. doi: 10.1093/hmg/10.3.189. [DOI] [PubMed] [Google Scholar]
- Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995;91:1512–1519. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- Reid DS, Tynan M, Braidwood L, Fitzgerald GR. Bidirectional tachycardia in a child. A study using His bundle electrography. Br Heart J. 1975;37:339–344. doi: 10.1136/hrt.37.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori SG, Napolitano C, Memmi M, Colombi B, Drago F, Gasparini M, DeSimone L, Coltorti F, Bloise R, Keegan R, Cruz Filho FE, Vignati G, Benatar A, DeLogu A. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- Otsu K, Fujii J, Periasamy M, Difilippantonio M, Uppender M, Ward DC, MacLennan DH. Chromosome mapping of five human cardiac and skeletal muscle sarcoplasmic reticulum protein genes. Genomics. 1993;17:507–509. doi: 10.1006/geno.1993.1357. [DOI] [PubMed] [Google Scholar]
- Swan H, Piippo K, Viitasalo M, Heikkila P, Paavonen T, Kainulainen K, Kere J, Keto P, Kontula K, Toivonen L. Arrhythmic disorder mapped to chromosome 1q42–q43 causes malignant polymorphic ventricular tachycardia in structurally normal hearts. J Am Coll Cardiol. 1999;34:2035–2042. doi: 10.1016/s0735-1097(99)00461-1. [DOI] [PubMed] [Google Scholar]
- Lahat H, Pras E, Olender T, Avidan N, Ben-Asher E, Man O, Levy-Nissenbaum E, Khoury A, Lorber A, Goldman B, Lancet D, Eldar M. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum Genet. 2001;69:1378–1384. doi: 10.1086/324565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JD, Krikler D, Hallidie-Smith KA. Familial polymorphic ventricular arrhythmias: a quarter century of successful medical treatment based on serial exercise-pharmacologic testing. J Am Coll Cardiol. 1999;34:2015–2022. doi: 10.1016/s0735-1097(99)00438-6. [DOI] [PubMed] [Google Scholar]
- Tester DJ, Kopplin LJ, Creighton W, Burke AP, Ackerman MJ. Pathogenesis of unexplained drowning: new insights from a molecular autopsy. Mayo Clin Proc. 2005;80:596–600. doi: 10.4065/80.5.596. [DOI] [PubMed] [Google Scholar]
- Marks AR. Clinical implications of cardiac ryanodine receptor/calcium release channel mutations linked to sudden cardiac death. Circulation. 2002;106:8–10. doi: 10.1161/01.cir.0000021746.82888.83. [DOI] [PubMed] [Google Scholar]
- Chen S, Zhang L, Bryant RM, Vincent GM, Flippin M, Lee JC, Brown E, Zimmerman F, Rozich R, Szafranski P, Oberti C, Sterba R, Marangi D, Tchou PJ, Chung MK, Wang Q. KCNQ1 mutations in patients with a family history of lethal cardiac arrhythmias and sudden death. Clin Genet. 2003;63:273–282. doi: 10.1034/j.1399-0004.2003.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai J, Imagawa T, Hakamat Y, Shigekawa M, Takeshima H, Numa S. Primary structure and functional expression from cDNA of the cardiac ryanodine receptor/calcium release channel. FEBS Lett. 1990;271:169–177. doi: 10.1016/0014-5793(90)80399-4. [DOI] [PubMed] [Google Scholar]
- Tunwell RE, Wickenden C, Bertrand BM, Shevchenko VI, Walsh MB, Allen PD, Lai FA. The human cardiac muscle ryanodine receptor-calcium release channel: identification, primary structure and topological analysis. Biochem J. 1996;318:477–487. doi: 10.1042/bj3180477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzato F, Fujii J, Otsu K, Phillips M, Green NM, Lai FA, Meissner G, MacLennan DH. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1990;265:2244–2256. [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983;245:C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Protasi F, Ramesh V. Shape, size, and distribution of Ca(2+) release units and couplons in skeletal and cardiac muscles. Biophys J. 1999;77:1528–1539. doi: 10.1016/S0006-3495(99)77000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakamata Y, Nakai J, Takeshima H, Imoto K. Primary structure and distribution of a novel ryanodine receptor/calcium release channel from rabbit brain. FEBS Lett. 1992;312:229–235. doi: 10.1016/0014-5793(92)80941-9. [DOI] [PubMed] [Google Scholar]
- Takeshima H, Nishimura S, Matsumoto T, Ishida H, Kangawa K, Minamino N, Matsuo H, Ueda M, Hanaoka M, Hirose T, Numa S. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989;339:439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- Serysheva II, Orlova EV, Chiu W, Sherman MB, Hamilton SL, van Heel M. Electron cryomicroscopy and angular reconstitution used to visualize the skeletal muscle calcium release channel. Nat Struct Biol. 1995;2:18–24. doi: 10.1038/nsb0195-18. [DOI] [PubMed] [Google Scholar]
- Brandt A, Schleithoff L, Jurkat-Rott K, Klingler W, Baur C, Lehmann-Horn F. Screening of the ryanodine receptor gene in 105 malignant hyperthermia families: novel mutations and concordance with the in vitro contracture test. Hum Mol Genet. 1999;8:2055–2062. doi: 10.1093/hmg/8.11.2055. [DOI] [PubMed] [Google Scholar]
- Loke J, MacLennan DH. Malignant hyperthermia and central core disease: disorders of Ca2+ release channels. Am J Med. 1998;104:470–486. doi: 10.1016/s0002-9343(98)00108-9. [DOI] [PubMed] [Google Scholar]
- Aizawa Y, Ueda K, Komura S, Washizuka T, Chinushi M, Inagaki N, Matsumoto Y, Hayashi T, Takahashi M, Nakano N, Yasunami M, Kimura A, Hiraoka M, Aizawa Y. A novel mutation in FKBP12.6 binding region of the human cardiac ryanodine receptor gene (R2401H) in a Japanese patient with catecholaminergic polymorphic ventricular tachycardia. Int J Cardiol. 2005;99:343–345. doi: 10.1016/j.ijcard.2003.11.050. [DOI] [PubMed] [Google Scholar]
- Brillantes AB, Ondrias K, Scott A, Kobrinsky E, Ondriasova E, Moschella MC, Jayaraman T, Landers M, Ehrlich BE, Marks AR. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994;77:513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Kaftan E, Marks AR, Ehrlich BE. Effects of rapamycin on ryanodine receptor/Ca(2+)-release channels from cardiac muscle. Circ Res. 1996;78:990–997. doi: 10.1161/01.res.78.6.990. [DOI] [PubMed] [Google Scholar]
- Marx SO, Gaburjakova J, Gaburjakova M, Henrikson C, Ondrias K, Marks AR. Coupled gating between cardiac calcium release channels (ryanodine receptors). Circ Res. 2001;88:1151–1158. doi: 10.1161/hh1101.091268. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, D’Armiento JM, Napolitano C, Memmi M, Priori SG, Lederer WJ, Marks AR. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, Coromilas J, Landry DW, Marks AR. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- George CH, Higgs GV, Lai FA. Ryanodine receptor mutations associated with stress-induced ventricular tachycardia mediate increased calcium release in stimulated cardiomyocytes. Circ Res. 2003;93:531–540. doi: 10.1161/01.RES.0000091335.07574.86. [DOI] [PubMed] [Google Scholar]