Abstract
Using a cytosol and nucleotide dependent assay that we previously developed, we have investigated the requirement for coat proteins in the in vitro production of trans-Golgi network (TGN)-derived vesicles from a Madin-Darby canine kidney (MDCK) cell Golgi fraction that contains the 35S-labeled, terminally glycosylated, envelope glycoprotein of vesicular stomatitis virus (VSV-G) accumulated in the TGN. We found that the TGN-derived vesicles, like those involved in intra-Golgi transport and in retrograde transport to the endoplasmic reticulum, contain a coatomer coat and that coatomer is required for their formation. Thus, after they are produced with GTPγS, the coated vesicles could be captured on beads containing anticoatomer antibody. Moreover, a cytosolic protein fraction depleted of coatomer could not support vesicle formation but it did so after purified coatomer was added. We also determined that P200/myosin II does not play an essential role in the in vitro generation of TGN-derived vesicles. Thus, removal of this protein from the cytosol, by differential salt precipitation or binding to phalloidin-induced actin filaments, had no effect on vesicle generation. Nevertheless, immunodepletion of cytosol using the anti-P200/myosin II AD7 antibody abolished vesicle generation and that antibody was capable of effectively immunocapturing coated vesicles, even when these were generated in the absence of P200/myosin II. These effects, however, are explained by the unexpected finding that the AD7 antibody interacts with undenatured coatomer.
To study the molecular interactions that underlie the formation of post-Golgi vesicles in the trans-Golgi network (TGN), several experimental systems have been developed in which vesicles containing secretory proteins or membrane proteins destined to the plasma membrane, such as the poly IgA receptor or the envelope glycoprotein of vesicular stomatitis virus (VSV-G), are produced in vitro from purified Golgi fractions (1–4), from subcellular fractions containing Golgi membranes (5, 6), or from semi-intact or permeabilized cells (7–10). Some of these studies have shown that the activation of a GTP-binding protein of the ADP-ribosylation factor (ARF) family is required for the assembly of the coat of TGN-derived vesicles, although the molecular composition of the coat has not yet been established (3, 4, 11, 12).
A morphological analysis of the vesicles produced in vitro from Madin-Darby canine kidney (MDCK) cell Golgi membranes (4) has shown that TGN-derived vesicles containing sialylated VSV-G protein molecules are clearly distinct from clathrin-coated post-Golgi vesicles and, in fact, strikingly resemble the coatomer-coated vesicles that mediate intra-Golgi transport (13). Coatomer coats are macromolecular complexes of seven coat protomer type I (COPI) polypeptides (see ref. 14), and are primarily concentrated at the entry face of the Golgi apparatus, but are also localized in the TGN (15) and on endosomes (16). However, several reports (12, 17, 18) have concluded that coatomer coats are not involved in the production of TGN-derived exocytic vesicles. Thus, microinjection of anticoatomer antibodies into cells was found to prevent transport of the VSV-G protein from the endoplasmic reticulum to the Golgi, but not to affect its delivery from the TGN to the cell surface (17). Another study found that a cytosol depleted of coatomer fully supported the formation of secretory vesicles from a TGN-containing membrane preparation of PC12 cells (12). Recently, it was also observed that, after purification, post-Golgi vesicles generated in vitro from MDCK Golgi fractions containing the VSV-G protein contained no detectable levels of the COPI polypeptide βCOP (18). In the latter case, however, the vesicles could be immunocaptured by a mAb (AD7) that recognizes a TGN-associated protein known as P200. This protein, recently found to be identical to non-muscle myosin II (18, 19), was originally proposed to be a coat component of TGN-derived vesicles because, like coatomer, it was rapidly released from Golgi membranes when cells were treated with brefeldin A, a drug that prevents the activation of ARF (20, 21). Two recent direct studies on the role of P200/myosin in the formation of post-Golgi vesicles and transport from the TGN to the plasma membrane have yielded contradictory results. One (18) concluded that P200/myosin II is required for vesicle generation, while the other (10) found no evidence for a role of P200/myosin in transport from the TGN to the plasma membrane, or for its presence in TGN-derived vesicles.
We have studied the coat requirements for post-Golgi vesicle generation from a purified Golgi fraction obtained from MDCK cells that contained labeled sialylated VSV-G glycoprotein molecules arrested in the TGN. In this paper we show that these vesicles contain a coatomer coat and that coatomer is, in fact, required for their formation. In addition, we demonstrate that P200/myosin is not required for vesicle formation in the in vitro system.
MATERIALS AND METHODS
Antibodies.
Hybridomas producing the mAbs AD7 against P200 (20) and M3A5 against βCOP (22) were gifts of Karl Matlin (Massachusetts General Hospital, Charlestown, MA) and Thomas Kreis (University of Geneva, Switzerland), respectively. A mAb (CM1A10) that recognizes the native coatomer (23, 24), apparently through its β′ subunit, was generously provided by James Rothman and Thomas Söllner (Memorial Sloan-Kettering Cancer Center, New York). A polyclonal anti-βCOP antibody was generated by immunizing rabbits with the EAGE-containing βCOP peptide (17). The antibody to human platelet myosin was purchased from Biomedical Technologies (Stoughton, MA). Anti-P200 IgG fractions were obtained from hybridoma culture supernatants by ammonium sulfate precipitation (50% saturation) followed by protein G-agarose affinity chromatography.
Preparation of Liver Cytosol Protein (LCP) and Affinity Purification of the Anti-P200 Antibody.
After ammonium sulfate (100% saturation) precipitation of the proteins in liver cytosol, P200 was not solubilized in the buffer (4) used to resuspend the proteins. However it was solubilized by incubating the sediment in buffer containing ATP (1 mM). P200 was electrophoretically purified and, after transfer to nitrocellulose, was used for affinity purification of the mAb.
Vesicle Generation Assays.
These were carried out as described, (3, 4) using Golgi fractions from VSV-infected MDCK cells and either rat liver cytosol or the cytosolic protein fraction (LCP) prepared from it by ammonium sulfate precipitation. The Golgi fractions contained radiolabeled sialylated VSV-G protein that had been allowed to accumulate in the TGN during incubation of the cells at 20°C for 2 hr prior to lysis. Vesicle generation proceeded during an incubation at 37°C for 60 min and was supported by either ATP (1 mM) or the poorly hydrolyzable GTP analogs GTPγS or guanylyl-imidodiphosphate (GMP-PNP) (100 μM), both of which gave equivalent results.
Immunocapture and Immunodepletion Experiments.
Aliquots (40 μl) of a suspension (1:1) of protein G-agarose (PGA) beads (Calbiochem) in PBS were mixed with 10 μg of CM1A10 anticoatomer, or anti-P200 antibodies, or with PBS as control, and incubated at 37°C for 60 min with intermittent shaking. After recovery by centrifugation, the beads were washed (5×) in PBS and used for immunocapture after vesicle production by adding them to the reaction mixtures (100 μl), which were reincubated at 37°C for 1 hr. For immunodepletion, PGA beads containing crosslinked (23) AD7 or CM1A10 antibodies were suspended (10 μl) in 30 μl of LCP (100 mg/ml) and incubated for periods up to 40 hr at 4°C or at 37°C. After removal of the beads, the supernatants were analyzed by Western blotting and used for vesicle generation.
RESULTS
A Rat LCP Fraction Lacking P200/Myosin II Fully Supports the Formation of TGN-Derived Vesicles.
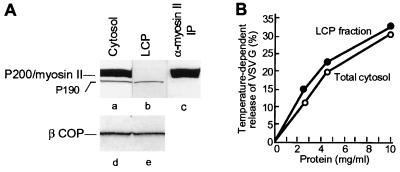
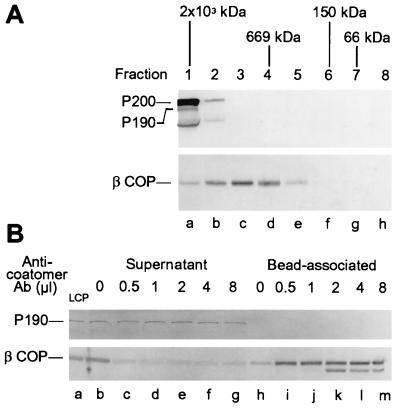
The rat liver cytosol used to support vesicle generation in our in vitro assay contains two polypeptides (Fig. 1A, lane a) that in Western blotting analysis are recognized by the AD7 anti-P200 mAb, a major one with a Mr of 200 kDa (P200/myosin II) that is immunoprecipitable with a specific anti-myosin II antibody and a much less abundant one of ≈190 kDa (P190) that is not (compare lanes a and c). Ammonium sulfate precipitation (see Materials and Methods) allowed us to obtain a LCP that was completely depleted of the P200/myosin II protein (Fig. 1A, compare lanes a and b) and, yet, fully supported the nucleotide-dependent release of TGN-derived vesicles (Fig. 1B). Vesicle generation was also unaffected when the cytosol was depleted of myosin by coprecipitation with actin filaments polymerized by the addition of phalloidin (18) (Fig. 2F). The Golgi fraction itself did not contain any detectable P200/myosin II and, even after washing with a buffer containing 0.25 M KCl, to remove any myosin that could be associated with the membranes (18), was capable of generating vesicles in the presence of LCP or myosin II depleted cytosol (not shown). We, therefore, conclude that P200/myosin II is not required for vesicle generation in the in vitro system.
Figure 1.
Rat liver cytosol contains two polypeptides, P200/myosin II and P190, that are recognized in immunoblots by the AD7 mAb: A cytosolic protein fraction (LCP) devoid of P200 efficiently supports the release of post-Golgi vesicles. (A) Equal aliquots (200 μg of protein) of total cytosol (lanes a and d), LCP (lanes b and e), and an immunoprecipitate from the total cytosol obtained with an anti-myosin II antibody (lane c) were analyzed by gel electrophoresis followed by Western blot with the AD7 anti-P200 (lanes a–c) or M3A5 anti-βCOP (lanes d and e) mAbs. (B) Aliquots of a Golgi fraction were incubated for 1 hr in standard reaction mixtures containing ATP (1 mM) with either total cytosol (○—○) or LCP (•—•) at the protein concentrations indicated. VSV-G protein release is expressed as percentage of the VSV-G radioactivity initially associated with the Golgi membranes (4).
Figure 2.
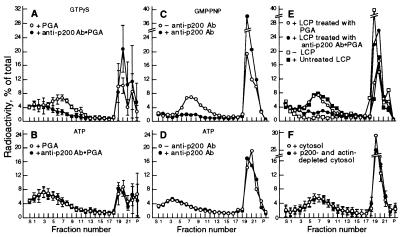
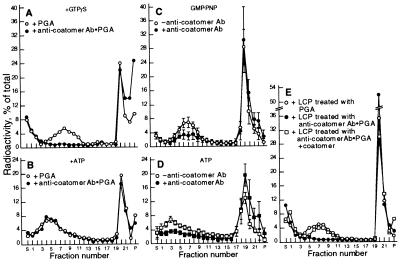
Effects of the anti-P200 antibody on the formation of TGN-derived vesicles. (A and B) After incubation for vesicle generation, with either GTPγS (A) or ATP (B), reaction mixtures (100 μl) were incubated for vesicle immunocapture with agarose beads containing (•—•) or lacking (○—○) bound anti-P200 antibody. The mixtures were then analyzed directly by velocity gradient centrifugation (4). For each gradient fraction, the mean and SD of four experiments are plotted. Note that in A, but not in B, the antibody-coated beads completely eliminated the vesicle peak. (C and D) LCP aliquots (1 mg protein in 25 μl), were preincubated for 30 min at 37°C with either the AD7 mAb (15 μg in 5 μl PBS), (•—•) or with PBS alone (○—○) before use in standard reaction mixtures containing either GMP-PNP to produce coated vesicles (C), or ATP to produce naked ones (D). (E) LCP aliquots (1 mg protein in 50 μl) adjusted to 0.1 M KCl, were incubated for 60 min at 37°C with either naked PGA beads (○—○), or with beads containing bound anti-P200 antibodies (•—•) to deplete immunoreactive proteins. The beads were removed by centrifugation (2 × 10 min at 10,000 × g) and the supernatants were used for vesicle generation with GMP-PNP. (F) Vesicle generation was carried out in the presence of GMP-PNP with a cytosol depleted of P200/myosin II by copolymerization with actin.
The AD7 Anti-P200/Myosin II Antibody Blocks Vesicle Formation Even in the Absence of P200.
The ammonium sulfate precipitation procedure used to prepare LCP, which removed the P200/myosin II, did not reduce, however, the concentration of the P190 polypeptide (Fig. 1A, compare lanes a and b) or of coatomer, as determined from the level of βCOP (Fig. 1A, lanes d and e). Initially, several observations seemed to indicate that P190 was a component of the coat of the in vitro generated TGN-derived vesicles and was required for their formation. Thus, vesicles containing the labeled sialylated VSV-G protein that were generated with the LCP fraction in the presence of GTPγS or GMP-PNP could be immunocaptured by the AD7 anti-P200 antibody bound to PGA beads (Fig. 2A), whereas naked vesicles produced with ATP could not (Fig. 2B). In control experiments (not shown) several other mAbs that recognize other antigens, such as cytochrome P450 2B1/2 and endolyn failed to capture the coated vesicles. In addition, when the anti-P200 antibody was included in a vesicle generation reaction mixture containing a nonhydrolyzable GTP analog, coated vesicles were not released from the rapidly sedimenting Golgi fraction (Fig. 2C), although electron microscopy showed that they were still produced but formed large aggregates that remained associated with the sedimentable Golgi membranes (Fig. 3b). However, the antibody had no effect on the amounts of naked vesicles produced and released when the reaction was supported by ATP (Fig. 2D). These experiments indicated that, although the anti-P200 antibody binds to a coat component of the TGN-derived vesicles and removes them from the reaction mixture, it does not prevent their formation nor the disassembly of their coat. Finally, treatment of LCP with anti-P200 antibodies bound to PGA beads, to deplete it of P190, eliminated its capacity to support the formation of coated vesicles in the presence of GTPγS or GMP-PNP (Fig. 2E).
Figure 3.
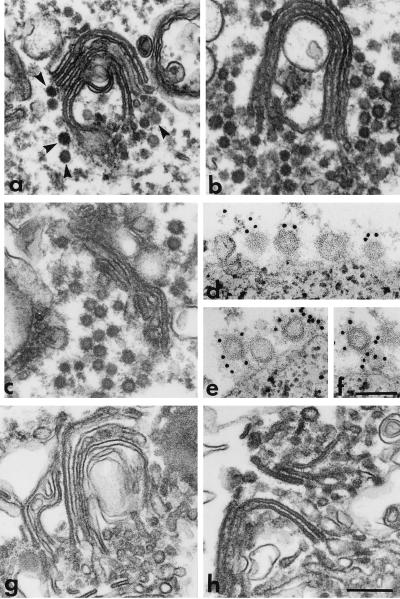
Electron microscopic analysis of the effects of anti-P200 and anticoatomer antibodies on post-Golgi vesicle production. (a–c) Golgi membranes were incubated in reaction mixtures containing GMP-PNP and intact LCP, without (a), or with either anti-P200 (b) or anticoatomer antibodies (c), or with LCP that had been treated for immunodepletion with anti-P200 (g) or anticoatomer antibodies (h) linked to PGA beads. After incubation at 37°C (a–c), or at 20°C (g and h), the Golgi cisternae were recovered by centrifugation (10,000 × g for 10 min) and processed for electron microscopy. Note that in the control sample (a), despite extensive vesicle release (see Fig. 2C, ○—○), some coated vesicles (arrowheads) still remain associated with the Golgi cisternae. Although the biochemical experiments show that in b and c vesicles are not released or released to a very limited extent (see Figs. 2C and 6C, •—•), the electron micrographs show that coated vesicles are still produced but in the presence of the antibodies they form large aggregates that remain associated with the Golgi membranes. Immunodepletion with either anti-P200 (g) or anticoatomer (h) antibodies renders LCP incapable of supporting the formation of nonclathrin coated vesicles. (d–f) Vesicles released as in a were captured on magnetic beads containing immobilized anti-P200 antibodies. The beads were then incubated with the EAGE anticoatomer antibody followed by protein A gold (10 nm). Portions of the surface of beads are shown. Note that vesicles bound to the beads via the anti-P200/myosin II antibody are also labeled with the anticoatomer antibody (gold particles). Bars = 0.25 μm (a, b, c, g, and h); 0.1 μm (d–f).
The AD7 Anti-P200 Antibody Recognizes Native Coatomer.
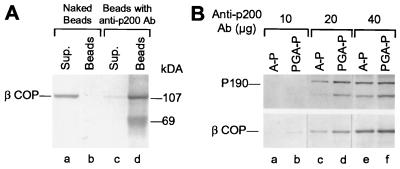
Electron microscopic examination of the Golgi fraction recovered after incubation with the LCP treated for depletion with the anti-P200 antibody revealed that this treatment almost completely suppressed the formation of nonclathrin-coated vesicles throughout the Golgi cisternae (Fig. 3g). This was quite surprising because we expected that the production of the coatomer coated vesicles thought to mediate intra-Golgi transport (see ref. 25) would not be affected by the anti-P200 antibody. This led us to investigate whether coatomer was also depleted from the LCP fraction by the anti-P200 antibody. We found that this was indeed the case: βCOP, was essentially completely removed from the LCP fraction by incubation with anti-P200 antibody-coated beads (Fig. 4A, compare lanes c and d). Because βCOP is found exclusively as a component of the coatomer (14), this finding indicated that coatomer was simultaneously immunocaptured with P190 by the anti-P200 antibody. To exclude the unlikely possibility that the anti-P200 mAb preparation contained a mixture of antibodies that separately recognized P190 and a coatomer component, we affinity purified the anti-P200 antibody by adsorption to immobilized purified P200/myosin. Using the affinity purified antibody at a series of concentrations, we found (Fig. 4B) that it removed from the LCP fraction, in parallel and with comparable efficiencies, both P190 (Fig. 4B, Top) and βCOP (Fig. 4B, Bottom), as expected from the presence of a single antibody. This still left open the possibility, however, that coatomer in the cytosolic fraction is linked to P190 so that immunoadsorption of P190 to anti-P200 antibody-coated beads also brings down the βCOP-containing coatomer. This possibility, however, could be dismissed on the basis of two experimental results. First, after gel filtration chromatography (Fig. 5A) most of the βCOP in the cytosol was recovered in fractions corresponding to ≈700 kDa—the particle weight reported for the coatomer complex (26)—and these fractions contained neither P200 nor P190, both of which eluted in fractions corresponding to an Mr of ≈2 × 103 kDa (Fig. 5A). In addition, coatomer could be nearly quantitatively immunoprecipitated by the AD7 antibody from column fractions that contained no detectable P190 and were far removed (e.g., fraction 5) from the position where P190 could have been present (not shown). Second, removal of coatomer by immunoadsorption to beads containing anticoatomer antibody did not remove any detectable P190 from the LCP fraction (Fig. 5B). These findings led us to conclude that the AD7 antibody—although incapable of recognizing any denatured coatomer subunit in a standard Western blotting analysis—efficiently recognizes independent epitopes in P200, P190, and in native coatomer.
Figure 4.
Anti-P200 mAb bound to PGA beads removes native coatomer from an LCP fraction. (A) LCP aliquots (200 μg) were incubated for 40 hr at 4°C with either naked PGA beads (lanes a and b), or with beads containing anti-P200 antibody (lanes c and d). The beads (lanes b and d), recovered by centrifugation and washed in immunoprecipitation buffer (20 mM Hepes⋅KOH, pH 7.2/100 mM KCl/1 mM DTT), and the supernatants combined with the washes (lanes a and c), were analyzed by SDS/PAGE and Western blotting with the anti-βCOP M3A5 mAb. (B) Immunoadsorption of coatomer by affinity purified anti-P200 antibody. Beads containing the indicated amounts of anti-P200 antibody, purified by binding to PGA (PGA-P, lanes b, d, and f), or by affinity purification on immobilized P200 (A-P, lanes a, c, and e), were incubated with 1 mg LCP for 20 hr at 4°C. After three washes with cold immunoprecipitation buffer, the bound proteins were analyzed by SDS/PAGE and Western blotting with the anti-P200 (Top) or the anti-βCOP M3A5 antibody (Bottom). The band below P190 detected by the AD7 antibody corresponds to a degradation product of P190.
Figure 5.
P190 and coatomer are not part of the same cytosolic protein complex. (A) Separation of P200 and P190 from coatomer by gel filtration on a Superose 6 column (60 cm × 1.5 cm) equilibrated with 20 mM Hepes⋅KOH (pH 7.3), 100 mM KCl, and 1 mM DTT. Aliquots (10 μg protein) of each column fraction (5 ml) were analyzed by PAGE and Western blotting using the anti-P200 (Upper) or the M3A5 anti-βCOP antibody (Lower). (B) P190 is not coimmunopreciptated with βCOP using an anticoatomer antibody. LCP aliquots (250 μg) were incubated for 20 hr at 4°C with PGA beads that had been preincubated with the indicated amounts of CM1A10 antibody. Bead-associated and supernatant proteins were analyzed by PAGE and Western blotting with the AD7 anti-P200 (Upper) or the M3A5 anti-βCOP (Lower) mAbs.
TGN-Derived Vesicles Produced in Vitro Contain a COPI Coat and Coatomer Is Required for Their Formation.
The results just described suggest that all the effects of the P200 antibody we observed on the generation and capture of TGN-derived vesicles could result from its binding to coatomer. We, therefore, examined directly the effects of anticoatomer antibodies on the same processes. We found that coated (Figs. 6A), but not naked (Fig. 6B), post-Golgi vesicles could be immunocaptured on beads containing anticoatomer antibody. This was the case (Fig. 6 A and B) with the CM1A10 monoclonal anticoatomer antibody that appears to recognize β′COP, as well as with an EAGE anti-βCOP antipeptide antibody (17) and, in the latter case, the immunocapture was prevented by preincubation of the beads containing the antibody with the peptide immunogen (data not shown). The CM1A10 anticoatomer and AD7 anti-P200 antibodies were also very effective in immunocapturing (59.5 ± 4.9 and 62.5 ± 3.8%, respectively) purified VSV-G-containing vesicles that were generated with LCP and GMP-PNP and, after collection from sucrose density gradients, had been further purified by flotation (3). The fact that the same vesicles can be recognized by both antibodies is visually demonstrated in Fig. 3 d–f, which shows that vesicles immunocaptured on beads coated with the anti-P200 antibody are labeled with the anticoatomer antibody.
Figure 6.
TGN-derived vesicles contain a coatomer coat. (A and B) After incubation for vesicle generation in the presence of either GTPγS (A) or ATP (B), reaction mixtures were incubated for immunocapture with PGA beads containing bound CM1A10 monoclonal anticoatomer antibody (•—•), or with control beads (○—○), and then analyzed for vesicle release by sucrose density centrifugation. (C and D) incubations for vesicle generation with a nonhydrolyzable GTP analog (C) or ATP (D) were carried out as described in Fig. 2 C and D, but using the CM1A10 anticoatomer antibody. (E) Coatomer depleted or mock depleted LCP (○—○) were used to support vesicle generation in the presence of GTPγS, without (•—•) or with (□—□) the addition of 60 μg purified coatomer (26).
Inclusion of the anticoatomer antibody in reaction mixtures containing nonhydrolyzable GTP analogs or ATP, greatly reduced the production of coated and naked vesicles, respectively (Fig. 6 C and D). The latter effect of the anticoatomer antibody is in marked contrast to that observed with the anti-P200 antibody, which did not prevent the release of naked vesicles. Electron microscopy (Fig. 3c) showed that Golgi fractions recovered from reaction mixtures containing the anticoatomer antibody had produced a substantial number of coated vesicles which, as was the case when P200 antibody was present (Fig. 3b), remained associated with the sedimentable membranes. These observations indicate that the anticoatomer antibody, although reducing the formation of the coated vesicles, also causes their aggregation and partially prevents their uncoating.
The requirement of coatomer for the production of TGN-derived vesicles was definitively demonstrated by the findings that cytosolic protein fractions depleted of coatomer totally failed to support vesicle production, but their capacity to do so was restored when purified coatomer was added to them (Fig. 6E). Electron microscopic examination (Fig. 3h) showed that depletion of coatomer rendered the cytosolic protein fraction unable to promote the formation of coated buds throughout the Golgi stacks during an incubation with nonhydrolyzable GTP analogs at 20°C, a condition that, with intact cytosol, prevents vesicle scission and leads to accumulation of coated buds (3).
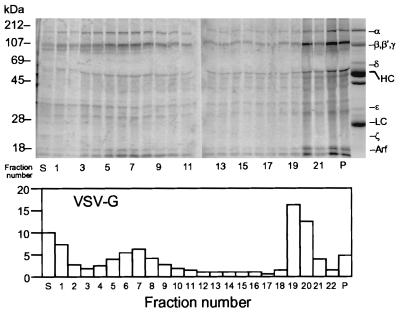
A direct demonstration that during their formation vesicles that contain the sialylated VSV-G protein acquire a COPI coat was obtained by utilizing a labeled cytosolic protein fraction prepared from MDCK cells grown in medium containing [35S]-methionine and [35S]-cysteine to support vesicle generation. With that fraction, coat assembly on Golgi membranes was carried out during an incubation with GTPγS at 20°C. The Golgi membranes were then recovered, to remove unbound labeled cytosolic proteins, and incubated for vesicle scission at 37°C in the presence of an unlabeled cytosolic protein fraction (3). It was found (Fig. 7) that a set of radioactive polypeptides with electrophoretic mobilities characteristic of the COPI subunits became associated with the membranes during coat assembly at 20°C. After vesicle scission, the set of labeled polypeptides was recovered from sucrose density gradients in the same fractions (nos. 4–8) as were the vesicles containing radioactive VSV-G protein in a parallel gradient. The amount of COP polypeptides that sedimented with the vesicles was markedly reduced when the nonhydrolyzable GTP analog was replaced by ATP (not shown), which allows disassembly of the vesicle coat.
Figure 7.
Post-Golgi vesicles formed in the presence of an 35S-labeled MDCK cytosolic protein fraction acquire labeled coatomer subunits. (Upper) An unlabeled Golgi fraction (400 μl; 25 μg/ml) from VSV-infected MDCK cells that had been incubated at 20°C for 2 hr was incubated for 1 hr at 20°C with 1 mM GMP-PNP and a radioactive MDCK cell cytosolic protein fraction (7.7 mg/ml). The Golgi membranes were recovered and incubated for vesicle scission (3) with nonradioactive LCP (15 mg/ml). The reaction mixture was then analyzed by sucrose density centrifugation. After adding 100 μg of BSA, protein in individual fractions (≈0.5 ml) was recovered by trichloroacetic acid precipitation (10%) and analyzed by PAGE and the radioactivity distribution determined in a PhosphorImager. Because of the large amount of protein, only one tenth of the top three fractions (S, 1, 2) was analyzed. The lane on the right of P is the Coomassie blue-stained pattern of COPI proteins recovered in an immunoprecipitate obtained from the LCP with anticoatomer antibody and serves as a reference to identify coatomer subunits in the gradient. HC, IgG heavy chain; LC, IgG light chain; Arf, ADP ribosylation factor. (Lower) Distribution of labeled VSV-G in a parallel gradient.
DISCUSSION
In this paper we investigated the participation of specific coat proteins in the in vitro generation of TGN-derived vesicles that contain the metabolically labeled, terminally glycosylated, VSV-G protein. We found that these vesicles contain a coatomer coat and that coatomer is, in fact, required for their formation. On the other hand, P200/myosin II is not required for vesicle formation in our in vitro system, in accord with a report (10) that P200 was not necessary for the transport of VSV-G to the cell surface in permeabilized MDCK cells. However, other authors (18), also using a semi-intact cell system of VSV-infected MDCK cells, found that depletion of P200/myosin II eliminated the capacity of the cytosol to promote vesicle release from the disrupted cells, which could be restored by supplying purified platelet myosin. In addition, inhibition of the myosin ATPase, or of its interaction with actin, by a variety of methods, also suppressed vesicle release. The results of those impressive experiments were interpreted as demonstrating a role of myosin in the formation of the TGN-derived VSV-G containing vesicles. They are also compatible, however, with a role of myosin in a post-Golgi transport process from the TGN to the cell surface, which could be required for release of the vesicles from the disrupted cells into the medium. The question of the possible role of myosin in vesicle generation per se, i.e., at the level of the TGN in the Golgi apparatus, was, however, directly addressed by the same authors (18) in experiments using a purified Golgi fraction in an in vitro system that resembles the one employed by us. Immunodepletion of P200/myosin II from cytosol using the immobilized AD7 antibody was found to markedly inhibit vesicle release, which was restored by the addition of purified myosin. We also found that treatment of the cytosol for immunodepletion with the AD7 antibody eliminated vesicle production, but in our hands addition of P200/myosin II failed to restore the activity of cytosol depleted with the AD7 antibody (data not shown) and we concluded that the inactivation of the cytosol was caused by removal of coatomer by the antibody.
Our finding that coatomer is a required component of the coat of TGN-derived vesicles also contrasts with the report (12) that TGN-derived constitutive secretory vesicles and immature secretory granules were formed in vitro from PC12 cell Golgi membranes even when more than 85% of the coatomer had been removed from the supporting cytosolic protein fraction. The MDCK and PC12 systems, however, also differ in other respects. Thus, whereas in the former GTPγS actually promotes vesicle formation (3, 4, 18), in the latter it suppresses it, which has been attributed to the stimulation of an inhibitory heterotrimeric G protein (27). It is possible that at least some of the differences between the two systems simply reflect the involvement of different types of vesicles in the transport of membrane or secretory proteins (ref. 28; but see ref. 1). Our findings are also at variance with the recent report that βCOP could not be detected in TGN-derived vesicles containing VSV-G protein obtained from semi-intact MDCK cells (18). In that work, however, the vesicles were purified by flotation through 1.2 M sucrose and it seems likely to us that this purification procedure led to vesicle uncoating, because the fully coated vesicles generated in our system have a density of ≈1.18 g/cm3, which corresponds to 1.4 M sucrose.
The behavior of the coatomer-coated TGN-derived vesicles we obtain in vitro differs in several respects from that reported for the COPI-coated vesicles involved in anterograde and retrograde intra-Golgi transport (see refs. 29 and 30). Most notably, whereas the TGN-derived vesicles are spontaneously released from Golgi membranes—as would be expected from post-Golgi vesicles that have no available docking sites in the cell free system—coatomer-coated vesicles involved in intra-Golgi transport, when produced in vitro, remain docked on Golgi cisterna, from where they can be released by incubation in media of high ionic strength (31). Second, whereas after coat assembly the scission from donor membranes of coatomer-coated vesicles involved in intra-Golgi transport requires palmitoyl CoA and no additional cytosolic proteins (29), scission of TGN-derived vesicles requires cytosolic proteins and is not promoted by palmitoyl CoA alone (3). Finally, the formation of COPI-coated intra-Golgi transport vesicles is not affected by N-ethylmaleimide treatment of the cytosol (32), whereas the formation of TGN-derived vesicles in our system is totally eliminated by the alkylating agent (4).
It is now clear that coatomer-coated vesicles are involved in multiple transport steps within the endomembrane system. In addition to their role in transport within the Golgi apparatus and from this organelle back to the endoplasmic reticulum (see refs. 25, 33, 34), evidence has been presented for their involvement in endoplasmic reticulum to Golgi transport (17, 35) and in transport between early and late endosomes (16, 36). In the latter case the coatomer involved appears to lack the gamma and delta subunits. This finding and the observation that coatomer subunits may undergo different degrees of phosphorylation (37) raise the possibility that distinct coatomer complexes function in different transport stages.
The formation of COPI-coated vesicles in the TGN seems well established, although their exact coat composition and destination are yet to be determined. A pool of coatomer that overlapped in its distribution with that of the VSV-G protein, was found to be associated with the TGN of VSV-infected cells, although it was not possible to colocalize the viral glycoprotein and the coatomer to the same buds or vesicles (15). In addition, it has been recently reported (38) that in some cells, COPI-coated vesicles associated with the TGN are morphologically distinct and significantly larger than those in the cis-Golgi region. It is noteworthy that proteins containing the KDEL signal that reach the TGN undergo retrograde transport from this late Golgi station (30, 39) presumably by coatomer-coated vesicles. It remains to be determined whether the TGN-derived COPI-coated vesicles containing the sialylated VSV-G proteins are involved in transport to the plasma membrane, or serve another function, such as the retrograde movement to more proximal regions of the endomembrane system.
Acknowledgments
We acknowledge the expert technical assistance of Heide Plesken, Iwona Gumper, and Jody Culkin, and the help of Myrna Cort and Antonio J.D. Rocha in preparing the manuscript. We are grateful to Drs. James Rothman and Thomas Söllner for their gift of the CM1A10 antibody and to Drs. Karl Matlin and Thomas Kreis for the AD7 and M3A5 hybridomas, respectively. This work was supported by National Institutes of Health Grant GM43583.
ABBREVIATIONS
- VSV-G
envelope glycoprotein of vesicular stomatitis virus
- TGN
trans-Golgi network
- MDCK
Madin-Darby canine kidney
- ARF
ADP-ribosylation factor
- LCP
liver cytosolic protein
- GMP-PNP
guanylyl-imidodiphosphate
- COPI
coat protomer type I
- PGA
protein G agarose
References
- 1.Salamero J, Sztul E S, Howell K E. Proc Natl Acad Sci USA. 1990;87:7717–7721. doi: 10.1073/pnas.87.19.7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones S M, Crosby J R, Salamero J, Howell K E. J Cell Biol. 1993;122:775–788. doi: 10.1083/jcb.122.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon J-P, Ivanov I E, Adesnik M, Sabatini D D. J Cell Biol. 1996;135:355–370. doi: 10.1083/jcb.135.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon J-P, Ivanov I E, Shopsin B, Hersh D, Adesnik M, Sabatini D D. J Biol Chem. 1996;271:16952–16961. doi: 10.1074/jbc.271.28.16952. [DOI] [PubMed] [Google Scholar]
- 5.Tooze S A, Huttner W B. Cell. 1990;60:837–847. doi: 10.1016/0092-8674(90)90097-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohashi M, Huttner W B. J Biol Chem. 1994;269:24897–24905. [PubMed] [Google Scholar]
- 7.Xu H, Shields D. J Cell Biol. 1993;122:1169–1184. doi: 10.1083/jcb.122.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musch A, Xu H, Shields D, Rodriguez-Boulan E. J Cell Biol. 1996;133:543–558. doi: 10.1083/jcb.133.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wandinger-Ness A, Bennett M K, Antony C, Simons K. J Cell Biol. 1990;111:987–1000. doi: 10.1083/jcb.111.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikonen E, Parton R G, Lafont F, Simons K. Mol Biol Cell. 1996;7:961–974. doi: 10.1091/mbc.7.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y-G, Shields D. J Biol Chem. 1996;271:5297–5300. doi: 10.1074/jbc.271.10.5297. [DOI] [PubMed] [Google Scholar]
- 12.Barr F A, Huttner W B. FEBS Lett. 1996;384:65–70. doi: 10.1016/0014-5793(96)00285-2. [DOI] [PubMed] [Google Scholar]
- 13.Orci L, Glick B S, Rothman J E. Cell. 1986;46:171–184. doi: 10.1016/0092-8674(86)90734-8. [DOI] [PubMed] [Google Scholar]
- 14.Lowe M, Kreis T E. J Biol Chem. 1996;271:30725–30730. doi: 10.1074/jbc.271.48.30725. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths G, Pepperkok R, Locker J K, Kreis T E. J Cell Sci. 1995;108:2839–2856. doi: 10.1242/jcs.108.8.2839. [DOI] [PubMed] [Google Scholar]
- 16.Whitney J A, Gomez M, Sheft D, Kreis T E, Mellman I. Cell. 1995;83:703–713. doi: 10.1016/0092-8674(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 17.Pepperkok, Scheel J, Horstmann H, Hauri H P, Griffths G, Kreis T E. Cell. 1993;74:71–82. doi: 10.1016/0092-8674(93)90295-2. [DOI] [PubMed] [Google Scholar]
- 18.Musch A, Cohen D, Rodriguez-Boulan E. J Cell Biol. 1997;138:291–306. doi: 10.1083/jcb.138.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikonen E, de Almeid J B, Fath K R, Burgess D R, Ashman K, Simons K, Stow J L. J Cell Sci. 1997;110:2155–2164. doi: 10.1242/jcs.110.18.2155. [DOI] [PubMed] [Google Scholar]
- 20.Narula N, McMorrow I, Plopper G, Doherty J, Matlin K S, Burke B, Stow J L. J Cell Biol. 1992;117:27–38. doi: 10.1083/jcb.117.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narula N, Stow J L. Proc Natl Acad Sci USA. 1995;92:2874–2878. doi: 10.1073/pnas.92.7.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allan V J, Kreis T E. J Cell Biol. 1986;103:2229–2239. doi: 10.1083/jcb.103.6.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer D J, Helms J B, Beckers C J, Orci L, Rothman J E. J Biol Chem. 1993;268:12083–12089. [PubMed] [Google Scholar]
- 24.Orci L, Palmer D J, Ravazzola M, Perrelet A, Amherdt M, Rothman J E. Nature (London) 1993;362:648–652. doi: 10.1038/362648a0. [DOI] [PubMed] [Google Scholar]
- 25.Rothman J E, Wieland F T. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- 26.Waters M G, Beakers C J M, Rothman J E. Methods Enzymol. 1992;219:331–337. doi: 10.1016/0076-6879(92)19033-3. [DOI] [PubMed] [Google Scholar]
- 27.Barr F A, Leyte A, Mollner S, Pfeuffer T, Tooze S A, Huttner W B. FEBS Lett. 1991;294:239–243. doi: 10.1016/0014-5793(91)81438-e. [DOI] [PubMed] [Google Scholar]
- 28.Saucan L, Palade G E. J Cell Biol. 1994;125:733–741. doi: 10.1083/jcb.125.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostermann J, Orci L, Tani K, Amherdt M, Ravazzola M, Elazar Z, Rothman J E. Cell. 1993;75:1015–1025. doi: 10.1016/0092-8674(93)90545-2. [DOI] [PubMed] [Google Scholar]
- 30.Orci L, Stamnes M, Ravazzola M, Amherdt M, Perrelet A, Söllner T H, Rothman J E. Cell. 1997;90:335–349. doi: 10.1016/s0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- 31.Malhotra V, Serafini T, Orci L, Shepherd J C, Rothman J E. Cell. 1989;58:329–336. doi: 10.1016/0092-8674(89)90847-7. [DOI] [PubMed] [Google Scholar]
- 32.Malhotra V, Orci L, Glick B S, Block M R, Rothman J E. Cell. 1988;54:221–227. doi: 10.1016/0092-8674(88)90554-5. [DOI] [PubMed] [Google Scholar]
- 33.Schekman R, Orci L. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- 34.Cosson P, Letourneur F. Curr Opin Cell Biol. 1997;9:484–487. doi: 10.1016/s0955-0674(97)80023-3. [DOI] [PubMed] [Google Scholar]
- 35.Peter F, Plutner H, Zhu H, Kreis T E, Balch W E. J Cell Biol. 1993;122:1155–1167. doi: 10.1083/jcb.122.6.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aniento F, Gu F, Parton R G, Gruenberg J. J Cell Biol. 1996;133:29–41. doi: 10.1083/jcb.133.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheff D, Lowe M, Kreis T E, Mellman I. J Biol Chem. 1996;271:7230–7236. doi: 10.1074/jbc.271.12.7230. [DOI] [PubMed] [Google Scholar]
- 38.Martinez-Menarguez J A, Geuze H J, Ballesta J. Eur J Cell Biol. 1996;71:137–143. [PubMed] [Google Scholar]
- 39.Miesenbock G, Rothman J E. J Cell Biol. 1995;129:309–319. doi: 10.1083/jcb.129.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]