Abstract
Although reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of melanocyte-associated mRNA can detect sentinel node melanoma metastases, most published assays are semi-quantitative methods of unknown sensitivity and precision, unsuitable for clinical use. We describe a single-step real-time quantitative RT-PCR assay for MART-1 and tyrosinase mRNAs, suitable for sentinel node analysis in a clinical setting. Using serial dilutions of melanoma cell line SK-MEL-28 RNA in water as a calibrator, we obtained linear calibration curves covering the range 0.5 to 10,000 arbitrary units (SK-MEL-28 melanoma cell equivalents). The sensitivity limit was 0.32 (MART-1) and 5 (tyrosinase) arbitrary units. Analytical imprecision was between 11% and 34%. MART-1 PCR efficiency was unaffected when samples were diluted with negative lymph node RNA rather than water, whereas tyrosinase PCR efficiency was halved. To evaluate the clinical suitability of our assay, we quantified melanocyte mRNAs in sentinel nodes with histologically verified micrometastases (n = 10) and benign nevus inclusions (n = 10), and in sentinel nodes without evidence of intranodal melanocytes (n = 10). We found significant differences in median melanocyte-derived mRNA levels comparing the three types of lymph nodes, suggesting that this quantitative molecular protocol may increase assay precision and be useful for the clinical evaluation of sentinel nodes.
Melanoma patients with lymph node metastases have a poor prognosis, their 5-year survival falling from about 95% to less than 50% compared with patients without lymph node involvement.1 The sentinel node (SN) technique has been introduced to improve the precision of staging, and in an attempt to identify patients who might benefit from regional lymph node clearance and from additional therapy. This technique involves excision of the first tumor draining lymph node (or nodes) and makes it possible to look for early metastatic disease, while this is still clinically occult.2,3 Because the number of tumor cells in SNs can be small, metastases may not be easily detected by conventional methods. At present, the gold standard for SN examination is routine histopathology combined with immunohistochemistry. However, this method lacks sensitivity because, for practical reasons, only a small percentage of the tissue is examined.4 Thus, there is a need for alternative or supplementary techniques to improve the precision of SN analysis. One such technique which has attracted particular attention because of its potentially high sensitivity compared with histopathology, is reverse transcriptase PCR (RT-PCR) analysis of melanocyte-associated mRNAs in SNs. Molecular RT-PCR analysis of melanocytic differentiation markers (such as tyrosinase and MART-1) has been reported in blood,5,6,7,8 bone marrow,9 and lymph nodes.10,11,12,13,14,15 However, there are several drawbacks to these techniques. Firstly, most of the assays are based on a nested PCR reaction making them laborious and time-consuming. Secondly and more importantly, they provide only semi-quantitative data regarding the presence or absence of a target, usually with highly variable results.16,17,18 Probably the single most important source of error when using molecular analysis of melanocyte-associated mRNA to stage SNs, is the detection of a “false-positive” signal from the benign nevus cell inclusions (BNIs) that are frequently present in these nodes, even in the absence of melanoma metastases.19
We believe that the ability to perform reliable quantitative analysis of metastatic melanoma load is likely to be an important step in improving the quality of the prognostic information available from SN analysis, in selecting patients for immunotherapy and experimental treatment, and, in the appropriate setting, in monitoring therapeutic efficacy. Although real-time RT-PCR can be performed quantitatively by subsequent analysis of a standard curve, proper evaluation of the conditions on which the method relies has not previously been reported. In this study, we have considered the hypothesis that quantitative analysis could improve the specificity of molecular examination, the premise being that SNs containing only BNIs would usually give a weaker positive RT-PCR signal compared with that from SNs harboring melanoma metastases. We describe a rapid and quantitative single-step real-time RT-PCR technique for tyrosinase and MART-1 mRNA analysis of SNs, applicable in a clinical setting. We report on the establishment of a calibration curve with a dynamic range appropriate for analyzing micrometastases, the effect on PCR efficiency of diluting samples in RNA derived from lymph nodes not containing any melanocytic cells (which we designate lymphoid matrix) rather than in water, and the sensitivity, the coefficient of variation (CV), and inter-assay variation of the analysis. Finally, in a pilot study on 30 randomly chosen SNs (10 with histologically verified melanoma micrometastases, 10 with histologically confirmed BNIs, and 10 with no histological evidence of intranodal melanocytes), we show that our technique can demonstrate statistically significant quantitative differences in melanocyte-associated mRNA for the three groups of lymph nodes.
Materials and Methods
Cell Culture
SK-MEL-28 melanoma cells (American Type Culture Collection, Manasas, VA) were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA) supplemented with 100 ml/L calf serum (Life Technologies, Gaithersburg, MD) and cultured at 37°C in a humidified atmosphere containing 5% CO2. After detachment, the cells were counted in a Bürker-Türk counting chamber. Total RNA was extracted from 106 cells using total RNA purification kit (Qiagen Mini Kits, Qiagen, Valencia, CA) according to the manufacturer’s instructions. Purified RNA was eluted in 50-μl distilled water to give a stock RNA corresponding to 20,000 SK-MEL-28 cell equivalents/μL.
Sentinel Nodes
Thirty lymph nodes (10 with histologically confirmed melanoma metastases from 7 patients, 10 with histologically confirmed BNIs from 9 patients, and 10 with no histological evidence of melanocytic cells from 5 patients) were included from patients with intermediate thickness (1 to 4 mm) melanoma, who underwent SN-dissection as part of a separate larger study on sentinel node diagnostics. This study was approved by the local ethical committee and written informed consent was obtained from all patients. Immediately after excision, the SN was sectioned in two halves. One half was examined histologically as described20 including extensive serial sectioning of the entire half lymph node, with hematoxylin and eosin staining supplemented by immunohistochemical staining. The other half of the lymph node was snap-frozen in liquid nitrogen and stored at −80°C before being used for real-time PCR analysis.
RNA Extraction (Tissue)
RNA from frozen SN specimens was isolated using Trizol reagent (GibcoBRL, Invitrogen, Taastrup, Denmark) according to the manufacturer’s instructions, with slight modifications. In brief, the tissue sample was homogenized in 1 ml Trizol, centrifuged (12,200 × g) for 5 minutes at room temperature, the supernatant was removed to fresh tubes, and 200 ml of chloroform was added. After a 5-minute incubation at room temperature and an additional 15-minute centrifuge at 4°C (12,200 × g), the supernatant (water phase) was removed to fresh tubes. RNA was precipitated from the supernatant by adding an equal volume of isopropyl alcohol. After 5 to 10 minutes of incubation at room temperature, the samples were centrifuged for 15 minutes at 4°C (12,200 × g), the supernatant was removed, and the RNA pellet was washed twice in 1 ml 75% ethanol with 5 minutes of centrifugation (7500 × g) between each wash. After the final wash, the RNA pellet was air-dried for 2 minutes at 70°C and then for 5 to 10 minutes at room temperature. Finally, the pellet was dissolved in 35-μl sterile water. The concentration, purity, and amount of total RNA were determined by UV spectrophotometry and OD 260/280 nm ratios >1.98 were obtained for all samples.
Real-Time Technology
Accumulation of PCR products was measured in real-time by using sequence-specific fluorescence resonance energy transfer (FRET) probes. Each probe carries a flourophore donor and acceptor. When the probes anneal to the target, the donor and acceptor flourophore are placed in close proximity and emit fluorescence equivalent to the amount of product generated, the process being monitored continuously by the LightCycler System (Roche Molecular Diagnostics, Penzberg, Germany).21 The threshold cycle (Ct value), which is the point at which target-derived fluorescence can be distinguished against background fluorescence, is determined. By constructing a standard curve, the Ct value can be translated into a quantitative result.22
Calibration Curve and Experimental Design
Serial dilutions in water of total RNA from 20,000 SK-MEL-28 cells were used to prepare a calibration curve for quantifying test mRNA. For MART-1 the range of RNA dilutions were equivalent to 1000, 100, 10, 1, and 0.5 SK-MEL-28 cells, respectively, and for tyrosinase 10,000, 1000, 100, 10, and 5 SK-MEL-28 cells, respectively. The amount of MART-1 (or tyrosinase) mRNA present in one SK-MEL-28 cell was defined as one arbitrary unit of melanoma cell equivalents (arb.u.) Each run consisted of five external calibrators (detailed above), one high-and one low-level positive control, and one negative control (without RNA template). For MART-1 the high- and low-level positive controls consisted of RNA from 100 and 1 SK-MEL-28 cell, respectively, and for tyrosinase RNA from 1000 and 10 SK-MEL-28 cells, respectively.
Primer and Probe Design
Primers were intron spanning to avoid amplification of genomic DNA and regions with known pseudogenes were avoided.23 Sequence-specific FRET probes were used. Primers and probes for tyrosinase were designed using the LightCycler Probe Design software (Roche Molecular Diagnostics) and gave an amplicon of 304 bp. MART-1 primers were as previously described,24 with the modification that only the inner set of the nested pair was used, giving an amplicon of 439 bp. Probe sequences for MART-1 were designed by TIB Molbiol (Berlin, Germany). Primers for β2-microglobulin (β2-M) were as previously described.25 Primer and probe sequences are listed in Table 1.
Table 1.
Primer and Probe Sequences
| mRNA | Product size (bp) | Primers (5′-to-3′-end) | Probes (5′-to-3′-end) |
|---|---|---|---|
| MART-1 | 439 | S: ATGCCAAGAGAAGATGCT | TTTCGTCTTCTACAATACCAACAGCCGATG-FL |
| AS: GGAGAACATTAGATGTCTG | LC Red640-GCAGTAAGACTCCCAGGATCACTGTCAG-P | ||
| *[Annealing: 55°C/primer concentration: 15 pmol] | [Probe concentration: 6 pmol] | ||
| Tyrosinase | 304 | S: ACAACAGCCATCAGTCT | TTGCTAGTCCACTTACTGGGATAGC-FL |
| AS: CCTGTACCTGGGACATT | LC Red640-ATGCCTCTCAAAGCAGCATG-P | ||
| [Annealing: 60°C/primer concentration: 2.5 pmol] | [Probe concentration: 6 pmol] | ||
| β2-M | 85 | S: TGACTTTGTCACAGCCCAAGATA | † |
| AS: AATCCAAATGCGGCATCTTC | |||
| [Annealing: 60°C/primer concentration: 15 pmol] |
Abbreviations: AS, antisense; β2-M, β2-Microglobulin; FL, fluorophore; LC Red 640, LightCycler Red 640 Flourophore; P, phosphate; S, sense.
Item in square brackets refer to PCR conditions (primer and probe concentrations and annealing temperature).
, Gel electrophoresis analysis.
Reverse Transcription
Two μg total RNA (from SNs or experiments with lymphoid RNA matrix) or 1 μl of each dilution (from calibrators, positive controls, and experiments without lymphoid RNA matrix) was reverse transcribed in a 20 μl reaction mixture containing 1X PCR buffer II (Applied Biosystems, Foster City, CA) supplemented with 6.3 mmol/L MgCl2, a total concentration of 0.3 mmol/L of each of the four deoxyribonucleoside triphosphates (dATP, dTTP, dGTP, and dCTP), 2.5 mmol/L 16mer oligo dT nucleotide, 20 units RNase inhibitor, and 50 units MULV reverse transcriptase (Applied Biosystems). Reverse transcription was performed in a Perkin-Elmer 9700 Thermocycler (Applied Biosystems) at 42°C for 30 minutes, followed by 5 minutes at 99°C. Two μl of the resulting cDNA were used immediately for real-time PCR, or the cDNA was stored at −20°C.
Real-Time Polymerase-Chain Reaction
MART-1 and tyrosinase mRNA was quantified by real-time PCR. Two μL of the reverse transcriptase product (cDNA) was used as template in a reaction mixture containing 4 mmol/L MgCl2, primers and probes (as shown in Table 1), and 2 μl probe mix (Roche Molecular Biochemicals, Indianapolis, IN; containing TaqDNA polymerase, reaction buffer, and deoxyribonucleoside triphosphates). The volume was adjusted to 20 μl with nuclease-free water. The samples were amplified in the LightCycler System (Roche) and the PCR was performed with an initial denaturation step at 95°C for 30 seconds, then 50 cycles (40 for tyrosinase) consisting of denaturation at 95°C, immediately followed by annealing (temperature given in Table 1) for 15 seconds, and extension at 72°C for 13 seconds. The LightCycler software was used for quantification.
Polymerase Chain Reaction
To control for false-negative results due to RNA degradation, all samples were analyzed for β2-M. Two μL cDNA was added to a mixture of 5 μl cresol, 2 μl 10X PCR buffer (Applied Biosystems), 0.2 mmol/L of each nucleotide, 1.25 units Taq-polymerase, and sense and antisense primer (concentrations given in Table 1) to a total volume of 20 μl. Samples were amplified in a Perkin-Elmer 9700 Thermocycler (Applied Biosystems) with an initial denaturation at 94° for 1 minute, followed by amplification as follows: 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. After 40 cycles, the PCR product was extended at 72°C for 7 minutes. Finally, PCR products were size-fractionated on a 2.0% agarose gel, stained with ethidium bromide, and visualized with UV light. Samples scored positive when a band of the appropriate size was visible on the gel.
Determination of Linearity and Matrix Effect
From the stock of SK-MEL-28 cell RNA, four different concentrations (each in five replicates) in the range 625 to 5 SK-MEL-28 cell RNA, (tenfold higher concentration of SK-MEL-28 cell RNA for tyrosinase than MART-1) were prepared for MART-1 and tyrosinase analyses, respectively. RNA was serially diluted in water (T1without lymphoid matrix to T4without lymphoid matrix) or with 2 μg RNA (T1with lymphoid matrix to T4with lymphoid matrix) from an RNA pool from lymph nodes determined by both histology and PCR to be negative for melanocytic cells. Samples were quantified twice by real-time RT-PCR analysis. For both genes, expected values (T1without lymphoid matrix to T4without lymphoid matrix) were plotted against observed values for samples with (T1with lymphoid matrix to T4with lymphoid matrix) and without RNA matrix (T1without lymphoid matrix to T4without lymphoid matrix). The expected values for T2 without lymphoid matrix, T3 without lymphoid matrix and T4 without lymphoid matrix were calculated from the mean value observed for T1without lymphoid matrix.
Statistics
Linearity was tested by linear regression analysis. Analytical day-to-day variation was determined by ANOVA. Non-parametric Mann-Whitney t-test was used to compare medians between SNs with melanoma metastases and BNI.
Results
Calibration Curve
We constructed the calibration curve by diluting RNA from SK-MEL-28 cells in water to cover the range of 0.5 to 1000 (MART) and 5 to 10,000 (tyrosinase) arb.u. The fitted regression line for the calibrators was generated by the LightCycler software and exhibited an excellent linearity in 15 different runs for MART-1 (r2 = 0.99; range, 0.98 to 1.0) and tyrosinase (r2 = 0.99; range, 0.98 to 1.0).
Imprecision and Sensitivity
Imprecision was evaluated by including high- and low-level controls derived from dilutions of SK-MEL-28 cell RNA in water in 15 independent runs. The CV was below 15% for the high controls for both MART-1 (14%, mean = 105 arb.u.) and tyrosinase (11%, mean = 1058 arb.u.), but was considerably higher for the low controls (Table 2). The sensitivity limit of the two assays was calculated as 2 SD from the low control and was 0.32 arb.u. for MART-1 and 5 arb.u. for tyrosinase.
Table 2.
Coefficients of Variation (CV) Calculated for MART-1 and Tyrosinase
| N = 15 | Mean | CV (interassay) | |
|---|---|---|---|
| MART-1 | high-level control | 105 | 14% |
| low-level control | 1 | 34% | |
| Tyrosinase | high-level control | 1058 | 11% |
| low-level control | 10 | 24% |
For MART-1, high- and low-level controls refer to RNA from 100 and 1 SK-MEL-28 melanoma cells, respectively. For tyrosinase, high- and low-level controls refer to RNA from 1,000 and 10 SK-MEL-28 melanoma cells, respectively. See also Material and Methods.
Matrix-Effect and Reproducibility
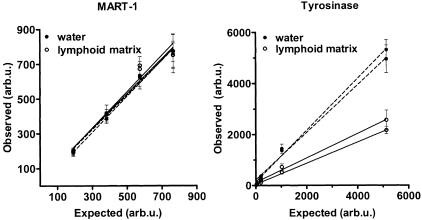
We evaluated the feasibility of using a single-step real-time RT-PCR-based assay for quantification of MART-1 and tyrosinase mRNA in sentinel nodes. SK-MEL-28 cell RNA was diluted either in water or in a solution containing 2 μg lymphoid RNA from a sentinel node tested negative by RT-PCR and histology. Dilution curves with and without the lymphoid RNA matrix were linear for both the MART-1 and tyrosinase assay (r2 = 0.99) with an intercept not different from zero (Figure 1 and Table 3). No matrix effect was observed for MART-1, whereas for tyrosinase the results were lower (by a factor of 0.5) for samples diluted in lymph node RNA compared with water. Thus, to correct for differences in PCR efficiencies caused by the RNA matrix, the quantitative result for tyrosinase mRNA should be doubled. As judged from the CV calculated from pooled data (five replicates run twice) for each of the four dilutions, the reproducibility for MART-1 for samples with lymphoid matrix compared to samples without lymphoid matrix was 6% to 11%. In contrast, the imprecision for tyrosinase was greater for the samples with lymphoid matrix (15% to 39%) compared with samples without lymphoid matrix (10% to 23%).
Figure 1.
Assay linearity and effect of analysis of melanoma cell (SK-MEL-28) titrations with (in 2 μg lymph node-derived RNA) and without (in water) lymphoid RNA matrix. Observed values for samples with and without lymphoid matrix are based on data from the quantitative real-time RT-PCR. Expected values are calculated from titrations in water (see Material and Methods). Data are presented for two experimental runs, quantifying MART-1 and tyrosinase mRNA. Values are given in arbitrary units (arb.u.) (defined in Materials and Methods) measured in water or in 2 μg lymphoid RNA. Each data point represents the mean of five replicates ± SD. Correlation of fit (r2) was ≥0.99 for all regression lines, except for MART-1with lymphoid matrix (r2 = 0.96). For tyrosinase, regression lines with and without lymphoid matrix were significantly different (P < 0.05), whereas no difference was shown for MART-1 regression lines.
Table 3.
Assay Linearity and Effect of Analysis of Melanoma Cell (SK-MEL-28) Titrations with and without Lymphoid RNA Matrix
| Dilutions without lymphoid matrix
|
Dilutions with lymphoid matrix
|
|||
|---|---|---|---|---|
| Slope (regression line) | Y-intercept (95% CI) | Slope (regression line) | Y-intercept (95% CI) | |
| MART-1 | 1.01 ± 0.07 | −145–170 | 1.02 ± 0.14 | −295–342 |
| Tyrosinase | 0.98 ± 0.05 | −330–690 | 0.45 ± 0.02 | −97–257 |
Regression lines for repeated measurements of four different concentrations of RNA from SK-MEL-28 melanoma cells (each in five replicates) diluted in water (without lymphoid matrix) and in lymph node RNA (with lymphoid matrix). See also description in Material and Methods.
Quantification of mRNA for MART-1 and Tyrosinase mRNA in Sentinel Nodes
SNs with Metastases
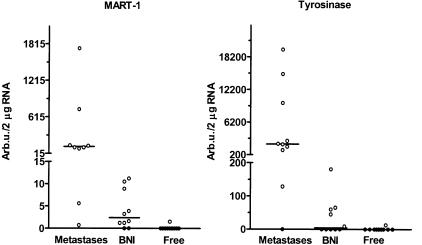
Ten SNs from seven melanoma patients were studied, in which histologically verified melanoma metastases were found in the lymph node half analyzed by histopathology. All of the frozen samples submitted from these nodes for molecular analysis were positive for MART-1 mRNA and 9 of 10 were positive for tyrosinase mRNA. The positive results were within the range of the calibration curve for both MART-1 (range 0.7 to 2300; median 126 arb.u./2 μg lymph node RNA) and tyrosinase (range, 129 to 20,000; median 2200 arb.u./2 μg lymph node RNA)(Figure 2).
Figure 2.
Quantification of melanocyte-associated differentiation marker mRNAs in sentinel nodes. MART-1 and tyrosinase mRNAs were quantified in frozen tissue from 30 nodes, classified after extensive histological examination of the paraffin-embedded half as follows: 10 with metastatic melanoma, 10 with benign nevus inclusions, and 10 with no histological evidence of intranodal melanocytes. Values are given in arbitrary units (arb.u.) (defined in Materials and Methods) present in 2 μg lymphoid RNA. Vertical lines represent median values. Medians for sentinel nodes without melanocytic cells were zero arb.u. for both MART-1 and tyrosinase.
SNs with BNIs
Ten SNs from nine melanoma patients were studied, in which histologically verified BNIs were found in the lymph node half analyzed by histopathology. In eight of these, MART-1 mRNA could be quantified in the frozen lymph node tissue (range, 0 to 11.2 and median 2.4 arb.u./2 μg lymph node RNA), whereas tyrosinase could be quantified in only five specimens (range, 0 to 181 and median 4.4 arb.u./2 μg lymph node RNA).
SNs Free of Melanocytic Cells
Ten SNs from five melanoma patients were studied, in which there was no histologically confirmed evidence of intranodal melanocytes. In one of these cases, both MART-1 and tyrosinase mRNAs could be quantified, although the level was low (MART-1: 1.53 and tyrosinase 13.0 arb.u./2 μg lymph node RNA). Median mRNA values comparing SNs with metastases with SNs with BNI, differed significantly for MART-1 (P = 0.002) and tyrosinase (P = 0.0007).
Specificity
Tissue processing, RNA extraction, reverse transcription, and PCR assay set-up were performed in separate designated rooms to prevent cross-contamination. To confirm the correct target size, PCR-products for MART-1 and tyrosinase analysis from selected samples were run on a 2% gel electrophoresis and revealed bands of the expected size. The identity of the generated PCR products was verified by DNA sequencing. β2-M could be detected in all samples, excluding false-negative results due to lack of amplifiable RNA. To control for false positivity caused by amplification of contaminating DNA, RT-PCR reactions without reverse transcription enzyme were performed on selected SNs with histologically verified micrometastases. These revealed no PCR product.
Discussion
The methodological challenge in SN diagnostics is to be able to detect specifically a minimal number of tumor cells, at a point at which the metastasis is not yet clinically overt. This requires highly accurate and sensitive methods. RT-PCR-based demonstration of MART-1 and tyrosinase mRNAs in SNs has been used as a marker for metastatic melanoma, and detects many more positive nodes compared with traditional histopathology.12,15,26 However, a major drawback with studies using this type of molecular analysis, is that they appear to greatly overestimate the fraction of patients that would be expected to develop tumor recurrence or nodal metastases,27 suggesting that non-neoplastic sources of melanocyte-associated mRNA are being detected. Furthermore, previously published RT-PCR protocols are often very time-consuming, due to a nested PCR design, most are at best only semi-quantitative techniques, and few have been adequately validated.
We report a highly sensitive one-step real-time PCR analysis for quantification of MART-1 and tyrosinase mRNAs in melanoma SNs. Our protocol is based on a single-step PCR and provides several advantages over the nested PCR design normally used. The PCR part of the analysis can be performed in less than 1 hour, which makes it suitable for use in a clinical setting. A single-step PCR design reduces the risk of false-positive results caused by post-PCR aerosolized amplicon contamination. In addition, it may be more accurate compared with nested PCR, since the entire analysis can be monitored cycle-by-cycle without post-PCR interruption, thus providing accurate determination of the threshold cycle on which the quantification is made.21 However, reducing a nested-PCR to a single-round PCR can have an impact on sensitivity. To optimize sensitivity, we increased the amount of RNA template from 0.1 μg to 2 μg. Using this approach, we could keep the number of cycles low (Ct <35 for the lowest calibrator) avoiding generation of non-specific transcripts.28 Using sequence-specific hybridization probes for detecting the PCR products further improved specificity.
Ideally, a quantitative molecular assay should be calibrated using samples that reflect the specimens to be investigated as closely as possible. Thus, an assay for detecting melanoma metastases in SNs should be based on a calibration curve prepared from a set of standard dilutions of melanoma-derived RNA in a background of RNA from melanocyte-negative lymph nodes. However, in a clinical setting, there are serious limitations to this approach. Firstly, it would be both difficult and expensive to routinely prepare so much negative lymph node RNA. Secondly, the inevitable assay variations introduced as a result of the varying degree of RNA degradation present in lymph nodes collected over time may present problems, especially when RNA serves as the standard.
To overcome these problems, we based our assay on a calibration curve prepared from dilutions of melanoma cell (SK-MEL-28) RNA in water. This approach is non-problematic if the PCR efficiencies in the test sample and the calibrators are the same. If, however, they are different, an error will be introduced that may become substantial following exponential PCR amplification. Repeated measurement of various dilutions of melanoma RNA in water and in purified lymph node RNA matrix gave excellent linearity (r2 ≥ 0.98) for the investigated genes. MART-1 analysis gave similar imprecision data irrespective of whether the melanoma RNA was diluted in water or in lymph node RNA (6% to 11%), whereas imprecision was slightly greater for tyrosinase samples measured in lymphoid RNA matrix compared with water (15% to 39% versus 10% to 23%). Thus, it appears that a water-based calibration curve can be readily used with our protocol for measuring MART-1 mRNA, while this will result in quantification of tyrosinase being underestimated by some 50%.
While a sensitive and reproducible method is a prerequisite for detecting melanocyte-associated mRNAs in SNs, lymph nodes found positive in this way do not necessarily contain metastatic melanoma. Benign nevocytes have been reported in up to 4% of melanoma SNs,19 and both these and other non-neoplastic cells sometimes found in lymph nodes (eg, Schwann cells)29 may express MART-1 and tyrosinase mRNAs. So far, no melanoma-specific target genes have been identified that can be used to ensure absolute specificity of molecular SN analysis. However, in the absence of such markers it is possible that the specificity of RT-PCR assays may be improved by using a quantitative technique. Although there is no evidence to suggest that quantitative RT-PCR would be able to discriminate completely between benign nevus cells and metastatic melanoma, it is possible it could be used to reduce the number of “false-positive” SNs sufficiently to allow the technique to be used in clinical practice. Furthermore, determining the metastatic melanoma load in a SN may have prognostic importance, and help identify clinically relevant micrometastases. Since quantitative molecular assays can measure the amount of RNA extracted from an entire specimen, they are likely to be more accurate for estimating tumor load than is a quantitative histological technique in which, at most, only a few percentage of the submitted tissue is examined.
We have not yet tested our quantitative RT-PCR technique in a large set of SN patients in which long-term clinical follow-up is available. However, our preliminary results in the 30 randomly selected SNs reported here, in which half of each node was submitted for molecular analysis, suggest that the method may be used in a clinical setting. Thus, melanocyte-associated mRNA was found and quantified in all 10 SNs (from seven patients) in which extensive histological examination of the other half had shown metastatic melanoma. One of these SNs expressed only mRNA for MART-1. This finding may be explained by tumor heterogeneity, often seen in melanomas,30,31 and it supports studies advocating analysis of more than one melanoma-associated marker.32 Similarly, in the 10 SNs in which BNIs were found on histology, MART-1 mRNA could also be quantified in most cases (8 of 10), while tyrosinase mRNA was present in only a minority (5 of 10). Interestingly, in both MART-1 and tyrosinase analyses, the median mRNA level for BNI nodes was significantly lower than that in metastatic melanoma SNs (MART-1: P = 0.002; tyrosinase: P = 0.0007). Although, there is an overlap in values comparing these two groups of SNs, the distribution suggests that it may be possible to establish a cut-off threshold that would separate SNs with BNIs from most SNs with melanoma metastases.
Of the SNs without histological evidence of intranodal melanocytic cells, 9 of 10 showed no expression of MART-1 and tyrosinase mRNA. In the single exception, both genes were expressed, but at very low levels. The most likely explanation for this is that quantitative molecular analysis was able to detect gene expression from small numbers of melanocytic cells (either benign or malignant) which were not seen on pathological examination. Given the limited amount of a SN that is actually examined by histopathology, this is not surprising. Indeed, the fact that we only found one such “false-negative” SN probably reflects the extensive histopathological technique used in these nodes.
In conclusion, we believe our single-step real-time RT-PCR assay is both easy and rapid to perform, and can be used in a clinical setting for accurate and reproducible quantitation of MART-1 and tyrosinase mRNAs in melanoma SNs. We plan to use this technique in a larger group of SNs to try to establish threshold values that can distinguish different types of SN, and to study the prognostic significance of MART-1 and tyrosinase mRNA levels on clinical follow up.
Footnotes
Supported by Forskningsinitiativet, County of Aarhus; the Clinical Research Unit of the Danish Cancer Society; the Aage Bang Foundation; the Danish Cancer Research Foundation; the Erland Richard Frederiksen Legate; the Max and Inge Wørzner Memorial Foundation; the Simon Fougner Hartmann Family Foundation; the Rigmor and Alex Trosborg Memorial Legate; the Sigvald and Edith Anelise Cecine Kristence Rasmussen Legate; and the Ludvig and Franciska Andersen Legate.
References
- de Braud F, Khayat D, Kroon BB, Valdagni R, Bruzzi P, Cascinelli N. Malignant melanoma. Crit Rev Oncol Hematol. 2003;47:35–63. doi: 10.1016/s1040-8428(02)00077-x. [DOI] [PubMed] [Google Scholar]
- Cochran AJ, Wen DR, Morton DL. Management of the regional lymph nodes in patients with cutaneous malignant melanoma. World J Surg. 1992;16:214–221. doi: 10.1007/BF02071523. [DOI] [PubMed] [Google Scholar]
- Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, Foshag LJ, Cochran AJ. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–399. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- van Diest PJ. Histopathological workup of sentinel lymph nodes: how much is enough? J Clin Pathol. 1999;52:871–873. doi: 10.1136/jcp.52.12.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry BJ, Myers K, Hersey P. MART-1 is expressed less frequently on circulating melanoma cells in patients who develop distant compared with locoregional metastases. J Clin Oncol. 1999;17:2562–2571. doi: 10.1200/JCO.1999.17.8.2562. [DOI] [PubMed] [Google Scholar]
- Curry BJ, Myers K, Hersey P. Utility of tests for circulating melanoma cells in identifying patients who develop recurrent melanoma. Recent Results Cancer Res. 2001;158:211–230. doi: 10.1007/978-3-642-59537-0_22. [DOI] [PubMed] [Google Scholar]
- Jung FA, Buzaid AC, Ross MI, Woods KV, Lee JJ, Albitar M, Grimm EA. Evaluation of tyrosinase mRNA as a tumor marker in the blood of melanoma patients. J Clin Oncol. 1997;15:2826–2831. doi: 10.1200/JCO.1997.15.8.2826. [DOI] [PubMed] [Google Scholar]
- Smith B, Selby P, Southgate J, Pittman K, Bradley C, Blair GE. Detection of melanoma cells in peripheral blood by means of reverse transcriptase and polymerase chain reaction. Lancet. 1991;338:1227–1229. doi: 10.1016/0140-6736(91)92100-g. [DOI] [PubMed] [Google Scholar]
- Blaheta HJ, Paul T, Sotlar K, Maczey E, Schittek B, Paul A, Moehrle M, Breuninger H, Bueltmann B, Rassner G, Garbe C. Detection of melanoma cells in sentinel lymph nodes, bone marrow a peripheral blood by reverse transcription-polymerase chain assay in patients with primary cutaneous melanoma; association with Breslow’s tumor thickness. Br J Dermatol. 2001;2:195–202. doi: 10.1046/j.1365-2133.2001.04334.x. [DOI] [PubMed] [Google Scholar]
- Bostick PJ, Morton DL, Turner RR, Huynh KT, Wang HJ, Elashoff R, Essner R, Hoon DS. Prognostic significance of occult metastases detected by sentinel lymphadenectomy and reverse transcriptase-polymerase chain reaction in early-stage melanoma patients. J Clin Oncol. 1999;17:3238–3244. doi: 10.1200/JCO.1999.17.10.3238. [DOI] [PubMed] [Google Scholar]
- Hochberg M, Lotem M, Gimon Z, Shiloni E, Enk CD. Expression of tyrosinase, MIA and MART-1 in sentinel lymph nodes of patients with malignant melanoma. Br J Dermatol. 2002;146:244–249. doi: 10.1046/j.1365-2133.2002.04579.x. [DOI] [PubMed] [Google Scholar]
- Li W, Stall A, Shivers SC, Lin J, Haddad F, Messina J, Glass LF, Lyman G, Reintgen DS. Clinical relevance of molecular staging for melanoma: comparison of RT-PCR and immunohistochemistry staining in sentinel lymph nodes of patients with melanoma. Ann Surg. 2000;231:795–803. doi: 10.1097/00000658-200006000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowsky A, Bellmann B, Ringk A, Winter H, Audring H, Fenske S, Sterry W. Detection of melanoma micrometastases in the sentinel lymph node and in non-sentinel nodes by tyrosinase polymerase chain reaction. J Invest Dermatol. 1999;113:554–559. doi: 10.1046/j.1523-1747.1999.00719.x. [DOI] [PubMed] [Google Scholar]
- Ribuffo D, Gradilone A, Vonella M, Chiummariello S, Cigna E, Haliassos N, Massa R, Silvestri I, Calvieri S, Frati L, Agliano AM, Scuderi N. Prognostic significance of reverse transcriptase-polymerase chain reaction-negative sentinel nodes in malignant melanoma. Ann Surg Oncol. 2003;10:396–402. doi: 10.1245/aso.2003.06.006. [DOI] [PubMed] [Google Scholar]
- Shivers SC, Wang X, Li W, Joseph E, Messina J, Glass LF, DeConti R, Cruse CW, Berman C, Fenske NA, Lyman GH, Reintgen DS. Molecular staging of malignant melanoma: correlation with clinical outcome. JAMA. 1998;280:1410–1415. doi: 10.1001/jama.280.16.1410. [DOI] [PubMed] [Google Scholar]
- Ghossein RA, Bhattacharya S, Coit DG. Reverse transcriptase polymerase chain reaction (RT-PCR) detection of melanoma-related transcripts in the peripheral blood and bone marrow of patients with malignant melanoma: what have we learned? Recent Results Cancer Res. 2001;158:63–77. doi: 10.1007/978-3-642-59537-0_7. [DOI] [PubMed] [Google Scholar]
- Gutzmer R, Kaspari M, Brodersen JP, Mommert S, Volker B, Kapp A, Werfel T, Kiehl P. Specificity of tyrosinase and HMB45 PCR in the detection of melanoma metastases in sentinel lymph node biopsies. Histopathology. 2002;41:510–518. doi: 10.1046/j.1365-2559.2002.01512.x. [DOI] [PubMed] [Google Scholar]
- Tsao H, Nadiminti U, Sober AJ, Bigby M. A meta-analysis of reverse transcriptase-polymerase chain reaction for tyrosinase mRNA as a marker for circulating tumor cells in cutaneous melanoma. Arch Dermatol. 2001;137:325–330. [PubMed] [Google Scholar]
- Carson KF, Wen DR, Li PX, Lana AM, Bailly C, Morton DL, Cochran AJ. Nodal nevi and cutaneous melanomas. Am J Surg Pathol. 1996;20:834–840. doi: 10.1097/00000478-199607000-00006. [DOI] [PubMed] [Google Scholar]
- Abrahamsen HN, Hamilton-Dutoit SJ, Larsen J, Steiniche T. Sentinel lymph nodes in malignant melanoma: extended histopathological evaluation improves diagnostic precision. Cancer. 2004;100:1683–1691. doi: 10.1002/cncr.20179. [DOI] [PubMed] [Google Scholar]
- Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods. 2001;25:386–401. doi: 10.1006/meth.2001.1261. [DOI] [PubMed] [Google Scholar]
- Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- Takeda A, Matsunaga J, Tomita Y, Tagami H, Shibahara S. Nucleotide sequence of the putative human tyrosinase pseudogene. Tohoku J Exp Med. 1991;163:295–297. doi: 10.1620/tjem.163.295. [DOI] [PubMed] [Google Scholar]
- Sorensen BS, Schmidt H, Von Der MH, Straten PT, Nexo E. Quantification of melanoma cell-specific MART-1 mRNA in peripheral blood by a calibrated competitive reverse transcription-PCR. Clin Chem. 2000;46:1923–1928. [PubMed] [Google Scholar]
- Bijwaard KE, Aguilera NS, Monczak Y, Trudel M, Taubenberger JK, Lichy JH. Quantitative real-time reverse transcription-PCR assay for cyclin D1 expression: utility in the diagnosis of mantle cell lymphoma. Clin Chem. 2001;47:195–201. [PubMed] [Google Scholar]
- Blaheta HJ, Ellwanger U, Schittek B, Sotlar K, Maczey E, Breuninger H, Thelen MH, Bueltmann B, Rassner G, Garbe C. Examination of regional lymph nodes by sentinel node biopsy and molecular analysis provides new staging facilities in primary cutaneous melanoma. J Invest Dermatol. 2000;114:637–642. doi: 10.1046/j.1523-1747.2000.00925.x. [DOI] [PubMed] [Google Scholar]
- McMasters KM. Molecular staging of melanoma: sensitivity, specificity, and the search for clinical significance. Ann Surg Oncol. 2003;10:336–337. doi: 10.1245/aso.2003.02.013. [DOI] [PubMed] [Google Scholar]
- Chelly J, Concordet JP, Kaplan JC, Kahn A. Illegitimate transcription: transcription of any gene in any cell type. Proc Natl Acad Sci USA. 1989;86:2617–2621. doi: 10.1073/pnas.86.8.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haninec P, Vachtenheim J. Tyrosinase protein is expressed also in some neural crest derived cells which are not melanocytes. Pigment Cell Res. 1988;1:340–343. doi: 10.1111/j.1600-0749.1988.tb00129.x. [DOI] [PubMed] [Google Scholar]
- Sarantou T, Chi DD, Garrison DA, Conrad AJ, Schmid P, Morton DL, Hoon DS. Melanoma-associated antigens as messenger RNA detection markers for melanoma. Cancer Res. 1997;57:1371–1376. [PubMed] [Google Scholar]
- Takeuchi H, Kuo C, Morton DL, Wang HJ, Hoon DS. Expression of differentiation melanoma-associated antigen genes is associated with favorable disease outcome in advanced-stage melanomas. Cancer Res. 2003;63:441–448. [PubMed] [Google Scholar]
- Kulik J, Nowecki ZI, Rutkowski P, Ruka W, Rochowska M, Skurzak H, Siedlecki JA. Detection of circulating melanoma cells in peripheral blood by a two-marker RT-PCR assay. Melanoma Res. 2001;11:65–73. doi: 10.1097/00008390-200102000-00008. [DOI] [PubMed] [Google Scholar]