Abstract
Previous studies performed on Sardinian patients affected by cystic fibrosis (CF) have led to the identification of molecular defects in 87 of 88 patients. Two mutations, the F508del and T338I, were quite prevalent and accounted for 50% and 20% of the molecular defects, respectively. T338I has been detected rarely in other populations, most likely because of the genetic isolation of Sardinians. In the present study, we have performed a molecular analysis of the CF gene in eight Sardinian patients in whom only a single mutation has been defined. Using DNA analyses (Southern blot, single nucleotide polymorphisms, microsatellite analyses, and Extra-Long polymerase chain reaction) selected to detect gross gene rearrangement and by using mRNA studies, we detected a novel mutation c.54-5811_164 + 2186del8108ins182 in six of the eight patients investigated. This mutation consists of a gross deletion of 8108 bp spanning exon 2 with an insertion of 182 bp at the deletion junction, between nucleotide 54-5811 of intron 1 (IVS1 nt16864) and nucleotide 164 + 2186 of intron 2 (IVS2 nt 2186). By including the novel mutation in our mutation panel we are now able to reach a 95% detection rate, thereby improving the process of carrier detection and genetic counseling in Sardinia.
Cystic fibrosis (CF; Online Mendelian Inheritance in Man #219700) is the most frequent severe autosomal recessive disorder in the European population.1 Indeed, CF affects approximately one in 2500 births, and approximately one in 25 individuals are heterozygotes, with marked regional variations.2 CF is caused by mutations of the cystic fibrosis transmembrane conductance regulator (CFTR or ABCC7; Online Mendelian Inheritance in Man #602421) gene, which is also involved in a broad spectrum of phenotypes including male infertility due to congenital bilateral absence of the vas deferens,3,4 disseminated bronchiectasis,5,6 and chronic pancreatitis.7,8 The mutational spectrum of this disease is comprised of more than 1300 different mutations of which 98% consists of point mutations or microdeletions/insertions.9 A number of cases, however, still remain uncharacterized, even after extensive screening of all 27 exons. CF mutations as yet unidentified may lie in introns or in regulatory regions, which are not routinely investigated, or may result from gene rearrangements such as large deletions that escape detection using current polymerase chain reaction (PCR)-based techniques. Recently, an Italian group showed that previously described CFTR gene rearrangements account for ∼20% of the undetected CF alleles in the Italian population.10 Some newer gross rearrangements, like the CFTRdele2,3 and the 3120 + 1kbdel8.6kb deletion, have been described recently and the respective deletion break-points characterized, allowing their inclusion in the mutation panel tested by conventional PCR using specific primers.11,12
In the Sardinian population, cystic fibrosis is characterized by the presence of two prevalent mutations, the F508del and T338I mutations, which account for ∼50% and 20% of the CF alleles, respectively. The T338I, described previously by our group,13 is a mild mutation characterized by pancreatic sufficiency, hyponatremia, hypochloremia, metabolic alkalosis, positive sweat test, and mild pulmonary involvement. This is consistent with the fact that CF patients with T338I and other severe mutations show a mild phenotype.
The list of the remaining mutations and their respective frequencies is reported in Table 1. However, a significant percentage of the Sardinian CF mutations (∼4.5%) still remain unidentified.
Table 1.
Mutations Identified in Sardinian Population
| Mutation | Chromosomes (%) | Exon/intron |
|---|---|---|
| F508del | 88 (50) | 10 |
| T338I | 34 (19.31) | 7 |
| 2183AA>G | 10 (5.68) | 13 |
| G542X | 9 (5.11) | 11 |
| c.54-5811_164+2186del-8108ins182 | 6 (3.4) | Intron 1/intron 2 |
| N1303K | 4 (2.27) | 21 |
| G1244E | 3 (1.7) | 20 |
| 1706del17bp | 2 (1.13) | 10 |
| 3849+10KbC>T | 2 (1.13) | Intron19 |
| 991del5bp | 2 (1.13) | 6b |
| S912X | 2 (1.13) | 15 |
| G576A | 1 (0.57) | 12 |
| 1717–1G>A | 1 (0.57) | Intron 10 |
| 621+1G>T | 1 (0.57) | Intron 4 |
| G85E | 1 (0.57) | 3 |
| L375F | 1 (0.57) | 8 |
| 4016insT | 1 (0.57) | 21 |
| H1054D | 1 (0.57) | 17 |
| 711+3A>G | 1 (0.57) | Intron 5 |
| D1270N − R74W | 1 (0.57) | 20–3 |
| 2184insA | 1 (0.57) | 13 |
| L997F | 1 (0.57) | 17a |
| R1066H | 1 (0.57) | 17a |
| 1001+3A>T | 1 (0.57) | Intron 6b |
| Defined | 175 (99.4) | |
| Undefined | 1(0.57) | |
| Total | 176 (99.97) |
The novel mutation detected in this study is evidenced in bold.
In this paper we describe the molecular characterization of the CF mutations in eight of 176 patients from Sardinia enrolled in our center, with one undefined CF allele, which was obtained by both DNA and RNA analyses.
Patients and Methods
Patients
This study includes eight unrelated CF patients of Sardinian origin affected by a classical form of cystic fibrosis. Clinical features of affected individuals include pancreatic insufficiency, chronic lung disease with Pseudomonas aeruginosa infections, and sweat test >60 mmol/L [Cl−].
Extensive molecular analysis (described in detail below) led to the definition of one mutation in each patient, of which the F508del in five, and the 1717–1G>A, the 991del5bp, and the 2183AA>G mutation, respectively, in the remaining three.
Methods
DNA Analysis
DNA extraction was performed in accordance with standard procedures.14 Informed consent was obtained in all cases before collecting both patient and parent samples. Screening for the most common CF mutations (F508del, T338I, G542X, N1303K, 2183AA->G, G1244E, 3849 + 10kbC->T, S912X) was performed by reverse dot blot hybridization on amplified DNA prepared in our laboratory. The 1706del17bp and 991del5bp mutations were detected by heteroduplex analysis with electrophoresis of PCR products of exons 10 and 6b in 8% polyacrilamide gel. Samples not identified by this first step were analyzed by Inno-Lipa CFTR 19 and 17+Tn Kit (Innogenetics, Gent, Belgium), which provides a multiparameter screening for 36 CFTR gene mutations thus expanding the tested CFTR mutational spectrum. This method allowed us to detect less common mutations (ie, 1717–1G>A, 621 + 1G>T, and G85E). Denaturing gradient gel-electrophoresis15 and/or denaturing high-performance liquid chromatography16 analysis of all 27 CFTR exons was performed in those patients who were not defined by the above approach. This further analysis allowed for the detection of the rare G576A L375F, 4016insT, H1054D, 711 + 3A>G, D1270N-R74W, 2184insA, L997F, R1066H, and 1001 + 3A>T mutations. Sequence of the primers and conditions used to perform denaturing gradient gel-electrophoresis/denaturing high-performance liquid chromatography analysis are available on request.
Intronic microsatellite, single nucleotide polymorphisms (SNPs), and sequence analyses have been performed on ABI PRISM 3100 (Applied Biosystems, Foster City, CA). Sequence of primer and PCR/cycle sequencing conditions are available on request. To detect CFTR large deletions in the region of exons 1 to 5, Southern blot analysis was performed in accordance with standard procedures.17 Genomic DNA was digested with the following restriction endonucleases: HindIII, EcoRI, BamHI, PstI, NcoI. Digested DNA was hybridized with CFTR cDNA probe spanning exons 1 to 5. To define the deletion breakpoint, Extra-Long PCR (XL-PCR) was performed with GeneAmp XL PCR kit (Applied Biosystems) in accordance with the manufacturer’s instructions.
PCR flanking the deletion breakpoints has been performed by duplex PCR assay. Primers BD forward 5′-TGCTAAATACCTTGTGGAATCAGA-3′ and BD reverse 5′-GGTTTGTACTGGCATAGCATTG-3′ which flank the deletion, amplify a 459-bp fragment in the presence of the deletion, whereas primers 2 forward 5′-GTGAATATCTGTTCCTCCTCTC-3′ and 2 reverse 5′-TGGTATCAAACTCCTGGTCTC-3′ generate a 338-bp product containing exon 2 that ensures an internal amplification control and can distinguish between homozigotes and heterozigotes for the deletion.
RNA Analysis
RNA was extracted from nasal epithelial cells and collected using cyto-brush from four patients, their parents, and five non-CF control subjects. cDNA synthesis was performed with the use of High Capacity cDNA Archive kit (Applied Biosystems) in accordance with the manufacturer’s instructions. The cDNA was amplified in six overlapping fragments. Sequence of primers and PCR conditions are available on request.
Results
Mutation analysis
Denaturing high-performance liquid chromatography analysis of all of the 27 exons in the eight patients allowed us to identify in a single patient the 1001 + 3A>T mutation in intron 6b (inherited from the mother), which was recently described in (http://www.genet.sickkids.on.ca/cgi-bin/WebObjects/MUTATION). This patient whose genotype was thus F508del/1001 + 3A>T died at 18 years of age as a consequence of the disease.
Novel Mutation
c.54–5811_164 + 2186del8108ins182 Mutation
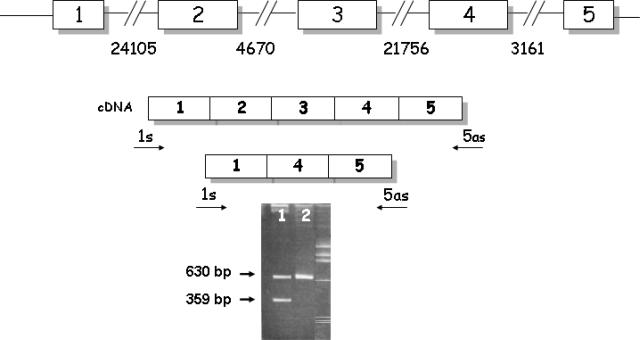
Three of the remaining undefined unrelated patients (CF1, CF2, and CF3) were studied at RNA level. Patient CF1 carried, in the characterized allele, the F508del mutation, patient CF2 carried the 991del5bp mutation, and patient CF3 carried the 1717–1G>A mutation. Electrophoresis of PCR cDNA fragments spanning exons 1 to 5 from the three patients showed two fragments of 630 and 359 bp, respectively (Figure 1).
Figure 1.
Electrophoretic pattern of PCR cDNA fragment spanning exons 1–5. 1: cDNA of CF patient. The 359-bp fragment represents the recombinant chromosome. 2: normal control.
The 630-bp fragment contained all five exons, whereas the 359-bp completely lacked exons 2 and 3, leading us to suspect gross DNA deletion to be the molecular defect responsible for CF in these patients. Southern blot analysis of genomic DNA performed in all three patients and two normal controls did not reveal, however, the presence of a CFTR gross deletion. Microsatellite analysis (GA, CA, and TG repeats located at nucleotides 6765, 10165, and 19282 of IVS1, respectively) performed in these patients as well as in their parents led us to detect the hemizygous state for the TG marker in all three patients, pinpointing to the presence of a deletion of a specific segment.
In fact, patient CF1 carried the 350-bp allele, her father the 350/356-bp alleles, and her mother carried the 344-bp allele; patient CF2 carried the 354-bp allele, his father the 346-bp allele, and his mother the 344/354-bp alleles; patient CF3 carried the 356-bp allele inherited from the father heterozygous for the 354/356-bp alleles, while his mother carried the 344 allele.
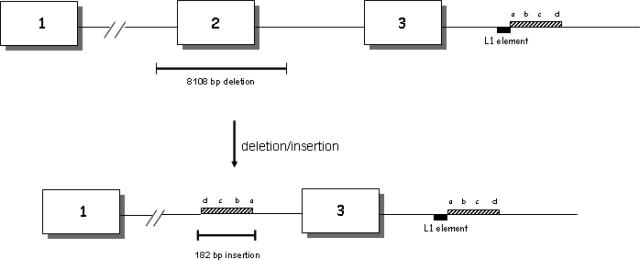
To identify the deletion breakpoint, we analyzed the SNPs mapping in this region, namely SNP1(C>G) located at nucleotide 14824 in intron 1 between the CA and TG markers (Genome Browser rs4148688), SNP2 (C>G) located at nucleotide 23401 in intron 1 between TG marker and exon 2 (Genome Browser rs4148689), and SNP3 (A>C) located at nucleotide 2632 in intron 3 (Genome Browser rs980574). This analysis showed heterozygosity for SNP1 and SNP3 in all three families. Whereas, SNP2, which was informative only in the CF1 family detected in the proband hemizygous for this marker, allowed us to limit the region of DNA to ∼17 kb, including the deletion. Extra-Long PCR amplified a fragment of roughly 3 kb in the patients, but not in the normal control. Sequence analysis of this fragment showed a gross deletion of 8108 bp spanning exon 2 with an insertion of 182 bp at the deletion junction, between the nucleotide 54-5811 of intron 1 (IVS1 nt16864) and nucleotide 164 + 2186 of intron 2 (IVS2 nt 2186). The inserted sequence is identical to part of the downstream sequence of intron 3 between IVS3 nt 6780 and IVS3 nt 6961, but inserted in an inverted orientation. BLAST (basic local alignment search tool) searching revealed that nucleotides 6780 to 6824 of the inserted sequence were complementary to part of a functional human LINE-1 (long interspersed nucleotide element-1, L1) retrotransposon (Figure 2).
Figure 2.
The new mutation c.54-5811_164 + 2186del8108ins182. Boxes containing lined bars represent the 182-bp inserted sequences of intron 3. The intronic sequence is represented by 5′-a-b-c-d-3′. After the inversion, the inserted sequence is 5′-d-c-b-a-3′. Boxes containing black bars represent L1 element sequences.
Once the deletion breakpoint was defined, we designed a PCR assay to perform a molecular screening for this deletion in all of the undefined patients. The new mutation c.54-5811_164 + 2186del8108ins182 was detected in six of seven still unidentified CF Sardinian chromosomes, thereby resulting in the fifth most common mutation in the Sardinian population with a frequency of 3.4% (Table 1).
Molecular Mechanism Proposed for c.54-5811_164 + 2186del8108ins182 Mutation
This mutation represents a complex indel mutation characterized by a gross deletion of 8108 bp and insertion of 182 bp. Analysis of the deletion breakpoint showed two deletion hot spot consensus sequences (CTT and GAATA), and two endonuclease recognition sites (GAAA and AATA) which are described to be involved in the mechanisms of retrotransposition. Moreover, the inserted sequence, which is identical to part of intron 3, contains part of a L1 sequence in its 3′ terminus. The L1 could retrotranspose by “target-primed reverse transcription”18 in which the first strand of DNA is cleaved by the retrotransposon endonuclease at the recognition site GAAA that lies 5′ to the break point, leading the annealing of the retrotransposon RNA at the nick. Then the retrotransposon reverse transcriptase synthesize from the sequence CTT. The L1 endonuclease cleaves the second DNA strand and the new sequence integrates at the double break. This series of events could give rise to the described complex rearrangement. However, a second mechanism, involving the recently described mechanism of intrachromosomal serial replication slippage (SRS) in trans, could also be involved.19
Discussion
In most populations, a significant percentage of CF alleles remain unidentified even after extensive studies of the CFTR gene by PCR-based procedures. In recent years, in addition to point mutations and short deletion/insertion, gross CFTR gene rearrangements including large deletion/insertion have been characterized and account for ∼16 to 24% of unidentified CF alleles, at least in European populations.10,20,21,22 Recently, a number of molecular approaches have been developed to detect these large rearrangements at DNA level, such as quantitative multiplex PCR of short fragments (QMPSF), and very recently multiplex ligation-dependent probe amplification (MLPA). However, even at present time, CFTR mRNA analysis represents an effective tool for the identification of unknown molecular defects of the CFTR gene with high mutation detection rate of gross gene deletion/insertion. In addition, mRNA analysis may allow researchers to define the pathogenic role of sequence variations not yet defined and splicing defects causing alternative products.
Herein, we describe the characterization of the molecular defect by CFTR mRNA analysis of eight patients affected by a classic form of CF, in which only one allele was molecularly defined. These studies led to identify a novel CFTR gene rearrangement consisting in a large deletion of 8108 bp and an insertion of 182 bp including exon 2 and a portion of introns 1 and 2. This mutation was found in six of the eight patients investigated, accounting for ∼75% of undefined CF alleles in Sardinians. Following this study, in our population, only one allele remains to be defined (because of difficulty in obtaining patient RNA). The novel c.54-5811_164 + 2186del8108ins182 mutation represents the fifth most common CF Sardinian molecular defect, accounting for 3.4% of the total (Table 1).
In conclusion, since the identification of the cystic fibrosis transmembrane regulator in 1989,23 we have characterized, either by DNA or RNA analysis, the molecular defect in 175 of 176 Sardinian CF chromosomes studied. The chromosomes investigated belong to 88 patients, who most likely represent the large majority of CF patients presently living in Sardinia. By including the novel mutation in our mutation panel we are now able to reach a 95%-detection rate, thereby improving the process of carrier detection and genetic counseling in Sardinia.
Note
Since this manuscript was submitted, Férec et al24 have independently described the c.54-5811_164 + 2186del8108ins182 mutation in one patient of Czech origin.
Acknowledgments
We thank all of the families included in this study for their kind cooperation.
Footnotes
Supported by grants from the Italian Cystic Fibrosis Research Foundation, adopted by Gruppo Alimentare Rossetto-Verona.
Research for this study was performed at the Dipartimento di Scienze Biomediche e Biotecnologie, Università degli Studi, Cagliari.
References
- Welsh MJ, Tsui LC, Boat TF, Beaudet AI. Cystic fibrosis. Scriver CR, Beaudet AI, Sly WS, Valle D, editors. New York: McGraw-Hill,; The Methabolic and Molecular Bases of Inherited Disease. 1995:3799–3876. [Google Scholar]
- Estivill X, Bancells C, Ramos C. The Biomed CF Mutation Analysis Consortium. Geographic distribution and regional origin of 272 cystic fibrosis mutations in European populations. Hum Mutat. 1997;10:135–154. doi: 10.1002/(SICI)1098-1004(1997)10:2<135::AID-HUMU6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Chillon M, Casals T, Mercier B, Brassas L, Lissens W, Silber S, Romey M, Ruiz-Romero J, Verlingue C, Claustres M, Nunes V, Ferec C, Estvill X. Mutations in the cystic fibrosis gene in patients with congenital absence of the vas deferens. N Engl J Med. 1995;332:1475–1480. doi: 10.1056/NEJM199506013322204. [DOI] [PubMed] [Google Scholar]
- Costes B, Girodon E, Ghanem N, Flori E, Jardin A, Soufir JC, Goossens M. Frequent occurrence of the CFTR intron 8 (TG)n5T allele in men with congenital absence of the vas deferens. Eur J Hum Genet. 1995;3:285–293. doi: 10.1159/000472312. [DOI] [PubMed] [Google Scholar]
- Pignatti PF, Bombieri C, Marigo C, Benetazzo M, Luisetti M. Increased incidence of cystic fibrosis gene mutations in adults with disseminated bronchiectasis. Hum Mol Genet. 1995;4:635–639. doi: 10.1093/hmg/4.4.635. [DOI] [PubMed] [Google Scholar]
- Girodon E, Cazeneuve C, Lebargy F, Chinet T, Costes B, Ghanem N, Martin J, Lemay S, Scheid P, Housset B, Bignon J, Goossens M. CFTR gene mutations in adults with disseminated bronchiectasis. Eur J Hum Genet. 1997;5:149–155. [PubMed] [Google Scholar]
- Cohn JA, Friedman KJ, Noone PG, Knowels MR, Silverman LM, Jowel PS. Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. N Engl J Med. 1998;339:653–658. doi: 10.1056/NEJM199809033391002. [DOI] [PubMed] [Google Scholar]
- Sharer N, Schawrz M, Malone G, Howarth A, Painter J, Super M, Brazanga J. Mutations of the cystic fibrosis gene in patients with chronic pancreatitis. N Engl J Med. 1998;339:645–652. doi: 10.1056/NEJM199809033391001. [DOI] [PubMed] [Google Scholar]
- Bobadilla JL, Macek M, Jr, Fine JP, Farrell PM. Cystic fibrosis: a worldwide analysis of CFTR mutations-correlations with incidence data and application to screening. Hum Mut. 2002;19:575–606. doi: 10.1002/humu.10041. [DOI] [PubMed] [Google Scholar]
- Bombieri C, Bonizzato A, Castellani C, Assael BM, Pignatti PF. Frequency of large CFTR gene rearrangements in Italian CF patients. Eur J Hum Genet. 2005;13:687–689. doi: 10.1038/sj.ejhg.5201387. [DOI] [PubMed] [Google Scholar]
- Dork T, Macek M, Jr, Mekus F, Tummler B, Tzountzouris J, Casals T, Krebsova A, Koudova M, Sakmaryova I, Macek M, Sr, Vavrova V, Zemkova D. Characterization of a novel 21-kb deletion, CFTR2,3dele (21kb), in the CFTR gene: a cystic fibrosis mutation of Slavic origin common in Central and East Europe. Hum Genet. 2000;106:259–268. doi: 10.1007/s004390000246. [DOI] [PubMed] [Google Scholar]
- Lerer I, Laufer-Cahana A, Rivlin JR, Augarten A, Abeliovich D. A large deletion mutation in the CFTR gene (3120+1kbdel8.6kb): a founder mutation in the Palestinian Arabs. Hum Mut. 1999;13:337. doi: 10.1002/(SICI)1098-1004(1999)13:4<337::AID-HUMU13>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Saba L, Leoni GB, Meloni A, Faà V, Cao A, Rosatelli MC. Two novel mutations in the transmembrane domains of the CFTR gene in subjects of Sardinian descent. Hum Mol Genet. 1993;2:1739–1740. doi: 10.1093/hmg/2.10.1739. [DOI] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polensky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acid Res. 1998;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferec C, Audrezet MP, Mercier B, Guillermit H, Moullier P, Quere I, Verlingue C. Detection of over 98% cystic fibrosis mutations in a Celtic population. Nat Genet. 1992;1:188–191. doi: 10.1038/ng0692-188. [DOI] [PubMed] [Google Scholar]
- Le Marechal C, Audrezet MP, Quere I, Raguenes O, Langonne S, Ferec C. Complete and rapid scanning of the cystic fibrosis transmembrane conductance regulator (CFTR) gene by denaturing high-performance liquid chromatography (D-HPLC): major implications for genetic counselling. Hum Genet. 2001;108:290–298. doi: 10.1007/s004390100490. [DOI] [PubMed] [Google Scholar]
- Southern EM. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Ostertag EM, Kazazian HH. Twin priming: a proposed mechanism for the creation of inversion in L1 retrotransposition. Genome Res. 2001;11:2059–2065. doi: 10.1101/gr.205701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JM, Chuzhanova N, Stenson PD, Ferec C, Cooper DN. Intrachromosomal serial replication slippage in trans gives rise to diverse genomic rearrangements involving inversions. Hum Mut. 2005;26:362–373. doi: 10.1002/humu.20230. [DOI] [PubMed] [Google Scholar]
- Audrezet MP, Chen JM, Raguenes O, Raguenes O, Chuzhanova N, Giteau K, Le Marechal C, Quere I, Cooper DN, Ferec C. Genomic rearrangements in the CFTR gene: extensive allelic heterogeneity and diverse mutational mechanisms. Hum Mut. 2004;23:343–357. doi: 10.1002/humu.20009. [DOI] [PubMed] [Google Scholar]
- Niel F, Martin J, Dastot-Le Moal F, Costes B, Boissier B, Delattre V, Goossens M, Giroson E. Rapid detection of CFTR gene rearrangements impacts on genetic counselling in cystic fibrosis. J Med Genet. 2004;41:1–7. doi: 10.1136/jmg.2004.022400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier-Porst F, Souche G, Bozon D. Identification and characterization of three large deletions and a deletion/polymorphism in the CFTR gene. Hum Mut. 2005;25:504. doi: 10.1002/humu.9335. [DOI] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, Drumm ML, Iannuzzi MC, Collins FS, Tsui LC. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Férec C, Casals T, Chuzhanova N, Macek M, Jr, Bienvenu T, Holubova A, King C, McDevitt T, Castellani C, Farrell PM, Sheridan M, Pantaleo SJ, Loumi O, Messaoud T, Cuppens H, Torricelli F, Cutting GR, Williamson R, Alonso Ramos MJ, Pignatti PF, Raguénès O, Cooper DN, Audrézet MP, Chen JM. Gross genomic rearrangements involving deletions in the CFTR gene: characterization of six new events from a large cohort of hitherto unidentified cystic fibrosis chromosomes and meta-analysis of underlying mechanisms. Eur J Hum Genet. 2006;22:1–10. doi: 10.1038/sj.ejhg.5201590. [DOI] [PubMed] [Google Scholar]