Abstract
Batten disease is an autosomal recessive disorder also known as juvenile neuronal ceroid lipofuscinosis. The most common mutation for this disease is an approximately 1-kbp deletion in the CLN3 gene, which accounts for about 80 to 85% of the mutation load. We developed a rapid assay for this mutation using the PCR to produce amplicons that are detected by nucleobase quenching of the fluorescent signal from a probe labeled with a fluorescent dye. The probe overlaps the deletion breakpoint and is completely base paired to the mutant amplicon. However, three bases at the 5′ end of the probe do not base pair with the wild-type amplicon. The alleles are distinguished by the different melting temperatures of the probe amplicon hybrids. Comparison of this new method with an allele-specific PCR and gel electrophoresis-based method showed 100% concordance in determination of the genotype for 30 specimens (11 homozygous mutant, 8 heterozygotes, and 11 homozygous normal). PCR followed by allele-specific melting curve analysis using nucleobase quenching has utility as a rapid method for detection of the most common mutation that causes Batten disease.
The neuronal ceroid lipofuscinoses are a group of autosomal recessive lysosomal-storage disorders characterized clinically by neurodegeneration and biochemically by the accumulation of hydrophobic pigments (ceroid and lipofuscin) in neurons and other cell types. The overall incidence is estimated to be 1 in 12,500 live births. For a review, please see reference 1.1 The juvenile form (Batten disease, BD) usually begins with vision loss between ages 4 and 10, followed by total blindness within 2 to 4 years. Other manifestations include seizures, loss of motor skills, progressive cognitive decline and dementia, resulting in a near vegetative state by young adulthood and death by age 30. Although there is evidence that autoimmunity may play a role in the pathogenesis of BD, there is still much to learn about this disease.2
The lack of specificity in the early stages and the rarity of the disease often result in a long delayed diagnosis for the proband. In its early stages, the retinal degeneration cannot be easily distinguished from other retinal degenerative diseases, particularly retinitis pigmentosa.3 During this period of uncertain diagnosis, the parents cannot obtain accurate genetic counseling. Prenatal diagnosis to prevent the birth of additional affected offspring is not available until an accurate diagnosis is obtained. Furthermore, future therapy that is capable of stopping the progression of the disease is unlikely to reverse the vision loss or other manifestations, which appear to be due to a loss of neurons. Therefore, the value of future therapeutic intervention is likely to be greatly enhanced by earlier diagnosis. For these reasons, early diagnosis of BD using molecular methods is useful now and may be even more so in the future.
BD is caused by inactivation of the CLN3 gene, located at chromosome 16, band p12.1.4 Studies in yeast demonstrate a role for the CLN3 protein in arginine transport.5 The most frequent mutation is an approximately 1-kbp deletion that removes exons 7 and 8, and creates a frame-shift. This mutation is in strong linkage disequilibrium with a rare haplotype indicating that it is descended from a founder mutation and not due to recurrent mutational events.4 The mutated gene encodes a protein in which the first 153 of 438 residues are intact, followed by 28 incorrect amino acids before premature termination.4 This mutation accounts for about 80 to 85% of the mutation load for BD.4,6 The goal of this work was to develop and validate a low-cost rapid assay that could be used to detect the common BD mutation.
Materials and Methods
Peripheral blood specimens were obtained from Batten disease patients and their families after informed consent under a protocol approved by the University of Rochester IRB. DNA was purified from peripheral blood or immortalized lymphocytes using the QiAmp System (Qiagen Inc., Valencia, CA). The PCR and acquisition of fluorescence data were done in the Roche LightCycler (Mannheim, Germany). The primers were designed to avoid repeated sequences and to produce amplicons with a similar size from the wild-type and common deletion alleles. The sequences of the primers were 3CLN-1, 5′-ACTCTGTGATGGACCAG; 3CLN-2, 5′-CCTAAGGTCAGGAAACC; and 3CLN-3, 5′-GTAACTTGTTCAACGCTACAA. The probe sequence was 5′-GTCCTTCAAGTCCCTAC and labeled on the 3′ terminus with 6-FAM. The PCR was done in Roche LightCycler capillary tubes in a volume of 20 μl with Roche FastStart DNA Master Hybridization Probes, MgCl2 to achieve a final concentration of 2.5 mmol/L, 0.1 μmol/L 3CLN-1, 0.3 μmol/L 3CLN-2, 1 μmol/L 3CLN-3, and 0.2 μmol/L probe. Primers and probe were obtained from Integrated DNA Technologies (Coralville, IA). The reaction was heated to 95°C for 10 minutes to activate the Taq DNA polymerase, then cycled 50 times between 95°C for 2 seconds, 57°C for 4 seconds, and 72°C for 16 seconds, with ramping rates at the maximum of 20°C/second except for the 57°C to 72°C transition which was done at 0.5°C/second. After PCR, the melting curve thermal profile was 95°C for 10 seconds, 40°C for two minutes, then 70°C with a 0.1°C/second temperature transition rate while continuously collecting fluorescence data. For validation we set up the assay described by Taschner et al,7 exactly as in their paper except that we used HotStar Taq DNA polymerase and the accompanying buffer (Qiagen, Inc.).
Results
The purpose of this work was to develop and validate a rapid and inexpensive method for the diagnosis of the most common Batten disease mutation, an approximately 1-kbp deletion in the CLN3 gene. Due to the rarity of the disease and the high anxiety level caused by progressive vision loss in a child, the test needs to be completed rapidly and so cannot be batched for greater efficiency. Methods that are cost efficient for large numbers of specimens are not useful in this situation. We decided to try the nucleobase quenching strategy described by Crockett and Wittwer8 to develop a method that is better suited for low-volume genotyping.
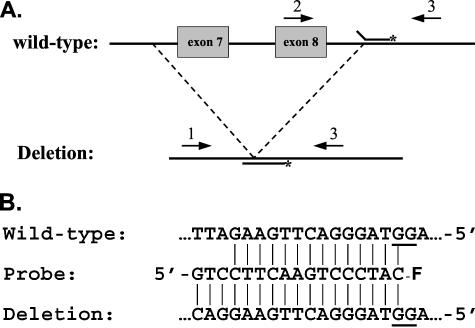
Figure 1 describes the overall strategy. The PCR was done with 3 primers as shown in Figure 1A. Primers 3CLN-1 and 3CLN-3 amplify the deleted allele to produce a 400-bp amplicon, while primers 3CLN-2 and 3CLN-3 produce a 373-bp product from the wild-type allele. The 1 and 3 primers can also produce a larger (1366 bp) product from wild-type DNA, but this is of no consequence for the assay. The probe was designed so that the fluorophore (6-FAM) is in proximity to deoxyguanosine residues on the opposite strand when the probe is base paired to the amplicon (Figure 1B). This causes a decrease in the fluorescent signal due to the quenching effect of the guanine base. The best relative position(s) for the G residues and the fluorophore for obtaining this quenching effect was determined by Crockett and Wittwer.8 We took advantage of their work in the design of our probe. When the probe dissociates due to thermal denaturation, the fluorescent signal increases due to elimination of the quenching effect of the guanine base. Thus, we distinguish the alleles using a melting curve strategy.
Figure 1.
Strategy. A: The positions of the primers (arrows) and probe on the wild-type and mutant CLN3 gene are shown. The fluorophore on the probe is indicated with an asterisk; not drawn to scale. B: The sequence of the probe is shown in between the complementary sequences of the wild-type and deletion alleles, with vertical lines connecting the base-paired residues. The –F on the 3′ end of the probe indicates the 6-FAM fluorophore and shows its position with respect to the G residues on the opposite strand. The probe is fully base-paired with the mutant sequence, but has three unmatched nucleotides at the 5′ end when annealed to a wild-type amplicon. The G residues that contribute to the quenching of the fluorescent signal are underlined in the normal and mutant sequences.
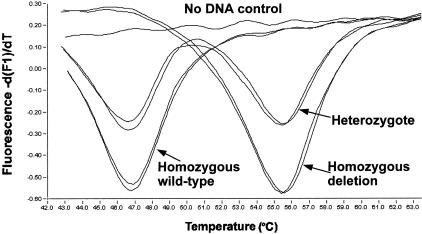
We optimized the reaction for Mg and primer concentrations. The reaction was done asymmetrically to get an excess of the strand that hybridizes with the probe. That increases the signal by avoiding competition between probe hybridization and amplicon renaturation. We also optimized the ratio between the two upstream primers to get approximately equal signal from the wild-type and deletion alleles from heterozgotes. The optimized conditions are described in the Materials and Methods section. Figure 2 shows melting troughs (or inverted peaks) for two template DNAs with each of the three genotypes that need to be distinguished. The probe is perfectly matched to the mutant sequence and gives a Tm of 55.6°C (SD, 0.3°C), while the Tm for the mismatched wild-type sequence is 47°C (SD, 0.3°C).
Figure 2.
Graph of -d(F1)/dT versus temperature showing the melting troughs that reveal the genotype. The direction of change in fluorescence intensity when the quenched probe dissociates from the amplicon (increase) is the opposite of that seen when using a dual probe fluorescence resonance energy transfer system to generate the fluorescent signal.14 For this reason we see a trough, or inverted peak, when using the software on the Roche LightCycler. The figure shows results for two template DNAs that are homozygous wild-type, two that are homozygous mutant, two heterozygotes, and one no template control.
The nucleobase quenching assay was validated by comparison with a genotyping method described by Taschner et al,7 which involves allele-specific PCR followed by gel electrophoresis. Their assay uses a downstream primer within the deleted region to amplify the normal allele, another downstream primer covering the breakpoint to amplify the deletion allele, and an upstream common primer. The alleles are distinguished by the size of the amplicons.7 Thirty specimens, including 11 homozygous for the deletion, 8 heterozygotes, and 11 normal homozygotes were genotyped. There was 100% agreement between the two methods (data not shown).
Discussion
The full spectrum of clinical findings that suggest a diagnosis of BD in a proband does not become apparent until blindness is total.9 Further studies that involve electroretinography and electron microscopic analysis of peripheral blood lymphocytes and/or skin biopsies are used to confirm the diagnosis.1 Diagnosis based on DNA analysis is also available.4,6,7,10,11,12 There is clearly a need for an early diagnosis of the disease if therapeutic options become available, or even to test candidate therapies. Easily available low-cost testing would facilitate the early diagnosis of BD. Toward this end, we undertook to develop a less expensive DNA-based test for the most common mutation that causes BD, an approximately 1-kbp deletion. The mutation detection system we developed and validated is based on the use of a real-time PCR platform. The advantages of such a system include rapid analysis, a reduction in the potential for contamination of future reactions because the PCR product is not removed from the tube, and the cost savings that accrue from the avoidance of post-PCR processing. Using this method, the time from completion of DNA preparation to a result is approximately 90 minutes with only about 15 minutes of hands-on labor, and the cost of the PCR reagents is approximately $2.50 per capillary. Other methods for diagnosis of the Batten disease common mutation use either gel analysis or radioactivity.4,6,7,10,11,12
To distinguish the alleles we made use of a single hybridization probe labeled with a single fluorescent dye. The wild-type and mutant alleles were clearly distinguishable by differences in melting temperature (Figure 2). The change in fluorescent signal on hybridization of the probe is mostly due to the quenching effect of the guanine base in the first overhang position of the opposite strand of the amplified DNA.8 The adjacent G residue in the last base-paired position also contributes to the quenching effect (Figure 1B).8 This strategy for genotyping was successfully applied by Crocket and Wittwer8 to a number of single nucleotide polymorphisms and was also used by Vaughn and Elenitoba-Johnson13 to discover a sequence variant in human herpesvirus 8. A single probe with only a single fluorescent label is less expensive than two dual-labeled probes used for 5′ nuclease assays, and also less expensive than the two labeled probes used for fluorescence resonance energy transfer assays.14,15 In contrast to Crockett and Wittwer8 we used 6-FAM (6-carboxyfluorescein) instead of fluorescein (fluorescein isothiocyanate) because the excitation and emission wavelengths are very similar, the 6-FAM fluorescence is quenched by G residues, and 6-FAM-labeled oligos are less expensive because they do not require additional purification before use (Integrated DNA Technologies, personal communication). The use of an inexpensive probe also contributes to lowering the cost of the analysis as well as lowering the cost of assay development when optimization of the probe sequence is needed.
The CLN3 1-kbp deletion was found to account for about 80 to 85% of the mutation load for this disease.4,6 In a large study, Munroe et al6 found that 74% of 188 unrelated affected individuals from 18 different countries were homozygous for this mutation, and a further 22% were compound heterozygotes for the deletion and another mutation. Thus, a test for only the CLN3 1-kbp deletion in a patient with some suspicion of BD would give either a diagnosis (homozygous result) or raise the level of suspicion (heterozygous result) in over 90% of patients who actually do have this disease. The remaining mutations that cause BD are very heterogeneous with over 31 mutations reported to date on the CLN3 mutation web site (http://www.ucl.ac.uk/ncl/CLN3.html). The next most frequent mutation after the common deletion was found in five families. With no other known high frequency CLN3 mutations, further molecular diagnosis of BD requires a variety of methods including mutation scanning and sequencing for micromutations and Southern blots for large deletions.6,11,12 The absence of the CLN3 1-kbp deletion in a patient with some suspicion of BD might also prompt further consideration of other diagnoses.
Acknowledgments
We thank the Batten Disease Support and Research Association and their families for participating in this study.
Footnotes
Supported in part by National Institutes of Health grant R01 NS44310 and the Luke and Rachel Batten Foundation.
References
- Weimer JM, Kriscenski-Perry E, Elshatory Y, Pearce DA. The neuronal ceroid lipofuscinoses. Neuromol Med. 2002;1:111–124. doi: 10.1385/NMM:1:2:111. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S, Ito M, Cooper JD, Brooks AI, Curran TM, Powers JM, Pearce DA. An autoantibody inhibitory to glutamic acid decarboxylase in the neurodegenerative disorder Batten disease. Hum Mol Genet. 2002;11:1421–1431. doi: 10.1093/hmg/11.12.1421. [DOI] [PubMed] [Google Scholar]
- Weleber RG. The dystrophic retina in multi-system disorders: the electroretinogram in neuronal ceroid lipofuscinoses. Eye. 1998;12:580–590. doi: 10.1038/eye.1998.148. [DOI] [PubMed] [Google Scholar]
- The International Batten Disease Consortium Isolation of a novel gene underlying Batten disease, CLN3. Cell. 1995;82:949–957. doi: 10.1016/0092-8674(95)90274-0. [DOI] [PubMed] [Google Scholar]
- Kim Y, Ramirez-Montealegre D, Pearce DA. A role in vacuolar arginine transport for yeast Btn1p and for human CLN3, the protein defective in Batten disease. Proc Natl Acad Sci USA. 2003;100:15458–15462. doi: 10.1073/pnas.2136651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munroe PB, Mitchison HM, O’Rawe AM, Anderson JW, Boustany R-M, Lerner TJ, Taschner PEM, de Vos N, Breuning MH, Gardiner RM, Mole SE. Spectrum of mutations in Batten disease gene, CLN3. Am J Hum Genet. 1997;61:310–316. doi: 10.1086/514846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschner PEM, de Vos N, Breuning MH. Rapid detection of the major deletion in the Batten disease gene CLN3 by allele-specific PCR. J Med Genet. 1997;34:955–956. doi: 10.1136/jmg.34.11.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett AO, Wittwer CT. Fluorescein-linked oligonucleotides for real-time PCR: using the inherent quenching of deoxyguanosine nucleotides. Anal Biochem. 2001;290:89–97. doi: 10.1006/abio.2000.4957. [DOI] [PubMed] [Google Scholar]
- Santavuori P, Lauronen L, Kirveskari E, Åberg L, Sainio K, Autti T. Neuronal ceroid lipofuscinoses in childhood. Neurol Sci. 2000;21:S35–S41. doi: 10.1007/s100720070038. [DOI] [PubMed] [Google Scholar]
- Järvelä I, Mitchison HM, Munroe PB, O’Rawe AM, Mole SE, Syvänen A-C. Rapid diagnostic test for the major mutation underlying Batten disease. J Med Genet. 1996;33:1041–1042. doi: 10.1136/jmg.33.12.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong NA, Wisniewski KE, Ju W, Moroziewicz DN, Jurkiewicz A, McLendon L, Jenkins EC, Brown WT. Molecular diagnosis of and carrier screening for the neuronal ceroid lipofuscinoses. Genet Test. 2000;4:243–248. doi: 10.1089/10906570050501452. [DOI] [PubMed] [Google Scholar]
- Zhong N. Molecular genetic testing for neuronal ceroid lipofuscinoses. Adv Genet. 2001;45:141–158. doi: 10.1016/s0065-2660(01)45008-5. [DOI] [PubMed] [Google Scholar]
- Vaughn CP, Elenitoba-Johnson KS. Intrinsic deoxyguanosine quenching of fluorescein-labeled hybridization probes: a simple method for real-time PCR detection and genotyping. Lab Invest. 2001;81:1575–1577. doi: 10.1038/labinvest.3780371. [DOI] [PubMed] [Google Scholar]
- Bernard PS, Ajioka RS, Kushner JP, Wittwer CT. Homogeneous multiplex genotyping of hemochromatosis mutations with fluorescent hybridization probes. Am J Pathol. 1998;153:1055–1061. doi: 10.1016/s0002-9440(10)65650-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ. Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal. 1999;14:143–149. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]