Abstract
Gastric cancer is a common tumor worldwide and a tremendous health burden. However, the underlying mechanisms of tumorigenesis in this cancer’s development are primarily undefined. Allelic imbalance (AI) of 8p has been reported in many cancers, yet, the target(s) of alteration and the importance of allelic imbalance on this chromosomal arm in gastric carcinoma development remained to be characterized. Our findings confirmed a high rate of AI on 8p in gastric cancers. Moreover, we demonstrated that AI on 8p, either overall or at marker D8S560, was associated with poorer survival in patients with gastric cancer. Finally, gastric cancers with a high rate of microsatellite instability were significantly associated with noncardia tumors and with female gender.
Gastric cancer occurs in 700,000 people worldwide each year, with ∼24,000 of these cases occurring in the United States. Five-year-survival rates are dismal, especially for late-stage disease, being almost zero when distant metastasis is present. Early detection improves the chance of survival, as does an increase in curative resection during the first course of treatment.1
Many genes have been implicated in the development of gastric cancer including individual transcription factors, genes involved in cell-cycle regulation, growth, and metastasis.2 Two different histological types of gastric cancer have been described: intestinal type (more well differentiated) and diffuse type (more poorly differentiated). These two tumor types have been shown to have different, distinct pathways involved in their initiation and progression. Cytogenetic differences have been noted between the two tumor types by traditional karyotyping.3 Molecular genetic differences have also been noted including various levels of gene expression.4,5,6 Despite the differences between these tumor types, no clear mechanism for tumor initiation and progression has been delineated.
The identification of additional genes involved in the gastric cancer pathway will be important in gaining a better understanding of the mechanisms that drive tumor development within the stomach. Allelic imbalance (AI) is one method that can be used for the identification of novel tumor suppressor genes. AI is useful in determining imbalance of alleles, which can result from either the loss of a chromosomal region (eg, deletion of a tumor suppressor gene) or gain of a chromosomal region (ie, trisomy or isochromosomes). Some cancers exhibit isochromosomes, which would be detected as an imbalance because of the loss of one arm and the subsequent gain of another. Iso 8q and trisomy 8 are common chromosomal changes in cancers.7,8,9,10,11,12,13 Thus, AI can detect genes involved in tumorigenesis either because of gains of activity or loss of function, but would not distinguish between 8p loss, 8 gain (trisomy), or iso 8q because of the nature of this assay.
Through AI analysis, several chromosomal regions have been identified as potential sites where novel genes involved in the etiology of gastric cancer might reside.2 Many of these regions have been implicated in other tumors as well, in particular the p arm of chromosome 8. Chromosome 8p has been found to be altered in other cancers including those arising from the colon,14,15,16,17,18,19,20,21,22,23,24 ovary,21 prostate,25,26,27,28,29 bladder,30 lung,21,31 breast,32 pancreas,33 and liver.21,34 These data support the hypothesis that gene(s) on the p arm of chromosome 8 have an important role in the development and/or progression of tumors from multiple sites.
Specifically in gastric cancer, multiple studies have detected changes in chromosome 8p.21,35,36,37 Our group found a high rate of AI on chromosome 8p using two representative markers in xenografted gastric tumors.35 Baffa and colleagues36 identified a region of loss on chromosome 8 in the p22-p21 region and made the observation that it was likely that more than one gene involved in gastric cancer exists on chromosome 8p. Vecchione and colleagues37 identified FEZ1, a potential tumor suppressor gene on 8p22, and showed that this gene is inactivated in some gastric cancers through genomic deletion, mutation, or hypermethylation. Furthermore, the authors were able to show that reduced protein expression of this gene was significantly correlated with diffuse histology. More work remains, however, to further define the loci on 8p that demonstrate AI, and the targets of inactivation at these sites that would be specific for clinicopathological phenotypes of gastric cancers.
One of the major obstacles in the identification of regions with AI in tumors is the presence of infiltrative normal tissue. Tissue from xenografts offers an excellent advantage in AI studies. Xenografted material is enriched for tumor cells, as normal cell growth is not supported by murine stroma. In xenografts, the tumor is supported by murine stroma, which usually fails to amplify in polymerase chain reaction-based assays with primers designed from nonintronic human DNA sequences. This allows for better AI detection; when an imbalance exists at a locus, the allele can be completely absent, compared to the microdissected tumor, which still has a residual normal peak present. Results from xenografted tumors can more easily reveal loss of an allele, and historically xenografts have led to the identification of important genes involved in cancer development, including the involvement of the DPC4 gene in colon and pancreatic cancer.38,39
This study extends the findings on chromosome 8p in mapping AI at a much greater resolution in a relatively large number of gastric tumors, both xenografted and primary tumors. Two hundred one samples arising from 174 patients were evaluated for microsatellite instability (MSI) and AI on chromosome 8 using paired normal and tumor DNAs.
Materials and Methods
Specimens
From 1995 through 2001, fresh tissue from 174 surgically resected gastric adenocarcinomas were collected from surgical pathology at the Universities of Indiana, Virginia, and Siena according to institutional review board protocols. Specimens were collected in standard RPMI media and stored on ice for establishing a xenograft or frozen immediately in liquid nitrogen and stored at −80°C for subsequent microdissection and DNA extraction. All tumor samples were at least 80% or greater for neoplastic cells before DNA extraction. Normal tissue from resected specimens or peripheral blood samples was also procured. Samples were obtained from patients at all stages of gastric cancer development (eg, stages I to IV) and included both intestinal and diffuse histopathological subtypes.
Xenografting and DNA Extraction
Small pieces of tumor tissue were soaked in Matrigel (Collaborative Biomed Research, Bedford, MA) then implanted subcutaneously into the flanks of immunodeficient mice (nu/nu from Harlan or SCID from Charles River, Wilmington, MA) for xenografting growth as described.35 First passage tumors were harvested when their diameter reached ∼1 cm and processed for histopathological confirmation. Genomic DNA was extracted according to a standard organic method as previously described.40
Marker Selection
Ten markers were selected to span the p arm of chromosome 8 and to represent regions where various tumor suppressor genes have been suggested in the literature to reside. Those regions include 8p23 (D8S262 and D8S1742), 8p22 (D8S254 and D8S261), 8p21.3 (D8S560, D8S136), 8p21.1-8p128,53,54,55,61 (D8S1820, D8S1055), and 8p11.2 (D8S255 and ANK1). Additionally, two markers were run on the q arm of chromosome 8, one in the 8q22 region (D8S1760), and one in the 8q24 region near the telomere (D8S1925). The selected markers and their locations can be found in Figure 1. Markers were selected based on high heterozygosity scores and smaller size. All known marker distances and localization came from the UCSC database (July 2003) (University of California, Santa Cruz, Santa Cruz, CA).
Figure 1.
Ideogram of chromosome 8. Marker localization: marker order based on data from the UCSC database (July, 2003). USCS location is denoted by the nucleotide starting position for each marker. UCSC location denotes the bp localization in relationship to the telomeric arm of chromosome 8p.
Polymerase Chain Reaction and Gel Electrophoresis
Each marker was tested to determine the optimal conditions for polymerase chain reaction analysis. Markers were amplified and their resulting products run on an ABI 3100 and interpreted using the Genotyper 2.5 software (Applied Biosystems, Foster City, CA). The main allele peaks for each patient were labeled with the relative peak amplitude (height), designating its signal intensity.
MSI Analysis
Primary and xenograft tumors were evaluated for MSI at each of their 12 markers on chromosome 8 as previously described45 by comparing their tumor-banding pattern to their normal. MSI was identified as additional peaks within the tumor sample when compared to the normal sample. Tumors were classified into one of three categories; microsatellite stable (MSS) in which no markers demonstrated instability; microsatellite instability low (MSI-L), in which at least one marker but <40% of the markers demonstrated instability; or microsatellite instability high (MSI-H), in which ≥40% of the markers demonstrated instability. Samples that did not have at least four informative amplified markers on chromosome 8 were not classified into any MSI category.
AI Scoring
All MSI-H tumors were excluded from AI evaluation. Within the MSS and MSI-L subclasses, loci were considered noninformative for AI evaluation purposes either if there was no amplification, if instability was demonstrated, if the sample was homozygous at that locus, or if the signal intensity was either too high or too low. Signal intensities needed to exceed 100 for both of the main peaks in the normal, and at least one peak in the tumor or the sample was considered to have no amplification. Similarly, no quantification was done if the signal exceeded 7000, to prevent artificial truncation of the signal from affecting the AI ratio. Informative alleles were then quantified by taking the ratio of the height of the normal alleles and dividing it by the ratio of the tumor alleles. If the resultant ratio was less than 1.0, the inverse of the ratio was used to make the numbers uniform and comparable. For the purpose of this study, a score of 1.0 to 1.9 was considered negative, and a score of 2.0 or greater was considered to demonstrate AI. Using information on whether samples showed AI at individual 8p markers, each sample was categorized as either exhibiting or not exhibiting AI on 8p overall based on whether they had at least one AI event at an 8p marker. A preliminary analysis was made, such that for each sample it was noted whether four informative markers on 8p were available and if at least one AI event was detected on 8p. Tumors lacking at least four amplified markers were excluded from overall AI evaluation to minimize the chances that samples would be inaccurately classified as not exhibiting AI on 8p. However, information on these samples was later used in analyses only requiring knowledge of AI status at individual markers and not a determination of overall AI status on 8p.
Statistical Methods of Analysis
Using data on primary tumor samples, chi-square tests of association and t-tests were used to examine possible associations between both AI and clinical pathological features, and MSI status and these features. All analyses are considered to be exploratory. In the AI-related association analyses, a significant level of α = 0.05 was used for tests involving overall AI status on 8p whereas those examining AI status at individual chromosome 8 markers were judged at a more restrictive significance level of α = 0.01. When possible, information from xenograft samples was incorporated in the analysis to assess the consistency of the results obtained.
Kaplan-Meier estimates of survival functions, 1-year-point estimates, and 95% confidence intervals were obtained separately for overall AI status on 8p, as well as each individual marker. Log-rank tests were used to assess the statistical significance of estimated survival differences related to AI status. Similar methods were used to examine possible survival differences among different MSI subtypes. Cox’s proportional hazard models were used to assess the relationship between AI status and survival while adjusting for patient age. The Wald test was used to assess statistical significance.
Using data on primary tumors and xenografts separately, cluster analysis was used as an exploratory measure to detect markers that were lost together in hopes of identifying patterns of regional loss. The clustering procedure was performed using the Splus function varclus46 with the similarity attribute set to “bothpos” (moved). This specified the usage of a similarity measure based on the proportion of tumor samples with AI at both markers for each possible bi-marker pair. Additionally, estimated rates of AI and 95% confidence intervals were calculated for each individual chromosome 8 marker.
Results
Primary and xenografted tumor samples from 174 patients with gastric carcinoma were selected to study AI on the p arm of chromosome 8. Samples were obtained from patients at all stages of gastric cancer development (eg, stage I to IV) and included histopathological subtypes of both intestinal and diffuse classifications. From the 174 patients, there were 162 primary tumors and 39 xenografts. Only 27 patients had both a primary tumor and xenograft. Of the 162 primary tumors, 11 had less than 4 amplified markers out of the 12 analyzed on chromosome 8. These samples were not classified for MSI and were removed from subsequent analyses. Of the remaining cases, 27 (18%) demonstrated MSI at ≥40% of the loci that amplified, and were classified as MSI-H, 16 (11%) demonstrated instability, but at <40% of successful markers, and were classified as MSI-L, and the remaining 108 (72%) tumors were labeled MSS. A similar distribution was observed for the 39 xenographs (Table 1).
Table 1.
Frequency of MSI Status for Primary and Xenograft Tumors
| MSI status | No. of primary tumors | No. of xenografts |
|---|---|---|
| MSS | 108 (72%) | 27 (71%) |
| MSI-L | 16 (11%) | 7 (18%) |
| MSI-H | 27 (18%) | 4 (11%) |
| Total | 151 | 38 |
The MSI status of primary gastric tumors was analyzed using the chi-square and t-tests to examine possible associations with both diagnostic and demographic criteria (Table 2). Because of the limited sample size in the MSI-L group, all tests involved comparisons between the MSI-H subclass and the combination of the MSS and MSI-L subclasses. MSI-H tumors were found to be significantly associated with tumors located in noncardia regions of the stomach (P = 0.006), and with the female gender (P = 0.024). MSI-H cases were also found to be more frequent in Italian patients than American patients (P = 0.007). These associations were then re-examined using data on both primary and xenograft samples, forgoing primary tumor data for xenograft data if both were available for a given patient. This was done to reassess previously detected significant associations in light of the fact that three patients had xenograft and primary samples that differed on their MSI classification (Table 3). In two of these, the misclassification was minor (MSS versus MSI-L) while it was more substantial in the third (MSS versus MSI-H). Addition of xenograft data did not affect the significant associations.
Table 2.
MSI Status and Clinical Pathologic Features for Primary Tumors
| Feature
|
MSI Status
|
P value
|
||
|---|---|---|---|---|
| MSS (n = 108) N (%) | MSI-L (n = 16) N (%) | MSI-H (n = 27) N (%) | ||
| Gender | ||||
| Male | 79 (73) | 12 (75) | 13 (48) | 0.024 |
| Female | 27 (25) | 4 (25) | 12 (44) | |
| Unknown | 2 (2) | 0 (0) | 2 (7) | |
| Age | ||||
| Median (range) | 67 (34–88) | 69 (41–88) | 69 (48–88) | 0.051† |
| Race | ||||
| Caucasian | 90 (83) | 16 (100) | 23 (85) | 0.27 |
| African-American | 14 (13) | 0 (0) | 1 (4) | (C versus A) |
| Other | 1 (1) | 0 (0) | 2 (7) | |
| Unknown | 3 (3) | 0 (0) | 1 (4) | |
| Region | ||||
| United States | 78 (72) | 10 (63) | 12 (44) | 0.007 |
| Italy | 29 (27) | 6 (38) | 15 (56) | |
| Unknown | 1 (1) | 0 (0) | 0 (0) | |
| Histology | ||||
| Intestinal | 56 (52) | 10 (63) | 19 (70) | 0.26 |
| Diffuse | 24 (22) | 3 (19) | 4 (15) | (I versus D) |
| Other | 2 (2) | 0 (0) | 0 (0) | |
| Unknown | 26 (24) | 3 (19) | 4 (15) | |
| Site | ||||
| Proximal | 45 (42) | 5 (31) | 3 (11) | 0.006 |
| Nonproximal | 60 (56) | 11 (69) | 22 (81) | |
| Unknown | 3 (3) | 0 (0) | 2 (7) | |
| Morphology | ||||
| ADEN | 44 (41) | 5 (31) | 13 (48) | 0.46 |
| SQA | 2 (2) | 0 (0) | 0 (0) | |
| SRC | 3 (3) | 0 (0) | 2 (7) | |
| Unknown | 59 (55) | 11 (69) | 12 (44) | |
| Stage | ||||
| 1 | 9 (8) | 1 (6) | 10 (37) | 0.14 |
| 2 | 27 (25) | 4 (25) | 3 (11) | (I/II versus III/IV) |
| 3 | 47 (44) | 8 (50) | 11 (41) | |
| 4 | 20 (19) | 3 (19) | 2 (7) | |
| Unknown | 5 (5) | 0 (0) | 1 (4) | |
| Grade | ||||
| PD | 20 (19) | 2 (13) | 6 (22) | 0.42 |
| MD | 23 (21) | 3 (19) | 4 (15) | (3 levels) |
| WD | 4 (4) | 1 (6) | 0 (0) | |
| Other | 1 (1) | 0 (0) | 0 (0) | |
| Unknown | 60 (56) | 10 (63) | 17 (63) | |
| H. Pylori | ||||
| Yes | 12 (11) | 2 (13) | 1 (4) | 0.17 |
| No | 31 (29) | 3 (19) | 10 (37) | |
| Unknown | 65 (60) | 11 (69) | 16 (59) | |
| Barretts | ||||
| Yes | 8 (7) | 2 (13) | 0 (0) | 0.13 |
| No | 20 (19) | 0 (0) | 5 (19) | |
| Unknown | 80 (74) | 14 (88) | 22 (81) | |
*Chi-square tests of association used for categorical variables.
t-test used.
Table 3.
Agreement on MSI Classification between 27 Cases with Both Primary and Xenographs
| Xenograph:
|
Primary
|
|||
|---|---|---|---|---|
| MSS | MSI-L | MSI-H | None | |
| MSS | 19 | 0 | 1 | 0 |
| MSI-L | 2 | 1 | 0 | 0 |
| MSI-H | 0 | 0 | 3 | 0 |
| None | 0 | 0 | 0 | 1 |
Survival functions were estimated for each MSI class using primary tumor survival data. Although there was a trend toward better survival, both log-rank and Wilcoxon tests failed to reveal a statistically significant survival advantage among the three groups (MSS, MSI-L, and MSI-H) either when treated as three separate groups (LRT, P = 0.24) or on combining samples in the MSS and MSI-L subclasses (LRT, P = 0.14) (Figure 2, plots A and B). Statistical power was low in these tests because of the relatively small sample sizes in the MSI-H and MSI-L subclasses. Addition and/or substitution of available data on xenograft samples did not alter the results.
Figure 2.
Survival analysis by MSI status. Plot A: Estimated survival rates by MSI status classification (MSS, solid line; MSI low, dotted line; MSI high, dashed line). Plot B: Estimated survival rates obtained on combining samples within the MSS and MSI low subtypes (MSS + MSI low, solid line; MSI high, dashed line). The numbers of patients at risk are labeled beneath the x axis at fixed intervals.
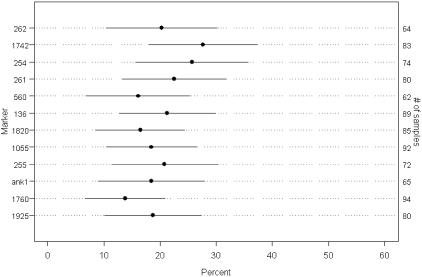
AI on 8p was evaluated using 158 samples in the MSS and MSI-L subclasses. Of these 158 samples, there were 102 primary tumors that were not paired to a xenograft, 12 unpaired xenograft tumors, and 22 xenograft/primary tumor pairs (44 samples). A calculated ratio of 2.0 or greater within the normal-tumor or normal-xenograft pair was considered to demonstrate AI. Overall, 46 of 116 (39.7%) primary tumors and 24 of 33 (72.7%) xenograft tumors assessed for AI on 8p demonstrated AI on at least one chromosome 8p locus. Primary tumors exhibited a frequency of AI at each individual marker on chromosome 8 in a range between 13.8 to 27.7% (Figure 3). Analysis of paired primary tumors and xenografts from patients whose samples had been classified as either MSS or MSI-L was performed to determine agreement on overall AI status on 8p as well as agreement on AI status at individual loci. Thirteen of the 20 patients (65%), with data on both a primary tumor and xenograft, had the same AI classification on 8p overall for both samples. On the marker level, of 167 markers for which both the primary tumor and xenograft were informative for AI status, 110 (65.9%) were found to agree on the AI classification. Moreover, 53 of the 57 (93.0%) markers for which AI status differed between primary tumor and xenograft, AI was observed in the xenograft but not in the primary tumor.
Figure 3.
AI rates and 95% confidence limits on primary tumors. Rates of AI observed among primary tumors on each of 12 markers for which AI was examined. Points and solid lines represent estimated AI rates and 95% confidence intervals, respectively.
Cluster analysis was performed to detect AI associations that might exist between markers or within regions of chromosome 8 for xenograft and primary tumor samples separately (Figure 4, plots A and B). Among xenograft tumors, AI occurred at both markers D8S261 and D8S136 in 77% of the samples (plot A). Both of these markers fall within the 8p22-8p21 region of chromosome 8. The highest similarity proportion was 24% in primary tumors, which occurred among markers D8S254 and D8S261 in the 8p22 region (plot B). This region has historically demonstrated frequent AI in gastric cancer.
Figure 4.
Cluster analysis. Cluster dendrograms in which markers are grouped based on similarity between patterns of AI. Xenograft (plot A) and primary tumor (plot B) samples were analyzed separately.
Using primary tumors, possible associations between AI status on 8p and patient characteristics were assessed, both overall and at each individual marker. Patient characteristics included ethnicity of patient, age, race, gender, tumor location, histology, stage, and grade. Geographic origin of patients was found to be associated with overall AI on 8p (P = 0.042). Primary tumor samples from patients obtained in Italy were less likely to show at least one AI event on the 8p arm than samples from patients in the United States. Also, patients with at least one AI event on 8p tended to be older (P = 0.009, n = 110) with mean ages being 68.6 years for those with AI on 8p and 63.2 years for those without AI on 8p, respectively. At α = 0.01, no tests of association between tumor or patient-level characteristics and AI status at individual chromosome 8 markers were found to be statistically significant.
Significant differences in survival rates were detected in primary tumor samples with available survival data according to overall AI status on 8p (P = 0.035, n = 62) (Figure 5 and Table 4). As expected, most survival loss events occurred within the first few years of diagnosis making the 5-year survival less than 25%, which is generally observed for this lethal cancer, leaving relatively few cases to study at this time point. Only one marker, D8S560, offered evidence of a difference in survival rates when all 12 markers were tested individually (P = 0.005, n = 38). In the cases of both overall AI status on 8p and AI status on 560, those with AI were found to have worse survival from time of surgery than those retaining 8p. It was noted that only 5 of the 38 (13%) samples used in the analysis of survival by AI status on 560 had actually exhibited AI at that marker.
Figure 5.
Survival by AI status on 8p for primary tumors. Estimated survival rates by overall AI status on 8p (no AI, solid line; AI, dashed line). The numbers of patients at risk are labeled beneath the x axis at fixed intervals.
Table 4.
8p AI and Survival
| Marker | AI
|
No AI
|
P values Log rank | ||||
|---|---|---|---|---|---|---|---|
| N/events | 1 year survival | 95% CI | N/events | 1 year survival | 95% CI | ||
| Overall 18p | 27/22 | .476 | (.319, .710) | 35/20 | .692 | (.550, .871) | 0.035 |
| 262 | 7/5 | .571 | (.301, 1.00) | 27/16 | .512 | (.347, .755) | 0.95 |
| 1742 | 11/9 | .545 | (.318, .936) | 38/28 | .530 | (.390, .721) | 0.66 |
| 254 | 10/7 | .686 | (.445, 1.00) | 27/14 | .768 | (.621, .950) | 0.28 |
| 261 | 14/11 | .408 | (.213, .784) | 32/21 | .618 | (.470, .814) | 0.085 |
| 560 | 5/5 | 0 | — | 33/23 | .653 | (.506, .842) | 0.005 |
| 136 | 11/8 | .455 | (.238, .868) | 37/22 | .655 | (.515, .834) | 0.53 |
| 1820 | 7/3 | .686 | (.403, 1.00) | 33/25 | .564 | (.416, .764) | 0.20 |
| 1055 | 10/8 | .500 | (.269, .929) | 38/26 | .637 | (.497, .816) | 0.12 |
| 255 | 7/6 | .429 | (.182, 1.00) | 34/20 | .656 | (.511, .843) | 0.14 |
| ank1 | 8/6 | .714 | (.447, 1.00) | 25/18 | .600 | (.436, .826) | 0.97 |
| 1760 | 7/4 | .714 | (.447, 1.00) | 41/30 | .612 | (.476, .788) | 0.51 |
| 1925 | 7/4 | .714 | (.447, 1.00) | 36/24 | .595 | (.451, .784) | 0.49 |
| Low | High | ||||||
| Microsatellite stability status | 66/46 | .605 | (.496, .739) | 12/5 | .750 | (.541, 1.00) | 0.14 |
Because of the age differences by AI status an age-adjusted Cox proportional hazard model was fitted to examine the relationship between survival and overall AI status on 8p. AI status was found to be a marginally significant predictor of survival (P = 0.058). Individuals with AI on the 8p arm were estimated to be at greater risk of dying than those not displaying AI (relative risk of 1.85). Similarly, in adjusting for age to assess the relationship between survival and AI status on marker 560, AI at marker 560 was related to survival (P = 0.047). Those with AI at marker 560 were estimated to be at greater risk of dying (relative risk of 3.27). Age was not significant predictor of survival in either model.
Discussion
The identification of regions that potentially harbor tumor suppressor genes is important to further characterize alterations critical for the development and progression of tumors. In gastric cancer, the 8p22 region has previously been identified as a region of interest.35,36,37 Recently, articles have been published identifying two different regions, 8p23 and 8p12-p21, that may contain putative tumor suppressor genes on chromosome 8 in different tumor types.47,48,49
In this AI study, we examined regions of chromosome 8p that have been identified as potentially harboring tumor suppressor genes. Each region was represented by two markers, thus reducing the likelihood of losing that region’s data because of homozygosity at one locus. To accurately assess AI status, it was necessary to first identify those patients with a phenotype of MSI-H from the rest of the tumors and remove them from AI evaluation.
The mechanism generating MSI-H tumors is thought to be different from tumors with proficient mismatch repair, so these tumors were identified and evaluated separately. An article by Schneider and colleagues50 suggested that MSI-L tumors might be phenotypically distinct from MSS tumors. No differences in survival were found either when the three categories of MSI (MSI-H, MSI-L, and MSS) were treated as separate groups or when the MSS and MSI-L subclasses were combined into a larger group, which was then compared to MSI-H. These analyses, however, were hindered by relatively small sample sizes in the MSI-L and MSI-H subclasses. Other studies have not observed a difference in MSI-L and MSS tumors.51,52,53 Thus, based on our results, which are in agreement with these other reports in the literature, we grouped the MSI-L and MSS groups together and compared to the MSI-H group for our subsequent evaluations.
In the examination of various clinicopathological features, we found MSI-H tumors to be significantly associated with several characteristics. Noncardia tumors were significantly associated with MSI-H tumors. This is in agreement with other reports published in the literature,54,55 although some studies have found no statistical association between tumor site and MSI-H phenotypes.50,51,56 Females were found to have MSI-H tumors more frequently than males. Most studies have not reported a gender difference associated with MSI-H status,41,50,51,55 although the data published by Olivera and colleagues,54 Ohmura and colleagues,42 and Ottini and colleagues43 seems to show a similar trend to what we have observed. This may be a sample size issue because MSI-H tumors represent only a small portion of the overall tumors studied.
Italian patients were also found to have a higher rate of MSI-H tumors than patients from America. This could be because of possible confounding because ethnicity was highly associated with tumor site. In our data, Italian patients had a greater number of distal tumors (96%) compared to United State patients (47%). Ethnic background has been suggested to be associated with differences in disease aggression and outcome in Asian populations.57,58 Dietary exposures have also been implicated in gastric tumor development.59,60 Perhaps ethnic differences or dietary influences might predispose a patient to develop tumors, which have a deficient mismatch repair pathway. We found no other characteristics to be significantly associated with MSI-H tumor phenotype.
We found that AI on 8p occurs in 39.7% of primary tumors and 72.7% of xenograft tumors examined. Primary tumor heterogeneity may be one explanation for the vastly higher detection of AI observed between the primary and xenografted tumors. It is possible that the area of primary tumor selected for xenograft growth had that imbalance present, whereas the vast majority of the primary tumor did not. Another thought is that during the growth of the xenograft within the murine environment, the tumor acquired that change. These two factors may also be combined, in that there may have been a selective outgrowth of a subpopulation that was present in a minority of primary tumor cells, but had a growth advantage within the xenograft population. Any of these scenarios could result in the skewing of results within the xenograft environment. Affirming this, we have previously microdissected multiple regions from the same tumor in colon, and have seen MSI status at a specific locus differ between the regions within the same tumor, but retain the same overall tumor phenotype, either MSI-H or MSI-L/MSS (unpublished data). Finally, the presence of normal human stromal tissue in the primary tumors may influence the AI ratio, whereas the xenograft tumors provide an unambiguous identification of altered DNA which will increase the sensitivity of our assay in these samples. Most of the observed differences between our primary tumors and xenografts were detection of AI in the xenograft but not in the primary tumor, which would be expected with sample sensitivity issues. There were, however, two tumors in which the xenograft did not show loss, but the primary tumor did. This represented a very small proportion of the cases with differences between the primary and xenograft tumors. Again, this might represent a growth advantage among a specific clone, or simply primary tumor heterogeneity, where in the region selected for xenograft growth had not yet acquired this change. In both cases, however, AI on all of chromosome 8p was seen for at least one locus, demonstrating that this imbalance is a common event, even among different clones in gastric cancer.
It should also be noted that several tumors exhibited differences in MSI status at both particular loci or (in one case) in overall MSI phenotype. DNA replication errors are known to occur sporadically at individual loci throughout the tumor genome, and can occasionally result in unrepaired errors, without having a defect in the mismatch repair pathway. These events are thought to be the cause of MSI-L tumors, and would most likely also account for the differences that we saw in the xenograft and primary tumors that were discrepant at individual loci. There was also one tumor that demonstrated a different MSI phenotype in the xenograft than in the primary tumor. The primary tumor demonstrated MSI at 80% of its loci, whereas the xenograft did not demonstrate an MSI-H phenotype. The primary tumor was intestinal type, which has been correlated with an MSI-H phenotype.52,53,55,61 Moreover, MSI-H tumors are thought to be of a less aggressive nature, possibly accounting for their presentation at a lower stage and better survival rate.41,44,50,53,55 A subclone existing within a tumor that has not lost its MMR function might be more aggressive and exhibit clonal dominance regarding growth within the murine environment compared to MMR-deficient clones. Overall, our results, in agreement with others in the literature, demonstrate xenografted tumors to be very reliable in exhibiting global molecular alterations consistent with the primary tumor that might otherwise go undetected because of normal stromal content.62,63
Overall survival was examined for AI on any region of chromosome 8p as well as at each individual marker of chromosome 8p. Survival was found to differ in overall AI status on 8p. Additionally, at a marker level, AI at marker D8S560 was found to be significantly associated with survival. In both cases, imbalance was indicative of a poorer outcome. During preliminary analysis, it was determined that mean age had differed across both overall AI status on 8p and AI status on marker 560 such that, in both instances, patients showing AI tended to be older. Using age-adjusted Cox proportional hazards models, the data suggested that the previously mentioned survival differences related to both overall AI status on 8p and AI status on marker 560 were not solely the result of age differences among the sample. Using this model, we found that individuals with AI on at least one 8p marker had a relative risk of 1.85 of dying when compared to AI retention. Additionally, a log-rank test was performed to assess survival differences between individuals who had or did not have AI at marker MYC located on chromosome 1 in addition to the two markers on chromosome 8q. Although the sample sizes of 7 and 13 were relatively small, our preliminary assessment did not indicate a survival difference (P = 0.60) (data not shown). Based on this preliminary assessment indicating a potential magnitude of survival difference with AI status on 8p that was not seen with markers on 8q or chromosome 1, further study is warranted.
Taken together, these results suggest that a gene on 8p may help determine the malignant potential of gastric cancers. Marker D8S261 was found to be marginally significant in regards to survival as well. Markers 261 and 560 create a flanked region of the DNA with 261 on the telomeric side and 560 on the centromeric side. Furthermore, when comparing the primary tumor cluster results to those of the xenografts, the regions that tended to be lost in conjunction with one another fell within the 8p22-8p21.3 regions, which is where D8S261 and D8S560 reside.
There are 10 genes known to reside between these two markers along with several hypothetical proteins (UCSC database, July 2003). Three of these genes, LZTS1, PCM1, and NAT1, have been reported associated with cancer.37 LZTS1 is the gene Vecchione and colleagues37 studied and observed both genetic and epigenetic changes associated with diffuse gastric cancers. PCM1 was found to form a fusion protein with the RET proto-oncogene in papillary thyroid cancer. By immunohistochemistry, it was demonstrated to show a decreased protein expression level, and altered localization.64 NAT1 is a gene that processes chemical carcinogens. Certain polymorphisms are associated with an increase in N-acetyltransferase activity leading to a possible increased risk factor in developing breast cancer.65 Further studies will define the true gene targets of alteration on 8p in gastric cancers.
In summary, we report a high rate of AI on chromosome 8p in gastric cancers. Analysis of both overall imbalance on 8p, and imbalance at marker D8S560 suggested an association between survival and AI status on 8p. We also confirm that MSI-H tumors are associated with noncardia tumors of the stomach, and report a significant association with females and Italian patients. Finally, we have identified a region of interest that exists in the 8p22-8p21.3 region that might serve to identify at least one important gene in gastric cancer. All of our results were obtained with a small population, however. Further analyses of 8p are anticipated to both confirm our results and to identify genes targeted for alteration in gastric cancers.
Footnotes
Supported by the National Cancer Institute (grant CA67900 to S.P.).
The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute, the Mayo Clinic, or the University of Virginia.
References
- Hanai A, Tsujuma H, Hiyama T, Fujimoto I. Gastric Cancer. Sugimura T, Sasako M, editors. New York: Oxford University Press; 1997:22–30. [Google Scholar]
- Yokozaki H, Yasui W, Tahara E. Genetic and epigenetic changes in stomach cancer. Int Rev Cytol. 2001;204:49–95. doi: 10.1016/s0074-7696(01)04003-7. [DOI] [PubMed] [Google Scholar]
- Saal K, Vollmers HP, Muller J, Kohler J, Hohn H, Muller-Hermelink HK. Cytogenetic differences between intestinal and diffuse types of human gastric carcinoma. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;64:145–150. doi: 10.1007/BF02915107. [DOI] [PubMed] [Google Scholar]
- Ramesh S, Nash J, McCulloch PG. Reduction in membranous expression of beta-catenin and increased cytoplasmic E-cadherin expression predict poor survival in gastric cancer. Br J Cancer. 1999;81:1392–1397. doi: 10.1038/sj.bjc.6693437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W, Schneiders A, Hommel G, Gabbert HE. Prognostic value of bcl-2 expression in gastric cancer. Anticancer Res. 1998;18:4699–4704. [PubMed] [Google Scholar]
- Vollmers HP, Dammrich J, Hensel F, Ribbert H, Meyer-Bahlburg A, Ufken-Gaul T, von Korff M, Muller-Hermelink HK. Differential expression of apoptosis receptors on diffuse and intestinal type stomach carcinoma. Cancer. 1997;79:433–440. doi: 10.1002/(sici)1097-0142(19970201)79:3<433::aid-cncr2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Castedo S, Correia C, David L, Sobrinho-Simoes M. Isochromosome 8q. A recurrent change in gastric carcinoma. Cancer Genet Cytogenet. 1991;54:137–138. doi: 10.1016/0165-4608(91)90044-u. [DOI] [PubMed] [Google Scholar]
- Virgin JB, Hurley PM, Nahhas FA, Bebchuk KG, Mohamed AN, Sakr WA, Bright RK, Cher ML. Isochromosome 8q formation is associated with 8p loss of heterozygosity in a prostate cancer cell line. Prostate. 1991;41:49–57. doi: 10.1002/(sici)1097-0045(19990915)41:1<49::aid-pros7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Jin Y, Jin C, Wennerberg J, Hoglund M, Mertens F. Cytogenetic and fluorescence in situ hybridization characterization of chromosome 8 rearrangements in head and neck squamous cell carcinomas. Cancer Genet Cytogenet. 2001;130:111–117. doi: 10.1016/s0165-4608(01)00476-9. [DOI] [PubMed] [Google Scholar]
- Mark HF, Afify AM, Werness BA, Das S, Mark S, Samy M. Trisomy 8 in stage I and stage III ovarian cancer detected by fluorescence in situ hybridization. Exp Mol Pathol. 1999;66:76–81. doi: 10.1006/exmp.1999.2241. [DOI] [PubMed] [Google Scholar]
- Afify A, Mark HF. Trisomy 8 in embryonal rhabdomyosarcoma detected by fluorescence in situ hybridization. Cancer Genet Cytogenet. 1999;108:127–132. doi: 10.1016/s0165-4608(98)00119-8. [DOI] [PubMed] [Google Scholar]
- Bullerdiek J, Leuschner E, Taquia E, Bonk U, Bartnitzke S. Trisomy 8 as a recurrent clonal abnormality in breast cancer? Cancer Genet Cytogenet. 1993;65:64–67. doi: 10.1016/0165-4608(93)90060-y. [DOI] [PubMed] [Google Scholar]
- Bofin AM, Ytterhus B, Fjosne HE, Hagmar BM. Abnormal chromosome 8 copy number in cytological smears from breast carcinomas detected by means of fluorescence in situ hybridization (FISH). Cytopathology. 2003;14:5–11. doi: 10.1046/j.1365-2303.2003.01132.x. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Fearon ER, Kern SE, Hamilton SR, Preisinger AC, Nakamura Y, White R. Allelotype of colorectal carcinomas. Science. 1989;244:207–211. doi: 10.1126/science.2565047. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Emi M, Ohata H, Kato Y, Nakajima T, Mori T, Nakamura Y. Evidence for the presence of two tumor suppressor genes on chromosome 8p for colorectal carcinoma. Cancer Res. 1993;53:1172–1174. [PubMed] [Google Scholar]
- Farrington SM, Cunningham C, Boyle SM, Wyllie AH, Dunlop MG. Detailed physical and deletion mapping of 8p with isolation of YAC clones from tumour suppressor loci involved in colorectal cancer. Oncogene. 1996;12:1803–1808. [PubMed] [Google Scholar]
- Fujiwara Y, Ohata H, Emi M, Okui K, Koyama K, Tsuchiya E, Nakajima T, Monden M, Mori T, Kurimasa A. A 3-Mb physical map of the chromosome region 8p21.3-p22, including a 600-kb region commonly deleted in human hepatocellular carcinoma, colorectal cancer, and non-small cell lung cancer. Genes Chromosom Cancer. 1994;10:7–14. doi: 10.1002/gcc.2870100103. [DOI] [PubMed] [Google Scholar]
- Yaremko ML, Wasylyshyn ML, Paulus KL, Michelassi F, Westbrook CA. Deletion mapping reveals two regions of chromosome 8 allele loss in colorectal carcinomas. Genes Chromosom Cancer. 1994;10:1–6. doi: 10.1002/gcc.2870100102. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Dunlop MG, Bird CC, Wyllie AH. Deletion analysis of chromosome 8p in sporadic colorectal adenomas. Br J Cancer. 1994;70:18–20. doi: 10.1038/bjc.1994.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Dunlop MG, Wyllie AH, Bird CC. Deletion mapping in colorectal cancer of a putative tumour suppressor gene in 8p22–p21.3. Oncogene. 1993;8:1391–1396. [PubMed] [Google Scholar]
- Emi M, Fujiwara Y, Nakajima T, Tsuchiya E, Tsuda H, Hirohashi S, Maeda Y, Tsuruta K, Miyaki M, Nakamura Y. Frequent loss of heterozygosity for loci on chromosome 8p in hepatocellular carcinoma, colorectal cancer, and lung cancer. Cancer Res. 1992;52:5368–5372. [PubMed] [Google Scholar]
- Kelemen PR, Yaremko ML, Kim AH, Montag A, Michelassi F, Westbrook CA. Loss of heterozygosity in 8p is associated with microinvasion in colorectal carcinoma. Genes Chromosom Cancer. 1994;11:195–198. doi: 10.1002/gcc.2870110308. [DOI] [PubMed] [Google Scholar]
- Gustafson CE, Wilson PJ, Lukeis R, Baker E, Woollatt E, Annab L, Hawke L, Barrett JC, Chenevix-Trench G. Functional evidence for a colorectal cancer tumor suppressor gene at chromosome 8p22–23 by monochromosome transfer. Cancer Res. 1996;56:5238–5245. [PubMed] [Google Scholar]
- Halling KC, French AJ, McDonnell SK, Burgart LJ, Schaid DJ, Peterson BJ, Moon-Tasson L, Mahoney MR, Sargent DJ, O’Connell MJ, Witzig TE, Farr GH, JR, Goldberg RM, Thibodeau SN. Microsatellite instability and 8p allelic imbalance in stage B2 and C colorectal cancers. J Natl Cancer Inst. 1999;91:1295–1303. doi: 10.1093/jnci/91.15.1295. [DOI] [PubMed] [Google Scholar]
- Matsuyama H, Pan Y, Skoog L, Tribukait B, Naito K, Ekman P, Lichter P, Bergerheim US. Deletion mapping of chromosome 8p in prostate cancer by fluorescent in situ hybridization. Oncogene. 1994;9:3071–3076. [PubMed] [Google Scholar]
- Trapman J, Sleddens HFBM, van der Weiden MM, Dinjen NM, Konig JJ, Schroder FH, Faber PW, Bosman FT. Loss of heterozygosity of chromosome 8 microsatellite loci implicates a candidate tumor suppressor gene between the loci D8S87 and D8S133 in human prostate cancer. Cancer Res. 1994;54:6061–6064. [PubMed] [Google Scholar]
- Macoska JA, Trybus TM, Benson PD, Sakr WA, Grignon DJ, Wojno KD, Pietruk T, Powell IJ. Evidence for three tumor suppressor gene loci on chromosome 8p in human prostate cancer. Cancer Res. 1995;55:5390–5395. [PubMed] [Google Scholar]
- Suzuki H, Emi M, Komiya A, Fujiwara Y, Yatani R, Nakamura Y, Shimazaki J. Localization of a tumor suppressor gene associated with progression of human prostate cancer within a 1.2-Mb region of 8p22–p21.3. Genes Chromosom Cancer. 1995;13:168–174. doi: 10.1002/gcc.2870130306. [DOI] [PubMed] [Google Scholar]
- Cunningham JM, Shan A, Wick MJ, McDonnell SK, Schaid DJ, Tester DJ, Qian J, Takahashi S, Jenkins RB, Bostwick DG, Thibodeau SN. Allelic imbalance and microsatellite instability in prostatic adenocarcinoma. Cancer Res. 1996;56:4475–4482. [PubMed] [Google Scholar]
- Knowles MA, Shaw ME, Proctor AJ. Deletion mapping of chromosome 8 in cancers of the urinary bladder using restriction fragment length polymorphisms and microsatellite polymorphisms. Oncogene. 1993;8:1364–1367. [PubMed] [Google Scholar]
- Ohata H, Emi M, Fujiwara Y, Higashino K, Nakagawa K, Futagami R, Tsuchiya E, Nakamura Y. Deletion mapping of the short arm of chromosome 8 in non-small cell lung carcinoma. Genes Chromosom Cancer. 1993;7:85–88. doi: 10.1002/gcc.2870070204. [DOI] [PubMed] [Google Scholar]
- Pykett MJ, Murphy ME, Harnish PR, Muenke M, Marks J, Goerge DL. Loss of chromosome 8p sequences in human breast carcinoma cell lines. Cancer Genet Cytogenet. 1994;76:23–28. doi: 10.1016/0165-4608(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Wasylyshyn ML, Neuman WL, Angriman I, Snyder LA, Montag AG, Westbrook CA, Michelassi F. Evidence for a new tumor-suppressor gene involved in gastrointestinal malignancies. Surgery. 1991;110:265–268. [PubMed] [Google Scholar]
- Emi M, Fujiwara Y, Ohata H, Tsuda H, Hirohashi S, Koike M, Miyaki M, Monden M, Nakamura Y. Allelic loss at chromosome band 8p21.3-p22 is associated with progression of hepatocellular carcinomas. Genes Chromosom Cancer. 1993;7:152–157. doi: 10.1002/gcc.2870070307. [DOI] [PubMed] [Google Scholar]
- Yustein AS, Harper JC, Petroni GR, Cummings OW, Moskaluk CA, Powell SM. Allelotype of gastric adenocarcinoma. Cancer Res. 1999;59:1437–1441. [PubMed] [Google Scholar]
- Baffa R, Santoro R, Bullrich F, Mandes B, Ishii H, Croce CM. Definition and refinement of chromosome 8p regions of loss of heterozygosity in gastric cancer. Clin Cancer Res. 2000;6:1372–1377. [PubMed] [Google Scholar]
- Vecchione A, Ishii H, Shiao YH, Trapasso F, Rugge M, Tamburrino JF, Murakumo Y, Alder H, Croce CM, Baffa R. Fez1/lzts1 alterations in gastric carcinoma. Clin Cancer Res. 2001;7:1546–1552. [PubMed] [Google Scholar]
- Thiagalingam S, Lengauer C, Leach FS, Schutte M, Hahn SA, Overhauser J, Willson JK, Markowitz S, Hamilton SR, Kern SE, Kinzler KW, Vogelstein B. Evaluation of candidate tumour suppressor genes on chromosome 18 in colorectal cancers. Nat Genet. 1996;13:343–346. doi: 10.1038/ng0796-343. [DOI] [PubMed] [Google Scholar]
- Hahn SA, Schutte M, Hoque AT, Moskaluk CA, da Costa LT, Rozenblum E, Weinstein CL, Fischer A, Yeo CJ, Hruban RH, Kern SE. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- Goelz S, Hamilton S, Vogelstein B. Purification of DNA from formaldehyde fixed and paraffin embedded human tissue. Biochem Biophys Res Commun. 1985;130:118–126. doi: 10.1016/0006-291x(85)90390-0. [DOI] [PubMed] [Google Scholar]
- Hayden JD, Cawkwell L, Quirke P, Dixon MF, Goldstone AR, Sue-Ling H, Johnston D, Martin IG. Prognostic significance of microsatellite instability in patients with gastric carcinoma. Eur J Cancer. 1997;33:2342–2346. doi: 10.1016/s0959-8049(97)00343-2. [DOI] [PubMed] [Google Scholar]
- Ohmura K, Tamura G, Endoh Y, Sakata K, Takahashi T, Motoyama T. Microsatellite alterations in differentiated-type adenocarcinomas and precancerous lesions of the stomach with special reference to cellular phenotype. Hum Pathol. 2000;31:1031–1035. doi: 10.1053/hupa.2000.16669. [DOI] [PubMed] [Google Scholar]
- Ottini L, Palli D, Falchetti M, D’Amico C, Amorosi A, Saieva C, Calzolari A, Cimoli F, Tatarelli C, De Marchis L, Masala G, Mariani-Costantini R, Cama A. Microsatellite instability in gastric cancer is associated with tumor location and family history in a high-risk population from Tuscany. Cancer Res. 1997;57:4523–4529. [PubMed] [Google Scholar]
- Choi S-W, Choi J-R, Chung Y-J, Kim K-M, Rhyu M-G. Prognostic implications of microsatellite genotypes in gastric carcinoma. Int J Cancer. 2000;89:378–383. doi: 10.1002/1097-0215(20000720)89:4<378::aid-ijc10>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Thibodeau SN, French AJ, Cunningham JM, Tester D, Burgart LJ, Roche PC, McDonnell SK, Schaid DJ, Vockley CW, Michels VV, Fahr GH, Jr, O’Connell MJ. Microsatellite instability in colorectal cancer: different mutator phenotypes and the principle involvement of hMLH1. Cancer Res. 1998;58:1713–1718. [PubMed] [Google Scholar]
- Harrell FE., Jr New York: Springer-Verlag; Regression Modeling StrategiesWith Applications to Linear Models, Logistic Regression, and Survival Analysis. 2001:66–67. [Google Scholar]
- Muscheck M, Sukosd F, Pesti T, Kovacs G. High density deletion mapping of bladder cancer localizes the putative tumor suppressor gene between loci D8S504 and D8S264 at chromosome 8p23.3. Lab Invest. 2000;80:1089–1093. doi: 10.1038/labinvest.3780114. [DOI] [PubMed] [Google Scholar]
- Washburn JG, Wojno KJ, Dey J, Powell IJ, Macoska JA. 8pter-p23 deletion is associated with racial differences in prostate cancer outcome. Clin Cancer Res. 2000;6:4647–4652. [PubMed] [Google Scholar]
- Pribill I, Speiser P, Leary J, Leodolter S, Hacker NF, Friedlander ML, Birnbaum D, Zeillinger R, Krainer M. High frequency of allelic imbalance at regions of chromosome arm 8p in ovarian carcinoma. Cancer Genet Cytogenet. 2001;129:23–29. doi: 10.1016/s0165-4608(01)00419-8. [DOI] [PubMed] [Google Scholar]
- Schneider BG, Bravo JC, Roa JC, Roa I, Kim MC, Lee KM, Plaisance KT, JR, McBride CM, Mera R. Microsatellite instability, prognosis and metastasis in gastric cancers from a low-risk population. Int J Cancer. 2000;89:444–452. doi: 10.1002/1097-0215(20000920)89:5<444::aid-ijc8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Wu C-W, Chen G-D, Jiang K-C, Li AF-Y, Chi C-W, Lo S-S, Chen J-Y. A genome-wide study of microsatellite instability in advanced gastric carcinoma. Cancer. 2001;92:92–101. doi: 10.1002/1097-0142(20010701)92:1<92::aid-cncr1296>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Chung YJ, Song JM, Lee JY, Seo EJ, Choi SW, Rhyu MG. Microsatellite instability-associated mutations associate preferentially with the intestinal type of primary gastric carcinomas in a high-risk population. Cancer Res. 1996;56:4662–4665. [PubMed] [Google Scholar]
- dos Santos NR, Seruca R, Constancia M, Seixas M, Sobrinho-Simoes M. Microsatellite instability at multiple loci in gastric carcinoma: clinicopathologic implications and prognosis. Gastroenterology. 1996;110:38–44. doi: 10.1053/gast.1996.v110.pm8536886. [DOI] [PubMed] [Google Scholar]
- Oliveira C, Seruca R, Seixas M, Sobrinho-Simoes M. The clinicopathological features of gastric carcinomas with microsatellite instability may be mediated by mutations of different “target genes.”. Am J Pathol. 1998;153:1211–1219. doi: 10.1016/s0002-9440(10)65665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seruca R, Santos NR, David L, Constancia M, Barroca H, Carneiro F, Seixas M, Peltomaki P, Lothe R, Sobrinho-Simoes M. Sporadic gastric carcinomas with microsatellite instability display a particular clinicopathologic profile. Int J Cancer. 1995;64:32–36. doi: 10.1002/ijc.2910640108. [DOI] [PubMed] [Google Scholar]
- Czopek J, Bialas M, Rudzki Z, Zazula M, Pituch-Noworolska A, Zembala M, Popiela T, Kulig J, Kolodziejczyk P, Stachura J. The relationship between gastric cancer cells circulating in the blood and microsatellite instability positive gastric carcinomas. Aliment Pharmacol Ther. 2002;16(Suppl 2):S128–S136. doi: 10.1046/j.1365-2036.16.s2.5.x. [DOI] [PubMed] [Google Scholar]
- Young JL, Ries LG, Pollack ES. Cancer patient survival among ethnic groups in the United States. J Natl Cancer Inst. 1984;73:341–352. doi: 10.1093/jnci/73.2.341. [DOI] [PubMed] [Google Scholar]
- Theuer CP, Kurosaki T, Ziogas A, Butler J, Anton-Culver H. Asian patients with gastric carcinoma in the United States exhibit unique clinical features and superior overall and cancer specific survival rates. Cancer. 2000;89:1883–1892. doi: 10.1002/1097-0142(20001101)89:9<1883::aid-cncr3>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- La Vecchia C, Franceschi S. Nutrition and gastric cancer. Can J Gastroenterol. 2000;14(Suppl D):51D–54D. doi: 10.1155/2000/869862. [DOI] [PubMed] [Google Scholar]
- Palli D. Epidemiology of gastric cancer: an evaluation of available evidence. J Gastroenterology. 2000;35(Suppl 12):84–89. [PubMed] [Google Scholar]
- Buonsanti G, Calistri D, Padovan L, Luinetti O, Fiocca R, Solcia E, Ranzani GN. Microsatellite instability in intestinal- and diffuse-type gastric carcinoma. J Pathol. 1997;182:167–173. doi: 10.1002/(SICI)1096-9896(199706)182:2<167::AID-PATH830>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Hahn SA, Seymour AB, Hoque AT, Schutte M, da Costa LT, Redston MS, Caldas C, Weinstein CL, Fischer A, Yeo CJ, Hruban RH, Kern SE. Allelotype of pancreatic adenocarcinoma using xenograft enrichment. Cancer Res. 1995;55:4670–4675. [PubMed] [Google Scholar]
- McQueen H, Wyllie A, Piris J, Foster E, Bird C. Stability of critical genetic lesions in human colorectal carcinoma xenografts. Br J Cancer. 1991;63:94–97. doi: 10.1038/bjc.1991.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforova MN, Caudill CM, Biddinger P, Nikiforov YE. Prevalence of RET/PTC rearrangements in Hashimoto’s thyroiditis and papillary thyroid carcinomas. Int J Surg Pathol. 2002;10:15–22. doi: 10.1177/106689690201000104. [DOI] [PubMed] [Google Scholar]
- Lee KM, Park SK, Kim SU, Doll MA, Yoo KY, Ahn SH, Noh DY, Hirvonen A, Hein DW, Kang D. N-acetyltransferase (NAT1, NAT2) and glutathione S-transferase (GSTM1, GSTT1) polymorphisms in breast cancer. Cancer Lett. 2003;196:179–186. doi: 10.1016/s0304-3835(03)00311-2. [DOI] [PubMed] [Google Scholar]