Abstract
Knowing the etiology of hearing loss in a person has implications for counseling and management of the condition. More than 50% of cases of early onset, nonsyndromic sensorineural hearing loss are attributable to genetic factors. However, deafness is a genetically heterogeneous condition and it is therefore currently not economically and practically feasible to screen for mutations in all known deafness genes. We have developed a microarray-based hybridization biochip assay for the detection of known mutations. The current version of the hearing loss biochip detects nine common mutations in the connexin 26 gene, four mutations in the pendrin gene, one mutation in the usherin gene, and one mutation in mitochondrial DNA. The biochip was validated using DNA from 250 people with apparent nonsyndromic, moderate to profound sensorineural hearing loss. The hearing loss biochip detected with 100% accuracy the mutations it was designed for. No false-positives or false-negative results were seen. The biochip can easily be expanded to test for additional mutations in genes associated with hearing impairment or other genetic conditions.
Approximately one child in 1000 is born with a prelingual hearing loss that can have significant impact on the infant’s speech, language, and communication skills, thereby incurring lifelong social, educational, and economic costs.1 Approximately 10% of the population is affected by hearing loss by the age of 60 years and ∼50% by the age of 80 years.2 More than half of prelingual deafness cases have a genetic basis.3 Mutations in many genes, probably more than 200, can cause deafness.4 So far, more than 40 genes associated with nonsyndromic hearing loss have been identified (Van Camp G, Smith RJH: Hereditary Hearing Loss Homepage. URL: http://webhost.ua.ac.be/hhh/). Mutations in the connexin 26 gene (GJB2) are responsible for more than half of autosomal recessive nonsyndromic hearing loss in some populations.5 Mutations in the pendrin gene (SLC26A4) can cause both nonsyndromic and syndromic (Pendred syndrome) deafness and might also contribute to a significant proportion of genetic hearing losses.6,7,8 The A1555G mitochondrial 12S rRNA mutation is associated with aminoglycoside-induced hearing loss. This mutation has been reported at a high frequency in Spanish and Japanese families with severe progressive deafness.9,10 Mutations in the usherin gene (USH2A) are responsible for the most common form of Usher syndrome, which is characterized by congenital deafness with onset of retinitis pigmentosa in late teens.11
The genetic heterogeneity of deafness has proved a challenge for genetic testing: analysis of multiple genes by conventional gel-based or sequencing methods is both time-consuming and expensive. The DNA microarray, or biochip, is a hybridization-based genotyping technique that offers simultaneous analysis of many genetic mutations. The parallelism offered by the microarray platform makes it ideally suited to genotyping of genetically heterogeneous conditions such as deafness. We have developed a proof-of-concept hearing loss chip that allows the parallel analysis of 15 common mutations or polymorphisms in the GJB2, SLC26A4, and USH2A genes and the mitochondrial (mt)DNA-encoded 12S rRNA. The chip can relatively easily be expanded to detect additional known mutations. In this study we have validated our hearing loss biochip by analyzing 250 patients with nonsyndromic hearing impairment and estimated the prevalence of these mutations in the Australian population. Availability of an inexpensive and extensive mutation screening test for mutations associated with hearing loss will result in improved diagnosis, more accurate genetic counseling, and eventually in improved management of hearing loss.
Materials and Methods
Patients
As part of clinical diagnostic testing for mutations in known deafness genes, DNA was isolated from the blood of 250 patients with moderate to profound hearing loss. All samples were tested for mutations in the GJB2 gene by DNA sequencing. All samples were also tested for the specific mutations in the pendrin, usherin, and mtDNA genes by assays based on restriction enzymes being able to cleave polymerase chain reaction (PCR) fragments at the mutation sites in PCR-amplified fragments. Internal restriction enzyme cleavage sites are present in the PCR fragments to provide positive controls for restriction enzyme cutting. The results of these tests were not accessible until after the hearing loss biochip analyses had been performed.
The Hearing Loss Biochip
The hearing loss biochip (version HL7) was designed to detect the following changes: the GJB2 W24X, 35delG, M34T, V37I, L90P, R143W, 167delT, 235delC, and 313del14 mutations or polymorphisms; the SLC26A4 L236P, E384G, T416P, and IVS8 + 1G>A mutations; the mtDNA A1555G mutation; and the USH2A (Usher2A syndrome) 2299delG mutation. The 15 mutations were selected based on basis their confirmed12 or predicted high prevalence in Australia, a country with large groups of immigrants, especiallyfrom Europe, the Middle East and Asia. The GJB2 35delG, M34T, W24X, L90P, and R143W mutations are common in Caucasians.13,14,15,16,17,18,19,20,21,22 V37I and 167delT are common in Asians and Ashkenazi Jews, respectively.23,24 The mtDNA change A1555G is common in people from Southern Europe.25 The Usherin 2299delG mutation is common in people of European background.26 The pendrin mutations L236P, IVS8 + 1, E384G, T416P are frequently found in Caucasian patients.27
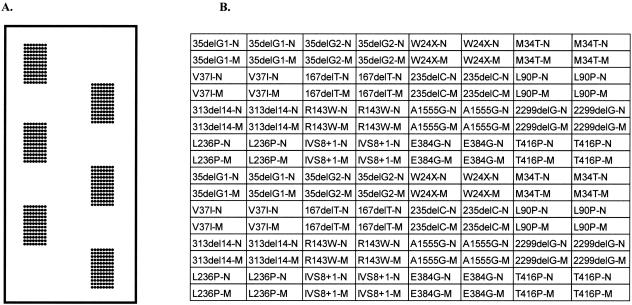
Allele-specific oligonucleotides to the normal and mutant sequences (Table 1) were printed on epoxy-coated glass slides (MWG Biotech, Ebersberg, Germany) using a MicroGrid Compact printer (BioRobotics, Apogent Discoveries, Hudson, NH). The 35delG deletion has occurred in a run of six guanine residues, which could make it difficult to detect by hybridization. For this reason, two sets of oligonucleotide were designed for this mutation to maximize the chance of effective allelic discrimination. The oligonucleotides were printed in duplicate spots with the mutation oligonucleotide printed directly below its corresponding normal oligonucleotide for each specified change. Each such set was printed in duplicate so that for each mutation there were four normal and mutation oligonucleotide spots. Six such blocks of 16 × 8 oligonucleotides were printed on each slide thereby allowing the testing of DNAs from six different people per slide (Figure 1).
Table 1.
Allele-Specific Oligonucleotides to the Normal and Mutant Sequences
| Name | Sequence |
|---|---|
| 35delG1-N | 5′-TTTTTTTTTTGATCCTGGGGGGTGTGAA-3′ |
| 35delG1-M | 5′-TTTTTTTTTTGATCCTGGGGGTGTGAA-3′-3′’ |
| 35delG2-N | 5′-TTTTTTTTTTATCCTGGGGGGTGTGAAC-3′ |
| 35delG2-M | 5′-TTTTTTTTTTATCCTGGGGGTGTGAAC-3′ |
| W24X-N | 5′-TTTTTTTTTTGAAAGATCTGGCTCACCGT-3′ |
| W24X-M | 5′-TTTTTTTTTTGAAAGATCTAGCTCACCGT-3′ |
| M34T-N | 5′-TTTTTTTTTTCGCATTATGATCCTCG-3′ |
| M34T-M | 5′-TTTTTTTTTTCGCATTACGATCCTCG-3′ |
| V37I-N | 5′-TTTTTTTTTTGATCCTCGTTGTGGCT-3′ |
| V37I-M | 5′-TTTTTTTTTTTGATCCTCATTGTGGCTG-3′ |
| 167delT-N | 5′-TTTTTTTTTTCAACACCCTGCAGCCAGG-3′ |
| 167delT-M | 5′-TTTTTTTTTTCAACACCCGCAGCCAGG-3′ |
| 235delC-N | 5′-TTTTTTTTTTTATGGGCCCTGCAGCT-3′ |
| 235delC-M | 5′-TTTTTTTTTTTATGGGCCTGCAGCT-3′ |
| L90P-N | 5′-TTTTTTTTTTCAGCGCTCCTAGTGGCCAT-3′ |
| L90P-M | 5′TTTTTTTTTTAGCGCTCCCAGTGGCCA-3′ |
| 313del14-N | 5′-TTTTTTTTTTAGGAAGTTCATCAAGGGGGA-3′ |
| 313del14-M | 5′-TTTTTTTTTTGAAGAAGAGGGGAGATAAAG-3′ |
| R143W-N | 5′-TTTTTTTTTTTCTTCTTCCGGGTCATC-3′ |
| R143W-M | 5′-TTTTTTTTTTATCTTCTTCTGGGTCATCT-3′ |
| A1555G-N | 5′-TTTTTTTTTTTTATAGAGGAGACAAGTCGTAA-3′ |
| A1555G-M | 5′-TTTTTTTTTTTTATAGAGGAGGCAAGTCGTAA-3′ |
| 2299delG-N | 5′-TTTTTTTTTTGGGCAGTGTGAGTGCAAAAA-3′ |
| 2299delG-M | 5′-TTTTTTTTTTGGCAGTGTAGTGCAAAA-3′ |
| L236P-N | 5′-TTTTTTTTTTGTCTCACAGCTAAAGATTGTC-3′ |
| L236P-M | 5′-TTTTTTTTTTGTCTCACAGCCAAAGATTGTC-3′ |
| IVS8 + 1-N | 5′-TTTTTTTTTTCCAAGGGGGTGAGTGTG-3′ |
| IVS8 + 1-M | 5′-TTTTTTTTTTCCAAGGGGATGAGTGTGG-3′ |
| E384G-N | 5′TTTTTTTTTGCTTCCTTAGGAATTCATTGCC-3′ |
| E384G-M | 5′-TTTTTTTTTTTTCCTTAGGGATTCATTGC-3′ |
| T416P-N | 5′-TTTTTTTTTTCAGGAGAGCACTGGAGGAA-3′ |
| T416P-M | 5′-TTTTTTTTTTCAGGAGAGCCCTGGAGGAA-3′ |
Oligonucleotides to wild-type sequences are indicated by a -N and to mutant sequences by a -M.
Figure 1.
A: Layout of the Hearing Loss Biochip (version HL7) showing the six identical blocks of 16 × 8 oligonucleotide spots. B: Organization of oligonucleotides in each block. Oligonucleotides to wild-type sequences are indicated by a -N and to mutant sequences by a -M.
Multiplex PCR Amplification
To test for the mutations using the hearing loss biochip the 250 patient DNAs were each amplified in three 25-μl multiplex PCR reactions using HotStarTaq polymerase (Qiagen, Valencia, CA) as recommended by the manufacturer. Biotin was incorporated into the probes by using a nucleotide mix in the PCR reactions to make the final concentrations of 0.2 mmol/L each of dATP, dCTP, and dGTP, 0.15 mmol/L dTTP, and 0.05 mmol/L biotin-dUTP. The primers are listed in Table 2. Reverse primers were made with phosphorothioate bonds to prevent degradation during T7 gene 6 exonuclease treatment when generating single-stranded probes (see below). PCR consisted of one cycle of denaturation for 5 minutes at 94°C followed by 40 cycles of denaturation for 30 seconds at 94°C, annealing for 30 seconds at 58°C, and extension for 30 seconds at 72°C, followed by a final extension step for 5 minutes at 72°C. The three multiplex PCR reactions were pooled and purified on a Qiagen MinElute column according to the manufacturer’s instructions. The fragments were eluted in 12 μl of 10 mmol/L Tris-Cl, pH 7.5, made single stranded by adding 3 μl of 5×T7 gene 6 exonuclease buffer and 0.5 μl of T7 gene 6 exonuclease (USB Corp., Cleveland, OH), followed by a 20-minute incubation at 37°C and heat inactivation at 90°C for 10 minutes. Products were stored at −20°C until use.
Table 2.
Oligonucleotide Primers Used in Multiplex PCR Reactions
| Gene (size of PCR product) | To detect mutation(s) | Forward primer | Reverse primer | |
|---|---|---|---|---|
| 10× Primer mix 1 | ||||
| Cx26 (286 bp) | W24X, 35delG, M34T, V37I | 5′-TCTTTTCCAGAGCAAACCGC-3′ | 5′-GsAsCsAsCsGAAGATCAGCTGCA-3′ | |
| MtDNA 12 S RNA (137 bp) | A1555G | 5′-CGTCACCCTCCTCAAGTATACTTC-3′ (2 μmol/L) | 5′-GsCsTsTsTsGTGTTAAGCTACACTCTGG-3′ (2 μmol/L) | |
| 10× Primer mix 2 | ||||
| SLC26A4 (249 bp) | L236P | 5′-GGTTTCTATCTCAGGCAAACAT-3′ | 5′-AsTsTsGsTsTTCTGGAATGAACAGTGACC-3′ | |
| SLC26A4 (139 bp) | IVS8 + 1G>A | 5′-TTCAGACGATAATTGCTACTG-3′ | 5′-GsAsCsTsGsACTTACTGACTTAATG-3′ | |
| SLC26A4 (215 bp) | E384G, T416P | 5′-GTAGGATCGTTGTCATCCAG-3′ | 5′-CsGsAsGsCsCTTCCTCTGTTGC-3′ | |
| 10× Primer mix 3 | ||||
| GJB2 (311 bp) | L90P, R143W, 167delT, 235delC, 313del14 | 5′-CTGCAGCTGATCTTCGTGTC-3′ | 5′-AsCsAsAsAsGCAGTCCACAGTGTT-3′ | |
| USH2A (159 bp) | 2299delG | 5′-ATGTGAGCCCTGCCAGTGTA-3′ | 5′-TsCsAsCsAsGGCCTTACAATTGGTG-3′ |
The 10× primer mixes are 4 μmol/L of each primer unless indicated. s, phosphorothioate bond.
Hybridization
Six 10-mm diameter round glass coverslips were mounted with glue onto a plain 64 × 24 mm-glass coverslip to match the regions of the six oligonucleotide blocks. The hybridization reaction contained 2 μl of pooled single-stranded PCR products and 2 μl of hybridization buffer (5×SSPE, 0.01% Triton X-100). It was heated to 90°C for 5 minutes and snap-cooled in ice. Six such hybridization mixtures (2.5 μl each) were pipetted onto the six 10-mm diameter coverslips. We have determined that 2.5 μl is sufficient to give good coverage of the hybridization grid without spreading beyond the coverslips, thereby avoiding cross-contamination. The hearing loss biochip slide (print side down) was then lowered onto the probe-containing coverslips with the array grids aligned to coverslips. The hybridization mixes were allowed to spread to the edges of the coverslips and the slide was carefully flipped over so that the coverslips were on top of the hearing loss biochip arrays. The hearing loss biochip was placed in a hybridization cassette containing 2× standard saline citrate (SSC) in the humidification wells and incubated in a 45°C water bath for 30 minutes. After hybridization the coverslips were removed, and the hearing loss biochip was washed at 45°C as follows: 2× SSC/0.1% sodium dodecyl sulfate (3 minutes), 0.5× SSC/0.1% sodium dodecyl sulfate (5 minutes), 2× SSC (1 minute), and 4× SSC/0.2% Tween (1 minute).
During the washing steps, 20 μl of streptavidin-Cy5 diluted 1:250 in blocking solution (4× SSC, 0.2% Tween 20/1% bovine serum albumin) was pipetted onto a 64 × 24-mm coverslip. After washing, the hearing loss biochip was drained briefly (but not allowed to dry out). The hearing loss biochip was placed on the streptavidin-containing coverslip and the solution allowed to spread to the edges of the coverslip. The slides were then incubated in a damp chamber in the dark at room temperature for 30 to 60 minutes. The coverslips were removed, and the hearing loss biochip washed twice in 4× SSC, 0.2% Tween at 45°C for 3 minutes and once in 0.1× SSC at room temperature for 2 minutes. The hearing loss biochip was drained briefly, dried by centrifugation at 50 × g for 3 minutes in a 50-ml Falcon tube (Benton Dickinson Labware, Franklin Lakes, NJ) with tissue padding in the bottom and stored in a dark, dry place until scanning.
Analysis of Results
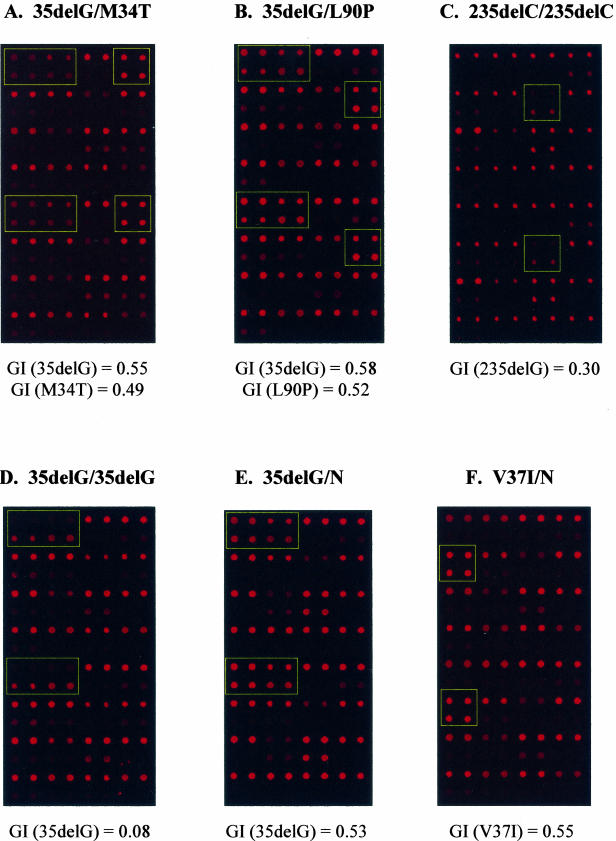
The hearing loss biochips were scanned in a Gene Pix 4000B (Axon Instruments; Molecular Devices Corp., Sunnyvale, CA) scanner using the red Cy5 channel (635 nm). Examples are shown in Figure 2. Spot intensities were quantitated using the Gene Pix Pro4.1 scanner software and the data imported into Microsoft Excel (Microsoft Corp., Redmond, WA). We have written a small software program to analyze the data and automatically call the genotype. Using the background subtracted median pixel intensity as the spot value (SV), the genotype index (GI) for each normal and mutant spot pair was calculated by the following program: GI = SVN/(SVN + SVM), where SVN = normal SV and SVM = mutant SV. GI values for replicate spot pairs were averaged and used to call genotype for each mutation. Cutoff GI values for genotypes for individual mutations are based on being within 2 SDs of the GI values determined after more than 20 hybridizations to samples with no or known mutations. Cutoff GI values are shown in Table 3. The signal-to-noise ratios for all mutations were better than 5:1.
Figure 2.
Examples of hybridization signals. A–F: Shown are some of the connexin 26 mutations in panels. The GIs calculated from the hybridization signals of mutant spots are also shown.
Table 3.
Genotype Index (GI) Values Used to Determine Genotypes for the 15 Mutations
| Mutation | Normal | Heterozygous | Homozygous mutation |
|---|---|---|---|
| 35delG | 0.75 to 1.00 | 0.40 to 0.65 | 0.01 to 0.17 |
| W24X | 0.89 to 1.00 | 0.48 to 0.80 | 0.09 to 0.33 |
| M34T | 0.81 to 1.00 | 0.33 to 0.55 | 0.07 to 0.25 |
| V37I | 0.82 to 1.00 | 0.38 to 0.59 | 0.00 to 0.28 |
| 167delT | 0.80 to 1.00 | 0.36 to 0.58 | 0.00 to 0.18 |
| 235delC | 0.81 to 1.00 | 0.46 to 0.62 | 0.00 to 0.36 |
| L90P | 0.87 to 1.00 | 0.38 to 0.60 | 0.00 to 0.28 |
| 313del14 | 0.82 to 1.00 | 0.63 to 0.80 | 0.00 to 0.58 |
| R143W | 0.80 to 1.00 | 0.41 to 0.67 | 0.00 to 0.31 |
| A1555G | (0.65 to 1.00) | (0.00 to 0.14) | |
| 2299delG | 0.91 to 1.00 | 0.53 to 0.85 | 0.21 to 0.37 |
| L236P | 0.70 to 1.00 | 0.27 to 0.65 | 0.00 to 0.17 |
| IVS8 + 1 | 0.82 to 1.00 | 0.29 to 0.63 | 0.00 to 0.19 |
| E384G | 0.91 to 1.00 | 0.50 to 0.81 | 0.00 to 0.40 |
| T416P | 0.90 to 1.00 | 0.37 to 0.80 | 0.00 to 0.27 |
The values for the mitochondrial A1555G mutation are in paren-theses because the level of this mutation can vary from 0 to 100% (heteroplasmy).
Results
The coding region of the human connexin 26 gene is contained within exon 2. This exon and its intron boundaries were sequenced in 250 patients with hearing loss as part of diagnostic investigations for a genetic cause of their hearing loss. DNA from these 250 patients was used to validate the hearing loss biochip. Of these 250 people, 30 had two GJB2 mutations, 46 had one GJB2 mutation, two had a single USH2A mutation, and one had a single SLC26A4 mutation (Table 4).
Table 4.
Mutations Detected in the 250 Individuals
| Genotype | Gene | Number of individuals |
|---|---|---|
| 35delG/35delG | GJB2 | 10 |
| W24X/W24X | GJB2 | 3 |
| 35delG/M34T | GJB2 | 2 |
| 235delC/V37I | GJB2 | 2 |
| 35delG/(W77R) | GJB2 | 2 |
| V37I/V37I | GJB2 | 2 |
| M34T/V37I | GJB2 | 1 |
| 35delG/235delC | GJB2 | 1 |
| 35delG/R143W | GJB2 | 1 |
| 35delG/167delT | GJB2 | 1 |
| 35delG/(T186A) | GJB2 | 1 |
| 167delT/(R32H) | GJB2 | 1 |
| V37I/(V95M) | GJB2 | 1 |
| 35delG/(H100Y) | GJB2 | 1 |
| 235delC/235delC | GJB2 | 1 |
| 35delG/N | GJB2 | 27 |
| M34T/N | GJB2 | 6 |
| V37I/N | GJB2 | 3 |
| L90P/N | GJB2 | 2 |
| 167delT/N | GJB2 | 1 |
| L236P/N | SLC26A4 | 1 |
| 2299delG/N | USH2AI | 2 |
| (W77R)/N | GJB2 | 1 |
| (R143Q)/N | GJB2 | 2 |
| (112-113delGT)/N | GJB2 | 1 |
| (M151L)/N | GJB2 | 2 |
| (269insT)/N | GJB2 | 1 |
Mutations in parentheses were not on the Biochip and therefore only identified by DNA sequencing. All other mutations were detected both by the Biochip and by DNA sequencing.
Mutations on the chip were detected with 100% accuracy. Both oligonucleotide sets designed to detect the GJB2 35delG mutation performed well. Of 30 people with two GJB2 mutations (based on sequencing) the hearing loss biochip detected both mutations in 24 people and one of the two mutations in six people. This version of the hearing loss biochip was not designed to detect the five mutations that were not identified: R32H, V95M, W77R (present in two patients), T186A, and H100Y.
Of 46 people with one GJB2 mutation (based on sequencing) the hearing loss biochip detected the single mutation in 39 people. Seven mutations were not on the hearing loss biochip: W77R, R143Q (present in two patients), 112-113delGT, M151L (present in two patients), and 269insT (Table 4). One person had a single SLC26A4 L236P mutation. Two people had a single USH2A 2299delG mutation. These results were confirmed by PCR followed by mutation-specific restriction enzyme digestion or DNA sequencing.
Discussion
Hearing loss is caused by genetic and/or environmental factors. A timely diagnosis of the genetic etiology for congenital deafness expedites early intervention (such as teaching sign language, fitting hearing aids or cochlear implants) and aids in management decisions and genetic counseling. However, the genetic heterogeneity of deafness has proved a challenge for genetic testing. Searching for causative mutations is in many cases not financially or practically feasible, because the mutations can be in one of several hundred genes, many of which are not yet identified. Demand for genetic testing for deafness has risen sharply, partly because of the identification of some of the genes associated with hearing loss and partly because of the introduction of Universal Neonatal Hearing Screening Programs in many countries. There is therefore a need for simpler, cheaper, and more comprehensive tests for mutations that cause hearing loss.
We have explored an oligonucleotide-array based approach to the analysis of mutations. This allows for the simultaneous screening for a large number of specific mutations in known genes. A screen can be done in less than 2 days. The mutations that are detected by the chip can easily be expanded or changed. Recurrent new mutations can be added or chips can be designed to detect common mutations in different ethnic populations. Sequence analysis of the relatively small and simple GJB2 gene is simple and might therefore be the preferred first test in searching for mutations in genes associated with deafness. The hearing loss biochip could therefore be designed to detect common mutations in genes other than GJB2 and used to screen samples in which no GJB2 mutations were found. In this study we have used the approach to investigate 15 known mutations in genes associated with deafness.
We have validated the hearing loss biochip on DNA from 250 deaf people. Sequencing had shown that 30 of these (12%) carried two GJB2 mutations. Ten people (4%) were homozygous, and nine patients (4%) were compound heterozygous for the 35delG mutation. The common 35delG mutation accounted for 29 of 60 alleles (48%) in the patients with two known GJB2 changes. The hearing loss biochip currently allows detection of nine GJB2 mutations or polymorphisms. The V37I, M34T, and L90P changes appear to be mutations associated with slight/mild to moderate hearing loss,14 although their contributions to hearing losses are still in dispute. All patients with two GJB2 mutations carried at least one mutation detectable by the biochip. However, six patients were compound heterozygotes with one mutation not represented on the hearing loss biochip. The T186A and 112-113delGT changes have not been reported before. The hearing loss biochip could be relatively easily expanded to include these and additional mutations. Identification of just one mutation in a patient using the hearing loss biochip could also justify sequencing the gene in question to search for a possible second mutation.
The incidence of deaf people with only one GJB2 mutation was 18% (46 of the 250 patients). This number is higher than previously observed in the Australian population.12 The hearing loss biochip detected 39 of these patients. In seven instances the mutation was not included on the hearing loss biochip. The M151L change has not previously been reported. The R143Q mutation has been associated with dominantly inherited hearing loss,19 but we did not have family histories from the two individuals with this mutation. Again the missed mutations could be added to the testing regime. Of the 46 patients in which only one GJB2 mutation was detected, 27 (59%) carried the 35delG mutation.
It has recently been shown that deletions in or near the GJB6 (connexin 30) gene combine with monoallelic GJB2 mutations to cause deafness.28 The patients were tested for the GJB6-D13S1830 deletion near the GJB6 gene. This deletion was not found in any of the 46 GJB2 carriers. The relatively large group of deaf people with only one detectable GJB2 mutation creates a significant problem when counseling the affected families because it is not clear if the mutation is part of the etiology of the hearing loss. Alternatively, the affected person might be a carrier of the recessive mutation and the hearing loss caused by environmental or other genetic factors.
The current version of the hearing loss biochip also detects four common mutations in the SLC26A4 gene, one mutation in the USH2A gene and the mitochondrial A1555G mutation. One of the patients carried the SLC26A4 L236P mutation and two patients carried the USH2A 2299delG mutation. One of the samples that was heterozygous for the USH2A 2299delG mutation is also a compound heterozygote for GJB2 mutations 35delG/M34T.
The GJB2 313del14, the SLC26A4 IVS8 + 1G>A, E384G, T416P, and the mtDNA A1555G mutations were not present in any of the 250 patient samples. However, we have subsequently shown that the hearing loss biochip reliably detects these mutations using DNA from patients known to carry the changes.
This oligonucleotide-based test can relatively easily be expanded to a much larger number of mutations and is of course not restricted to genes associated with hearing loss. Testing for mutations in the GJB2 gene is often the only diagnostic test available for genetic hearing loss, because the gene is small and can therefore be easily and inexpensively analyzed. Using the hearing loss biochip it will be possible to quickly screen for known mutations in other genes implicated in hearing loss. Of special importance are genes involved in syndromic deafness for which knowledge about mutations in these genes may have implications for management of the patient, such as genes for Usher syndrome or Jervell and Lange-Nielsen syndrome. In cases in which only one mutation is detected in a deafness gene, further analysis will be needed to confirm the presence or absence of a second recessive mutation. All of our 30 patients with two GJB2 mutations had at least one mutation detected by the current version of the hearing loss biochip. By adding oligonucleotides for the detection of additional mutations in other deafness genes to the hearing loss biochip it is possible to reduce the number of patients missed because more mutations can be detected.
Based on array technology and oligonucleotide hybridization we have designed a novel method for analyzing patient DNAs for known mutations in deafness genes. The current version of the hearing loss biochip detects 15 mutations, but it can easily be expanded to test a much larger number of mutations. It is also possible to use a multiarray slide system so that, for example, 16 DNAs can be screened on one slide. Furthermore, the PCR and hybridization procedures have the potential to be automated on commercial microarray or lab-on-a-chip platforms and therefore become part of routine genetic screening procedures. We did not find any false-positives or false-negative results in our validation of the hearing loss biochip. This microarray-based method therefore provides a relatively inexpensive, robust, and rapid approach to testing for known mutations.
Acknowledgments
We thank Prof. R. Smith and Dr. C. Nishimura for helpful discussions.
Footnotes
Supported by The Garnett Passe and Rodney Williams Memorial Foundation, the Murdoch Childrens Research Institute, J. and J. Calvert-Jones, and the ANZ Charitable Services.
H.-H.M. Dahl is a National Health and Medical Research Council principal research fellow.
References
- Yoshinaga-Itano C. Benefits of early intervention for children with hearing loss. Otolaryngol Clin North Am. 1999;32:1089–1102. doi: 10.1016/s0030-6665(05)70196-1. [DOI] [PubMed] [Google Scholar]
- Davis AC. London: Whurr,; Hearing in Adults. 1995 [Google Scholar]
- Morton NE. Genetic epidemiology of hearing impairment. Ann NY Acad Sci. 1991;630:16–31. doi: 10.1111/j.1749-6632.1991.tb19572.x. [DOI] [PubMed] [Google Scholar]
- Petit C. Genes responsible for human hereditary deafness: symphony of a thousand. Nat Genet. 1996;14:385–391. doi: 10.1038/ng1296-385. [DOI] [PubMed] [Google Scholar]
- Kenneson A, Van Naarden Braun K, Boyle C. GJB2 (connexin 26) variants and nonsyndromic sensorineural hearing loss: a HuGE review. Genet Med. 2002;4:258–274. doi: 10.1097/00125817-200207000-00004. [DOI] [PubMed] [Google Scholar]
- Park HJ, Shaukat S, Liu XZ, Hahn SH, Naz S, Ghosh M, Kim HN, Moon SK, Abe S, Tukamoto K, Riazuddin S, Kabra M, Erdenetungalag R, Radnaabazar J, Khan S, Pandya A, Usami SI, Nance WE, Wilcox ER, Griffith AJ. Origins and frequencies of SLC26A4 (PDS) mutations in east and south Asians: global implications for the epidemiology of deafness. J Med Genet. 2003;40:242–248. doi: 10.1136/jmg.40.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp P. Pendred’s syndrome: identification of the genetic defect a century after its recognition. Thyroid. 1999;9:65–69. doi: 10.1089/thy.1999.9.65. [DOI] [PubMed] [Google Scholar]
- Hutchin T, Coy NN, Conlon H, Telford E, Bromelow K, Blaydon D, Taylor G, Coghill E, Brown S, Trembath R, Liu XZ, Bitner-Glindzicz M, Mueller R. Assessment of the genetic causes of recessive childhood non-syndromic deafness in the UK—implications for genetic testing. Clin Genet. 2005;68:506–512. doi: 10.1111/j.1399-0004.2005.00539.x. [DOI] [PubMed] [Google Scholar]
- Estivill X, Govea N, Barcelo E, Badenas C, Romero E, Moral L, Scozzri R, D’Urbano L, Zeviani M, Torroni A. Familial progressive sensorineural deafness is mainly due to the mtDNA A1555G mutation and is enhanced by treatment of aminoglycosides. Am J Hum Genet. 1998;62:27–35. doi: 10.1086/301676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami S, Abe S, Akita J, Namba A, Shinkawa H, Ishii M, Iwasaki S, Hoshino T, Ito J, Doi K, Kubo T, Nakagawa T, Komiyama S, Tono T, Komune S. Prevalence of mitochondrial gene mutations among hearing impaired patients. J Med Genet. 2000;37:38–40. doi: 10.1136/jmg.37.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eudy JD, Yao S, Weston MD, Ma-Edmonds M, Talmadge CB, Cheng JJ, Kimberling WJ, Sumegi J. Isolation of a gene encoding a novel member of the nuclear receptor superfamily from the critical region of Usher syndrome type IIa at 1q41. Genomics. 1998;50:382–384. doi: 10.1006/geno.1998.5345. [DOI] [PubMed] [Google Scholar]
- Dahl HH, Saunders K, Kelly TM, Osborn AH, Wilcox S, Cone-Wesson B, Wunderlich JL, Du Sart D, Kamarinos M, Gardner RJ, Dennehy S, Williamson R, Vallance N, Mutton P. Prevalence and nature of connexin 26 mutations in children with non-syndromic deafness. Med J Aust. 2001;175:191–194. doi: 10.5694/j.1326-5377.2001.tb143093.x. [DOI] [PubMed] [Google Scholar]
- Denoyelle F, Weil D, Maw MA, Wilcox SA, Lench NJ, Allen-Powell DR, Osborn AH, Dahl HH, Middleton A, Houseman MJ, Dode C, Marlin S, Boulila-ElGaied A, Grati M, Ayadi H, BenArab S, Bitoun P, Lina-Granade G, Godet J, Mustapha M, Loiselet J, El-Zir E, Aubois A, Joannard A, Levilliers J, Garabedian EN, Mueller RF, Mac Gardner RJ, Petit C. Prelingual deafness: high prevalence of a 30delG mutation in the connexin 26 gene. Hum Mol Genet. 1997;6:2173–2177. doi: 10.1093/hmg/6.12.2173. [DOI] [PubMed] [Google Scholar]
- Snoeckx RL, Huygen PLM, Feldmann D, Marlin S, Denoyelle F, Waligora J, Mueller-Malesinska M, Pollak A. GJB2 mutations and degree of hearing loss: a multi-center study. Am J Hum Genet. 2005;77:945–957. doi: 10.1086/497996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minarik G, Ferak V, Ferakova E, Ficek A, Polakova H, Kadasi L. High frequency of GJB2 mutation W24X among Slovak Romany (Gypsy) patients with non-syndromic hearing loss (NSHL). Gen Physiol Biophys. 2003;22:549–556. [PubMed] [Google Scholar]
- Toth T, Kupka S, Haack B, Riemann K, Braun S, Fazakas F, Zenner HP, Muszbek L, Blin N, Pfister M, Sziklai I. GJB2 mutations in patients with non-syndromic hearing loss from Northeastern Hungary. Hum Mutat. 2004;23:631–632. doi: 10.1002/humu.9250. [DOI] [PubMed] [Google Scholar]
- Tekin M, Duman T, Bogoclu G, Incesulu A, Comak E, Ilhan I, Akar N. Spectrum of GJB2 mutations in Turkey comprises both Caucasian and Oriental variants: roles of parental consanguinity and assortative mating. Hum Mutat. 2003;21:552–553. doi: 10.1002/humu.9137. [DOI] [PubMed] [Google Scholar]
- Pampanos A, Economides J, Iliadou V, Neou P, Leotsakos P, Voyiatzis N, Eleftheriades N, Tsakanikos M, Antoniadi T, Hatzaki A, Konstantopoulou I, Yannoukakos D, Gronskov K, Brondum-Nielsen K, Grigoriadou M, Gyftodimou J, Iliades T, Skevas A, Petersen MB. Prevalence of GJB2 mutations in prelingual deafness in the Greek population. Int J Pediatr Otorhinolaryngol. 2002;65:101–108. doi: 10.1016/s0165-5876(02)00177-5. [DOI] [PubMed] [Google Scholar]
- Loffler J, Nekahm D, Hirst-Stadlmann A, Gunther B, Menzel HJ, Utermann G, Janecke AR. Sensorineural hearing loss and the incidence of Cx26 mutations in Austria. Eur J Hum Genet. 2001;9:226–230. doi: 10.1038/sj.ejhg.5200607. [DOI] [PubMed] [Google Scholar]
- Brobby GW, Muller-Myhsok B, Horstmann RD. Connexin 26 R143W mutation associated with recessive nonsyndromic sensorineural deafness in Africa. N Engl J Med. 1998;338:548–550. doi: 10.1056/NEJM199802193380813. [DOI] [PubMed] [Google Scholar]
- Abe S, Usami S, Shinkawa H, Kelley PM, Kimberling WJ. Prevalent connexin 26 gene (GJB2) mutations in Japanese. J Med Genet. 2000;37:41–43. doi: 10.1136/jmg.37.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelmann C, Amedofu GK, Albrecht K, Muntau B, Gelhaus A, Brobby GW, Horstmann RD. Pattern of connexin 26 (GJB2) mutations causing sensorineural hearing impairment in Ghana. Hum Mutat. 2001;18:84–85. doi: 10.1002/humu.1156. [DOI] [PubMed] [Google Scholar]
- Wattanasirichaigoon D, Limwongse C, Jariengprasert C, Yenchitsomanus PT, Tocharoenthanaphol C, Thongnoppakhun W, Thawil C, Charoenpipop D, Pho-iam T, Thongpradit S, Duggal P. High prevalence of V37I genetic variant in the connexin-26 (GJB2) gene among non-syndromic hearing-impaired and control Thai individuals. Clin Genet. 2004;66:452–460. doi: 10.1111/j.1399-0004.2004.00325.x. [DOI] [PubMed] [Google Scholar]
- Lerer I, Sagi M, Malamud E, Levi H, Raas-Rothschild A, Abeliovich D. Contribution of connexin 26 mutations to nonsyndromic deafness in Ashkenazi patients and the variable phenotypic effect of the mutation 167delT. Am J Med Genet. 2000;95:53–56. doi: 10.1002/1096-8628(20001106)95:1<53::aid-ajmg11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Gallo-Teran J, Morales-Angulo C, del Castillo I, Villamar M, Moreno-Pelayo MA, Garcia-Mantilla J, Moreno F. Incidence of A1555G mutations in the mitochondrial DNA and 35delG in the GJB2 gene (connexin-26) in families with late onset non-syndromic sensorineural hearing loss from Cantabria. Acta Otorrinolaringol Esp. 2002;53:563–571. doi: 10.1016/s0001-6519(02)78349-0. [DOI] [PubMed] [Google Scholar]
- Dreyer B, Tranebjaerg L, Brox V, Rosenberg T, Moller C, Beneyto M, Weston MD, Kimberling WJ, Cremers CW, Liu XZ, Nilssen O. A common ancestral origin of the frequent and widespread 2299delG USH2A mutation. Am J Hum Genet. 2001;69:228–234. doi: 10.1086/321269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle B, Reardon W, Herbrick JA, Tsui LC, Gausden E, Lee J, Coffey R, Grueters A, Grossman A, Phelps PD, Luxon L, Kendall-Taylor P, Scherer SW, Trembath RC. Molecular analysis of the PDS gene in Pendred syndrome. Hum Mol Genet. 1998;7:1105–1112. doi: 10.1093/hmg/7.7.1105. [DOI] [PubMed] [Google Scholar]
- del Castillo I, Villamar M, Moreno-Pelayo MA, del Castillo FJ, Alvarez A, Telleria D, Menendez I, Moreno F. A deletion involving the connexin 30 gene in nonsyndromic hearing impairment. N Engl J Med. 2002;346:243–249. doi: 10.1056/NEJMoa012052. [DOI] [PubMed] [Google Scholar]