Abstract
Laser microdissection allows isolation of tiny samples from tissue sections for analysis of gene expression by real-time quantitative polymerase chain reaction (PCR). Although immunohistochemical labeling is often required to identify target structures, it drastically degrades mRNA so that shortened protocols are needed. Here, we present a novel method that allows fluorescence double labeling to be performed in only one incubation of 5 minutes. Fab fragments directly coupled to fluorochromes are linked to primary antibodies before these complexes are applied to sections. We quantified the influences of fixatives, labeling solutions, and incubation time on the mRNA yield and compared our method with previously proposed protocols. While tissue components, ie, vimentin and Ki67 antigen, were sufficiently stained after only 5 minutes of incubation, the new method produced a minute loss of mRNA that did not significantly differ from that of untreated sections. In contrast, incubation times of 15 and 30 minutes reduced the mRNA yield by 99.8 to 99.9%. Furthermore, incubation periods longer than 5 minutes critically affected the ratio between the target and housekeeping genes tested by factors of up to 10.6. In conclusion, the novel method described here reduces mRNA loss and potential ratio shifts to a level that does not significantly differ from that of unlabeled samples.
Molecular interactions that are restricted to specific microcompartments are of increasing significance for the understanding of biological processes, eg, the initiation of immune responses. In the past few years, novel techniques have been developed in the field of molecular pathology that can be applied to minute amounts of a substance, eg, some dozens of identical mRNA molecules, so that these are identified specifically and analyzed quantitatively (for review, see Refs.1,2). Because such extremely sensitive detection methods allow tiny tissue samples to be analyzed,3,4 techniques are needed for the effective isolation of relevant tissue microcompartments or even single cells. Laser microdissection uses conventional tissue sections on membrane-covered glass slides, optical microscopy, and a UV laser beam to separate samples that, in most cases, are by far smaller than one cubic millimeter.5,6 Cell groups and regions of interest are identified according to morphological or histochemical peculiarities. This can be achieved by classical staining, eg, using toluidine blue or hematoxylin and eosin, as it is often done in diagnostic histopathology.5,7,8,9 In many cases, however, immunohistochemical labeling is required because numerous cell types and tissue components cannot readily be identified using morphology alone.10,11 Such situations comprise lymphocyte subsets and functional compartments in an ongoing inflammation in immunological research, potential tumor cells in pathology, neuronal and glial cell types in neurobiology, and numerous other fields.
Conventional protocols for immunohistochemical labeling normally comprise two or three incubations and about the same number of rinsing steps, generally lasting for several hours.12,13 It is well known that such long incubation times dramatically deteriorate the nucleic acids to be detected later because of diffusion and enzymatic degradation, especially when mRNA is analyzed.10,14,15 This reduces the potential sensitivity by several orders of magnitude.16,17 In addition, it is normally required to determine ratios between target and housekeeping genes, because potential influences such as sample volume, efficiency of reverse transcription, and variances of the polymerase chain reaction (PCR) considerably affect the amount of cDNA copies detected.2,4,16 However, it is still unclear whether such ratios are stable or to what extent they depend on the tissue processing.
To minimize mRNA loss and potential ratio shifts, the number of incubation steps and the incubation time per step should be reduced. Compared with conventional bright field microscopy, fluorescence techniques do not require separate steps for the generation of visible dyes and can easily be combined with laser microdissection systems. In conventional immunohistochemistry, at least two separate steps for the primary and the secondary antibody are required. Because complete antibody molecules possess two antigen binding sites, mixtures of primary and secondary antibodies would agglutinate before binding to tissue epitopes. An elegant way to circumvent this has recently been published18,19 using monovalent Fab antibody fragments instead of complete secondary antibodies. Fab fragments coupled to fluorescent dyes are pre-incubated with primary antibodies before the resulting complexes are applied to tissue sections in a single incubation.
In the present study, we established a new ultra-short Fab fragment method for use in laser microdissection and determined the minimum incubation time necessary to sufficiently identify target structures in tissue sections after single and double labeling. Using real-time reverse transcriptase-PCR (RT-PCR), we quantified the effects of fixatives, labeling solutions and incubation time on mRNA yield and on the mRNA ratios of a target gene (vimentin) and a housekeeping gene (EF-1a).
Materials and Methods
Tissue Processing
Tissue samples of the appendix of male adult rabbits (2.5 to 3 kg; Charles River, Sulzfeld, Germany) were frozen in liquid nitrogen immediately after removal and stored at −80°C. Serial sections (10 μm in thickness) were mounted on glass slides covered with a membrane of polyethylene naphthalate 1.35 μm in thickness (PALM Microlaser Technologies, Bernried, Germany) and stored at −80°C.
Fixatives
Tissues sections were fixed in three different solutions previously described by other groups20,21,22 to identify the optimal fixation for immunolabeling and laser microdissection: 95% ethanol, a 1:1 mixture of methanol:acetone, and Carnoy’s medium (60% ethanol, 30% chloroform, and 10% acetic acid). Sections were fixed in one of these solutions at −20°C for 2 minutes before labeling.
Staining
Sections were stained in a solution of toluidine blue O (0.1% in water that was treated with diethyl pyrocarbonate [DEPC]; Sigma-Aldrich, Munich, Germany) for survey microscopy according to the following protocol: 75% ethanol for 1 minute, DEPC-treated water for 1 minute, toluidine blue solution for 5 minutes, DEPC-treated water for 15 seconds, and ethanol two times for 30 seconds. Stained slides were air-dried at room temperature and stored at −80°C. All solutions used for staining, rinsing, and immunolabeling were prepared using DEPC-treated water.
Immunolabeling
The optimized basic protocol for all immunolabelings started with fixation in 95% ethanol at −20°C for 2 minutes followed by seven dippings (about 10 seconds in total) in phosphate-buffered saline (PBS). Immunolabelings were performed as one-step methods at room temperature using either a dye-coupled antibody (direct technique) or primary antibodies conjugated to dye-coupled Fab antibody fragments (Fab fragment technique).
For the direct technique, an anti-vimentin antibody (clone V9, dilution 1:200; Sigma-Aldrich) chemically conjugated to the red fluorescent dye Cy3 was applied for 5, 10, 15, 30, or 60 minutes. For the Fab fragment technique, 2 μl of unconjugated anti-vimentin antibody (clone V9) was incubated with 15 μl of anti-mouse Fab fragments coupled with the red fluorescent dye Alexa-Fluor 555 for 10 minutes (Zenon antibody labeling kit; Molecular Probes, Leiden, The Netherlands). To block excessive anti-mouse Fab fragments, 15 μl of mouse immunoglobulin (blocking reagent of Zenon antibody labeling kit) was added and incubated for 10 minutes. A defined volume of PBS was then added to achieve the final dilution (1:200) of the antibody-Fab-dye complexes. Ethanol-fixed sections on membrane-covered slides were incubated with this solution for 5 minutes at room temperature, dipped twice in PBS, air-dried at room temperature, and immediately used for sample acquisition.
One-step double labelings were performed using an anti-vimentin mouse monoclonal antibody (clone V9) and an anti-Ki67 mouse monoclonal antibody (clone Mib-5; Dako, Hamburg, Germany). Using the Fab fragment technique, the vimentin antibody was coupled to Alexa Fluor 488 (final dilution in the mixture 1:100) and the Ki67 antibody to Alexa Fluor 555 (final dilution in the mixture 1:8). To achieve such low dilutions, both the Fab solution and the blocking reagent were concentrated in a vacuum centrifuge to 30% of their previous volume before they were mixed with the monoclonal antibodies. The two antibody-Fab-dye complexes were then mixed, some PBS was added to achieve the final dilutions, and the sections were incubated with this solution for 5 minutes. To estimate the influence of the incubation time on mRNA ratios, additional sections were incubated for 15, 30, or 60 minutes. Labeled slides were air-dried at room temperature and used immediately for microscopy and sample acquisition.
Microscopy and Sample Acquisition
Labeled sections were examined in a microdissection system equipped with fluorescence filter sets for UV, blue, and green excitation and a pulsed 337-nm UV laser (PALM MicroBeam; PALM Microlaser Technologies). Using the laser microdissection and laser-pressure catapulting modes, small areas of the sections (5,000 to 500,000 μm2) were excised and analyzed by real-time PCR (Figure 1). To avoid possible detrimental influences of the laser light and potential loss of tissue fragments during laser-pressure catapulting, one tissue section from each of the differently treated slides was excised using a sterile scalpel and directly transferred to digestion buffer RLT (RNeasy Minikit; Qiagen, Hilden, Germany). Quantitative measurements presented are thus based on tissue that was not exposed to UV light. Mean threshold cycle (Ct) values are based on six independently processed samples per group and displayed as means ± SD.
Figure 1.
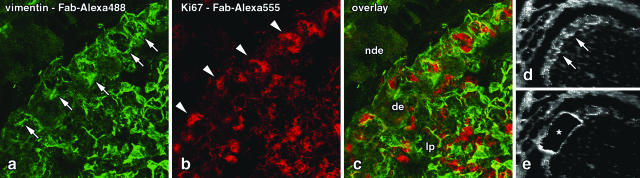
a–c: Fluorescence double labeling of vimentin (green) and the proliferation marker Ki67 (red) in rabbit appendix using the Fab fragment technique. The section was incubated for 5 minutes with a mixture of an anti-vimentin antibody coupled to Alexa Fluor 488-labeled anti-mouse Fab fragments and an anti-Ki67 antibody coupled to Alexa Fluor 555-labeled anti-mouse Fab fragments. a and b show single channel fluorescences; the overlay of the two channels is depicted in c. The presence of vimentin is used to identify the membranous (M) cells (arrows) in the dome epithelium (de). In addition, fibroblasts and some other cells of the lamina propria (lp) contain vimentin and are thus labeled, whereas the opposed non-dome epithelium (nde) is devoid of vimentin-containing cells. The nuclei of proliferating cells (red, arrowheads), most of which are lymphocytes, are located in the cytoplasmic pockets of the M cells and in the lamina propria. Imaging was done in a confocal laser scanning microscope to depict the full quality of the labeling independent of optical limitations. d and e: Fluorescence view of rabbit appendix before (d) and after (e) laser microdissection of an epithelial region (asterisk) containing M cells. The section was labeled for vimentin in a single incubation of only 5 minutes. The quality of the micrographs is limited because they were taken in a low quality video camera of the microdissection system, and because optical imaging was done through the supporting membrane, which scatters light and produces autofluorescence. The latter is additionally induced by the laser beam at the margins of the microdissected area. a–c, 250:1; d and e, 70:1.
RNA Extraction and Reverse Transcription
Total RNA was extracted using the RNeasy Minikit (Qiagen) following the protocols of the supplier. The extracted RNA was concentrated to a final volume of approximately 8 μl using a vacuum centrifuge and digested using DNase 1 (Sigma-Aldrich). Reverse transcription was performed using Superscript II reverse transcriptase (Invitrogen, Karlsruhe, Germany) and random hexamers according to the manufacturer’s manual. In addition to real-time quantitative PCR (described below), the RNA extracted from immunolabeled and microdissected samples was analyzed using gel electrophoresis. This second, independent technique showed sharply bounded bands for rRNA, indicating that the contained RNA was of high quality.
Real-Time Quantitative PCR
Taq-Man assays for vimentin and the housekeeping gene EF-1a23,24 were designed using Primer Express software (Applied Biosystems, Foster City, CA) and synthesized by IBA (probes; Göttingen, Germany) and MWG Biotech AG (primers; Ebersberg, Germany). Sequences for primers and probes were from 3′ to 5′ as follows: vimentin forward, GCACGATGAGGAAATCCAGGA; vimentin reverse, AGGTCAGGCTTGGAAACATCC; vimentin probe, FAM-TGCAGGCCCAGATCCAGGAACAGC-TAMRA; EF-1a forward, TGTTGAGAGCTTCTCTGACTATCC; EF-1a reverse, CTCCAGCAGCCTTCTTGTCC; and EF-1a probe, FAM-CCTCTGGGTCGTTTCGCTGTCCGT-TAMRA.
Amplicon sizes were 110 bp for EF-1a and 84 bp for vimentin; sequences were taken from GenBank (accession numbers AY465353 for vimentin and U09823 for EF-1a). Optimized runs were performed on an ABI 7000 sequence detection system (Applied Biosystems) and started with 10 minutes at 95°C followed by 40 cycles with 15 seconds at 95°C and 1 minute at 54°C. PCR reactions were performed at the following conditions: 300 nmol of each primer, 250 nmol of the respective probe, 2 μl of sample cDNA, 12.5 μl of PCR master mix (Eurogentec, Seraing, Belgium), and 10.9 μl of DEPC-treated water to yield a final volume of 25 μl. Samples and no template controls were included in each run as duplexes, and each run was performed twice. Tenfold dilutions of cDNA were subjected to PCR, and standard curves were generated based on these results using PCR software (Applied Biosystems) to prove the kinetics of the reactions.
Ratios between vimentin mRNA yields and EF-1a mRNA yields were calculated on the base of Ct values of six samples per group and two independently performed PCR runs (ratio = 2Ct EF-1a − Ct vimentin). Ratios represent relative gene expressions and are displayed as number of vimentin copies per 100 copies of EF-1a. Data were statistically analyzed using the Mann-Whitney-Wilcoxon rank sum test.
Results
Identification and Isolation of Tissue Structures
Toluidine blue-stained cryo-sections allowed the major compartments of the gut-associated lymphoid tissue to be identified in bright field microscopy. Specific cell types (eg, the membranous M cells) or functional states (eg, proliferation) were assessed using immunofluorescence. Laser microdissection of areas larger than a single cell requires a supporting membrane through which fluorescence illumination and optical imaging is done. Independent of the protocols used for section processing, this polymeric membrane possessed a strong autofluorescence under UV excitation (at ∼350 nm) so that blue fluorescent dyes could not be identified on the bright background. Excitation with blue light (at ∼470 nm) resulted in a moderate green autofluorescence, whereas excitation with green light (at ∼546 nm) gave the best results with only little background fluorescence and good overall contrast (Figure 1). Therefore, single labelings were performed using the red dyes Cy3 or Alexa Fluor 555, whereas double labelings were performed using Alexa Fluor 555 combined with the green fluorescent dye Alexa Fluor 488. Identification and laser microdissection of tissue structures was limited in both bright field and fluorescence microscopy by insufficient flatness of the supporting membrane, so that the position of the focal plane varied locally and had to be re-adjusted steadily. To confirm that mRNA was sufficiently preserved after immunolabeling, small tissue areas were isolated from labeled sections using laser microdissection and laser-pressure catapulting (Figure 1), and the content of EF-1a mRNA was determined in real-time RT-PCR. The resulting Ct values after a 5-minute labeling ranged between 22.3 ± 0.32 for sample sizes of 500,000 μm2 (about 5,000 cells) and 38.5 ± 1.03 for 5,000 μm2 (about 50 cells).
Reduction of Incubation Time
It was reported that the incubation time required for staining or immunolabeling of tissue sections critically affects the mRNA yield in RT-PCR.10,14,15 We therefore drastically reduced the total processing time by introducing the ultra-short one-step Fab fragment technique. Preliminary tests revealed that incubation times of only 5 minutes (7 minutes of total processing time including rinsing and drying) gave sufficient results if the concentration of the primary antibody was increased by factors of 10 to 20. Under these conditions, the labeling of rabbit M cells using the Fab-labeled anti-vimentin antibody (V9-Fab-Alexa 555) resulted in a strong fluorescence of individual cells (M cells) in the dome epithelium, whereas other parts of the epithelium as well as the pure supporting membrane showed a moderate or weak background fluorescence (Figure 1). The fluorescence intensity of the 5-minute Fab fragment method was comparable with that of a two-step protocol in which an unconjugated primary antibody was followed by a rinsing step and a Cy3-coupled secondary antibody. In addition, the direct technique, in which the V9 antibody was covalently coupled to Cy3 (5 minutes incubation; 7 minutes of total processing time), resulted in a similar but slightly brighter fluorescence.
One-Step Double Labeling
The Fab fragment technique also allowed double labelings to be performed in only one step of 5 minutes. Double-labeled sections showed vimentin-containing structures, such as M cells, in the green channel (V9-Fab-Alexa 488) and proliferating nuclei, such as those of the crypts, in the red channel (Ki67-Fab-Alexa 555; Figure 1). Using single and double immunofluorescence, relevant areas of the labeled sections could easily be isolated by laser microdissection and collected in PCR tubes. Triple labeling was not possible because of the technical limitations regarding autofluorescence of the supporting membrane as described above.
Quantitation of mRNA
The Ct measured in real-time PCR is reciprocally and logarithmically related to the absolute number of mRNA copies. Thus, an increase by 1 Ct unit equals a loss of 50% specific mRNA in the sample.
Sensitivity and Specificity of Real-Time PCR
The slopes of standard curves recorded for vimentin and EF-1a sequences ranged between −3.33 and −3.28. According to Pfaffl,25 these values correspond to reaction efficiencies between 99.7 and 101.8%. SD based on mean Ct values of the duplicates of each run were below 1.0, and the coefficient of variation based on Ct values of two corresponding runs was below 0.05 for all measurements. According to Bustin,2 such values confirm that our quantitative real-time PCR data are highly reproducible. No-template controls and controls containing genomic DNA did not yield any signal in real-time PCR.
Effect of Different Fixatives
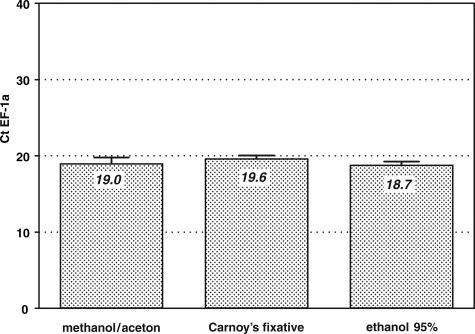
The three different solutions used to fix the tissue sections before labeling and laser microdissection revealed almost identical yields of EF-1a mRNA in real-time PCR (Figure 2). Because ethanol gave the best preservation of tissue structures and was easy to store and handle, it was selected for all following labelings and quantitations. Statistical tests in which Ct values of EF-1a mRNA after ethanol treatment were compared with Ct values produced after treatment with the other two fixatives showed that ethanol was slightly superior to Carnoy’s solution (P < 0.05) but not different from methanol/acetone.
Figure 2.
Effect of different fixatives on mRNA recovery of the housekeeping gene EF-1a. Cryo-sections of rabbit appendix were incubated with a Cy3-conjugated anti-vimentin antibody for 5 minutes (one-step direct method). Each bar represents the mean Ct value and the SD of six identically treated samples. Although the different fixatives influenced the mRNA preservation only marginally, ethanol gave significantly better mRNA yields than Carnoy’s medium (P < 0.05).
Effect of Incubation Time
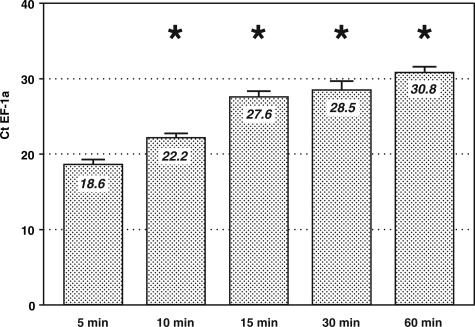
To determine the influence of incubation in antibody solution, the duration of this step was varied, whereas other parameters such as fixation, rinsing, and drying remained constant. Compared with the shortest incubation time that generated sufficient immunolabeling (5 minutes), prolonged incubation times drastically reduced the amount of mRNA detected in real-time PCR (Figure 3). Although EF-1a mRNA was detected at Ct 18.6 ± 0.6 after 5 minutes of incubation in V9-Cy3 antibody solution, 10 and 15 minutes of incubation in the same solution resulted in Ct values of 22.2 ± 0.6 and 27.6 ± 0.8, respectively. This represents an average loss of 0.72 Ct units (39% mRNA) or more within every minute of additional incubation. Incubation for 30 or 60 minutes led to a further loss of mRNA copies (Ct 28.5 ± 1.2; Ct 30.8 ± 0.7). A 60-minute incubation in antibody solution, as typically used in standard immunolabeling protocols, reduced the mRNA yield by 12.2 Ct units (factor 4700) compared with the 5-minute incubation, indicating that only 0.02% of specific mRNA was preserved in the samples after tissue processing. The differences in Ct units between the 5-minute incubation and all longer incubation times tested were statistically significant (P < 0.05).
Figure 3.
Effect of the incubation period on EF-1a mRNA recovery. Cryo-sections of rabbit appendix were fixed in ethanol for 2 minutes and incubated with antibody solution for different periods. Prolongation of the incubation period from 5 to 60 minutes leads to a drastic loss of detectable mRNA copies, corresponding to significantly increased Ct values in real-time PCR (P < 0.05; asterisks). The difference between the 5- and 15-minute samples is 9.0 Ct units, indicating that within only 10 minutes of additional incubation, there is a loss of 99.8% of specific mRNA. Bars represent means and SD of six samples per group.
Influence of Labeling and Staining Protocols
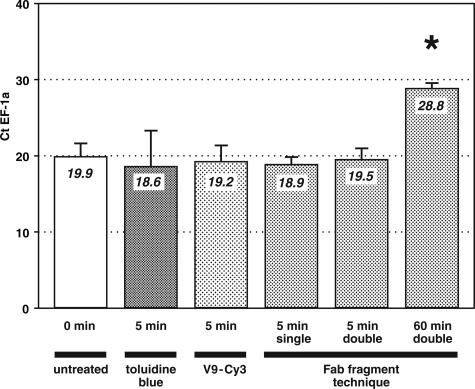
Staining with toluidine blue as well as direct immunolabeling, Fab fragment single labeling, and Fab fragment double labeling required about 5 minutes of incubation and about 7 minutes in total of contact with aqueous solutions. Ct values for EF-1a obtained after these four ultra-short treatments were compared with those determined for sections that had not been in contact with any solution (Figure 4, untreated). Although a 60-minute incubation in antibody solution (Ct 28.8 ± 0.7) reduced the mRNA yield by 9.9 Ct units (factor 950), the 5-minute incubation protocols did not produce significant loss of specific mRNA compared with untreated tissue (Ct 19.9 ± 1.8).
Figure 4.
Impact of different staining and labeling protocols on the amount of EF-1a mRNA detected in cryo-sections of rabbit appendix using quantitative real-time RT-PCR. All methods that comprise a single incubation of only 5 minutes (bars 2 to 5) yielded comparable mRNA recovery and did not significantly differ from untreated controls. In contrast, an incubation of 60 minutes led to a significant loss of detectable EF-1a copies (P < 0.05; asterisk). The difference of 9.3 Ct units, which equals a factor of more than 600, between the 5-minute and the 60-minute incubations corresponds to a quantitative loss of 99.8% EF-1a mRNA. Bars represent means and SD of six samples per group.
Preservation of mRNA Ratios
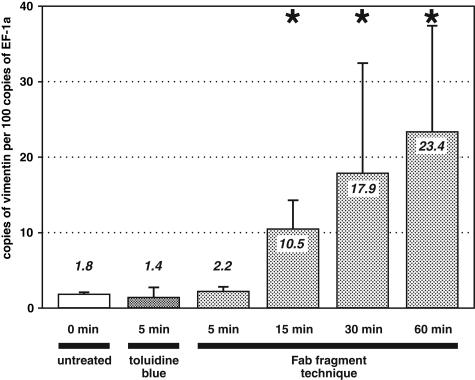
To confirm whether the staining and labeling protocols affect different mRNA species in a comparable manner, ratios between the number of copies of vimentin mRNA (gene of interest) and EF-1a mRNA (housekeeping gene) were determined (Figure 5). Ratios are displayed as number of copies of our target gene (vimentin) per 100 copies of our housekeeping gene (EF-1a). Sections stained with toluidine blue (mean ratio 1.4 ± 1.3) or immunolabeled for 5 minutes (mean ratio 2.2 ± 0.6) did not differ from untreated sections (mean ratio 1.8 ± 0.3), indicating that these ultra-short protocols did not alter the ratio significantly (P < 0.05). In contrast, an incubation of 60 minutes significantly shifted the ratio by a factor of 13 (mean ratio 23.4 ± 14.1 compared with 1.8 ± 0.3 found in untreated sections; P < 0.05). Thus, the 60-minute labeling produced a false-high result for the target gene (Figure 5). Reduced incubation times of 15 and 30 minutes likewise produced a significant alteration of the ratio between the analyzed sequences (P < 0.05; Figure 5, asterisks). The shift was smaller than after a 60-minute incubation (10.5 ± 3.8 and 17.9 ± 14.6 compared with 23.4 ± 14.1) but still produced a false-high result for the gene of interest (Figure 5).
Figure 5.
Influence of different staining and labeling techniques on ratios between the target gene vimentin and the housekeeping gene EF-1a. Although samples that underwent labeling or staining techniques that require just 5 minutes of incubation show ratios almost identical to those obtained from untreated tissues, we found a significant shift of ratios in tissue sections that were incubated for 15, 30, or 60 minutes compared with untreated tissue (P < 0.05; asterisks). Each bar represents ratios and SD of six identically treated samples.
Discussion
Laser microdissection of tissue sections and subsequent analysis of gene expressions has become a powerful tool in research and diagnosis in the past few years. If combined with real-time PCR, this technique yields an unrivalled sensitivity and thus allows tiny samples or even single cells to be excised and analyzed.5,6 In most cases, staining or immunolabeling is needed to identify target structures in complex tissues. Conventional protocols for such labelings comprise numerous incubation and rinsing steps and typically last 1 hour or more, so that mRNA is washed out or damaged.10,14,15 Several attempts have been made to reduce the time period in which the sections are in contact with aqueous solutions,10,14,16,22,26 and it must be assumed that the total incubation time is still the most crucial point regarding mRNA recovery.16,17 We therefore developed an elegant and ultra-fast labeling method, compared it with other staining protocols, and quantitatively determined the influence of several critical factors.
The results show that 1) sufficient immunolabeling can be performed within 5 minutes of incubation, 2) the mRNA loss is minute and not significantly different from that of untreated or toluidine blue-stained sections, and 3) fluorescence double labeling can successfully be performed using the new method. Nevertheless, we also found that a number of parameters drastically affect the mRNA yield and thus must be considered when developing standardized protocols for laser microdissection and mRNA quantitation. Although the composition of the fixative only marginally influenced the Ct values measured, prolonged incubation in aqueous solutions (ie, staining, antibody incubation, and rinsing steps) dramatically reduced the mRNA yield. Compared with the shortest protocol (5 minutes of incubation in antibody solution), just 5 additional minutes of incubation generated a signal reduction of 3.6 Ct units, which equals a loss of 92% specific mRNA. It was shown previously that a reduction of total incubation time down to about 30 minutes or less considerably improves the mRNA yield.10,14,16,22,26 Nevertheless, we here demonstrate that even incubation periods of only 15 minutes still damage the vast majority of all specific mRNA molecules and thus should be avoided. Interestingly, an incubation of 1 hour, which is still a short period compared with conventional immunohistochemical protocols, resulted in a loss of 99.98% of the detected mRNA sequences. It thus must be assumed that labeling protocols that last 15 to 30 minutes10,14,16,22,26 reduce the sensitivity of the method by factors of 100 to 1000. In contrast, samples labeled within only 5 minutes using the Fab fragment method evaluated here did not significantly differ in their mRNA yield from untreated samples. We therefore suggest that our ultra-short labeling method preserves mRNA at a still unrivalled level.
The Fab fragment technique not only allows single and double labelings to be performed within ultra-short incubation periods, but also produced fluorescence labelings comparable in intensity with classical methods. It must be noted, however, that the primary antibodies should possess a high avidity and that they should be applied at higher concentrations than in conventional two-step protocols. Fluorescence appears to represent the only immunohistochemical method that does not require an additional incubation for visualizing antibodies bound to target epitopes. We here demonstrate that antibodies covalently coupled to highly efficient fluorochromes (eg, the V9-Cy3 conjugate included in the present study) might be useful for fast one-step labelings, but unfortunately, most primary antibodies are not available as fluorochrome conjugates, and it is known that the sensitivity of such conjugates is relatively low.18 The Fab fragment method presented here allows any mouse antibody to be coupled to various fluorochromes of the Alexa family, which are known to be uncommonly bright and stable.27 In addition, the technique can be used to combine mouse IgG antibodies of the same isotype (eg, IgG1/IgG1) in one double labeling and even in the same incubation step, as recently demonstrated.18,19 It must be noted, that, in some cases, the solution of fluorochrome-labeled Fab fragments might require a concentration step (eg, in a vacuum centrifuge), because further antibody, fragment, and blocking solutions add solvent to the final volume and must be taken into consideration when calculating dilutions and molar ratios.
Our experiments revealed that fluorescence microscopy if combined with laser microdissection still suffers from a number of technical limitations, most of which are related to the supporting film. Its plastic material exhibited autofluorescence, especially under UV and blue excitation, displayed insufficient flatness, and reduced the performance of the optical imaging because of turbidity. The supporting film might be replaced by special coating layers on the glass surface, which then mediate laser catapulting effects, but no such systems are available yet. Recently, Micke et al9 demonstrated that a thin fluid layer applied onto the tissue section before laser microdissection may improve imaging quality in conventionally stained sections. In our hands, this cover fluid only marginally improved contrast and brightness in fluorescence microscopy (data not shown), so that we decided not to include the method in the present study.
Because mRNA is washed out and enzymatically degraded in aqueous solutions, recovery of nucleic acids is the most critical problem whenever tissue sections are processed before sample acquisition. Factors that possibly affect mRNA preservation comprise fixation, staining or labeling, UV irradiation during laser microdissection, RNA extraction, and reverse transcription. It was shown previously that crosslinking fixatives such as aldehydes considerably decrease the mRNA recovery.21,28 We show here that a simple fixation in ethanol gave better results than Carnoy’s solution, which has previously been published to represent an appropriate fixative regarding recovery of nucleic acids.20 It is known that ultraviolet irradiation damages nucleic acids,29,30 but the influence of the 337-nm laser pulses applied during microdissection and laser-pressure catapulting has not yet been determined systematically. To exclude possible artifacts induced by the ultra-violet laser beam, the tissue samples analyzed in the present study by quantitative real-time PCR were isolated mechanically and thus not exposed to UV light.
Because mRNA recovery and reverse transcription vary considerably, ratios between the target and one or more housekeeping genes are normally calculated.2,4This concept implies that the mRNA sequences contained in an individual sample are identically altered by experimental conditions such as fixation, efficiency of the reverse transcription, amounts of tissue investigated, and the total processing time. However, our data show that prolongation of the tissue processing time, even by only a few minutes, affects the ratios between the mRNA sequences measured. Total processing times of 15 or 30 minutes, as previously proposed for different protocols,10,14,16,22,26 produced a false-high ratio for our target gene vimentin and shifted the ratio between target and housekeeping gene by factors of 4.8 and 8.1, respectively. In contrast, a 5-minute single or double labeling using the ultra-short Fab complex method did not significantly alter the ratio compared with that of untreated samples. Similar artifacts in the quantitation of gene expressions have recently been shown for the influence of aldehyde fixation and paraffin-embedding procedures.28 It is important to note that the shift of ratios cannot be attributed to the target or the housekeeping gene alone. Thus we strongly recommend ultra-short labeling techniques when performing quantitative gene expression studies on microdissected tissue.
In conclusion, the present study shows that immunohistochemical labeling before laser microdissection and quantitative real-time PCR not only reduces the mRNA yield at a rate of 39% per minute or more but also alters the measured ratios between target and housekeeping genes by factors of up to 13. The ultra-short one-step method introduced here reduces both effects to a level that does not significantly differ from that of unlabeled samples, provided that the total processing time of the tissue sections does not exceed 7 minutes.
Acknowledgments
We gratefully acknowledge the technical assistance of D. Stöckmann, M.-L. Leppin, H. Manfeldt, and C. Örün.
Footnotes
Supported by the University of Lübeck.
References
- Freeman WM, Walker SJ, Vrana KE. Quantitative RT-PCR: pitfalls and potential. Biotechniques. 1999;26:112–125. doi: 10.2144/99261rv01. [DOI] [PubMed] [Google Scholar]
- Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- Wang T, Brown MJ. mRNA quantification by real time TaqMan polymerase chain reaction: validation and comparison with RNase protection. Anal Biochem. 1999;269:198–201. doi: 10.1006/abio.1999.4022. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F: Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002, research0034.1–research0034.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink L, Seeger W, Ermert L, Hanze J, Stahl U, Grimminger F, Kummer W, Bohle RM. Real-time quantitative RT-PCR after laser-assisted cell picking. Nat Med. 1998;4:1329–1333. doi: 10.1038/3327. [DOI] [PubMed] [Google Scholar]
- Schütze K, Lahr G. Identification of expressed genes by laser-mediated manipulation of single cells. Nat Biotechnol. 1998;16:737–742. doi: 10.1038/nbt0898-737. [DOI] [PubMed] [Google Scholar]
- De Preter K, Vandesompele J, Heimann P, Kockx MM, Van Gele M, Hoebeeck J, De Smet E, Demarche M, Laureys G, Van Roy N, De Paepe A, Speleman F. Application of laser capture microdissection in genetic analysis of neuroblastoma and neuroblastoma precursor cells. Cancer Lett. 2003;197:53–61. doi: 10.1016/s0304-3835(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Okuducu AF, Janzen V, Hahne JC, Ko Y, Wernert N. Influence of histochemical stains on quantitative gene expression analysis after laser-assisted microdissection. Int J Mol Med. 2003;11:449–453. [PubMed] [Google Scholar]
- Micke P, Bjornsen T, Scheidl S, Stromberg S, Demoulin JB, Ponten F, Ostman A, Lindahl P, Busch C. A fluid cover medium provides superior morphology and preserves RNA integrity in tissue sections for laser microdissection and pressure catapulting. J Pathol. 2004;202:130–138. doi: 10.1002/path.1496. [DOI] [PubMed] [Google Scholar]
- Fend F, Emmert-Buck MR, Chuaqui R, Cole K, Lee J, Liotta LA, Raffeld M. Immuno-LCM: laser capture microdissection of immunostained frozen sections for mRNA analysis. Am J Pathol. 1999;154:61–66. doi: 10.1016/S0002-9440(10)65251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fend F, Kremer M, Quintanilla-Martinez L. Laser capture microdissection: methodical aspects and applications with emphasis on immuno-laser capture microdissection. Pathobiology. 2000;68:209–214. doi: 10.1159/000055925. [DOI] [PubMed] [Google Scholar]
- Grumbach IM, Veh RW. The SA/rABC technique: a new ABC procedure for detection of antigens at increased sensitivity. J Histochem Cytochem. 1995;43:31–37. doi: 10.1177/43.1.7822761. [DOI] [PubMed] [Google Scholar]
- Whiteland JL, Nicholls SM, Shimeld C, Easty DL, Williams NA, Hill TJ. Immunohistochemical detection of T-cell subsets and other leukocytes in paraffin-embedded rat and mouse tissues with monoclonal antibodies. J Histochem Cytochem. 1995;43:313–320. doi: 10.1177/43.3.7868861. [DOI] [PubMed] [Google Scholar]
- Fink L, Kinfe T, Stein MM, Ermert L, Hanze J, Kummer W, Seeger W, Bohle RM. Immunostaining and laser-assisted cell picking for mRNA analysis. Lab Invest. 2000;80:327–333. doi: 10.1038/labinvest.3780037. [DOI] [PubMed] [Google Scholar]
- Lindeman N, Waltregny D, Signoretti S, Loda M. Gene transcript quantitation by real-time RT-PCR in cells selected by immunohistochemistry-laser capture microdissection. Diagn Mol Pathol. 2002;11:187–192. doi: 10.1097/00019606-200212000-00001. [DOI] [PubMed] [Google Scholar]
- Gjerdrum LM, Abrahamsen HN, Villegas B, Sorensen BS, Schmidt H, Hamilton-Dutoit SJ. The influence of immunohistochemistry on mRNA recovery from microdissected frozen and formalin-fixed, paraffin-embedded sections. Diagn Mol Pathol. 2004;13:224–233. doi: 10.1097/01.pdm.0000134779.45353.d6. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Asamoto M, Tsujimura K, Shirai T. Specific differences in gene expression profile revealed by cDNA microarray analysis of glutathione S-transferase placental form (GST-P) immunohistochemically positive rat liver foci and surrounding tissue. Carcinogenesis. 2004;25:439–443. doi: 10.1093/carcin/bgh030. [DOI] [PubMed] [Google Scholar]
- Brown JK, Pemberton AD, Wright SH, Miller HR. Primary antibody-Fab fragment complexes: a flexible alternative to traditional direct and indirect immunolabeling techniques. J Histochem Cytochem. 2004;52:1219–1230. doi: 10.1369/jhc.3A6200.2004. [DOI] [PubMed] [Google Scholar]
- Ino H. Application of antigen retrieval by heating for double-label fluorescent immunohistochemistry with identical species-derived primary antibodies. J Histochem Cytochem. 2004;52:1209–1217. doi: 10.1369/jhc.3A6205.2004. [DOI] [PubMed] [Google Scholar]
- Foss RD, Guha-Thakurta N, Conran RM, Gutman P. Effects of fixative and fixation time on the extraction and polymerase chain reaction amplification of RNA from paraffin-embedded tissue: comparison of two housekeeping gene mRNA controls. Diagn Mol Pathol. 1994;3:148–155. doi: 10.1097/00019606-199409000-00003. [DOI] [PubMed] [Google Scholar]
- Goldsworthy SM, Stockton PS, Trempus CS, Foley JF, Maronpot RR. Effects of fixation on RNA extraction and amplification from laser capture microdissected tissue. Mol Carcinog. 1999;25:86–91. [PubMed] [Google Scholar]
- Kohda Y, Murakami H, Moe OW, Star RA. Analysis of segmental renal gene expression by laser capture microdissection. Kidney Int. 2000;57:321–331. doi: 10.1046/j.1523-1755.2000.00824.x. [DOI] [PubMed] [Google Scholar]
- Gruber AD, Levine RA. In situ assessment of mRNA accessibility in heterogeneous tissue samples using elongation factor-1 alpha (EF-1 alpha). Histochem Cell Biol. 1997;107:411–416. doi: 10.1007/pl00007903. [DOI] [PubMed] [Google Scholar]
- Leverkoehne I, Horstmeier BA, von Samson-Himmelstjerna G, Scholte BJ, Gruber AD. Real-time RT-PCR quantitation of mCLCA1 and mCLCA2 reveals differentially regulated expression in pre- and postnatal murine tissues. Histochem Cell Biol. 2002;118:11–17. doi: 10.1007/s00418-002-0420-4. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink L, Kinfe T, Seeger W, Ermert L, Kummer W, Bohle RM. Immunostaining for cell picking and real-time mRNA quantitation. Am J Pathol. 2000;157:1459–1466. doi: 10.1016/S0002-9440(10)64784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchuk-Voloshina N, Haugland RP, Bishop-Stewart J, Bhalgat MK, Millard PJ, Mao F, Leung WY, Haugland RP. Alexa dyes, a series of new fluorescent dyes that yield exceptionally bright, photostable conjugates. J Histochem Cytochem. 1999;47:1179–1188. doi: 10.1177/002215549904700910. [DOI] [PubMed] [Google Scholar]
- Von Smolinski D, Leverkoehne I, Samson-Himmelstjerna G, Gruber AD. Impact of formalin-fixation and paraffin-embedding on the ratio between mRNA copy numbers of differently expressed genes. Histochem Cell Biol. 2005;124:177–188. doi: 10.1007/s00418-005-0013-0. [DOI] [PubMed] [Google Scholar]
- De With A, Greulich KO. Wavelength dependence of laser-induced DNA damage in lymphocytes observed by single-cell gel electrophoresis. J Photochem Photobiol B. 1995;30:71–76. doi: 10.1016/1011-1344(95)07151-q. [DOI] [PubMed] [Google Scholar]
- Mohanty SK, Rapp A, Monajembashi S, Gupta PK, Greulich KO. Comet assay measurements of DNA damage in cells by laser microbeams and trapping beams with wavelengths spanning a range of 308 nm to 1064 nm. Radiat Res. 2002;157:378–385. doi: 10.1667/0033-7587(2002)157[0378:camodd]2.0.co;2. [DOI] [PubMed] [Google Scholar]