Abstract
Current preoperative diagnostic procedures for thyroid nodules rely mainly on the cytological interpretation of fine-needle aspirates (FNAs). DNA microarray analysis has been shown to reliably distinguish benign and malignant thyroid nodules in surgically resected specimens, but its diagnostic potential in thyroid FNA has not been examined. In the present study, the expression profiles of 50 benign thyroid lesions and papillary thyroid carcinoma tissue samples were compared, generating a list of 25 differentially expressed genes from this training set. A test set of 22 FNA specimens was evaluated by unsupervised hierarchical cluster analysis using this gene list, and the results were compared to FNA cytology. FNA specimens were found to fall into three clusters: malignant (n = 10), benign (n = 7), and indeterminate (n = 5). The benign and malignant groups showed complete concordance with the final histological diagnosis except for one histologically benign lesion, which was rediagnosed as follicular variant of papillary thyroid carcinoma on histological review. Paired analysis between FNA and matched tissues samples illustrated adequate sampling with FNA. These results illustrate that microarray analysis of FNA is feasible and has the potential to improve the accuracy of FNA in categorizing benign from malignant lesions beyond routine cytological evaluation.
Thyroid nodules are clinically detectable in 4 to 7% of the population and in one-half of autopsy specimens.1 Furthermore, a substantial increase in diagnosis of thyroid “incidentalomas” has been seen with the introduction and increasing use of screening and diagnostic ultrasound for parathyroid (40%), carotid (10% of screening carotid duplexes), and thyroid (67%) disease.2,3 For these nodules, either palpated or incidentally detected, cytological evaluation of fine-needle aspiration (FNA) specimens is currently the standard procedure to triage patients for surgical resection.
Although FNA has greatly increased the preoperative diagnostic accuracy of thyroid nodules throughout the past few decades, significant limitations remain. The majority of FNAs performed are classified as benign, while 5 to 10% are classified as malignant.4,5,6,7 However, a subset of 10 to 20% of FNAs are found to be nondiagnostic, frequently secondary to cystic or hemorrhagic fluid and resultant hypocellularity in the aspirate.8,9 An additional 10 to 20% of FNAs are classified as indeterminate or suspicious, diagnoses that typically include follicular neoplasms and atypical lesions (suggestive of, but not diagnostic for, malignancy). Only one in five of these cases diagnosed as indeterminate will prove to be malignant at surgery. The inability to classify follicular lesions by cytology (which requires nodule architecture for diagnosis), varying extent and spectra of nuclear pleomorphism allowing for subjectivity during histological examination, lack of specific classification algorithms, and fear of liability have all been cited for the difficulty in categorizing this intermediate group.10,11
Overall, the sensitivity and specificity of thyroid FNA ranges from 65 to 98% and 72 to 100% depending on how suspicious and nondiagnostic specimens are classified.7 False-negatives, attributed to sampling error or misdiagnosis, have been reported in 5% of cases. The true false-negative rate, however, cannot be accurately determined because nondiagnostic FNAs have not been included in analyses, and only 10% of those cases with negative or benign FNAs undergo subsequent surgery. Cited false-positive rates range from 3 to 6%; however, this rate significantly increases when suspicious nodules are included.5,7 Moreover, 7% of nondiagnostic aspirates are subsequently found to be malignant.9
Transcriptional profiling has revolutionized oncology research throughout the past decade with the advent of microarray technology. Our laboratory and others have been successful in classifying ex vivo postoperative tumor samples into both benign and malignant as well as discriminating tumor subclassifications using various statistical analyses (ie, support vector machines, hierarchical clustering, and k means analysis) of differential expression between groups.12,13,14,15 Although great progress has been made in identifying common mutations through this process, a consistent and accurate malignant signature has not been borne out. Efforts at using singular discriminating molecular markers using immunohistochemistry or genetic analysis [ie, polymerase chain reaction (PCR)] preoperatively have not provided reliable distinction between benign and malignant lesions.16,17,18,19,20 These results are not surprising given the heterogeneity of thyroid tumors. BRAF mutations, commonly found in papillary thyroid carcinoma (PTC) and PAX-PPARγ translocations found in follicular thyroid carcinoma (FTC), although indicative of disease, are not sensitive screening tests for all differentiated thyroid carcinomas.21,22,23 Groups have turned to the development of panels of genes using quantitative PCR and immunohistochemistry. Although some report sensitivities and specificities superior to that of cytology, some include only follicular neoplasms in the analysis and most did not validate these tests on an adequate number of FNA specimens and larger cohorts.24,25,26,27,28,29 Furthermore, immunohistochemical analysis on FNA cytology specimens, although potentially useful, suffers from the lack of established diagnostic markers in addition to the likely pitfalls of subjective interpretation and technical variability between pathological laboratories.
Given the considerations above, the microarray platform has emerged as a potential preoperative diagnostic test. In addition to cost and labor considerations, however, earlier microarray assay systems required large amounts of RNA, precluding its use with thyroid FNA specimens. These limitations have been surpassed by recent technological developments. In this study, we sought to assess the feasibility of microarray analysis of FNAs in the diagnosis of thyroid nodules. Below we report the classification of benign to malignant thyroid nodules using microarray analysis of FNA biopsies, focusing on the distinction between most commonly encountered lesions [ie, follicular adenoma (FA) and hyperplastic nodules (HYP) versus PTC, including follicular variant of PTC (FVPTC)]. The results were correlated to the cytological diagnosis on FNA and the final histological diagnosis on the resection specimens.
Materials and Methods
Patient Selection and Sample Procurement
FNA and tissue specimens were obtained from all clinically significant thyroid nodules identified at the time of diagnostic and/or therapeutic partial or total thyroidectomy performed by one of the authors (T.J.F.) from 2002 to 2005 at New York Presbyterian Hospital–Weill Cornell Medical College. FNAs of the nodules were performed on the ex vivo specimens with five passes of a 23-gauge needle with a 10-ml syringe and aspirated 10 times with RLT lysis buffer (Qiagen Inc., Valencia, CA). The suspended FNA specimens and 2 × 2-mm blocks of matched tissue samples were then snap-frozen in liquid nitrogen and stored at −80οC until processing. The time between devascularization of tissue and freezing was 20 to 30 minutes. Diagnoses were confirmed by comparison to final pathology report. Adult patients carrying the pathological diagnosis of HYP, FA, PTC, and FVPTC were chosen retrospectively from the database. All tissues were obtained with the informed consent of each patient and in accordance with approved protocols and guidelines of our internal review board.
RNA Isolation, Purification, Labeling, and Hybridization for Gene Expression Analysis
Tissue Samples
The training set consisted of 50 tissue samples: 10 HYP, 16 FA, 11 PTC, and 13 FVPTC. Preparation of total RNA from frozen tissue was performed as previously described.12,30 Briefly, thyroid tissue was homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA). Following the manufacturer’s protocol, total RNA extraction and clean-up were performed using the RNeasy mini kit (Qiagen Inc.). Integrity of RNA was assessed using spectrophotometry. Samples were processed following the Affymetrix protocol (Affymetrix Inc., Santa Clara, CA). In brief, 8 μg of total RNA was amplified, biotin labeled, and hybridized to the Affymetrix Hu95Av2 GeneChip (Affymetrix Inc.). RNA from nine tissue samples matched to the FNA specimens included in the study was isolated and purified as above. To control for other variables, RNA amplification and labeling were performed with the Ovation biotin RNA amplification and labeling system (NuGEN Technologies, Inc., San Carlos, CA) in the same manner as the FNA samples, as described in detail below, and hybridized to HG-U133A GeneChips (Affymetrix Inc.). Each sample was hybridized to a test chip to validate RNA quality and customary GeneChip internal controls were observed.
Fine-Needle Aspirates
Ex vivo FNA biopsy patients different from those included in the training set were included in the test set: five HYP, six FA, seven PTC, and four FVPTC. The Qiagen MicroKit was used for RNA extraction using the manufacturer’s protocol except for the substitution of bacterial ribosomal RNA as carrier RNA for the elution step (Qiagen Inc.). The optional DNase step was omitted. Quantity and integrity of RNA yield was assessed using the NanoDrop (NanoDrop Technologies, Wilmington, DE) and Bioanalyzer 2100 and RNA 6000 Nano/Pico LabChip (Agilent Technologies, Palo Alto, CA). Clean-up was performed with Zymo RNA clean-up (Zymo Research, Orange, CA) for those samples with an OD260/280 < 1.8.
RNA amplification and labeling were performed per the Ovation biotin system protocol (NuGEN Technologies, Inc.). Briefly, 25 ng of total RNA was reverse transcribed using a chimeric cDNA/mRNA primer. A second complimentary cDNA strand was then synthesized via binding of DNA polymerase at sites of fragmented mRNA on the anti-sense strand. Next, amplification of the target transcriptome with SPIA enzyme was performed as follows: RNA degradation in first strand primer, SPIA DNA/RNA primer binding, extension, and replication. Amplified DNA was then purified with Zymo Research DNA Clean and Conentrator-25 (Zymo Research), cleaved to produce 50- to 100-bp fragments, and biotinylated. Purified DNA was assessed for yield and integrity assessed by the Bioanalyzer 2100 and RNA 6000 Nano LabChip (Agilent Technologies). All samples (2.2 μg) were hybridized to the HG-U133A Affymetrix GeneChip array following the Affymetrix standard protocol (Affymetrix Inc.). Each sample was hybridized to a test chip to validate RNA quality, and customary GeneChip internal controls were observed.
Statistical Analysis of Microarray Data
Data were imported to Genetraffic UNO (Stratagene, La Jolla, CA) using RMA (robust multilevel analysis) for probe level analysis and normalization. Benign (HYP and FA) tissue samples hybridized to the HG-U95Av2 GeneChip (Affymetrix Inc.) were compared to malignant (PTC and FVPTC) specimens, generating a list of differentially expressed genes in the training set. This list was then filtered to a significance of P < 0.01 with Bonferroni multiple test correction and twofold differential expression. Corresponding HG-U133A GeneChip probe IDs were imported from NetAffx (Affymetrix Inc.) and used in an unsupervised hierarchical cluster analysis of the FNA test set. Matched tissue specimens were included in a secondary cluster analysis.
Results
RNA Isolation and Amplification Yield from FNA
For the 22 FNA samples, total RNA yield was 137 ng to 5.8 μg (mean, 1.7 μg) and an average concentration of 129 ng/μl. Mean OD260/280 was 2.0 (1.84 to 2.13), verifying that samples had little protein contamination. The average ratio of 28S:18S rRNA for the group was 1.4 (0.9 to 1.8). Ratios >1.5 indicate RNA free of significant degradation. After amplification as described above, pre- and post-fragmentation average complementary DNA yield was 7.3 and 4.4 μg, respectively.
Genes Differentiating Benign from Malignant Thyroid Nodules
Comparison of 26 benign and 24 malignant tissue samples in the training set revealed 25 significantly differentially expressed genes (Table 1). Multiple genes are well-documented previously as being differentially expressed in thyroid cancer including TPO, TFF3, KRT19, TIMP1, FN1, and CITED1.15,28,31,32,33
Table 1.
Probe Sets with Differential Expression Between Benign (FA + HYP) and Malignant (PTC + FVPTC) Thyroid Tissue in Training Set
| UniGene symbol | UniGene name | Fold change* | P value |
|---|---|---|---|
| TPO | Thyroid peroxidase | −9.6 | 0.0001 |
| TFF3 | Trefoil factor 3 (intestinal) | −9.1 | 0.0063 |
| TFF3 | Trefoil factor 3 (intestinal) | −4.6 | 0.0008 |
| FCGBP | Fc fragment of IgG binding protein | −3.6 | 0.0064 |
| HGD | Homogentisate 1,2-dioxygenase | −3.1 | 0.0068 |
| MATN2 | Matrilin 2 | −2.9 | 0.0023 |
| RAP1GA1 | RAP1, GTPase-activating protein 1 | −2.5 | 0.0035 |
| HMGA2 | High mobility group AT-hook 2 | 2.3 | 0.0045 |
| TIPARP | TCDD-inducible poly (ADP-ribose) polymerase | 2.5 | 0.0064 |
| QPCT | Glutaminyl-peptide cyclotransferase | 2.6 | 0.0026 |
| PSD3 | Pleckstrin and Sec7 domain containing 3 | 2.7 | 0.0041 |
| DUSP4 | Dual specificity phosphatase 4 | 3.0 | 0.0001 |
| ABCC3 | ATP-binding cassette, sub-family C (CFTR/MRP), member 3 | 3.1 | 0.0068 |
| DPP4 | Dipeptidylpeptidase 4 (CD26, adenosine deaminase complexing protein 2) | 3.2 | 0.0023 |
| TIMP1 | Tissue inhibitor of metalloproteinase 1 | 3.5 | 0.0089 |
| KRT19 | Keratin 19 | 3.9 | 0.0010 |
| GALIG | Galectin-3 internal gene | 4.0 | 0.0030 |
| PROS1 | Protein S α | 4.1 | 0.0020 |
| SERPINA1 | Serine (or cysteine) proteinase inhibitor, clade A (α-1 antiproteinase, antitrypsin), member 1 | 5.1 | 0.0081 |
| FN1 | Fibronectin 1 | 5.2 | 0.0001 |
| FN1 | Fibronectin 1 | 6.0 | 0.0008 |
| CITED1/ MSG1 | Cbp/p300-interacting transactivator, with Glu/Asp-rich carboxy-terminal domain, 1 | 6.1 | 0.0000 |
| LRP4 | Low-density lipoprotein receptor-related protein 4 | 6.4 | 0.0000 |
| TACSTD2/ TROP2/ GA733–1 | Tumor-associated calcium signal transducer 2 | 7.5 | 0.0020 |
| SH2D1A | SH2 domain protein 1A | 7.7 | 0.0001 |
Fold change is expressed in relation to malignant tumors.
Hierarchical Clustering of FNA Samples
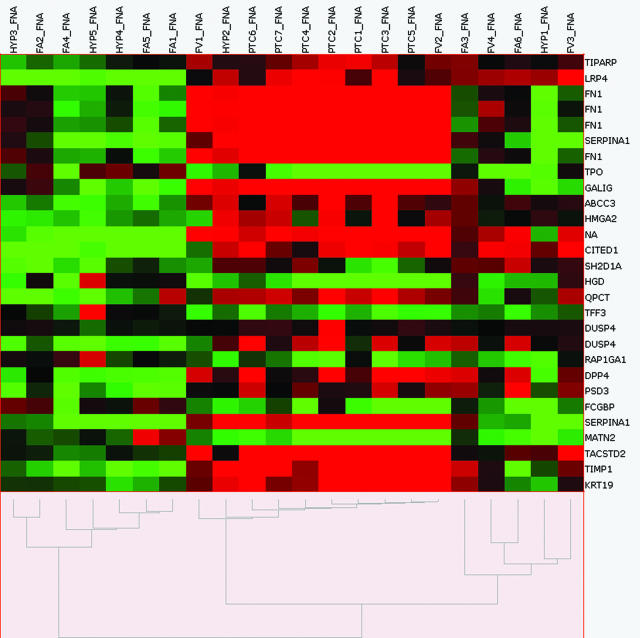
Using the gene list generated from the training set, the 22 FNA test set was clustered in an unsupervised manner (Figure 1). This analysis clustered the test set into three main groups: one malignant group (n = 10), one benign group (n = 7), and one indeterminate group (two FA, two FVPTC, and one HYP).
Figure 1.
Unsupervised hierarchical cluster analysis of individual hybridizations in the FNA test set using 25 differentially expressed genes (29 probe sets) established from the training set. Columns represent individual samples, rows represent genes, red indicates relative overexpression, and green indicates relative underexpression. FV, follicular variant of PTC.
These results were compared with the preoperative FNA diagnosis and the final histological diagnosis of the resected specimens (Table 2). Sixteen of the 17 cases predicted to be either benign or malignant by microarray analysis were confirmed by pathological diagnosis. One case, HYP2, was initially interpreted histologically as nodular hyperplasia. Review of this case by two independent, blinded pathologists and additional immunohistochemical analysis determined this case to be FVPTC. Thus, the classification of benign or malignant by microarray analysis of FNA was 100% concordant to the histological diagnosis. In comparison, the preoperative FNA diagnosis was correct in 13 of 17 of these cases (76%), inconclusive (indeterminate) in three cases, and incorrect in one malignancy case, diagnosed as benign by cytology.
Table 2.
Patient Demographics and Comparison of Diagnostic Methods
| Sample | Age | Sex | Preoperative FNA | Pathologic diagnosis | Cluster grouping* |
|---|---|---|---|---|---|
| FA3 | 39 | F | Indeterminate | FA | Indeterminate |
| FV3 | 63 | F | Indeterminate | FVPTC | Indeterminate |
| FV4 | 24 | F | Indeterminate | FVPTC | Indeterminate |
| FA6 | 34 | F | Indeterminate | FA | Indeterminate |
| HYP1 | 33 | F | Indeterminate | HYP | Indeterminate |
| HYP2 | 24 | F | Indeterminate | HYP→FVPTC | Malignant |
| HYP3 | 67 | M | Benign | HYP | Benign |
| HYP4 | 34 | F | Benign | HYP | Benign |
| HYP5 | 47 | F | Benign | HYP | Benign |
| FA1 | 50 | M | Indeterminate | FA | Benign |
| FA2 | 36 | F | Benign | FA | Benign |
| FA4 | 47 | F | Indeterminate | FA | Benign |
| FA5 | 53 | F | Benign | FA | Benign |
| FV1 | 48 | F | Malignant | FVPTC | Malignant |
| PTC1 | 46 | F | Malignant | PTC | Malignant |
| PTC2 | 43 | M | Malignant | PTC | Malignant |
| PTC3 | 27 | F | Malignant | PTC | Malignant |
| PTC4 | 37 | F | Malignant | PTC | Malignant |
| FV2 | 53 | F | Malignant | FVPTC | Malignant |
| PTC5 | 78 | F | Malignant | PTC | Malignant |
| PTC7 | 50 | F | Benign | PTC | Malignant |
| PTC6 | 42 | F | Malignant | PTC | Malignant |
Indeterminate diagnoses include lesions suspicious for papillary carcinoma and follicular neoplasms.
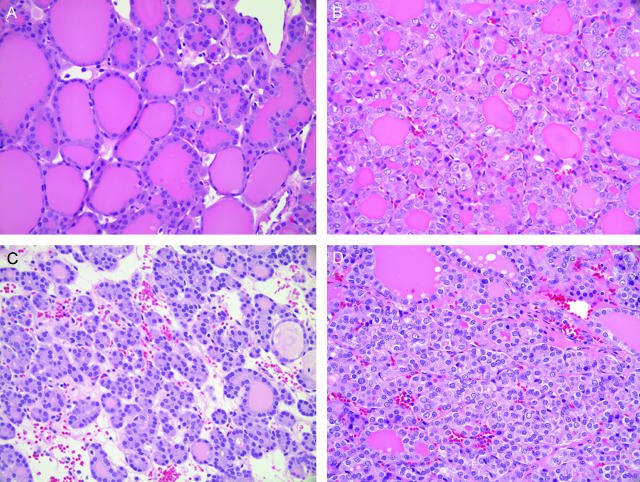
Of note is the identification of five FNA cases that appeared to show expression profiles in between the benign and malignant groups (Figure 1). Final tissue histological diagnoses of these five indeterminate cases were two FA, two FVPTC, and one HYP. Interestingly, all five cases were deemed suspicious on preoperative FNA (Table 2). The histological sections of these five cases were reviewed (by T.S. and Y.T.C.), and the diagnoses were confirmed. Four of the five showed cytological heterogeneity within the tumors (Figure 2), including partial nuclear features of PTC (ie, nuclear clearing and grooves) in patchy distribution within the lesion.
Figure 2.
Sections illustrating cytological heterogeneity within indeterminate tumors. Adjacent areas of lesion from patient FV3 (A, B) and patient FA3 (C, D). H&E stain. Original magnifications, ×40.
Assessment of Sampling Error with Matched Tissue Clustering
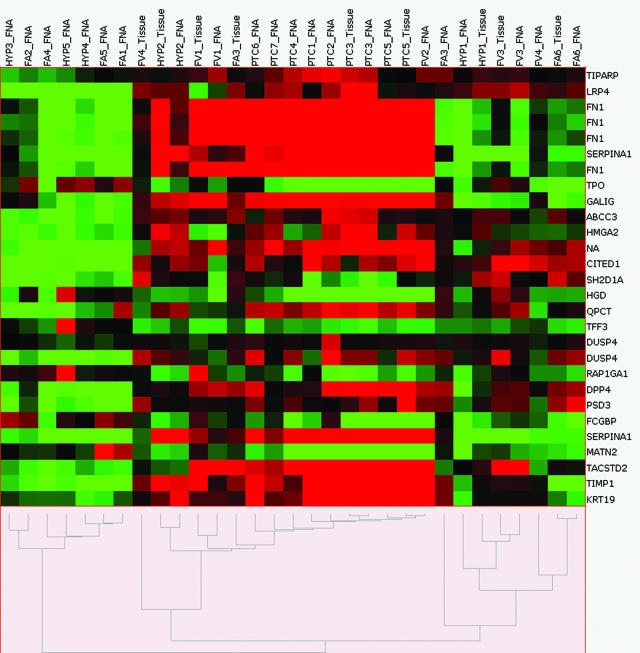
To validate adequate sampling with FNA, a direct comparison of FNA and matched tissue was undertaken in nine cases (four malignant and five indeterminate). As illustrated in Figure 3, seven of the nine matched tissue specimens were classified (ie, benign versus malignant) identically, with most coupling with their FNA counterparts as the next neighbor. The matched tissue samples of two of the intermediate samples (one FA and one FVPTC), however, clustered in the malignant group.
Figure 3.
Unsupervised hierarchical cluster analysis of FNA samples and nine matched tissues (test set) using 25 differentially expressed genes (28 probe sets) established from the training set. Columns represent individual samples, rows represent genes, red indicates relative overexpression, and green indicates relative underexpression.
Discussion
In this study, we showed that microarray analysis of FNAs may represent a feasible and useful approach to molecular diagnosis of thyroid nodules. In comparison to FNA diagnosis by cytology, the microarray data showed higher concordance rate to the final tissue diagnosis. As is summarized in Table 2, the classification of benign or malignant by microarray analysis of FNA was 100% concordant to the histological diagnosis versus 76% concordance with the preoperative cytological FNA diagnosis. Eight of twenty-two FNAs were deemed indeterminate, including follicular and suspicious for PTC lesions, on preoperative cytology. Although five of these eight maintained an intermediate classification with our method, the remaining three could have resulted in a change in disease management. Two suspicious lesions by FNA definitely clustered with the benign group, while microarray analysis of the third, HYP2, uncovered a misdiagnosis. Furthermore, we illustrated analogous expression profiles of matched FNA and tissue specimens, verifying that the sample of genetic material acquired by FNA is a representative sample of the underlying tumor.
Importantly, the majority of the 25 significantly differentially expressed genes determined by our training set, including TPO, TFF3, KRT19, TIMP1, FN1, and CITED1, are well corroborated in literature as being differentially expressed in thyroid carcinoma.15,28,31,32,33 Comparing expression profiles of eight PTC and matched normal thyroid tissue, Huang and colleagues15 found a substantial number of the same differentially regulated genes. Consistent with our results, they found that CITED1, KRT19, DPP4, SERPINA1, and FN1 were overexpressed in all of the malignant tissues, whereas TPO and TFF3 were underexpressed in seven of eight tumors. Follow-up work verified CITED1 (involved in co-regulation transcriptional factors) and KRT19 protein overexpression by immunohistochemistry.31,33,34 Proteins involved in normal thyroid metabolism, including thyroid peroxidase (TPO), are frequently underexpressed in the carcinomas. FN1, TPO, and TFF3 were also among a panel of genes found to be consistently differentially expressed in PTC versus matched controls by quantitative PCR.27 Fibronectin (FN1), an extracellular matrix protein involved in cell adhesion, migration, and metastasis, was previously shown to be up-regulated in thyroid FNAs, although contamination of FN1-secreting fibroblasts excludes this as an independent marker of malignancy.35,36 The consistency of these expressional profiles in our FNA samples supports the reliability of this method.
In addition to diagnostic implications, it is intriguing that our cluster analysis shows three distinct groups: one clearly benign, one clearly malignant, and one indeterminate grouping. All five cases in the indeterminate group were suspicious on FNA cytology. Because all suspicious lesions are excised surgically, the microarray data would not pose a clinical dilemma in these cases. We hypothesize that this indeterminate grouping could be the result of sampling error or could indicate that these tumors are true biological intermediates. Microarray data on the resected tumor tissue were available for all five of these cases. Three coupled with their FNA counterpart, excluding sampling error, and two clustered with the malignant group (one FA and one FVPTC, FV4, and FV3 in Figure 3). This discrepancy between FNA and tissue profiling suggests that the intermediate finding in the mismatched FVPTC FNA sample might be an artifact attributable to a sampling error. Possible reasons for sampling error in FNA can include varied sampling in a phenotypically heterogeneous tumor (ie, expression of a marker gene can be uneven in different areas of the tumor), the contamination by normal thyroid tissue, or an unexpected predominance of benign stromal tissues in the FNA sample. On the other hand, this tissue/FNA mispairing could also be a mere statistical artifact. Visual comparison of the FA3 tissue and FNA data in Figure 3, for instance, revealed highly similar expression profiles for the majority of the probe sets, although the two samples did not pair in this analysis. It is possible that the differential expression of a few genes, for example, the up-regulation of fibronectin (FN1) in the matched tissue specimen, could have skewed the analysis and resulted in this discrepancy in pairing. Thus, part of this discrepancy might be related to statistical reasons that hopefully will be improved by increased sample sizes and improved analytical tools in the future. However, tumor heterogeneity within thyroid tumors is a well-known and unavoidable confounding obstacle to any molecular diagnostics, and the interpretation of molecular results should therefore always be made in conjunction with accompanying histopathology and cytopathology results.
As mentioned, all five of the indeterminate samples were deemed so on preoperative FNA. Histological review showed partial features of PTC in the majority of these cases, hinting that at least some cases in this indeterminate group might truly represent borderline lesions between FA/HYP and PTC. Hierarchical clustering of the original 50 tissue training set samples with the same 25-gene list indeed revealed a similar indeterminate group (data not shown); because sampling error is not an issue here, this observation also argues for the presence of a true borderline group. Although a source of debate, supporters of this concept introduced by Rosai and colleagues38 argue that ambiguous lesions, encapsulated tumors with follicular architecture containing incompletely developed PTC-type nuclear changes, be classified as “well-differentiated tumors of uncertain malignant potential (WDT-UMP)”.37 Studies investigating these tumors have shown intermediate protein expression of markers of malignancy between FA and carcinoma.28,39 For instance, Papotti and colleagues39 showed the heterogeneous distribution of HBME-1 and Galectin-3 in lesions fitting the definition of WDT-UMP. In this study, two of the intermediate lesions had the histopathological diagnosis of FA and two of FVPTC. Are these intermediate lesions, frequently diagnosed as FA or FVPTC, steps in a progression from benign to malignant? If so, one would expect the behavior of FVPTCs to differ from classical PTC. Although few statistically significant differences have been found between the clinical courses of FVPTC and PTC, studies suggest that FVPTC may be a more indolent variant. Although reports have documented distant metastases from encapsulated FVPTC, a trend toward increased incidence of metastases in PTC patients versus FVPTC and improved cancer-specific survival has been seen.40,41 Other groups found decreased incidence of cervical lymph node metastases in the FVPTC compared to PTC.42,43 Because this concept of progression from benign lesions to PTC has not been universally accepted, identifying and analyzing more cases in this category would be valuable. This investigation is currently ongoing. The ambiguities of current morphological classification systems for these intermediate lesions, as well as the presence of this intermediate grouping in this study, underscore the need for a better understanding of the molecular pathogenesis of these nodules and ultimately the potential utility of molecular diagnostics.
One limitation of this study is that follicular carcinomas were not included. Although suspicious or follicular neoplasm constituted a significant percentage of indeterminate FNAs historically, the incidence and prevalence of FTC in the United States is waning. Since the inception of this study, we have not seen a single case of widely invasive FTC and only three minimally invasive FTC, preventing us from addressing this issue. Some of the genes that distinguished the benign versus malignant group (eg, CITED1) are recognized hallmarks of PTC and would not be expected to be overexpressed in follicular carcinoma.31,44 On the other hand, follicular carcinoma and PTC have been shown to share certain gene expression profiles, such as the overexpression of galectin-3 and decreased expression of TFF3.45,46,47 The determination of how FTCs will classify will require the accrual of an adequate sample size.
Issues often cited as obstacles to using microarray technology as a diagnostic test are its relative high cost and technical complexity. Although this might be true at present, commercialization, automation, and possibly the use of miniarrays will likely eliminate or alleviate at least some of these obstacles. Additionally, some argue that our current statistical tools for assessing the vast amount of data accrued from microarray experiments, including normalization and significance analyses, are deficient. We are currently investigating other analytical methods for class prediction. Finally, ex vivo specimens were used in this study. It will be necessary to confirm the accuracy of this method on preoperative FNA biopsies because this may introduce increased heterogeneity of the samples. It is likely that these obstacles can be overcome and that microarray analysis of FNA may be an exciting and promising addition to the armamentarium of thyroid nodule diagnosis.
Footnotes
Supported by the G. Thomas Shires Faculty Scholar Award.
References
- Vander JB, Gaston EA, Dawber TR. The significance of nontoxic thyroid nodules. Final report of a 15-year study of the incidence of thyroid malignancy. Ann Intern Med. 1968;69:537–540. doi: 10.7326/0003-4819-69-3-537. [DOI] [PubMed] [Google Scholar]
- Steele SR, Martin MJ, Mullenix PS, Azarow KS, Andersen CA. The significance of incidental thyroid abnormalities identified during carotid duplex ultrasonography. Arch Surg. 2005;140:981–985. doi: 10.1001/archsurg.140.10.981. [DOI] [PubMed] [Google Scholar]
- Castro MR, Gharib H. Continuing controversies in the management of thyroid nodules. Ann Intern Med. 2005;142:926–931. doi: 10.7326/0003-4819-142-11-200506070-00011. [DOI] [PubMed] [Google Scholar]
- Hegedus L. The thyroid nodule. N Engl J Med. 2004;351:1764–1771. doi: 10.1056/NEJMcp031436. [DOI] [PubMed] [Google Scholar]
- Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328:553–559. doi: 10.1056/NEJM199302253280807. [DOI] [PubMed] [Google Scholar]
- Caruso D, Mazzaferri EL. Fine needle aspiration biopsy in the management of thyroid nodules. Endocrinologist. 1991;1:194–202. [Google Scholar]
- Gharib H, Goellner JR. Fine-needle aspiration biopsy of the thyroid: an appraisal. Ann Intern Med. 1993;118:282–289. doi: 10.7326/0003-4819-118-4-199302150-00007. [DOI] [PubMed] [Google Scholar]
- Goellner JR, Gharib H, Grant CS, Johnson DA. Fine needle aspiration cytology of the thyroid, 1980 to 1986. Acta Cytol. 1987;31:587–590. [PubMed] [Google Scholar]
- Chow J, Gharib H, Goellner JR, Van Heerden JA. Nondiagnostic thyroid fine-needle aspiration cytology: management dilemmas. Thyroid. 2001;11:1147–1151. doi: 10.1089/10507250152740993. [DOI] [PubMed] [Google Scholar]
- Franc B, de la Salmoniere P, Lange F, Hoang C, Louvel A, de Roquancourt A, Vilde F, Hejblum G, Chevret S, Chastang C. Interobserver and intraobserver reproducibility in the histopathology of follicular thyroid carcinoma. Hum Pathol. 2003;34:1092–1100. doi: 10.1016/s0046-8177(03)00403-9. [DOI] [PubMed] [Google Scholar]
- Hirokawa M, Carney JA, Goellner JR, DeLellis RA, Heffess CS, Katoh R, Tsujimoto M, Kakudo K. Observer variation of encapsulated follicular lesions of the thyroid gland. Am J Surg Pathol. 2002;26:1508–1514. doi: 10.1097/00000478-200211000-00014. [DOI] [PubMed] [Google Scholar]
- Finley DF, Zhu B, Barden CB, Fahey TJ., III Discrimination of benign and malignant thyroid nodules by molecular profiling. Ann Surg. 2004;240:425–436. doi: 10.1097/01.sla.0000137128.64978.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti C, Zeiger MA, Costouros NG, Umbricht C, Westra W, Smith D, Somervell H, Bevilacqua G, Alexander H, Libutti S, Costourous N. Using gene expression profiling to differentiate benign versus malignant thyroid tumors. Cancer Res. 2004;64:2898–2903. doi: 10.1158/0008-5472.can-03-3811. [DOI] [PubMed] [Google Scholar]
- Chevillard S, Ugolin N, Vielh P, Ory K, Levalois C, Elliott D, Clayman GL, El-Naggar AK. Gene expression profiling of differentiated thyroid neoplasms: diagnostic and clinical implications. Clin Cancer Res. 2004;10:6586–6597. doi: 10.1158/1078-0432.CCR-04-0053. [DOI] [PubMed] [Google Scholar]
- Huang Y, Prasad M, Lemon WJ, Hampel H, Wright FA, Kornacker K, LiVolsi V, Frankel W, Kloos RT, Eng C, Pellegata NS, de la Chapelle A. Gene expression in papillary thyroid carcinoma reveals highly consistent profiles. Proc Natl Acad Sci USA. 2001;98:15044–15049. doi: 10.1073/pnas.251547398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolazzi A, Gasbarri A, Papotti M, Bussolati G, Lucante T, Khan A, Inohara H, Marandino F, Orlandi F, Nardi F, Vecchione A, Tecce R, Larsson O. Application of an immunodiagnostic method for improving preoperative diagnosis of nodular thyroid lesions. Lancet. 2001;357:1644–1650. doi: 10.1016/s0140-6736(00)04817-0. [DOI] [PubMed] [Google Scholar]
- Martins L, Matsuo SE, Ebina KN, Kulcsar MA, Friguglietti CU, Kimura ET. Galectin-3 messenger ribonucleic acid and protein are expressed in benign thyroid tumors. J Clin Endocrinol Metab. 2002;87:4806–4810. doi: 10.1210/jc.2002-020094. [DOI] [PubMed] [Google Scholar]
- Takano T, Miyauchi A, Matsuzuka F, Yoshida H, Kuma K, Amino N. Ubiquitous expression of galectin-3 mRNA in benign and malignant thyroid tumors. Cancer Lett. 2003;199:69–73. doi: 10.1016/s0304-3835(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Mase T, Funahashi H, Koshikawa T, Imai T, Nara Y, Tanaka Y, Nakao A. HBME-1 immunostaining in thyroid tumors especially in follicular neoplasm. Endocr J. 2003;50:173–177. doi: 10.1507/endocrj.50.173. [DOI] [PubMed] [Google Scholar]
- Miettinen M, Karkkainen P. Differential reactivity of HBME-1 and CD15 antibodies in benign and malignant thyroid tumours. Preferential reactivity with malignant tumours. Virchows Arch. 1996;429:213–219. doi: 10.1007/BF00198336. [DOI] [PubMed] [Google Scholar]
- Nikiforova MN, Biddinger PW, Caudill CM, Kroll TG, Nikiforov YE. PAX8-PPARgamma rearrangement in thyroid tumors: RT-PCR and immunohistochemical analyses. Am J Surg Pathol. 2002;26:1016–1023. doi: 10.1097/00000478-200208000-00006. [DOI] [PubMed] [Google Scholar]
- Marques AR, Espadinha C, Catarino AL, Moniz S, Pereira T, Sobrinho LG, Leite V. Expression of PAX8-PPAR gamma 1 rearrangements in both follicular thyroid carcinomas and adenomas. J Clin Endocrinol Metab. 2002;87:3947–3952. doi: 10.1210/jcem.87.8.8756. [DOI] [PubMed] [Google Scholar]
- Kimura ET, Nikiforov YE, Zhaowen Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–1457. [PubMed] [Google Scholar]
- Kebebew E, Peng M, Reiff E, Duh QY, Clark OH, McMillan A. ECM1 and TMPRSS4 are diagnostic markers of malignant thyroid neoplasms and improve accuracy of fine needle aspiration biopsy. Ann Surg. 2005;242:353–361. doi: 10.1097/01.sla.0000179623.87329.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F, Shen L, Aldred MA, Morrison CD, Frilling A, Saji M, Schuppert F, Broelsch CE, Ringel MD, Eng C. Genetic classification of benign and malignant thyroid follicular neoplasia based on a three-gene combination. J Clin Endocrinol Metab. 2005;90:2512–2521. doi: 10.1210/jc.2004-2028. [DOI] [PubMed] [Google Scholar]
- Cerutti JM, Delcelo R, Amadei MJ, Nakabashi C, Maciel RM, Peterson B, Shoemaker J, Riggins GJ. A preoperative diagnostic test that distinguishes benign from malignant thyroid carcinoma based on gene expression. J Clin Invest. 2004;113:1234–1242. doi: 10.1172/JCI19617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada A, Mankovskaya S, Saenko V, Rogounovitch T, Mine M, Namba H, Nakashima M, Demidchik Y, Demidchik E, Yamashita S. Diagnostic usefulness of PCR profiling of the differentially expressed marker genes in thyroid papillary carcinomas. Cancer Lett. 2005;224:289–301. doi: 10.1016/j.canlet.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Prasad ML, Pellegata NS, Huang Y, Nagaraja HN, de la Chapelle A, Kloos RT. Galectin-3, fibronectin-1, CITED-1, HBME1 and cytokeratin-19 immunohistochemistry is useful for the differential diagnosis of thyroid tumors. Mod Pathol. 2005;18:48–57. doi: 10.1038/modpathol.3800235. [DOI] [PubMed] [Google Scholar]
- Rosen J, He M, Umbricht C, Alexander HR, Dackiw AP, Zeiger MA, Libutti SK. A six-gene model for differentiating benign from malignant thyroid tumors on the basis of gene expression. Surgery. 2005;138:1050–1057. doi: 10.1016/j.surg.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Barden CB, Shister KW, Zhu B, Guiter G, Greenblatt DY, Zeiger MA, Fahey TJ., III Classification of follicular thyroid tumors by molecular signature: results of gene profiling. Clin Cancer Res. 2003;9:1792–1800. [PubMed] [Google Scholar]
- Prasad ML, Pellegata NS, Kloos RT, Barbacioru C, Huang Y, de la Chapelle A. CITED1 protein expression suggests papillary thyroid carcinoma in high throughput tissue microarray-based study. Thyroid. 2004;14:169–175. doi: 10.1089/105072504773297830. [DOI] [PubMed] [Google Scholar]
- Takano T, Miyauchi A, Yoshida H, Kuma K, Amino N. Decreased relative expression level of trefoil factor 3 mRNA to galectin-3 mRNA distinguishes thyroid follicular carcinoma from adenoma. Cancer Lett. 2005;219:91–96. doi: 10.1016/j.canlet.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Giordano TJ, Kuick R, Thomas DG, Misek DE, Vinco M, Sanders D, Zhu Z, Ciampi R, Roh M, Shedden K, Gauger P, Doherty G, Thompson NW, Hanash S, Koenig RJ, Nikiforov YE. Molecular classification of papillary thyroid carcinoma: distinct BRAF, RAS, and RET/PTC mutation-specific gene expression profiles discovered by DNA microarray analysis. Oncogene. 2005;24:6646–6656. doi: 10.1038/sj.onc.1208822. [DOI] [PubMed] [Google Scholar]
- Beesley MF, McLaren KM. Cytokeratin 19 and galectin-3 immunohistochemistry in the differential diagnosis of solitary thyroid nodules. Histopathology. 2002;41:236–243. doi: 10.1046/j.1365-2559.2002.01442.x. [DOI] [PubMed] [Google Scholar]
- Takano T, Miyauchi A, Matsuzuka F, Kuma K, Amino N. Expression of oncofetal fibronectin messenger ribonucleic acid in fibroblasts in the thyroid: a possible cause of false positive results in molecular-based diagnosis of thyroid carcinomas. J Clin Endocrinol Metab. 2000;85:765–768. doi: 10.1210/jcem.85.2.6344. [DOI] [PubMed] [Google Scholar]
- Takano T, Miyauchi A, Yokozawa T, Matsuzuka F, Liu G, Higashiyama T, Morita S, Kuma K, Amino N. Accurate and objective preoperative diagnosis of thyroid papillary carcinomas by reverse transcription-PCR detection of oncofetal fibronectin messenger RNA in fine-needle aspiration biopsies. Cancer Res. 1998;58:4913–4917. [PubMed] [Google Scholar]
- Williams ED. Guest editorial: two proposals regarding the terminology of thyroid tumors. Int J Surg Pathol. 2000;8:181–183. doi: 10.1177/106689690000800304. [DOI] [PubMed] [Google Scholar]
- Rosai J, Carcangiu ML, DeLellis RA. Rosai J, editor. Washington DC: Armed Forces Institute of Pathology Press,; Atlas of Tumor Pathology. 1992:49–62. [Google Scholar]
- Papotti M, Rodriguez J, De Pompa R, Bartolazzi A, Rosai J. Galectin-3 and HBME-1 expression in well-differentiated thyroid tumors with follicular architecture of uncertain malignant potential. Mod Pathol. 2005;18:541–546. doi: 10.1038/modpathol.3800321. [DOI] [PubMed] [Google Scholar]
- Baloch ZW, LiVolsi VA. Encapsulated follicular variant of papillary thyroid carcinoma with bone metastases. Mod Pathol. 2000;13:861–865. doi: 10.1038/modpathol.3880153. [DOI] [PubMed] [Google Scholar]
- Passler C, Prager G, Scheuba C, Niederle BE, Kaserer K, Zettinig G, Niederle B. Follicular variant of papillary thyroid carcinoma: a long-term follow-up. Arch Surg. 2003;138:1362–1366. doi: 10.1001/archsurg.138.12.1362. [DOI] [PubMed] [Google Scholar]
- Zidan J, Karen D, Stein M, Rosenblatt E, Basher W, Kuten A. Pure versus follicular variant of papillary thyroid carcinoma: clinical features, prognostic factors, treatment, and survival. Cancer. 2003;97:1181–1185. doi: 10.1002/cncr.11175. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Gandhi M, Nikiforova MN, Fischer AH, Nikiforov YE. Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. Am J Clin Pathol. 2003;120:71–77. doi: 10.1309/ND8D-9LAJ-TRCT-G6QD. [DOI] [PubMed] [Google Scholar]
- Aldred MA, Huang Y, Liyanarachchi S, Pellegata NS, Gimm O, Jhiang S, Davuluri RV, de la Chapelle A, Eng C. Papillary and follicular thyroid carcinomas show distinctly different microarray expression profiles and can be distinguished by a minimum of five genes. J Clin Oncol. 2004;22:3531–3539. doi: 10.1200/JCO.2004.08.127. [DOI] [PubMed] [Google Scholar]
- Takano T, Miyauchi A, Yoshida H, Kuma K, Amino N. High-throughput differential screening of mRNAs by serial analysis of gene expression: decreased expression of trefoil factor 3 mRNA in thyroid follicular carcinomas. Br J Cancer. 2004;90:1600–1605. doi: 10.1038/sj.bjc.6601702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet JF, Hurbain I, Prengel C, Utzmann O, Scetbon F, Bernaudin JF, Fajac A. Galectin-3 immunodetection in follicular thyroid neoplasms: a prospective study on fine-needle aspiration samples. Br J Cancer. 2005;93:1175–1181. doi: 10.1038/sj.bjc.6602822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggiorato E, De Pompa R, Volante M, Cappia S, Arecco F, Dei Tos AP, Orlandi F, Papotti M. Characterization of thyroid ‘follicular neoplasms’ in fine-needle aspiration cytological specimens using a panel of immunohistochemical markers: a proposal for clinical application. Endocr Relat Cancer. 2005;12:305–317. doi: 10.1677/erc.1.00944. [DOI] [PubMed] [Google Scholar]