Abstract
The limitations of classical diagnostic methods for invasive fungal infections (IFIs) have led to the development of molecular techniques to aid in the detection of IFIs. Despite good published performance, interlaboratory reproduction of these assays is variable, and no consensus has been reached for an optimal method. This publication describes the first multicenter study of polymerase chain reaction methods, for the detection of Aspergillus and Candida species, currently used in the UK and Ireland by distribution and analysis of multiple specimen control panels. All three Candida methods were comparable, achieving a satisfactory level of detection (10 cfu), and the method of preference was dependent on the requirements of the particular laboratory. The results for the five Aspergillus assays were more variable, but two methods (2Asp and 4Asp) were superior (101 conidia). Formally, the overall performances of the two Aspergillus assays were comparable (κ statistic = 0.77). However, on the Roche LightCycler, there was a clear sample-type effect that greatly reduced the detection limit of the 4Asp method when testing whole blood samples. Therefore, the preferred Aspergillus method relied on the amplification platform available to the user. This study represents the initial process to achieve a consensus method for the diagnosis of IFIs.
Improvements in medical intervention have led to significant increases in cases of invasive fungal infections (IFIs) in a growing immunocompromised population.1 Early diagnosis is paramount, with delays or missed diagnosis increasing mortality.1 However, diagnosis of most IFIs is difficult, particularly for invasive aspergillosis, with classical culture techniques providing poor sensitivity. Radiological investigations are a useful adjunct but provide nonspecific and transient results,2 and histopathology, despite providing definitive evidence of IFI, cannot always identify the causative organism.
The British Society for Medical Mycology has proposed standards of care for patients with IFI,1 and the European Organization for Research and Treatment of Cancer has published consensus diagnostic criteria for IFIs.3 Both documents comment on the use of serological methods for the diagnosis of IFIs (eg, Aspergillus antigen enzyme-linked immunosorbent assay), but the use of molecular methods is discussed only briefly because of limited evaluation of published methods resulting in little standardization of methodology. Our research here represents a multicenter evaluation of molecular methods used in the UK and Ireland for the detection of Aspergillus and Candida with the aim of widespread future collaboration to achieve an accepted consensus method to aid in the diagnosis of IFI.
The UK-Irish Fungal PCR Consensus Group: A History
In 2001, seven centers in the UK formed the UK-Irish Fungal PCR Consensus Group (Table 1) with the aim of developing a consensus for a robust and reproducible polymerase chain reaction (PCR)-based test for the diagnosis of IFIs. At the time there were many reports on the development and application of molecular methods for the diagnosis of IFIs, but with wide variation in extraction and amplification techniques there was little consensus. The group decided to distribute a series of quality control panels to compare existing extraction and amplification methods in terms of sensitivity and specificity, focusing on tests used at the time in the UK and Ireland for the detection of Aspergillus and Candida DNA (Table 2).
Table 1.
The Members of the UK Fungal PCR Consensus Group
| Center* | Members |
|---|---|
| Birmingham Health Protection Agency (HPA) | Mr. Steve Wilson |
| Mycology Reference Laboratory, HPA Southwest | Dr. Chris J. Linton and Dr. Elizabeth M. Johnson |
| NPHS Microbiology Cardiff and School of Medicine, Cardiff University | Dr. P. Lewis White, Mr. Mike D. Perry, and Dr. Rosemary A. Barnes |
| University of Dublin, Trinity College, Dublin | Professor Tom Rogers, Dr. Brian O’Connell, Dr. Michael J. Carr, and Dr. Breida Boyle |
| Dulwich HPA | Dr. Melvyn Smith |
| Glasgow Royal Infirmary | Dr. Brian Jones, Professor Tessa Holyoake, Dr. Lorna McLintock, and Mrs. Elaine Allan |
| Mycology Reference Centre, Leeds General Infirmary | Dr. Richard Barton and Dr. Richard P. Hobson |
| University Hospitals of Leicester | Dr. Patrick T. Kimmitt, Dr. Kumar Rajakumar, and Ms. Nita Pancholi |
| Molecular Diagnostics, Northwest HPA Manchester Laboratory | Dr. Malcolm Guiver |
| Royal Free Hospital, London | Mrs. Shila Seaton, Dr. Beatriz L. Gomez, and Dr. Chris C. Kibbler |
Original members in bold.
In 2002 a panel (D02) of ethylenediaminetetraacetic acid (EDTA) whole blood spiked with known quantities (0 to 105) of Aspergillus fumigatus conidia or Candida albicans blastospores was distributed, and the results revealed wide variation. The panel concluded that both extraction methods published by Loeffler and colleagues4,5 worked satisfactorily, but the automated method was preferred, being a shorter and more robust procedure, and because some groups had experienced specimen contamination when using spin columns.
The Candida assays6,7,8,9 were broadly comparable, despite considerable variation in design (Table 2), and capable of detecting Candida to a lower limit of 10 cfu/ml blood (Table 3). The lower limits of detection of the Aspergillus assays were more variable, although three Aspergillus assays [methods 1,6 2Asp10 and 4Asp11 (Table 2)] performed optimally with detection limits of 10 to 100 conidia/ml of blood (Table 3).
Table 2.
The Extraction and Amplification Methods Evaluated
| Group(s) | Method | Extraction method | Apsergillus PCR | Candida PCR |
|---|---|---|---|---|
| Birmingham Glasgow | 1 | Bead beating/Maganapure4 | Pan-fungal LC hybridization probe assay*6 | Pan-fungal LC hybridization probe assay*6 |
| Bristol | 2Asp | Freeze-thaw/Qiagen | Not done | |
| Cardiff | 2Asp 2Can | Lyticase/Qiagen†5 | Aspergillus-specific LC hydrolysis probe assay‡10 | Candida-specific LC hydrolysis probe assay§7,8 |
| Dulwich | 3Asp | Bead beating/Qiagen | In-house method | Not done |
| Manchester | 4Asp 3Can | Bead beating/Maganapure4 | Aspergillus-specific TQ (hydrolysis) probe assay¶11 | Candida-specific TQ (hydrolysis) probe assay||9 |
| Leeds | 4a Asp | Not done** | Sybr Green assay†† | Not done |
Method 1 was a pan-fungal assay developed using the oligonucleotides designed by the Tübingen group.12,20 It was capable of detecting other fungal pathogens (eg, Aspergillus), and different genera were differentiated by melt-curve analysis.
Modified as described by White et al.7
Method used Aspergillus-specific primers designed by Williamson et al13 and Aspergillus-specific probe.
Method used pan-fungal primers with a Candida-specific probe. It was capable of detecting, but not differentiating, the main causes of invasive candidal infection (C. albicans, C. dubliniensis, C. glabrata, C. kefyr, C. krusei, C. parapsilosis, and C. tropicalis).
Method used pan-fungal primers with an Aspergillus-specific probe.
The system was Candida-specific but had the advantage of differentiating between Candida species (C. albicans, C. kefyr, C. glabrata, C. parapsilosis, C. tropicalis, and C. krusei) by using species-specific oligonucleotides in a multiplexed reaction.
Only involved in distribution D03.
Using primers designed by Kami et al.11
Table 3.
The Results of the First Two Quality Control Distributions
| Group(s) | Method | Detection limit* (D02)
|
Detection limit* (D03)
|
|
|---|---|---|---|---|
| Candida (cfu/ml) | Aspergillus (conidia/ml) | Aspergillus DNA† (10-fold dilutions) | ||
| Birmingham | 1 | 105 | 104 | −2 |
| Glasgow | 101 | 102 | −1 | |
| Bristol | 2Asp | n.a. | 101‡ | −3 |
| Cardiff | 2Asp | n.a. | n.p. | −3 |
| 2Can | 101 | n.a. | n.a. | |
| Dulwich | 3Asp | n.a. | 105 | −1 |
| Manchester | 4Asp | n.a. | 102 | −3 |
| 3Can | 101 | n.a. | n.a. | |
| Leeds | 4a Asp | n.p. | n.p. | −2 |
Detection limit determined using duplicate results.
Genomic DNA was extracted from ∼5000 A. fumigatus conidia: 250 pg of DNA, assuming 1 conidia correlates to 50 fg of DNA.20 Therefore −3 dilution correlates to 250 fg of DNA or 5 A. fumigatus conidia.
Nested version of the assay used.
n.p., test not performed; n.a., test not applicable to target.
With the Candida assays showing consistent and comparable performances, the subsequent research of the UK-Irish Fungal PCR Consensus Group focused on the Aspergillus assays only. In subsequent distributions it was decided to evaluate amplification methods only; therefore, DNA extracts, rather than suspensions of viable fungi, were distributed.
In 2003, a panel of serially diluted genomic DNA extracted from A. fumigatus (D03) was distributed to the seven centers, and five assays were evaluated (Table 2). There was broad agreement that the D03 distribution highlighted two assays, with method 2Asp and method 4Asp having the lowest limits of detection within the group of participating laboratories (Table 3).
With only minimal interlaboratory evaluation of the two optimal Aspergillus assays [methods 2Asp and 4Asp (Table 2)] and the 4Asp assay originally designed for use with Applied Biosciences TaqMan (TQ; Applied Biosystems, Warrington, UK), it was decided to further compare the performance of these assays between centers using the amplification system(s) available in the participating laboratories [Roche LightCycler (LC; Roche, Lewes, UK), n = 7; TQ, n = 2; and Corbett Rotor-Gene (CR; Corbett Research LTD, Cambridge, UK), n = 3]. To minimize variables, both DNA extracts and the oligonucleotides were distributed to the original seven centers as well as an additional two centers from the UK and one from the Republic of Ireland (Table 1). The findings of this distribution (D04) are discussed below.
Materials and Methods
DNA Extraction
D04 consisted of 16 samples comprising 8 positive specimens [genomic DNA extracted from known quantities of A. fumigatus (10 to 5000 conidia) spiked into either 1 ml water or 1 ml EDTA whole human blood] and 8 negative specimens (molecular grade water). DNA was extracted as described previously4 using bead beating (acid-washed glass beads, 180 μm; Sigma, Poole, UK) and the MagnaPure total nucleic acid kit (Roche, Lewes, UK). For the blood samples, additional red and white cell lysis steps were applied.12 For each concentration eight extractions were performed and eluted in 100-μl volumes that were subsequently pooled. Fifty-μl aliquots of pooled DNA suspensions were distributed to the 10 participating laboratories.
PCR Amplification
Oligonucleotides (Eurogentec, Southampton, UK) were distributed before the DNA panel at concentrations of 100 μmol/L. Both Aspergillus PCR assays were performed on the platforms available to the participating laboratories (Table 4), including the LC, TQ, and CR. In two laboratories the specimens were analyzed on more than one amplification platform (Table 4). PCR on the LC was performed with the LC Fast Start DNA master hybridization probes kit (Roche, UK). PCR on the TQ was performed with TQ Universal PCR master mix. PCR on the CR was performed with either the LC Fast Start DNA master hybridization probes kit (one laboratory) or with the ABgene Q-PCR mastermix (two laboratories). Molecular grade water-negative controls were included in every run.
Table 4.
The Results of the Third Quality Control Distribution (D04) Assessing the Performances of the Aspergillus 2Asp and 4Asp Assays
| Group | Platform | Detection limit (conidia/ml)
|
|||
|---|---|---|---|---|---|
| 2Asp assay
|
4Asp assay
|
||||
| Water* | Blood† | Water* | Blood† | ||
| Birmingham | LC | 50 | n.d. | 50 | n.d. |
| Bristol | LC | 10 | 25 | 10 | n.d. |
| Cardiff | LC | 10 | 25 | 10 | n.d. |
| CR | 10 | 25 | n.p. | n.p. | |
| Dublin | TQ | 10 | 25 | 10 | 25 |
| Dulwich | LC | 50 | 75 | 50 | n.d. |
| Glasgow | LC | 10 | 75 | 10 | n.d. |
| Leeds | LC | 50 | 75 | 10 | n.d. |
| Leicester | CR | 50 | 25 | 10 | 75 |
| Manchester | TQ | n.p. | n.p. | 10 | 25 |
| LC | 10‡ | 75 | 10c | n.d. | |
| Royal Free | CR | 10 | 25 | 50 | 25 |
DNA extracted from 5000 to 10 conidia/ml water.
DNA extracted from 75 to 25 conidia/ml EDTA whole blood.
Did not detect the 50 conidia/ml water.
n.d., Aspergillus DNA not detected; n.p., test not performed.
Method 2Asp
PCR was performed with the Aspergillus-specific primers ASF1 5′-GCA CGT GAA ATT GTT GAA AGG-3′, ADR1 5′-CAG GCT GGC CGC ATT G-3′ targeting the 28S rRNA gene13 and an Aspergillus-specific hydrolysis probe ASP 28P 5′-FAM-CAT TCG TGC CGG TGT ACT TCC CCG-TAMRA-3′.10 Briefly, the PCR mix contained 1× reaction mix, primers (final concentration, 0.75 μmol/L), probe (0.4 μmol/L), MgCl2 (4 mmol/L), and DNA template in a total volume of 20 μl. PCR conditions were one cycle of 95°C for 10 to 15 minutes followed by up to 60 cycles of 95°C for 5 seconds (15 seconds on TQ and CR) and 60°C for 30 seconds (1 minute on TQ and CR) while acquiring fluorescence data each cycle.
Method 4Asp
PCR was performed using pan-fungal primers (5′-TTG GTG GAG TGA TTT GTC TGC T-3′ and 5′-TCT AAG GGC ATC ACA GAC CTG-3′) targeting the 18S rRNA gene and an Aspergillus-specific TaqMan (hydrolysis) probe (5′-FAM-TCG GCC CTT AAA TAG CCC GGT CCG C-TAMRA-3′) as previously described.11 Briefly, the PCR mix contained 1× reaction mix, primers (0.3 μmol/L), probe (0.2 μmol/L), MgCl2 (5 mmol/L), and DNA template in a total volume of 20 μl. PCR conditions were one cycle of 95°C for 15 minutes followed by up to 60 cycles of 95°C for 15 seconds and 60°C for 1 minute while acquiring fluorescence data each cycle. On the TQ the precycling conditions were one cycle at 50°C for 2 minutes followed by one cycle of denaturation at 95°C for 10 minutes; however, the subsequent cycling conditions were as before.
Sequencing
To identify the products of PCR amplification, some of the PCR amplicons representing a high and low fungal load (1000 and 75 conidia/ml A. fumigatus spiked blood) and two negative blood samples were purified using the QIAquick PCR product purification kit (Qiagen, UK), before cycle sequencing using the Big Dye 3 terminator cycle sequencing kit (Perkin-Elmer, UK) and sequencing using the ABI Prism 3100 genetic analyzer (Perkin-Elmer). The identity of the PCR amplicon sequences were determined by BLAST search,14 and sequences were compared by multiple alignment using DNAsis version 2.5 (Hitachi Software Engineering Company).
Statistical Analysis
To determine the significance of the results and the differences between the results, 95% confidence intervals were generated using the methods as described by Newcombe (single proportion,15 paired difference,16 and unpaired difference17). A κ statistic was generated to test assay agreement, in which values greater than 0.75 represented excellent agreement between assays.18
Results
Because different centers performed the two assays a varying number of times (one to three replicates), it was decided to analyze only the first set of results returned for each test per platform from each center to remove bias.
Assay Performance on the Roche LC
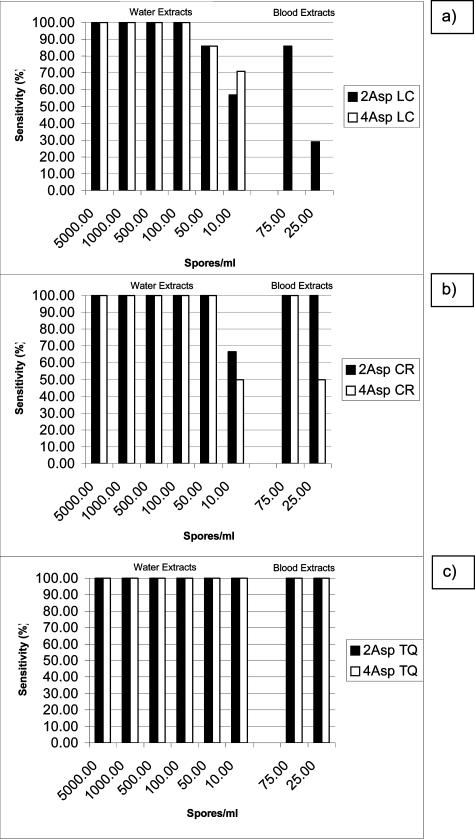
Most tests were performed on the LC with 7 of the 10 centers returning data for this platform (Table 4). Formally, the overall agreement between the two assays on the LC was excellent (κ value, 0.77). However, on the LC sensitivity, specificity, and positive and negative predictive values (PPV and NPV) of the 2Asp assay were superior in comparison with the values for the 4Asp assay, and 95% confidence intervals confirmed that the increases in sensitivity and specificity were significant (Table 5). Also, there was a clear sample type effect with the 4Asp assay failing to detect any of the spiked blood samples on the LC platform, whereas the 2Asp assay achieved sensitivities of 28.6% (difference, 28.6%; 95% CI, 12.3 to 64.1) and 85.7% (difference, 85.7%; 95% CI, 34.5 to 97.4) for the 25 conidia/ml and 75 conidia/ml DNA load, respectively (Figure 1).
Table 5.
The Overall Performances of the Aspergillus Assays on the Roche LightCycler, Corbett Rotor-Gene, and Applied Biosystems TaqMan for D04
| Platform | 2Asp (95% CI) | 4Asp (95% CI) | Difference (2Asp to 4Asp, 95% CI) | |
|---|---|---|---|---|
| LightCycler | Sensitivity (%) | 82.1 (70.1 to 90.0) | 69.6 (56.7 to 80.1) | 12.5 (1.8 to 23.3) |
| (n = 7 centers) | Specificity (%) | 91.1 (80.7 to 96.1) | 80.4 (68.2 to 88.7) | 10.7 (0.3 to 21.9) |
| PPV (%) | 90.2 (79.0 to 95.7) | 78.0 (64.8 to 87.3) | 12.2 | |
| NPV (%) | 83.6 (72.4 to 90.8) | 72.6 (60.4 to 82.1) | 11.0 | |
| Rotor-Gene | Sensitivity (%) | 95.8 (79.8 to 99.3) | 87.5 (64.0 to 96.5) | 8.3 (−10.1 to 32.1) |
| (n = 3 centers) | Specificity (%) | 100 (86.2 to 100) | 87.5 (64.0 to 96.5) | 12.5 (−4.0 to 36.0) |
| PPV (%) | 100 (85.7 to 100) | 87.5 (64.0 to 96.5) | 12.5 | |
| NPV (%) | 96.0 (80.5 to 99.3) | 87.5 (64.0 to 96.5) | 8.5 | |
| TaqMan | Sensitivity (%) | 100 (67.6 to 100) | 100 (80.6 to 100) | 0 (−32.4 to 19.4) |
| (n = 2 centers) | Specificity (%) | 87.5 (52.9 to 97.8) | 81.3 (57.0 to 93.4) | 6.2 (−30.4 to 32.6) |
| PPV (%) | 88.9 (56.5 to 98.0) | 84.2 (62.4 to 94.5) | 4.7 | |
| NPV (%) | 100 (64.6 to 100) | 100 (78.5 to 100) | 0 |
Figure 1.
Sensitivities of the Aspergillus PCR assays using different PCR platforms with different fungal loads and sample types. a: Roche LightCycler, b: Corbett Rotor–Gene, and c: Applied Biosystems TaqMan.
Assay Performance on the Corbett Rotor–Gene and Applied Biosystems TaqMan
Other platforms used to analyze the panel were the CR (three centers) and the TQ (two centers) systems (Table 4). The overall agreement between the two assays on these systems was good although a κ value was not generated because of the limited number of tests performed. Sensitivity, specificity, PPV, and NPV for both assays are shown in Table 5, with both methods performing to similar standards. Furthermore the sample-type effect present on the LC was less apparent or absent on the CR and TQ platforms, respectively, although the number of tests performed was limited (Figure 1).
Investigating the Sample-Type Effect
To further investigate the LC sample-type effect, the 4Asp assay was performed using varied cycling parameters and a range of primer, probe, and MgCl2 concentrations with the aim of optimizing the assay on the LC in the center distributing D04 (Cardiff). These changes had no effect on the sample type difference with neither the 75 conidia/ml nor 25 conidia/ml blood extract detected (results not shown).
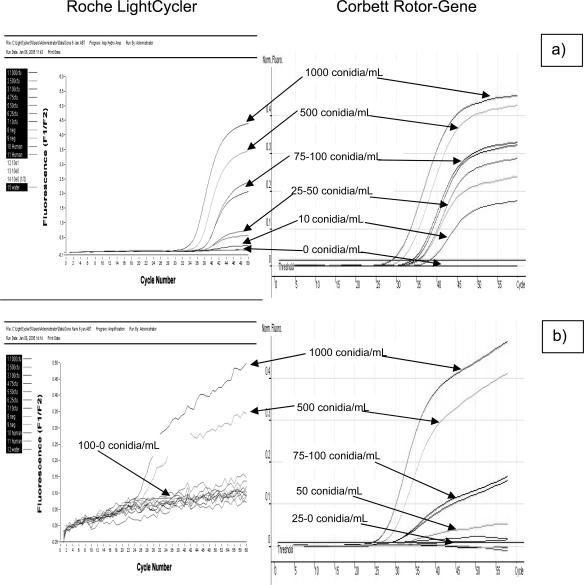
To determine a threshold value for the 4Asp assay on the LC, DNA was extracted from a larger panel of EDTA whole blood spiked with varying concentrations of Aspergillus conidia (0 to 1000 conidia/ml). The extracts were tested using both assays on the LC. The detection limit for the 2Asp assay was 10 conidia/ml whereas the 4Asp assay was only able to detect a limit of 500 conidia/ml on the LC with a marked reduction in the quality of signal (Figure 2). Testing this panel using both assays on the CR platform confirmed previous findings, with the 2Asp assay able to detect all of the Aspergillus-positive extracts and the 4Asp assay showing an improved detection threshold of 50 conidia/ml (Figure 2).
Figure 2.
Detection limits for the Aspergillus assays using the Roche LightCycler platform and Corbett Rotor–Gene with DNA extracted from EDTA whole blood spiked with Aspergillus conidia. a: 2Asp assay and b: 4Asp assay.
Gel electrophoresis of the 4Asp LC products revealed that consistent PCR amplification was occurring, but this was independent of fungal load, whereas the 2Asp LC amplicons correlated with fungal load. Because a consistent volume was used for the extractions (1 ml of EDTA blood), this suggested that the 4Asp on the LC was amplifying human DNA. To investigate this, four of the 4Asp PCR amplicons (1000 conidia/ml and 75 conidia/ml spiked blood samples and 2 negative blood samples) were purified and sequenced. BLAST searches revealed >94.0% sequence similarity between all four amplicons and identified a section of the human 18S rRNA gene (Figure 3). Alignment of the 4Asp oligonucleotides with the human and A. fumigatus 18S rRNA sequences confirmed that the primers would amplify both human and Aspergillus DNA, although the probe was specific for Aspergillus (Figure 3). Alignment of the 2Asp oligonucleotides, targeting the Aspergillus 28S rRNA gene, suggested that they should not hybridize to the human 28S rRNA gene; however, this nonspecific amplification occurred in blood extracts with little or no Aspergillus DNA present (results not shown).
Figure 3.
Sequence similarity between a: 4Asp forward primer; b: 4Asp probe; c: A. fumigatus 18S rRNA gene partial sequence (accession no. AF548063, bp 1212 to 1367); d: Homo sapiens 18S rRNA gene partial sequence (accession no. X03205, bp 1346 to 1508); e–h: 4Asp LC products; and i: 4Asp reverse primer.
Discussion
This research describes collaboration between 10 laboratories to evaluate methods currently in use and under research in the UK for the detection of Aspergillus spp. and Candida spp. Before the consensus group, many individual centers had evaluated certain methods in choosing protocols for use in their laboratory. The selected methods were assessed in this study, and it is therefore possible that some of the excluded methods12,19,20 may have not received a sufficient assessment.
The Candida assays [methods 1, 2Can and 3Can (Table 2)] consistently achieved good detection limits (10 cfu/ml blood; Table 3) and specificities whereas the Aspergillus assays were more variable, particularly in terms of lower detection limits (Table 3). The performances of the two optimal Aspergillus assays varied with sample type and platform (Figure 1). The general interlaboratory reproducibility of both assays was very good, although numbers were limited for the CR and TQ. For both assays there was a drop in sensitivity (<100%) in detecting less than 100 conidia/ml on the LC, although this was improved on the CR and TQ. The 2Asp assay was more specific than the 4Asp, although this was only statistically significant on the LC. The 2Asp assay was able to detect Aspergillus DNA extracts from blood and water using the LC, CR, or TQ with equal sensitivity, whereas the 4Asp was only able to detect both extract types on the CR and TQ. Using the LC the 4Asp method could detect Aspergillus DNA extracted from water to a similar efficiency to that of the 2Asp assay. In fact crossing point analysis indicated that the 4Asp method was actually more efficient at amplification (results not shown).
When using blood extracts the detection threshold of the 4Asp assay was significantly reduced on the LC, possibly as a result of amplification of human DNA. Sequencing of these amplicons revealed high sequence similarity with the human 18S rRNA gene (Figure 3). Because the same extracts, reagents, and conditions were used on the different platforms, this should also occur on the other platforms. Sequencing of PCR products amplified on the CR revealed that human DNA had also been amplified (results not shown) but despite this the system was still able to detect Aspergillus DNA extracted from 50 conidia. PCR products amplified using the TQ have not been sequenced.
The reasons for these differences are not clear. Optimizing the assay for the LC had little effect, and results for the water extracts reveal that the 4Asp method was as efficient on the LC as it was on other platforms. Sample inhibition of the 4Asp assay when testing the blood extracts on the LC is unlikely because the 2Asp used the same samples and no inhibition occurred on other platforms. Differences in PCR master-mix composition were excluded because the same master-mix had been used on the LC and CR.
With the same samples used on the three platforms, it is possible that human DNA is being amplified using the CR and TQ systems but in addition to the amplification of the Aspergillus target. The reason for the lack of amplification of Aspergillus DNA on the LC is not clear but is possibly a result of enhanced cycling efficiency of the LC and glass capillaries amplifying the large quantities of human DNA in preference to the smaller amounts of Aspergillus DNA. On the other platforms, the putative reduced cycling efficiency may have allowed the primers further time to mix before binding and thus the opportunity to bind to the target Aspergillus DNA. However, further critical analysis would need to be performed before this or any reason was accepted. Amplification of human DNA also occurred using the 2Asp assay but, in the absence of Aspergillus DNA and sequencing, revealed this to be nonspecific. Furthermore, with increasing fungal load the nonspecific amplification diminished.
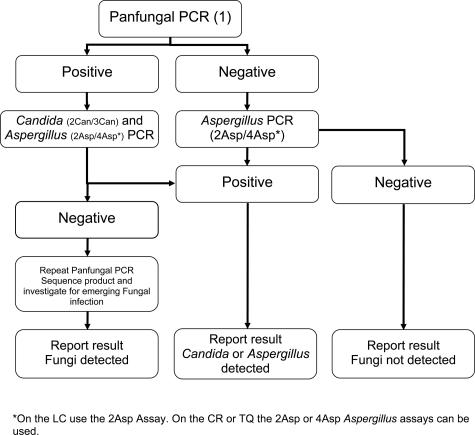
Despite being excluded after the D03 distribution panel, the primers used in method 1 have been extensively used and described in the literature,4,5,12,20 and the assay has the advantage of being pan-fungal. Because the other assays target Aspergillus alone, clinicians may be hesitant in withholding empirical treatment in patients with negative Aspergillus PCR results because the emerging infections caused by other fungal pathogens (eg, Zygomycetes, Fusarium spp., and Scedosporium spp.) are increasing.21,22 However, reduced sensitivity is a possible result of greater target diversity. By combining the pan-fungal and species-specific methods, it may be possible to develop a pre-emptive and exclusion strategy covering a range of IFIs (Figure 4). Combining pan-fungal method 1 with one of the Aspergillus methods and a Candida assay in a carefully constructed screening protocol will help in the diagnosis of invasive aspergillosis, invasive candidal infections, and other IFIs (Figure 4). A positive pan-fungal PCR result would be followed by the Aspergillus and Candida PCR assays or, if necessary, sequencing to identify the pathogen. Using the current methods, a negative pan-fungal PCR result would need to be followed by the more sensitive Aspergillus PCR assays to definitely exclude invasive aspergillosis. However, this will probably be addressed by the future development of enhanced pan-fungal assays.
Figure 4.
Schematic proposing a structure for a pan-fungal PCR using the current methods as described and assessed.
In conclusion, the UK-Irish Fungal PCR Consensus Group found real-time Candida PCR to be consistent, achieving good lower limits of detection and specificity. All three Candida assays can be recommended with the final choice being dependent on particular requirements of the specific laboratory (eg, real-time platform available, species level differentiation, or a combined Candida/pan-fungal assay). The Aspergillus methods were more variable, with two assays in particular performing better than the others. For the detection of Aspergillus in EDTA whole blood, we recommend the use of the 2Asp assay for the LC and either of the methods for the CR and TQ platforms. For enhanced sensitivity it is possible to perform a nested version of the 2Asp assay.10,13 The requirement for comparative interlaboratory studies of different Aspergillus PCR assays to provide a gold standard method has been recently highlighted.23 This research represents the start of an ongoing commitment to achieve a consensus method to improve the difficult diagnosis of IFIs that, with the possible involvement of further laboratories, may provide a method to be included in future consensus criteria for defining IFIs.
Acknowledgments
We thank the initial support of Elan pharmaceuticals for help in establishing the group and Pfizer Pharmaceuticals UK for their continued support of the group and its research.
Footnotes
Supported by Elan Pharmaceuticals and Pfizer UK Ltd.
Related Commentary on page 297
References
- Denning DW, Kibbler CC, Barnes RA. British Society for Medical Mycology proposed standards of care for patients with invasive fungal infections. Lancet Infect Dis. 2003;3:230–240. doi: 10.1016/s1473-3099(03)00580-2. [DOI] [PubMed] [Google Scholar]
- Caillot D, Couaillier J-F, Bernard A, Casasnovas O, Denning DW, Mannone L, Lopez J, Couillault G, Piard F, Vagner O, Guy H. Increasing volume and changing characteristics of invasive pulmonary aspergillosis on sequential thoracic computed tomography scans patients with neutropenia. J Clin Oncol. 2001;19:253–259. doi: 10.1200/JCO.2001.19.1.253. [DOI] [PubMed] [Google Scholar]
- Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, Denning DW, Donnelly JP, Edwards JE, Erjavec Z, Fiere D, Lortholary O, Maertens J, Meis JF, Patterson TF, Ritter J, Selleslag D, Shah PM, Stevens DA, Walsh TJ. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- Löeffler J, Schimdt K, Herbart H, Schumacher U, Einsele H. Automated extraction of genomic DNA from medically important yeast species and filamentous fungi by using the MagNA Pure LC system. J Clin Microbiol. 2002;40:2240–2243. doi: 10.1128/JCM.40.6.2240-2243.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löeffler J, Herbart H, Schumacher U, Reitze H, Einsele H. Comparison of different methods for extraction of DNA of fungal pathogens from cultures and blood. J Clin Microbiol. 1997;35:3311–3312. doi: 10.1128/jcm.35.12.3311-3312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordanides NE, Allan EK, McLintock LA, Copland M, Devaney M, Stewart K, Parker AN, Johnson PR, Holyoake TL, Jones BL. A prospective study of real-time panfungal PCR for the early diagnosis of invasive fungal infection in haemato-oncology patients. Bone Marrow Transplant. 2005;35:389–395. doi: 10.1038/sj.bmt.1704768. [DOI] [PubMed] [Google Scholar]
- White PL, Shetty A, Barnes RA. Detection of seven Candida species using the Light-Cycler system. J Med Microbiol. 2003;52:229–238. doi: 10.1099/jmm.0.05049-0. [DOI] [PubMed] [Google Scholar]
- White PL, Archer AE, Barnes RA. A comparison of non-culture based methods for the diagnosis of systemic fungal infections with an emphasis on invasive Candida infections. J Clin Microbiol. 2005;43:2181–2187. doi: 10.1128/JCM.43.5.2181-2187.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiver M, Levi K, Oppenheim BA. Rapid identification of Candida species by TaqMan PCR. J Clin Pathol. 2001;54:362–366. doi: 10.1136/jcp.54.5.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PL, Linton CJ, Perry MD, Johnson EM, Barnes RA. The evolution and evaluation of a whole blood PCR assay for the detection of invasive aspergillosis in haematology patients in a routine clinical setting. Clin Infect Dis. 2006;42:479–486. doi: 10.1086/499949. [DOI] [PubMed] [Google Scholar]
- Kami M, Fukui T, Ogawa S, Kazuyama Y, Machida U, Tanaka Y, Kanda Y, Kashima T, Yamazaki Y, Hamaki T, Mori S, Akiyama H, Mutou Y, Sakamaki H, Osumi K, Kimura S, Hirai H. Use of real-time PCR on blood samples for diagnosis of invasive aspergillosis. Clin Infect Dis. 2001;33:1504–1512. doi: 10.1086/323337. [DOI] [PubMed] [Google Scholar]
- Löeffler J, Henke N, Herbart H, Schmidt D, Hagmeyer L, Schumacher U, Einsele H. Quantification of fungal DNA by using fluorescence resonance energy transfer and the Light Cycler system. J Clin Microbiol. 2000;38:586–590. doi: 10.1128/jcm.38.2.586-590.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson ECM, Leeming JP, Palmer HP, Steward CG, Warnock D, Marks DI, Millar MR. Diagnosis of invasive aspergillosis in bone marrow transplant recipients by polymerase chain reaction. Br J Haematol. 2000;108:132–139. doi: 10.1046/j.1365-2141.2000.01795.x. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programmes. Nucleic Acid Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe RG. Two sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Newcombe RG. Improved confidence intervals for the difference between binomial proportions based on paired data. Stat Med. 1998;17:2635–2650. [PubMed] [Google Scholar]
- Newcombe RG. Interval estimation for the difference between independent proportions: comparison of 11 methods. Stat Med. 1998;17:873–890. doi: 10.1002/(sici)1097-0258(19980430)17:8<873::aid-sim779>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Kirkwood BR, Sterne JAC. Boston: Blackwell Publishing Co.; Measurement error: assessment and implications. Medical Statistics. (ed 2) 2003:429–446. [Google Scholar]
- Pham AS, Tarrand JT, May GS, Lee MS, Kontoyiannis DP, Han XY. Diagnosis of invasive mold infection by real-time quantitative PCR. Am J Clin Pathol. 2003;119:38–44. doi: 10.1309/RQ05-PP9N-EG6D-ADXR. [DOI] [PubMed] [Google Scholar]
- Einsele H, Herbart H, Roller G, Loffler J, Rothenhofer I, Muller CA, Bowden RA, van Burik J, Engelhard D, Kanz L, Schumacher U. Detection and identification of fungal pathogens in blood by using molecular probes. J Clin Microbiol. 1997;35:1353–1360. doi: 10.1128/jcm.35.6.1353-1360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- Van Burik JA, Myerson D, Schreckhise RW, Bowden RA. Panfungal PCR for detection of fungal infection in human blood specimens. J Clin Microbiol. 1998;36:1169–1175. doi: 10.1128/jcm.36.5.1169-1175.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchheidt D, Hummel M. Aspergillus polymerase chain reaction (PCR) diagnosis. Med Mycol. 2005;43:S139–S145. doi: 10.1080/13693780500051596. [DOI] [PubMed] [Google Scholar]