Full Text
The Full Text of this article is available as a PDF (126.4 KB).
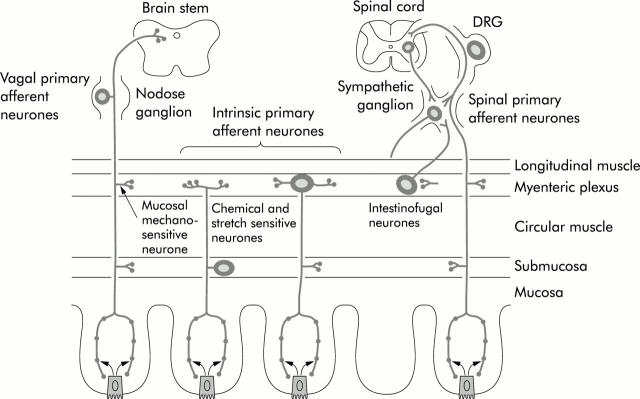
Figure 1 .
Arrangement of the primary afferent neurones within the intestine. DRG, dorsal root ganglion. Reproduced with permission from Furness and colleagues.1
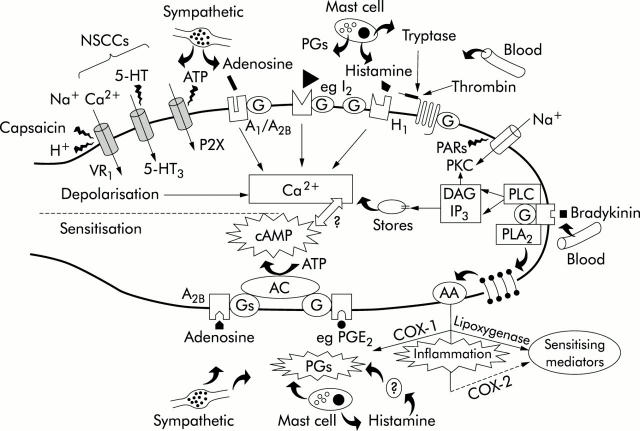
Figure 2 .
Potential receptor mechanisms underlying activation (depolarisation) and sensitisation of visceral sensory afferents. Some mediators, for example, serotonin (5-HT) acting on 5-HT3 receptors, cause activation while some (prostaglandin E2 (PGE2)) cause sensitisation. Others, for example adenosine, cause both stimulation and sensitisation. Bradykinin has a self sensitising action, stimulating discharge through activation of phospholipases (PLs) and enhancing excitability via PGs. Inflammatory mediators can be released from a variety of cell types (for example, sympathetic varicosities, mast cells, and blood vessels) present in or around the afferent nerve terminal. 5-HT, adenosine triphosphate (ATP), and capsaicin can directly activate non-selective cation channels (NSCCs) while adenosine, histamine, PGs (not PGE2), and proteases such as tryptase and thrombin act on G protein coupled receptors leading to a Ca2+ dependent modulation of ion channel activity. Sensitisation may be mediated via cyclic adenosine 3,5-monophosphate (cAMP). Adenosine and PGE2 can generate cAMP directly through G protein coupled stimulation of adenyl cyclase (AC). In contrast, histamine may act indirectly through generation of PGs. The actions of cAMP downstream are currently unknown but may involve modulation of ion channels, interaction with other second messengers, such as Ca2+, or even changes in receptor expression. COX, cyclooxygenase; DAG, diacylglycerol; IP3, inositol 1,4,5-triphosphate; PARs, protease activated receptors; PLC, phospholipase C; PLA2, phospholipase A2; PKC, protein kinase C. Modified from Kirkup and colleagues.12
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berthoud H. R., Neuhuber W. L. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000 Dec 20;85(1-3):1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- Berthoud H. R., Powley T. L. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J Comp Neurol. 1992 May 8;319(2):261–276. doi: 10.1002/cne.903190206. [DOI] [PubMed] [Google Scholar]
- Eastwood C., Grundy D. Opioid-receptor-mediated excitation of rat mesenteric afferent fibres supplying the rat jejunum. Neurogastroenterol Motil. 2000 Dec;12(6):517–522. doi: 10.1046/j.1365-2982.2000.00226.x. [DOI] [PubMed] [Google Scholar]
- Eastwood C., Maubach K., Kirkup A. J., Grundy D. The role of endogenous cholecystokinin in the sensory transduction of luminal nutrient signals in the rat jejunum. Neurosci Lett. 1998 Oct 2;254(3):145–148. doi: 10.1016/s0304-3940(98)00666-1. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Kunze W. A., Clerc N. Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am J Physiol. 1999 Nov;277(5 Pt 1):G922–G928. doi: 10.1152/ajpgi.1999.277.5.G922. [DOI] [PubMed] [Google Scholar]
- Gebhart G. F. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications IV. Visceral afferent contributions to the pathobiology of visceral pain. Am J Physiol Gastrointest Liver Physiol. 2000 Jun;278(6):G834–G838. doi: 10.1152/ajpgi.2000.278.6.G834. [DOI] [PubMed] [Google Scholar]
- Gershon M. D. Review article: roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther. 1999 May;13 (Suppl 2):15–30. [PubMed] [Google Scholar]
- Holzer P. Neural emergency system in the stomach. Gastroenterology. 1998 Apr;114(4):823–839. doi: 10.1016/s0016-5085(98)70597-9. [DOI] [PubMed] [Google Scholar]
- Joshi S. K., Gebhart G. F. Visceral pain. Curr Rev Pain. 2000;4(6):499–506. doi: 10.1007/s11916-000-0074-7. [DOI] [PubMed] [Google Scholar]
- Keates A. C., Castagliuolo I., Qiu B., Nikulasson S., Sengupta A., Pothoulakis C. CGRP upregulation in dorsal root ganglia and ileal mucosa during Clostridium difficile toxin A-induced enteritis. Am J Physiol. 1998 Jan;274(1 Pt 1):G196–G202. doi: 10.1152/ajpgi.1998.274.1.G196. [DOI] [PubMed] [Google Scholar]
- Lembo T., Munakata J., Mertz H., Niazi N., Kodner A., Nikas V., Mayer E. A. Evidence for the hypersensitivity of lumbar splanchnic afferents in irritable bowel syndrome. Gastroenterology. 1994 Dec;107(6):1686–1696. doi: 10.1016/0016-5085(94)90809-5. [DOI] [PubMed] [Google Scholar]
- Phillips R. J., Powley T. L. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev. 2000 Nov;34(1-2):1–26. doi: 10.1016/s0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- Randich A., Gebhart G. F. Vagal afferent modulation of nociception. Brain Res Brain Res Rev. 1992 May-Aug;17(2):77–99. doi: 10.1016/0165-0173(92)90009-b. [DOI] [PubMed] [Google Scholar]
- Raybould H. E. Nutrient tasting and signaling mechanisms in the gut. I. Sensing of lipid by the intestinal mucosa. Am J Physiol. 1999 Oct;277(4 Pt 1):G751–G755. doi: 10.1152/ajpgi.1999.277.4.G751. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E. Central connections of the sensory and motor nuclei of the vagus nerve. J Auton Nerv Syst. 1983 Oct;9(1):13–26. doi: 10.1016/0165-1838(83)90129-7. [DOI] [PubMed] [Google Scholar]
- Sengupta J. N., Saha J. K., Goyal R. K. Stimulus-response function studies of esophageal mechanosensitive nociceptors in sympathetic afferents of opossum. J Neurophysiol. 1990 Sep;64(3):796–812. doi: 10.1152/jn.1990.64.3.796. [DOI] [PubMed] [Google Scholar]
- Sternini C., De Giorgio R., Furness J. B. Calcitonin gene-related peptide neurons innervating the canine digestive system. Regul Pept. 1992 Nov 20;42(1-2):15–26. doi: 10.1016/0167-0115(92)90020-u. [DOI] [PubMed] [Google Scholar]
- Watkins L. R., Maier S. F. The pain of being sick: implications of immune-to-brain communication for understanding pain. Annu Rev Psychol. 2000;51:29–57. doi: 10.1146/annurev.psych.51.1.29. [DOI] [PubMed] [Google Scholar]
- Zagorodnyuk V. P., Brookes S. J. Transduction sites of vagal mechanoreceptors in the guinea pig esophagus. J Neurosci. 2000 Aug 15;20(16):6249–6255. doi: 10.1523/JNEUROSCI.20-16-06249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]