Abstract
A real-time reverse transcriptase-polymerase chain reaction (RT-PCR) method for detection of cytokeratin 20-positive cells in blood characterized by two novel features was developed and tested on 99 patients with colorectal cancer, 110 with breast cancer, and 150 healthy subjects. To optimize the specificity and sensitivity of the method, two novel features were used. First, a primer overlapping two adjacent exons was generated to inhibit nonspecific amplification both in healthy donors and cancer patients; second, a non-end-point first-round amplification was used to increase sensitivity. The number of first-round cycles was chosen to reach the highest level of sensitivity while conserving quantitative characteristics. PCR efficiency increased from 88.9% in single-round RT-PCR to 99.0% in nested real-time RT-PCR. To establish sensitivity and specificity of the method, HT29 cells were serially diluted with normal blood. Detection limit improved from 100 HT29 cells (single-round RT-PCR) to 1 to 10 cells (nested real-time RT-PCR) per 3 ml of whole blood. None of the healthy subjects was positive, whereas 22 and 29% of all colorectal and breast cancer patients, respectively, had cytokeratin 20 cell equivalents in blood. The association between cytokeratin 20 cell equivalents and metastasis was statistically significant for breast (P = 0.026) but not colorectal cancer patients (P = 0.361). Negativity of all 150 healthy controls examined confers diagnostic potential to the method.
Cytokeratin mRNAs are potential markers for detection of epithelial cells in blood. Several reports indicate that cytokeratin 20(CK20) mRNA in blood acts as a specific cancer cell marker in patients with frequent cancer forms of epithelial origin such as breast1,2,3,4,5 and colorectal cancer.6,7,8,9 Many breast cancer patients develop metastasis after locoregional and systemic treatment even in the absence of dissemination as assessed by conventional diagnostic tools. Approximately 30 to 50% of colorectal cancer patients who have undergone curative resection have recurrences with fatal outcome.10,11 Most recurrences occur in patients with TNM (tumor, nodes, and metastases) stage II and III cancers, but patients with stage I lesions also have appreciable risk. In these patients, cancer cells were disseminated either before or during surgery of the primary tumor.12,13,14 Although the relationship between circulating tumor cells and the development of recurrent cancer is not fully understood, it is generally assumed that enhanced dissemination of cancer cells in blood contributes significantly to the development of metastasis.15,16,17 The detection of circulating metastatic cells would be of great value for the assessment of the metastatic risk.18 Currently, the most powerful prognostic information in cancer patients is obtained from conventional histological assessment of regional lymph nodes.19,20,21,22,23 Because 20 to 30% of colorectal cancer patients without metastasis in lymph nodes die from distant metastases or local recurrence within 5 years24 and 15% of ”node-negative“ breast cancer patients will probably develop metastasis,25 there is a strong need for specific and sensitive methods capable of detecting circulating cancer cells. The assay of tumor-specific mRNA by quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) appears to be the most sensitive method to identify and detect minimal numbers of cancer cells in peripheral blood,6,26,27,28,29,30 but the interpretation of results is often difficult because of a variable number of positive normal subjects. The present available data do not allow determination of whether CK20-positive (CK20+) cells found in blood of normal subjects are due to CK20 expression of few normal epithelial cells released in the circulation or due to technical artifacts such as DNA co-amplification. Moreover, current methods are often not quantitative,1,31 have low sensitivity,6,31 possess high risk of cross-contamination, and have low specificity.2,7,32,33,34,35 Very few reports compared results obtained in different cancer types by the same methodology36 to ascertain whether differences were due to the cancer type or to the method used.
Aiming to increase sensitivity and abrogate false-positive results, we have developed a real-time RT-PCR method for detection of CK20 cell equivalents in blood provided with two novel features: a primer overlapping two adjacent exons to inhibit nonspecific amplifications and a non-end-point first-round amplification to increase sensitivity. The method was tested in healthy subjects and in breast and colorectal cancer patients at different stages of tumor development. None of the 150 healthy subjects tested positive, whereas 22% of the 99 patients with colorectal cancer and 29% of the 110 patients with breast cancer had CK20 cell equivalents in their blood. The association between CK20 cell equivalents and metastasis was statistically significant for breast but not colorectal cancer patients.
Materials and Methods
cDNA Preparation from Venous Blood and Tumor Cell Line HT29 and MCF-7
Venous blood samples were obtained from 99 patients with colorectal cancer and 110 patients with breast cancer at different stages of tumor development. No particular effort was made to stratify the participants into well-defined groups with respect to stage because this was not the aim of this study. All patients had given informed consent for the study. One hundred fifty (74 males and 76 females) healthy volunteers (from Associazione Volontari Italiani del Sangue Blood Bank, Torino, Italy) aged 30 to 60 years served as controls. All patients and controls were not affected by reactive/inflammatory condition. The first 5 ml of blood were discarded to avoid contamination with skin cells.37 The next 10 ml of blood were collected in vacutainer tubes with sodium citrate and used exclusively within 3 hours. Blood samples were diluted 1:3 in RPMI 1640 (Invitrogen, Carlsbad, CA), and mononuclear cells were separated using Ficoll density separation (Amersham Biosciences, Uppsala, Sweden). Tumor cell line HT29 (human colon adenocarcinoma) (Istituto Zooprofilattico B. Umbertini, Brescia, Italy) was maintained in continuous culture in McCoy’s media (Invitrogen) containing 10% fetal bovine serum and antibiotics at 37°C with 5% CO2. Tumor cell line MCF-7 (human breast adenocarcinoma) purchased from DSMZ (Braunschweig, Germany) was maintained in continuous culture in RPMI 1640 (Invitrogen) containing 10% fetal bovine serum and antibiotics at 37°C with 5% CO2. RNA was extracted from 1 × 106 HT29 or MCF-7 cells and 3 × 106 mononuclear cells from healthy donors and patients by phenol-chloroform precipitation and microparticle RNA capture (Nurex, Sassari, Italy). cDNA was prepared by adding 30 μl of extracted RNA to 48 μl of reaction mixture containing 300 ng of oligo-dT and 600 U of Moloney-murine leukemia virus reverse transcriptase (both Invitrogen).
Primers and Standard
Different primer pairs (Invitrogen) and one Taqman probe were used for CK20 PCR amplification (Table 1). The Taqman probe was labeled on the 5′ end with 6-carboxyfluorescein as the reporter and on the 3′ end with 6-carboxytetramethylrhodamine as the quenching dye. Oligonucleotide sequences were identified using Beacon Designer Software (PREMIER Biosoft International, Palo Alto, CA) and designed to differentiate between cDNA- and DNA-derived PCR products. Serial dilutions of cDNA from known numbers of HT29 cells were used to generate a standard curve.
Table 1.
Primer Pairs Amplicons Analyzed by Real-Time PCR for Detection of CK20 Gene (GenBank accession no. NM_019010)
| Sequence of selected primer pairs* | Length of amplicon (bp) | Predicted Tm of amplicon | Measured Tm of amplicon | ||
|---|---|---|---|---|---|
| Primer A | 5′-TCTTTGATGACCTAACCCTACA-3′ | Exon 3 | |||
| Primer B | 5′-ATTGACAGTGTTGCCCAGAT-3′ | Exon 4 | Primers A and B: 137 | 83.4 | 85.5 |
| Taqman probe | 5′-CAGGAGGAAGTCGATGGCCTACAC-3′ | Exon 3/4 | |||
| Primer C | 5′-CAGACACACGGTGAACTATGG-3′ | Exon 1 | |||
| Primer D | 5′-GATCAGCTTCCACTGTTAGACG-3′ | Exon 3 | Primers C and D: 371 | ||
| Primer E | 5′-GCAAATCAAGCAGTGGTACGAAAC-3′ | Exon 1 | Primers E and F: 110 | 85.7 | 87 |
| Primer F | 5′-GCAGTTGAGCATCCTTAATCTGAC-3′ | Exon 1/2 | Exon overlapping | ||
| Primer G | 5′-AATTTGCAGGACACACCGAGCA-3′ | Exon 2 | Primers E and G: 136 | 87 | 88 |
Primers A, C, and E are forward; primers B, D, F, and G are reverse.
Real-Time RT-PCR Using Taqman Probe or SYBR-Green and HT29 Cells
Real-time RT-PCR of CK20 mRNA was performed on the iCycler instrument (Bio-Rad, Hercules, CA). CK20-specific primers A and B spanning 137-bp sequence and the Taqman probe hybridizing to the target sequence were used. PCR amplification (50 cycles, 94°C, 30 seconds; 58°C, 30 seconds; and 72°C, 30 seconds) was performed in 50 μl of reaction mixture using serial dilutions of HT29 cells cDNA. Ten μL of template cDNA was added to the amplification mixture containing 200 nmol/L primer pair A and B, 2.5 U of Platinum TaqDNA Polymerase (Invitrogen), 3 mmol/L MgCl2, 200 μmol/L dNTP mixture (Applied Biosystems, Foster City, CA), and 200 nmol/L probe or 3.25 μl of SYBR-Green (Sigma-Aldrich, St. Louis, MO) diluted 1:10,000. DNA polymerase was pre-activated for 2 minutes at 94°C.
Single-Round Real-Time RT-PCR (S-PCR) Using SYBR-Green and HT29 Cells
S-PCR (50 cycles, 94°C, 30 seconds; 64°C, 30 seconds; and 72°C, 30 seconds) was performed in 50 μl of reaction mixture using serial dilutions of HT29 cells cDNA. Ten μL of template cDNA was added to the amplification mixture containing 200 nmol/L primer pair E and F, 2.5 U of Platinum TaqDNA Polymerase, 3 mmol/L MgCl2, 200 μmol/L dNTP mixture, and 3.25 μl of SYBR-Green diluted 1:10,000. DNA polymerase was pre-activated for 2 minutes at 94°C.
Nested Real-Time RT-PCR (N-PCR) Using SYBR-Green on HT29 Cells and Clinical Samples
N-PCR first-round amplification (20 or 35 cycles, 94°C, 30 seconds; 65°C, 30 seconds; and 72°C, 30 seconds) was performed in 100 μl of reaction mixture. Twenty microliters of cDNA obtained from mononuclear cells were added to the amplification mixture containing 100 nmol/L primer pair C and D,1 5 U of Platinum TaqDNA Polymerase, 3 mmol/L MgCl2, and 200 μmol/L dNTP mixture. One microliter of this mixture was re-amplified (35 cycles, 94°C, 30 seconds; 64°C, 30 seconds; and 72°C, 30 seconds) in 50 μl of reaction mixture using 200 nmol/L primer pair E and G or E and F, 2.5 U of Platinum TaqDNA Polymerase, 3 mmol/L MgCl2, 200 μmol/L dNTP mixture, and 3.25 μl of SYBR-Green diluted 1:10,000. DNA polymerase was pre-activated for 2 minutes at 94°C. A standard curve with four dilutions of HT29 cells cDNA was included in each respective PCR run to quantify the number of CK20 cell equivalents in clinical samples. All samples were analyzed in triplicate. To avoid contaminations, precautions included separate rooms and laboratory accessories for blood sampling, RNA isolation, PCR first round, and PCR second round.
Spiking Experiments and Cell Treatments
Varying numbers of HT29 cells (1 to 104 cells) or MCF-7 cells were added to 3 ml of blood and separated with mononuclear cells using Ficoll density separation. Alternatively, epithelial cells were separated from blood by Epithelial Enrich Ber-EP4-coated microsize immunobeads (Dynal Biotech, Success, NY) as described in the data sheet. RNA was purified as described above or in combination with a DNase treatment (Ambion, Austin, TX). RNA was also extracted and purified from cells after the removal of nuclei by treating cells with a 50 mmol/L Tris buffer, pH 8.0, containing 140 mmol/L NaCl, 1.5 mmol/L MgCl2, 1 mmol/L dithiothreitol, 0.5% Igepal CA-630 (Sigma-Aldrich), and Recombinant Ribonuclease Inhibitor (Invitrogen). Cells were incubated on ice for 5 minutes, lysates were centrifuged at 4°C for 2 to 3 minutes at 300 × g, and supernatants were collected for RNA extraction.
Sequence Analysis
Direct sequencing of PCR products from HT29 cells and CK20+ cells of colorectal cancer patient was performed on the ABI Prism 310 DNA sequencer (Applied Biosystems) using primers E and F (Table 1). Sequencing reactions were conducted with the big dye terminator sequencing ready reaction kit (Perkin-Elmer, Boston, MA).
Statistical Analysis
The association between metastasis and a detectable number of CK20 cell equivalents in blood samples was tested in 99 colorectal and 110 breast cancer patients. The presence of CK20 cell equivalents was analyzed as a dichotomous response variable (present/absent) in the two cancer groups, and the association with metastasis tested via the χ2 test statistic. The probability distributions of the number of CK20 cell equivalents in metastatic versus nonmetastatic patients were then compared through a nonparametric test (Wilcoxon-Mann-Whitney rank sum test) in both colorectal and breast cancer patients. Results were considered statistically significant for a = 0.05.
Results
Comparison between Taqman Probe and SYBR-Green Detection Methods Using HT29 Cells
To compare Taqman probe and SYBR-Green detection methods, cDNA from HT29 cells was used at different dilutions corresponding to a range between 1 and 105 cells. The detection limit of SYBR-Green detection method, expressed as minimal number of detectable cells, was at least 10-fold lower than that of the Taqman probe method (not shown).
Comparison between S-PCR and N-PCR Using HT29 Cells
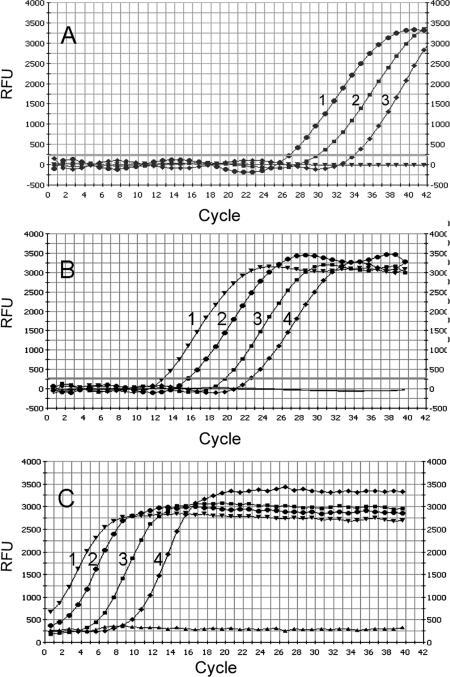
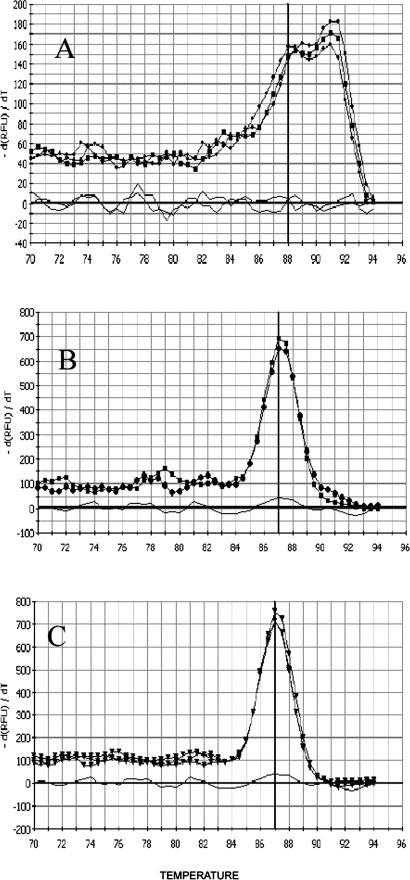
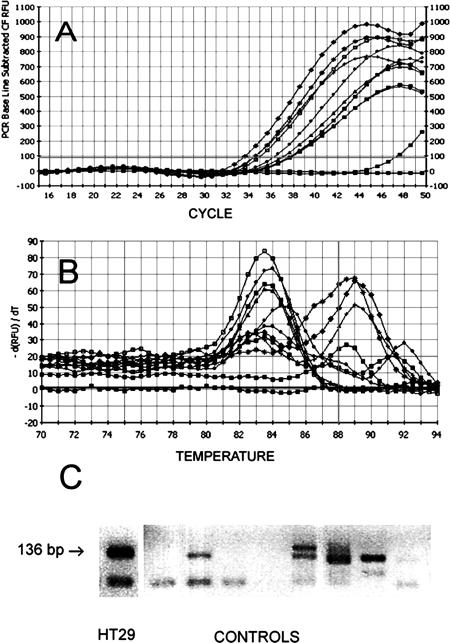
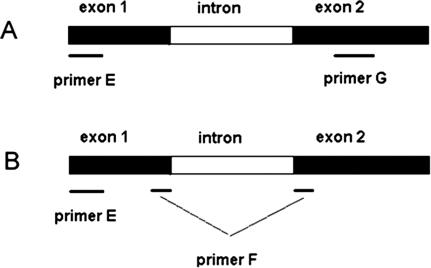
Because of its higher sensitivity, SYBR-Green was used to monitor real-time RT-PCR reactions instead of Taqman probe. Confirmation of results by melting curve analysis was an additional advantage conferred by SYBR-Green usage. S-PCR and N-PCR, with 20 cycles (20N-PCR) and 35 cycles (35N-PCR), were performed using serial dilutions of cDNA from HT29 cells. Detection limits of 20N-PCR and 35N-PCR were identical. The detection limits of 20N-PCR (Figure 1B) and 35N-PCR (Figure 1C) were at least 10-fold lower than the detection limit obtained by S-PCR (Figure 1A). Sensitivity data are shown in Table 2. 35N-PCR did not allow cell quantification because PCR curves were not comprised in the instrumental quantifiable range (Figure 1C). 20N-PCR showed 99.0 ± 3.8% efficiency (slope, −3.35 ± 0.09; intercept, 29.105 ± 0.97; correlation coefficient, 0.998 ± 0.0008) in eight independent test runs, whereas S-PCR efficiency was 88.8 ± 10% (slope, −3.65 ± 0.3; intercept, 39.22 ± 2.4; correlation coefficient, 0.994 ± 0.004) in six independent test runs. The specificity of the N-PCR method was tested on HT29 cells and on mononuclear cells obtained from healthy donors. Using HT29 cells, conventional intron-spanning primers (Table 1, primers E and G) often revealed two separate peaks by melting curve analysis, one corresponding to the expected melting temperature (Tm) and one corresponding to higher Tm (Figure 2A). This observation may indicate that the PCR reaction was influenced by the co-amplification of traces of residual DNA, as confirmed by experiments in which the reverse transcription step was performed in the absence of reverse transcriptase. Moreover, 20N-PCR analysis on lympho-monocytes isolated from healthy donors followed by 32 to 34 PCR cycles consistently revealed amplification signals (Figure 3A). In addition, melting curve analysis also revealed nonspecific amplifications (Figure 3B). The presence of nonspecific amplification products was also clearly documented by gel electrophoretic analysis (Figure 3C). To inhibit nonspecific amplifications, primer G was substituted by primer F designed to overlap exons 1 and 2 (Table 1; Figure 4) and containing only 5 bases on exon 1. Of note, when primer F contained more than 10 bases complementary to exon 1, some DNA amplification still occurred (not shown). Absence of DNA amplification using primer E in combination with primer F was confirmed by the absence of any amplification signal when the reverse transcription step was omitted (not shown).
Figure 1.
CK20 amplification plots of serially diluted HT29 cells. Serially diluted HT29 cells (1, 104 cells; 2, 103 cells; 3, 102 cells; 4, 101 cells) amplified by S-PCR (A), 20N-PCR (B), and 35N-PCR (C). Numbers of cycles were plotted against fluorescence expressed as relative fluorescence units.
Table 2.
Detection Limit* of S-PCR and N-PCR
| Sample | A. HT29 | B. HT29/blood | C. HT29/blood | D. HT29/blood | E. HT29/blood |
|---|---|---|---|---|---|
| Treatments | – | – | Immunocapture | DNase treatment | mRNA enrichment |
| S-PCR | 10 to 100 | 100 | 100 | 100 | 100 |
| 20N-PCR | 1 to 10 | 1 to 10 | 10 to 100 | 1 to 10 | 1 to 10 |
| 35N-PCR | 1 to 10 | 1 to 10 | 10 to 100 | 1 to 10 | 1 to 10 |
Detection limit is expressed as the minimum number of cells detected in triplicate experiments.
A, HT29 cells; B, D, and E, HT29 cells added to 3 ml of blood and isolated by Ficoll density separation; C, HT29 cells isolated by immunocapture; D, DNase treatment; and E, mRNA enrichment by nuclei removal.
Figure 2.
Melting peak analysis of 20N-PCR products. The melting peaks result from plotting the negative first derivative of measured fluorescence emission (y axis) at a given temperature (x axis). Curves were obtained with pure HT29 cells (A and B); with CK20+ clinical samples (C); with intron-spanning primers E and G (A); and with primer E and exon-overlapping primer F (B and C).
Figure 3.
Nonspecific amplifications by conventional intron spanning primers. Amplification plots obtained with cDNA of lympho-monocytes from healthy donors (A); melting curves obtained from the same PCR reactions (B); gel electrophoresis analysis (2% agarose gel stained with ethidium bromide) of amplified material from lympho-monocytes from healthy donors (controls) and HT29 cells (C).
Figure 4.
Schematic positions of the intron-spanning primers E and G (A) and exon-overlapping primer F (B) used for N-PCR.
In HT29 cells, a single peak, corresponding to the specific Tm, indicated absolute specificity of the combination between primers E and F (Figure 2B). Observed Tm was 87.3 ± 0.2°C in 14 independent test runs. Amplification specificity was further confirmed by sequencing the PCR products, which were found to be homologous with the CK20 sequence from the National Center for Biotechnology Information database. The sensitivity obtained with primers E and F was identical to the sensitivity obtained with primers E and G. The analytical reproducibility of 20N-PCR with primers E and F was tested with varying HT29 cell numbers, and PCR assays were performed on the same cDNA in six triplicate experiments: means, SD, and variation coefficient (CV) are shown in Table 3. Melting curve analysis was also tested in all CK20+ clinical samples (see below) and consistently showed one specific peak (Figure 2C).
Table 3.
Analytical Variance at Varying Number of HT29 Cells
| HT29 cells | Ct* | CV |
|---|---|---|
| 10,000 | 13.0 ± 0.29 | 0.022 |
| 1,000 | 16.2 ± 0.56 | 0.035 |
| 100 | 19.5 ± 0.37 | 0.019 |
| 10 | 22.1 ± 0.74 | 0.034 |
Threshold cycles (Ct) are expressed as means± SD.
20N-PCR assays performed on the same cDNA in six triplicate experiments.
Experiments Using HT29 or MCF-7 Cells Spiked in Blood
To mimic a diagnostic situation of metastatic cells in clinical samples, different dilutions of HT29 or MCF-7 cells were spiked in 3 ml of blood obtained from healthy controls. The detection limit of 20N-PCR ranged between 1 and 10 HT29 or MCF-7 cells, indicating no interference due to the addition of a large excess of blood (Table 2, columns A and B). It should be noted that immunocapture of epithelial cell (Table 2, column C) increased the detection limit (10 to 100 cells) in comparison with Ficoll separation (1 to 10 cells) (Table 2, column B), indicating a lower assay sensitivity of the first method. mRNA enrichment by nuclei removal and DNase treatment (Table 2, columns D and E) did not further increase the sensitivity of 20N-PCR. As expected, S-PCR was at least 10-fold less sensitive than 20N-PCR (Table 2, column B). Blood containing a low number of spiked HT29 cells was also used to assess the reproducibility of the RNA extraction method. RNA was extracted from blood containing 10 HT29 cells/3 ml in four independent experiments and HT29 cells quantified against a standard curve as described. Calculated interassay CV of the four experiments was 34.2%.
Analysis of Clinical Samples
The 20N-PCR using primers E and F was selected to analyze clinical samples because of its high sensitivity and specificity. Ninety-nine colorectal cancer patients, 110 breast cancer patients, and 150 healthy controls were assayed for circulating cells expressing CK20. Tumor staging was performed in all colorectal and breast cancer patients (Table 4). All analyzed controls showed completely negative amplification plots. Conversely, CK20+ samples were found in colorectal and breast cancer patients (Table 5). In duplicate experiments, all results were confirmed. Metastatic patients showed higher percentages of positive samples and higher numbers of circulating CK20 cell equivalents compared with nonmetastatic patients. It should be noted that the number of CK20 cell equivalents was quantified using a HT29 cells standard curve; differential CK20 expression could, therefore, interfere with the cell quantification. The association between presence of CK20 cell equivalents and metastasis was statistically significant for breast cancer patients (P = 0.026) but not for colorectal cancer patients (P = 0.361). Graphical inspection of the distributions of CK20 cell equivalents in blood samples of metastatic and nonmetastatic patients showed that data were not normally distributed, with a large number of zero counts in both cancer groups and a few large CK20 cell equivalents counts in metastatic patients. CK20 cell equivalents appeared higher in metastatic patients for both cancer types, although statistical significance was achieved only for breast cancer (P = 0.032) and not for colorectal cancer (P = 0.339). In all CK20+ samples, PCR amplification products displayed specific melting profiles, confirming the reliability of the method (Figure 2C). To confirm the above results, all samples were also assayed by a potentially more sensitive, although nonquantitative, analytical method (35N-PCR). All positive samples were confirmed, and no additional positive sample was detected by 35N-PCR, suggesting that 20N-PCR was already maximally sensitive. Negative controls containing all components except cDNA were run in parallel with each series of RT-PCR and did not show any detectable signals, indicating the absence of contamination during these studies.
Table 4.
Union Internationale Contre le Cancer Stage in CK20+ and CK20− Colorectal and Breast Cancer Patients
| I | II | IIIA | IIIB | IV | Total | ||
|---|---|---|---|---|---|---|---|
| Colorectal cancer | |||||||
| CK20+ | 8 (36.4%) | 0 | 0 | 1 (4.5%) | 13 (59%) | 22 (100%) | |
| CK20− | 18 (23.4%) | 3 (3.9%) | 12 (15.6%) | 7 (9%) | 37 (48%) | 77 (100%) | |
| Breast cancer | IIA | IIB | |||||
| CK20+ | 6 (18.7%) | 10 (31.2%) | 5 (15.6%) | 3 (9.4%) | 0 | 8 (25%) | 32 (100%) |
| CK20− | 40 (51.2%) | 14 (18%) | 11 (14.1%) | 5 (6.4%) | 1 (1.3%) | 7 (9%) | 78 (100%) |
Table 5.
Case and Control Sample Analysis
| Controls (150)
|
Breast cancer (110)
|
Colorectal cancer (99)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | N-M | M | N-M | |||||||
| 15 | 95 | 50 | 49 | |||||||
| CK20+ patients | yes | no | yes | no | yes | no | yes | no | yes | No |
| 0 | 150 | 8 | 7 | 24 | 71 | 13 | 37 | 9 | 40 | |
| Mean no. of CK20 cell equivalents
|
0
|
5.5
|
0.9
|
29.2
|
4.4
|
|||||
| Median no. of CK20 cell equivalents | 0 | 0.1 | 0 | 0 | 0 | |||||
Distribution of subjects by health status (control, breast cancer, colorectal cancer), presence (M) or absence (N-M) of metastases, and presence/absence of CK20 cell-equivalents (yes/no). Mean and median number of CK20 cell equivalents detected in 3 ml of blood.
Discussion
Despite great diagnostic and prognostic interest, the specific presence of metastatic cells in blood of cancer patients is still under debate. CK20 is considered a marker of neoplastic epithelial cells, but circulating CK20+ cells were described to be present or absent in the blood of both cancer patients and healthy subjects (7,32,33,34,35,38). These apparently conflicting results were not surprising because we (G. Giribaldi, unpublished data) and others39 frequently obtained false-positive results by using conventional nested RT-PCR methods. Here, the detection limit was improved by a first-round amplification of cDNA by 20 PCR cycles before real-time PCR analysis. First-round PCR amplification conditions were chosen to allow accurate real-time PCR quantification as indicated by the increase in PCR efficiency from 88.9% in S-PCR to 99.0% in 20N-PCR. Under those conditions, the Ct shift of approximately five cycles indicates a theoretical 32-fold increase in sensitivity in comparison with S-PCR. An additional 10-fold decrease of the detection limit was obtained using an intercalating fluorescent dye instead of an oligonucleotide fluorescent probe. The substantial increase in sensitivity made it mandatory to increase specificity to avoid the interference by any nonspecific amplification. Specificity was greatly enhanced using a primer overlapping two adjacent exons that could not anneal to any genomic DNA sequence because its complementary target could be generated only after mRNA splicing. Such novel primer design completely abrogated nonspecific amplifications both in positive samples and negative controls. The present method eliminated the DNase treatment of extracted RNA and allowed the immediate reverse transcription of mRNA into the more stable cDNA. Accuracy was not influenced by dilution of HT29 cells in a large excess of blood, although the detection limit of conventional PCR assays was reportedly influenced by the dilution of target mRNA in blood.6,31,40 Detection limit was not improved by immunocapture of epithelial cells41,42,43 or by increasing first-round cycles,44,45 suggesting that the sensitivity of the method to detect few RNA copies was already maximal. It should be noted that real-time RT-PCR was performed in a closed system that allowed easier control of DNA contamination compared with conventional PCR. In fact, blanks were always negative in all experiments, indicating lack of cross-contamination by amplified DNA. Despite improved sensitivity, all control samples tested negative for CK20+ cells. This result appears to indicate that the CK20 positivity reported in healthy subjects by others7,32,33,34,35 could more plausibly be of artifactual origin. Twenty-two percent and 29% of all colorectal and breast cancer patients, respectively, had CK20 cell equivalents in their blood. In breast cancer patients, the association between metastasis and CK20 cell equivalents in blood was statistically significant. Our results appear to be in accordance with results from others showing a correlation between the number of circulating cancer cells and breast cancer progression.46 A similar association, albeit not statistically significant, was observed in colorectal cancer patients. Results were highly reproducible and constantly confirmed in duplicate experiments. The range of variation of CK20 cell equivalent counts was different in the two case series. CK20 cell equivalents were found per 3 ml of blood as follows: 0 to 40 in nonmetastatic and 0 to 45 in metastatic breast cancer patients, 0 to 147 in nonmetastatic and 0 to 650 in metastatic colorectal cancer patients. We have no explanation for these differences. Possibly, loss of cytokeratin expression may justify apparent loss of sensitivity in less differentiated tumors47 in the most advanced cases. Varying CK20 expression in different cancer cells may lead to wrong numbers of CK20 cell equivalents, which is relative to their CK20 mRNA content.
In conclusion, we optimized a real-time RT-PCR method, increasing its sensitivity and specificity in comparison with previous PCR methods. No CK20 cell equivalents were present in the blood of healthy donors, whereas a variable number of CK20 cell equivalents was detected in the blood of breast and colorectal cancer patients. Because of the absence of false positives, appearance of CK20 cell equivalents in blood is a strong indication of colorectal or breast cancer. The appearance of CK20 cell equivalents in blood of patients affected by other tumors remains to be investigated. The prognostic relevance of present data are currently under investigation by a long-term follow-up study of all two-case series patients. We hope that the long-term follow-up of the patients affected by initial tumor stages will give information on the predictive value of circulating CK20 cell equivalents in the disease progression. Finally, it should be noted that the present method characterized by improved specificity and low detection limits could be easily adapted for detection of minimal residual disease in leukemia and lymphoma as well as for detection of any type of circulating tumor cells where reliable mRNA markers are available.
Acknowledgments
We thank the doctors and staff of Associazione Volontari Italiani del Sangue Blood Bank (Torino, Italy) for providing human blood from the healthy controls.
Footnotes
Supported by the Oncology Special Project, Compagnia di San Paolo/FIRMS, by the Italian Association for Cancer Research, and by the Ricerca sanitaria finalizzata, Regione Piemonte (Italy).
G.G. and S.P. contributed equally to this work.
References
- Bostick PJ, Chatterjee S, Chi DD, Huynh KT, Giuliano AE, Cote R, Hoon DS. Limitations of specific reverse-transcriptase polymerase chain reaction markers in the detection of metastases in the lymph nodes and blood of breast cancer patients. J Clin Oncol. 1998;16:2632–2640. doi: 10.1200/JCO.1998.16.8.2632. [DOI] [PubMed] [Google Scholar]
- Champelovier P, Mongelard F, Seigneurin D. CK20 gene expression: technical limits for the detection of circulating tumor cells. Anticancer Res. 1999;19:2073–2078. [PubMed] [Google Scholar]
- Hu XC, Chow LW. Fine needle aspiration may shed breast cells into peripheral blood as determined by RT-PCR. Oncology. 2000;59:217–222. doi: 10.1159/000012164. [DOI] [PubMed] [Google Scholar]
- Bae JW, Choi HK, Kim HG, Park SH. The detection of circulating breast cancer cells in peripheral blood by reverse transcriptase-polymerase chain reaction. J Korean Med Sci. 2000;15:190–198. doi: 10.3346/jkms.2000.15.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong R, Beyser K, Basten O, Kalbe A, Rueschoff J, Tabiti K. Quantitative reverse transcription-polymerase chain reaction detection of cytokeratin 20 in noncolorectal lymph nodes. Clin Cancer Res. 2000;7:3423–3429. [PubMed] [Google Scholar]
- Schuster R, Max N, Mann B, Heufelder K, Thilo F, Grone J, Rokos F, Buhr HJ, Thiel E, Keilholz U. Quantitative real-time RT-PCR for detection of disseminated tumor cells in peripheral blood of patients with colorectal cancer using different mRNA markers. Int J Cancer. 2004;108:219–227. doi: 10.1002/ijc.11547. [DOI] [PubMed] [Google Scholar]
- Wyld DK, Selby P, Perren TJ, Jonas SK, Allen-Mersh TG, Wheeldon J, Burchill SA. Detection of colorectal cancer cells in peripheral blood by reverse-transcriptase polymerase chain reaction for cytokeratin 20. Int J Cancer. 1998;79:288–293. doi: 10.1002/(sici)1097-0215(19980619)79:3<288::aid-ijc14>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Moll R, Lowe A, Laufer J, Franke WW. Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol. 1992;140:427–447. [PMC free article] [PubMed] [Google Scholar]
- Burchill SA, Bradbury MF, Pittman K, Southgate J, Smith B, Selby P. Detection of epithelial cancer cells in peripheral blood by reverse transcriptase-polymerase chain reaction. Br J Cancer. 1995;71:278–281. doi: 10.1038/bjc.1995.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- August DA, Ottow RT, Sugarbaker PH. Clinical perspective of human colorectal cancer metastasis. Cancer Metastasis Rev. 1984;3:303–302. doi: 10.1007/BF00051457. [DOI] [PubMed] [Google Scholar]
- Hughes KS, Simon R, Songhorabodi S, Adson MA, Ilstrup DM, Fortner JG, Maclean BJ, Foster JH, Daly JM, Fitzherbert D. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery. 1996;100:278–284. [PubMed] [Google Scholar]
- Kienle P, Koch M, Autschbach F, Benner A, Treiber M, Wannenmacher M, von Knebel Doeberitz M, Buchler M, Herfarth C, Weitz J. Decreased detection rate of disseminated tumor cells of rectal cancer patients after preoperative chemoradiation: a first step towards a molecular surrogate marker for neoadjuvant treatment in colorectal cancer. Ann Surg. 2003;238:324–330. doi: 10.1097/01.sla.0000086547.27615.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitz J, Koch M, Kienle P, Schrodel A, Willeke F, Benner A, Lehnert T, Herfarth C, von Knebel Doeberitz M. Detection of hematogenic tumor cell dissemination in patients undergoing resection of liver metastases of colorectal cancer. Ann Surg. 2000;232:66–72. doi: 10.1097/00000658-200007000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitz J, Kienle P, Lacroix J, Willeke F, Benner A, Lehnert T, Herfarth C, von Knebel Doeberitz M. Dissemination of tumor cells in patients undergoing surgery for colorectal cancer. Clin Cancer Res. 1998;4:343–348. [PubMed] [Google Scholar]
- Guller U, Zajac P, Schnider A, Bosch B, Vorburger S, Zuber M, Spagnoli GC, Oertli D, Maurer R, Metzger U, Harder F, Heberer M, Marti WR. Disseminated single tumor cells as detected by real-time quantitative polymerase chain reaction represent a prognostic factor in patients undergoing surgery for colorectal cancer. Ann Surg. 2002;236:768–776. doi: 10.1097/00000658-200212000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham JE, Hewett PJ, Sage RE, Finch JL, Nuttall JD, Kotasek D, Dobrovic A. Molecular detection of blood-borne epithelial cells in colorectal cancer patients and in patients with benign bowel disease. Int J Cancer. 2000;89:8–13. [PubMed] [Google Scholar]
- Stathopoulou A, Vlachonikolis I, Mavroudis D, Perraki M, Kouroussis Ch, Apostolaki S, Malamos N, Kakolyris S, Kotsakis A, Xenidis N, Reppa D, Georgoulias V. Molecular detection of cytokeratin-19-positive cells in the peripheral blood of patients with operable breast cancer: evaluation of their prognostic significance. J Clin Oncol. 2002;20:3404–3412. doi: 10.1200/JCO.2002.08.135. [DOI] [PubMed] [Google Scholar]
- Liefers GJ, Cleton-Jansen AM, van de Velde CJ, Hermans J, van Krieken JH, Cornelisse CJ, Tollenaar RA. Micrometastases and survival in stage II colorectal cancer. N Engl J Med. 1998;339:372–379. doi: 10.1056/NEJM199807233390403. [DOI] [PubMed] [Google Scholar]
- Stosiek P, Kasper M, Karsten U, Goertchen R. Detection of cancer metastases in regional lymph nodes: comparative histological and immunohistological investigations with the broad-range anticytokeratin monoclonal antibody A45-B/B3. Neoplasma. 1991;38:43–47. [PubMed] [Google Scholar]
- Bosman FT. Molecular pathology of colorectal cancer. Cytogenet Cell Genet. 1999;86:112–117. doi: 10.1159/000015362. [DOI] [PubMed] [Google Scholar]
- Lassmann S, Bauer M, Rosenberg R, Nekarda H, Soong R, Ruger R, Hofler H, Werner M. Identification of occult tumor cells in node negative lymph nodes of colorectal cancer patients by cytokeratin 20 gene and protein expression. Int J Colorectal Dis. 2004;19:87–94. doi: 10.1007/s00384-003-0530-z. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Siddiqi S, Ahmed S, Hands R, Dorudi S. Quantification of cytokeratin 20, carcinoembryonic antigen and guanylyl cyclase C mRNA levels in lymph nodes may not predict treatment failure in colorectal cancer patients. Int J Cancer. 2004;108:412–417. doi: 10.1002/ijc.11596. [DOI] [PubMed] [Google Scholar]
- Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER, Cruz AB, Foster R, Gardner B, Lerner H, Margolese R. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer: an NSABP update. Cancer. 1983;52:1551–1557. doi: 10.1002/1097-0142(19831101)52:9<1551::aid-cncr2820520902>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Goldberg RM, Fleming TR, Tangen CM, Moertel CG, Macdonald JS, Haller DG, Laurie JA. Surgery for recurrent colon cancer: strategies for identifying resectable recurrence and success rates after resection: Eastern Cooperative Oncology Group, the North Central Cancer Treatment Group, and the Southwest Oncology Group. Ann Intern Med. 1998;129:27–35. doi: 10.7326/0003-4819-129-1-199807010-00007. [DOI] [PubMed] [Google Scholar]
- Gilbey AM, Burnett D, Coleman RE, Holen I. The detection of circulating breast cancer cells in blood [Review]. J Clin Pathol. 2004;57:903–911. doi: 10.1136/jcp.2003.013755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Ichikawa Y, Tanaka K, Kamiyama M, Hamaguchi Y, Ishikawa T, Yamaguchi S, Togo S, Ike H, Ooki S, Shimada H. Real-time PCR (TaqMan PCR) quantification of carcinoembryonic antigen (CEA) mRNA in the peripheral blood of colorectal cancer patients. Anticancer Res. 2003;23:1271–1276. [PubMed] [Google Scholar]
- Hampton R, Walker M, Marshall J, Juhl H. Differential expression of carcinoembryonic antigen (CEA) splice variants in whole blood of colon cancer patients and healthy volunteers: implication for the detection of circulating colon cancer cells. Oncogene. 2002;21:7817–7823. doi: 10.1038/sj.onc.1205906. [DOI] [PubMed] [Google Scholar]
- Weigelt B, Bosma AJ, Hart AA, Rodenhuis S, van ’t Veer LJ. Marker genes for circulating tumour cells predict survival in metastasized breast cancer patients. Br J Cancer. 2003;88:1091–1094. doi: 10.1038/sj.bjc.6600868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aerts J, Wynendaele W, Paridaens R, Christiaens MR, van den Bogaert W, van Oosterom AT, Vandekerckhove F. A real-time quantitative reverse transcriptase polymerase chain reaction (RT-PCR) to detect breast carcinoma cells in peripheral blood. Ann Oncol. 2001;12:39–46. doi: 10.1023/a:1008317512253. [DOI] [PubMed] [Google Scholar]
- Stathopoulou A, Gizi A, Perraki M, Apostolaki S, Malamos N, Mavroudis D, Georgoulias V, Lianidou ES. Real-time quantification of CK-19 mRNA-positive cells in peripheral blood of breast cancer patients using the lightcycler system. Clin Cancer Res. 2003;9:5145–5151. [PubMed] [Google Scholar]
- Ko Y, Klinz M, Totzke G, Gouni-Berthold I, Sachinidis A, Vetter H. Limitations of the reverse transcription-polymerase chain reaction method for the detection of carcinoembryonic antigen-positive tumor cells in peripheral blood. Clin Cancer Res. 1998;4:2141–2146. [PubMed] [Google Scholar]
- Dimmler A, Gerhards R, Betz C, Gunther K, Reingruber B, Horbach T, Baumann I, Kirchner T, Hohenberger W, Papadopoulos T. Transcription of cytokeratins 8, 18, and 19 in bone marrow and limited expression of cytokeratins 7 and 20 by carcinoma cells: inherent limitations for RT-PCR in the detection of isolated tumor cells. Lab Invest. 2001;81:1351–1361. doi: 10.1038/labinvest.3780349. [DOI] [PubMed] [Google Scholar]
- Vlems FA, Diepstra JH, Cornelissen IM, Ruers TJ, Ligtenberg MJ, Punt CJ, van Krieken JH, Wobbes T, van Muijen GN. Limitations of cytokeratin 20 RT-PCR to detect disseminated tumour cells in blood and bone marrow of patients with colorectal cancer: expression in controls and downregulation in tumour tissue. J Clin Pathol Mol Pathol. 2002;55:156–163. doi: 10.1136/mp.55.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zippelius A, Pantel K. RT-PCR-based detection of occult disseminated tumor cells in peripheral blood and bone marrow of patients with solid tumors: an overview [Review]. Ann NY Acad Sci. 2000;906:110–123. doi: 10.1111/j.1749-6632.2000.tb06600.x. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Gyselman VG, Siddiqi S, Dorudi S. Cytokeratin 20 is not a tissue-specific marker for the detection of malignant epithelial cells in the blood of colorectal cancer patients. Int J Surg Investig. 2000;2:49–57. [PubMed] [Google Scholar]
- Chausovsky G, Luchansky M, Figer A, Shapira J, Gottfried M, Novis B, Bogelman G, Zemer R, Zimlichman, Klein A. Expression of cytokeratin 20 in the blood of patients with disseminated carcinoma of the pancreas, colon, stomach, and lung. Cancer. 1999;86:2398–2405. [PubMed] [Google Scholar]
- Vlems F, Soong R, Diepstra H, Punt C, Wobbes T, Tabiti K, van Muijen G. Effect of blood sample handling and reverse transcriptase-polymerase chain reaction assay sensitivity on detection of CK20 expression in healthy donor blood. Diagn Mol Pathol. 2002;11:90–97. doi: 10.1097/00019606-200206000-00005. [DOI] [PubMed] [Google Scholar]
- Ikeguchi M, Ohro S, Maeda Y, Fukuda K, Yamaguchi K, Shirai H, Kondo A, Tsujitani S, Kaibara N. Detection of cancer cells in the peripheral blood of gastric cancer patients. Int J Mol Med. 2003;11:217–221. [PubMed] [Google Scholar]
- Zippelius A, Lutterbuse R, Riethmuller G, Pantel K. Analytical variables of reverse transcription-polymerase chain reaction-based detection of disseminated prostate cancer cells. Clin Cancer Res. 2000;6:2741–2750. [PubMed] [Google Scholar]
- Max N, Willhauck M, Wolf K, Thilo F, Reinhold U, Pawlita M, Thiel E, Keilholz U. Reliability of PCR-based detection of occult tumour cells: lessons from real-time RT-PCR. Melanoma Res. 2001;11:371–378. doi: 10.1097/00008390-200108000-00007. [DOI] [PubMed] [Google Scholar]
- de Cremoux P, Extra JM, Denis MG, Pierga JY, Bourstyn E, Nos C, Clough KB, Boudou E, Martin EC, Muller A, Pouillart P, Magdelenat H. Detection of MUC1-expressing mammary carcinoma cells in the peripheral blood of breast cancer patients by real-time polymerase chain reaction. Clin Cancer Res. 2000;6:3117–3122. [PubMed] [Google Scholar]
- Park S, Lee B, Kim I, Choi I, Hong K, Ryu Y, Rhim J, Shin J, Park SC, Chung H, Chung J. Immunobead RT-PCR versus regular RT-PCR amplification of CEA mRNA in peripheral blood. J Cancer Res Clin Oncol. 2001;127:489–494. doi: 10.1007/s004320100239. [DOI] [PubMed] [Google Scholar]
- Ladanyi A, Soong R, Tabiti K, Molnar B, Tulassay Z. Quantitative reverse transcription-PCR comparison of tumor cell enrichment methods. Clin Chem. 2001;47:1860–1863. [PubMed] [Google Scholar]
- Max N, Wolf K, Thiel E, Keilholz U. Quantitative nested real-time RT-PCR specific for tyrosinase transcripts to quantitate minimal residual disease. Clin Chim Acta. 2002;317:39–46. doi: 10.1016/s0009-8981(01)00737-9. [DOI] [PubMed] [Google Scholar]
- Schamhart D, Swinnen J, Kurth KH, Westerhof A, Kusters R, Borchers H, Sternberg C. Numeric definition of the clinical performance of the nested reverse transcription-PCR for detection of hematogenous epithelial cells and correction for specific mRNA of non-target cell origin as evaluated for prostate cancer cells. Clin Chem. 2003;49:1458–1466. doi: 10.1373/49.9.1458. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- Franzen B, Linder S, Alaiya AA, Eriksson E, Uruy K, Hirano T, Okuzawa K, Auer G. Analysis of polypeptide expression in benign and malignant human breast lesions: down-regulation of cytokeratins. Br J Cancer. 1996;74:1632–1638. doi: 10.1038/bjc.1996.600. [DOI] [PMC free article] [PubMed] [Google Scholar]