Abstract
Pharmacogenomics aims to identify the inherited basis for interindividual differences in drug response, and translate this to molecular diagnostics that can be used to individualise drug therapy. This review uses a number of published examples of inherited differences in drug metabolising enzymes, drug transporters, and drug targets (for example, receptors) to illustrate the potential importance of inheritance in determining the efficacy and toxicity of medications in humans. It seems that this field is at the early stages of developing a powerful set of molecular diagnostics that will have profound utility in optimising drug therapy for individual patients.
Full Text
The Full Text of this article is available as a PDF (191.6 KB).
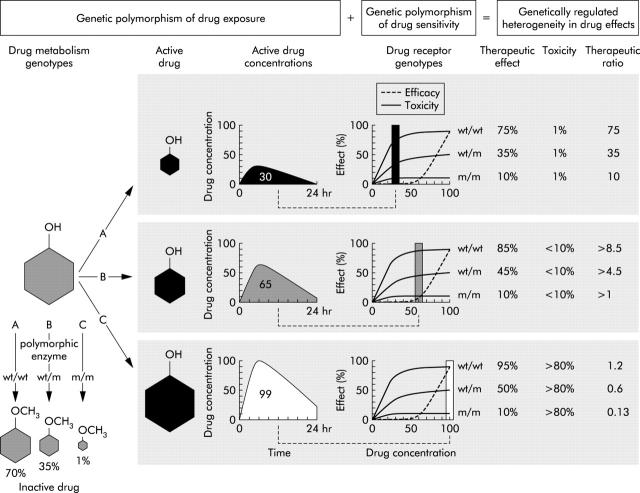
Figure 1 .
Polygenetic determinants of drug response (reproduced with the publisher's permission, from Evans and Johnson, Annu Rev Genomics Hum Genet 2001;2:9–39).
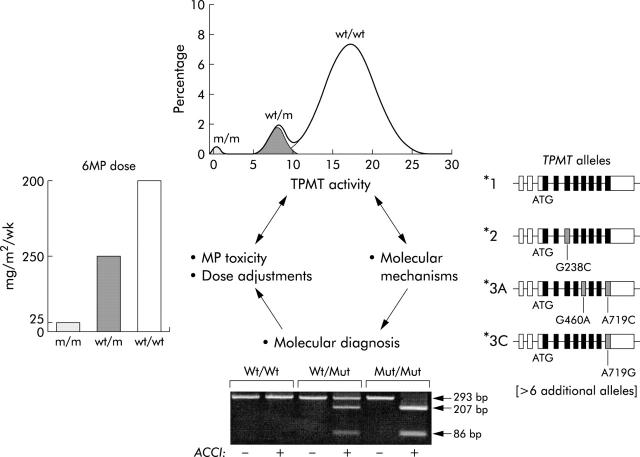
Figure 2 .
Genetic polymorphism of thiopurine methyltransferase and its importance in determining response to thiopurine medications (azathioprine, mercaptopurine, and thioguanine) (reproduced with publisher's permission, from Krynetski and Evans Am J Hum Genet 1998;63:11–16).
Figure 3 .
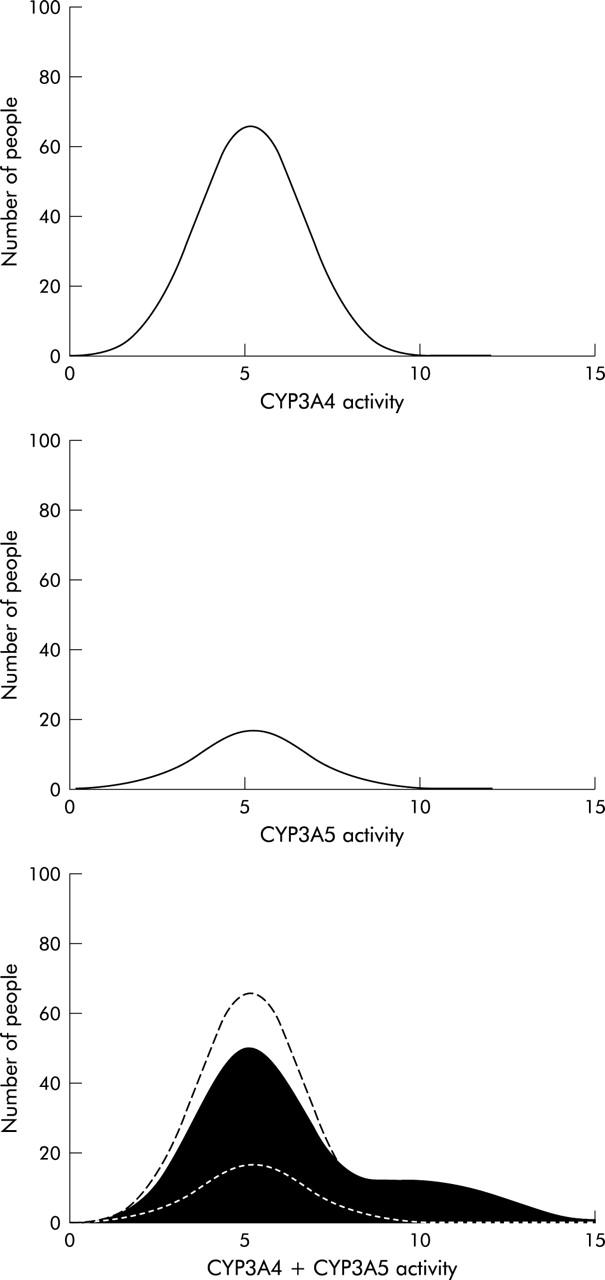
The cytochromes P450 CYP3A4 and CYP3A5 genetic polymorphism. The top panel depicts the distribution of CYP3A4 activity in the white population, assuming that 100% of individuals express CYP3A4, with a 10-fold range of activity in the population. The middle panel depicts CYP3A5 expression, assuming that 25% of the white population express 3A5, with a 10-fold range of activity. The bottom panel depicts the CYP3A4 and CYP3A5 distributions (dashed lines) and the composite distribution for drugs metabolised equally well by both enzymes.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALVING A. S., CARSON P. E., FLANAGAN C. L., ICKES C. E. Enzymatic deficiency in primaquine-sensitive erythrocytes. Science. 1956 Sep 14;124(3220):484–485. doi: 10.1126/science.124.3220.484-a. [DOI] [PubMed] [Google Scholar]
- Aarbakke J., Janka-Schaub G., Elion G. B. Thiopurine biology and pharmacology. Trends Pharmacol Sci. 1997 Jan;18(1):3–7. doi: 10.1016/s0165-6147(96)01007-3. [DOI] [PubMed] [Google Scholar]
- Aithal G. P., Day C. P., Kesteven P. J., Daly A. K. Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet. 1999 Feb 27;353(9154):717–719. doi: 10.1016/S0140-6736(98)04474-2. [DOI] [PubMed] [Google Scholar]
- Ambrosone C. B., Sweeney C., Coles B. F., Thompson P. A., McClure G. Y., Korourian S., Fares M. Y., Stone A., Kadlubar F. F., Hutchins L. F. Polymorphisms in glutathione S-transferases (GSTM1 and GSTT1) and survival after treatment for breast cancer. Cancer Res. 2001 Oct 1;61(19):7130–7135. [PubMed] [Google Scholar]
- Ameyaw M. M., Collie-Duguid E. S., Powrie R. H., Ofori-Adjei D., McLeod H. L. Thiopurine methyltransferase alleles in British and Ghanaian populations. Hum Mol Genet. 1999 Feb;8(2):367–370. doi: 10.1093/hmg/8.2.367. [DOI] [PubMed] [Google Scholar]
- Ameyaw M. M., Regateiro F., Li T., Liu X., Tariq M., Mobarek A., Thornton N., Folayan G. O., Githang'a J., Indalo A. MDR1 pharmacogenetics: frequency of the C3435T mutation in exon 26 is significantly influenced by ethnicity. Pharmacogenetics. 2001 Apr;11(3):217–221. doi: 10.1097/00008571-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Anderer G., Schrappe M., Brechlin A. M., Lauten M., Muti P., Welte K., Stanulla M. Polymorphisms within glutathione S-transferase genes and initial response to glucocorticoids in childhood acute lymphoblastic leukaemia. Pharmacogenetics. 2000 Nov;10(8):715–726. doi: 10.1097/00008571-200011000-00006. [DOI] [PubMed] [Google Scholar]
- Arranz M. J., Munro J., Birkett J., Bolonna A., Mancama D., Sodhi M., Lesch K. P., Meyer J. F., Sham P., Collier D. A. Pharmacogenetic prediction of clozapine response. Lancet. 2000 May 6;355(9215):1615–1616. doi: 10.1016/s0140-6736(00)02221-2. [DOI] [PubMed] [Google Scholar]
- Ballantyne C. M., Herd J. A., Stein E. A., Ferlic L. L., Dunn J. K., Gotto A. M., Jr, Marian A. J. Apolipoprotein E genotypes and response of plasma lipids and progression-regression of coronary atherosclerosis to lipid-lowering drug therapy. J Am Coll Cardiol. 2000 Nov 1;36(5):1572–1578. doi: 10.1016/s0735-1097(00)00918-9. [DOI] [PubMed] [Google Scholar]
- Ban N., Takahashi Y., Takayama T., Kura T., Katahira T., Sakamaki S., Niitsu Y. Transfection of glutathione S-transferase (GST)-pi antisense complementary DNA increases the sensitivity of a colon cancer cell line to adriamycin, cisplatin, melphalan, and etoposide. Cancer Res. 1996 Aug 1;56(15):3577–3582. [PubMed] [Google Scholar]
- Basile V. S., Masellis M., Badri F., Paterson A. D., Meltzer H. Y., Lieberman J. A., Potkin S. G., Macciardi F., Kennedy J. L. Association of the MscI polymorphism of the dopamine D3 receptor gene with tardive dyskinesia in schizophrenia. Neuropsychopharmacology. 1999 Jul;21(1):17–27. doi: 10.1016/S0893-133X(98)00114-6. [DOI] [PubMed] [Google Scholar]
- Black A. J., McLeod H. L., Capell H. A., Powrie R. H., Matowe L. K., Pritchard S. C., Collie-Duguid E. S., Reid D. M. Thiopurine methyltransferase genotype predicts therapy-limiting severe toxicity from azathioprine. Ann Intern Med. 1998 Nov 1;129(9):716–718. doi: 10.7326/0003-4819-129-9-199811010-00007. [DOI] [PubMed] [Google Scholar]
- Black A. J., McLeod H. L., Capell H. A., Powrie R. H., Matowe L. K., Pritchard S. C., Collie-Duguid E. S., Reid D. M. Thiopurine methyltransferase genotype predicts therapy-limiting severe toxicity from azathioprine. Ann Intern Med. 1998 Nov 1;129(9):716–718. doi: 10.7326/0003-4819-129-9-199811010-00007. [DOI] [PubMed] [Google Scholar]
- Blum M., Demierre A., Grant D. M., Heim M., Meyer U. A. Molecular mechanism of slow acetylation of drugs and carcinogens in humans. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5237–5241. doi: 10.1073/pnas.88.12.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Evers R., Kool M., Wijnholds J. A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst. 2000 Aug 16;92(16):1295–1302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- Brandt J. T., Isenhart C. E., Osborne J. M., Ahmed A., Anderson C. L. On the role of platelet Fc gamma RIIa phenotype in heparin-induced thrombocytopenia. Thromb Haemost. 1995 Dec;74(6):1564–1572. [PubMed] [Google Scholar]
- Brinkmann U., Roots I., Eichelbaum M. Pharmacogenetics of the human drug-transporter gene MDR1: impact of polymorphisms on pharmacotherapy. Drug Discov Today. 2001 Aug 15;6(16):835–839. doi: 10.1016/s1359-6446(01)01892-x. [DOI] [PubMed] [Google Scholar]
- Brøsen K., Gram L. F. Clinical significance of the sparteine/debrisoquine oxidation polymorphism. Eur J Clin Pharmacol. 1989;36(6):537–547. doi: 10.1007/BF00637732. [DOI] [PubMed] [Google Scholar]
- Caraco Y., Sheller J., Wood A. J. Pharmacogenetic determination of the effects of codeine and prediction of drug interactions. J Pharmacol Exp Ther. 1996 Sep;278(3):1165–1174. [PubMed] [Google Scholar]
- Chen C. L., Liu Q., Pui C. H., Rivera G. K., Sandlund J. T., Ribeiro R., Evans W. E., Relling M. V. Higher frequency of glutathione S-transferase deletions in black children with acute lymphoblastic leukemia. Blood. 1997 Mar 1;89(5):1701–1707. [PubMed] [Google Scholar]
- Choo E. F., Leake B., Wandel C., Imamura H., Wood A. J., Wilkinson G. R., Kim R. B. Pharmacological inhibition of P-glycoprotein transport enhances the distribution of HIV-1 protease inhibitors into brain and testes. Drug Metab Dispos. 2000 Jun;28(6):655–660. [PubMed] [Google Scholar]
- Cockcroft J. R., Gazis A. G., Cross D. J., Wheatley A., Dewar J., Hall I. P., Noon J. P. Beta(2)-adrenoceptor polymorphism determines vascular reactivity in humans. Hypertension. 2000 Sep;36(3):371–375. doi: 10.1161/01.hyp.36.3.371. [DOI] [PubMed] [Google Scholar]
- Cohen B. M., Ennulat D. J., Centorrino F., Matthysse S., Konieczna H., Chu H. M., Cherkerzian S. Polymorphisms of the dopamine D4 receptor and response to antipsychotic drugs. Psychopharmacology (Berl) 1999 Jan;141(1):6–10. doi: 10.1007/s002130050799. [DOI] [PubMed] [Google Scholar]
- Dahl M. L., Bertilsson L. Genetically variable metabolism of antidepressants and neuroleptic drugs in man. Pharmacogenetics. 1993 Apr;3(2):61–70. doi: 10.1097/00008571-199304000-00001. [DOI] [PubMed] [Google Scholar]
- Dahl M. L., Johansson I., Palmertz M. P., Ingelman-Sundberg M., Sjöqvist F. Analysis of the CYP2D6 gene in relation to debrisoquin and desipramine hydroxylation in a Swedish population. Clin Pharmacol Ther. 1992 Jan;51(1):12–17. doi: 10.1038/clpt.1992.2. [DOI] [PubMed] [Google Scholar]
- Davies S. M., Robison L. L., Buckley J. D., Tjoa T., Woods W. G., Radloff G. A., Ross J. A., Perentesis J. P. Glutathione S-transferase polymorphisms and outcome of chemotherapy in childhood acute myeloid leukemia. J Clin Oncol. 2001 Mar 1;19(5):1279–1287. doi: 10.1200/JCO.2001.19.5.1279. [DOI] [PubMed] [Google Scholar]
- Desmeules J., Gascon M. P., Dayer P., Magistris M. Impact of environmental and genetic factors on codeine analgesia. Eur J Clin Pharmacol. 1991;41(1):23–26. doi: 10.1007/BF00280101. [DOI] [PubMed] [Google Scholar]
- Diasio R. B., Beavers T. L., Carpenter J. T. Familial deficiency of dihydropyrimidine dehydrogenase. Biochemical basis for familial pyrimidinemia and severe 5-fluorouracil-induced toxicity. J Clin Invest. 1988 Jan;81(1):47–51. doi: 10.1172/JCI113308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishy V., Sofowora G. G., Xie H. G., Kim R. B., Byrne D. W., Stein C. M., Wood A. J. The effect of common polymorphisms of the beta2-adrenergic receptor on agonist-mediated vascular desensitization. N Engl J Med. 2001 Oct 4;345(14):1030–1035. doi: 10.1056/NEJMoa010819. [DOI] [PubMed] [Google Scholar]
- Drazen J. M., Yandava C. N., Dubé L., Szczerback N., Hippensteel R., Pillari A., Israel E., Schork N., Silverman E. S., Katz D. A. Pharmacogenetic association between ALOX5 promoter genotype and the response to anti-asthma treatment. Nat Genet. 1999 Jun;22(2):168–170. doi: 10.1038/9680. [DOI] [PubMed] [Google Scholar]
- Drysdale C. M., McGraw D. W., Stack C. B., Stephens J. C., Judson R. S., Nandabalan K., Arnold K., Ruano G., Liggett S. B. Complex promoter and coding region beta 2-adrenergic receptor haplotypes alter receptor expression and predict in vivo responsiveness. Proc Natl Acad Sci U S A. 2000 Sep 12;97(19):10483–10488. doi: 10.1073/pnas.97.19.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS D. A., MANLEY K. A., McKUSICK V. A. Genetic control of isoniazid metabolism in man. Br Med J. 1960 Aug 13;2(5197):485–491. doi: 10.1136/bmj.2.5197.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS D. A., MANLEY K. A., McKUSICK V. A. Genetic control of isoniazid metabolism in man. Br Med J. 1960 Aug 13;2(5197):485–491. doi: 10.1136/bmj.2.5197.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelbaum M., Bertilsson L., Säwe J., Zekorn C. Polymorphic oxidation of sparteine and debrisoquine: related pharmacogenetic entities. Clin Pharmacol Ther. 1982 Feb;31(2):184–186. doi: 10.1038/clpt.1982.29. [DOI] [PubMed] [Google Scholar]
- Eichhammer P., Albus M., Borrmann-Hassenbach M., Schoeler A., Putzhammer A., Frick U., Klein H. E., Rohrmeier T. Association of dopamine D3-receptor gene variants with neuroleptic induced akathisia in schizophrenic patients: a generalization of Steen's study on DRD3 and tardive dyskinesia. Am J Med Genet. 2000 Apr 3;96(2):187–191. doi: 10.1002/(sici)1096-8628(20000403)96:2<187::aid-ajmg13>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Evans W. E., Hon Y. Y., Bomgaars L., Coutre S., Holdsworth M., Janco R., Kalwinsky D., Keller F., Khatib Z., Margolin J. Preponderance of thiopurine S-methyltransferase deficiency and heterozygosity among patients intolerant to mercaptopurine or azathioprine. J Clin Oncol. 2001 Apr 15;19(8):2293–2301. doi: 10.1200/JCO.2001.19.8.2293. [DOI] [PubMed] [Google Scholar]
- Evans W. E., Horner M., Chu Y. Q., Kalwinsky D., Roberts W. M. Altered mercaptopurine metabolism, toxic effects, and dosage requirement in a thiopurine methyltransferase-deficient child with acute lymphocytic leukemia. J Pediatr. 1991 Dec;119(6):985–989. doi: 10.1016/s0022-3476(05)83063-x. [DOI] [PubMed] [Google Scholar]
- Evans W. E., Horner M., Chu Y. Q., Kalwinsky D., Roberts W. M. Altered mercaptopurine metabolism, toxic effects, and dosage requirement in a thiopurine methyltransferase-deficient child with acute lymphocytic leukemia. J Pediatr. 1991 Dec;119(6):985–989. doi: 10.1016/s0022-3476(05)83063-x. [DOI] [PubMed] [Google Scholar]
- Evans W. E., Johnson J. A. Pharmacogenomics: the inherited basis for interindividual differences in drug response. Annu Rev Genomics Hum Genet. 2001;2:9–39. doi: 10.1146/annurev.genom.2.1.9. [DOI] [PubMed] [Google Scholar]
- Evans W. E., Relling M. V. Pharmacogenomics: translating functional genomics into rational therapeutics. Science. 1999 Oct 15;286(5439):487–491. doi: 10.1126/science.286.5439.487. [DOI] [PubMed] [Google Scholar]
- Evans William E., McLeod Howard L. Pharmacogenomics--drug disposition, drug targets, and side effects. N Engl J Med. 2003 Feb 6;348(6):538–549. doi: 10.1056/NEJMra020526. [DOI] [PubMed] [Google Scholar]
- Felix C. A., Walker A. H., Lange B. J., Williams T. M., Winick N. J., Cheung N. K., Lovett B. D., Nowell P. C., Blair I. A., Rebbeck T. R. Association of CYP3A4 genotype with treatment-related leukemia. Proc Natl Acad Sci U S A. 1998 Oct 27;95(22):13176–13181. doi: 10.1073/pnas.95.22.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fojo A. T., Ueda K., Slamon D. J., Poplack D. G., Gottesman M. M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987 Jan;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta T., Ohashi K., Kamata T., Takashima M., Kosuge K., Kawasaki T., Hanai H., Kubota T., Ishizaki T., Kaneko E. Effect of genetic differences in omeprazole metabolism on cure rates for Helicobacter pylori infection and peptic ulcer. Ann Intern Med. 1998 Dec 15;129(12):1027–1030. doi: 10.7326/0003-4819-129-12-199812150-00006. [DOI] [PubMed] [Google Scholar]
- Furuya H., Fernandez-Salguero P., Gregory W., Taber H., Steward A., Gonzalez F. J., Idle J. R. Genetic polymorphism of CYP2C9 and its effect on warfarin maintenance dose requirement in patients undergoing anticoagulation therapy. Pharmacogenetics. 1995 Dec;5(6):389–392. doi: 10.1097/00008571-199512000-00008. [DOI] [PubMed] [Google Scholar]
- Goldstein J. A., Faletto M. B., Romkes-Sparks M., Sullivan T., Kitareewan S., Raucy J. L., Lasker J. M., Ghanayem B. I. Evidence that CYP2C19 is the major (S)-mephenytoin 4'-hydroxylase in humans. Biochemistry. 1994 Feb 22;33(7):1743–1752. doi: 10.1021/bi00173a017. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Fernandez-Salguero P. Diagnostic analysis, clinical importance and molecular basis of dihydropyrimidine dehydrogenase deficiency. Trends Pharmacol Sci. 1995 Oct;16(10):325–327. doi: 10.1016/s0165-6147(00)89065-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Skoda R. C., Kimura S., Umeno M., Zanger U. M., Nebert D. W., Gelboin H. V., Hardwick J. P., Meyer U. A. Characterization of the common genetic defect in humans deficient in debrisoquine metabolism. Nature. 1988 Feb 4;331(6155):442–446. doi: 10.1038/331442a0. [DOI] [PubMed] [Google Scholar]
- Grant D. M., Hughes N. C., Janezic S. A., Goodfellow G. H., Chen H. J., Gaedigk A., Yu V. L., Grewal R. Human acetyltransferase polymorphisms. Mutat Res. 1997 May 12;376(1-2):61–70. doi: 10.1016/s0027-5107(97)00026-2. [DOI] [PubMed] [Google Scholar]
- Gratze G., Fortin J., Labugger R., Binder A., Kotanko P., Timmermann B., Luft F. C., Hoehe M. R., Skrabal F. beta-2 Adrenergic receptor variants affect resting blood pressure and agonist-induced vasodilation in young adult Caucasians. Hypertension. 1999 Jun;33(6):1425–1430. doi: 10.1161/01.hyp.33.6.1425. [DOI] [PubMed] [Google Scholar]
- HUGHES H. B., BIEHL J. P., JONES A. P., SCHMIDT L. H. Metabolism of isoniazid in man as related to the occurrence of peripheral neuritis. Am Rev Tuberc. 1954 Aug;70(2):266–273. doi: 10.1164/art.1954.70.2.266. [DOI] [PubMed] [Google Scholar]
- HUGHES H. B., BIEHL J. P., JONES A. P., SCHMIDT L. H. Metabolism of isoniazid in man as related to the occurrence of peripheral neuritis. Am Rev Tuberc. 1954 Aug;70(2):266–273. doi: 10.1164/art.1954.70.2.266. [DOI] [PubMed] [Google Scholar]
- Hansen T., Echwald S. M., Hansen L., Møller A. M., Almind K., Clausen J. O., Urhammer S. A., Inoue H., Ferrer J., Bryan J. Decreased tolbutamide-stimulated insulin secretion in healthy subjects with sequence variants in the high-affinity sulfonylurea receptor gene. Diabetes. 1998 Apr;47(4):598–605. doi: 10.2337/diabetes.47.4.598. [DOI] [PubMed] [Google Scholar]
- Hayes J. D., Strange R. C. Potential contribution of the glutathione S-transferase supergene family to resistance to oxidative stress. Free Radic Res. 1995 Mar;22(3):193–207. doi: 10.3109/10715769509147539. [DOI] [PubMed] [Google Scholar]
- Hoffmeyer S., Burk O., von Richter O., Arnold H. P., Brockmöller J., Johne A., Cascorbi I., Gerloff T., Roots I., Eichelbaum M. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A. 2000 Mar 28;97(7):3473–3478. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoit B. D., Suresh D. P., Craft L., Walsh R. A., Liggett S. B. beta2-adrenergic receptor polymorphisms at amino acid 16 differentially influence agonist-stimulated blood pressure and peripheral blood flow in normal individuals. Am Heart J. 2000 Mar;139(3):537–542. doi: 10.1016/s0002-8703(00)90099-1. [DOI] [PubMed] [Google Scholar]
- Hon Y. Y., Fessing M. Y., Pui C. H., Relling M. V., Krynetski E. Y., Evans W. E. Polymorphism of the thiopurine S-methyltransferase gene in African-Americans. Hum Mol Genet. 1999 Feb;8(2):371–376. doi: 10.1093/hmg/8.2.371. [DOI] [PubMed] [Google Scholar]
- Hwu H. G., Hong C. J., Lee Y. L., Lee P. C., Lee S. F. Dopamine D4 receptor gene polymorphisms and neuroleptic response in schizophrenia. Biol Psychiatry. 1998 Sep 15;44(6):483–487. doi: 10.1016/s0006-3223(98)00134-6. [DOI] [PubMed] [Google Scholar]
- Höllt Volker. A polymorphism (A118G) in the mu-opioid receptor gene affects the response to morphine-6-glucuronide in humans. Pharmacogenetics. 2002 Jan;12(1):1–2. doi: 10.1097/00008571-200201000-00001. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M., Oscarson M., McLellan R. A. Polymorphic human cytochrome P450 enzymes: an opportunity for individualized drug treatment. Trends Pharmacol Sci. 1999 Aug;20(8):342–349. doi: 10.1016/s0165-6147(99)01363-2. [DOI] [PubMed] [Google Scholar]
- Israel E., Drazen J. M., Liggett S. B., Boushey H. A., Cherniack R. M., Chinchilli V. M., Cooper D. M., Fahy J. V., Fish J. E., Ford J. G. Effect of polymorphism of the beta(2)-adrenergic receptor on response to regular use of albuterol in asthma. Int Arch Allergy Immunol. 2001 Jan-Mar;124(1-3):183–186. doi: 10.1159/000053705. [DOI] [PubMed] [Google Scholar]
- Jacobsen P., Rossing K., Rossing P., Tarnow L., Mallet C., Poirier O., Cambien F., Parving H. H. Angiotensin converting enzyme gene polymorphism and ACE inhibition in diabetic nephropathy. Kidney Int. 1998 Apr;53(4):1002–1006. doi: 10.1111/j.1523-1755.1998.00847.x. [DOI] [PubMed] [Google Scholar]
- Jia H., Hingorani A. D., Sharma P., Hopper R., Dickerson C., Trutwein D., Lloyd D. D., Brown M. J. Association of the G(s)alpha gene with essential hypertension and response to beta-blockade. Hypertension. 1999 Jul;34(1):8–14. doi: 10.1161/01.hyp.34.1.8. [DOI] [PubMed] [Google Scholar]
- Johansson I., Lundqvist E., Bertilsson L., Dahl M. L., Sjöqvist F., Ingelman-Sundberg M. Inherited amplification of an active gene in the cytochrome P450 CYP2D locus as a cause of ultrarapid metabolism of debrisoquine. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11825–11829. doi: 10.1073/pnas.90.24.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joober R., Benkelfat C., Brisebois K., Toulouse A., Turecki G., Lal S., Bloom D., Labelle A., Lalonde P., Fortin D. T102C polymorphism in the 5HT2A gene and schizophrenia: relation to phenotype and drug response variability. J Psychiatry Neurosci. 1999 Mar;24(2):141–146. [PMC free article] [PubMed] [Google Scholar]
- Kaiser R., Könneker M., Henneken M., Dettling M., Müller-Oerlinghausen B., Roots I., Brockmöller J. Dopamine D4 receptor 48-bp repeat polymorphism: no association with response to antipsychotic treatment, but association with catatonic schizophrenia. Mol Psychiatry. 2000 Jul;5(4):418–424. doi: 10.1038/sj.mp.4000729. [DOI] [PubMed] [Google Scholar]
- Kapitany T., Meszaros K., Lenzinger E., Schindler S. D., Barnas C., Fuchs K., Sieghart W., Aschauer H. N., Kasper S. Genetic polymorphisms for drug metabolism (CYP2D6) and tardive dyskinesia in schizophrenia. Schizophr Res. 1998 Jul 27;32(2):101–106. doi: 10.1016/s0920-9964(98)00038-3. [DOI] [PubMed] [Google Scholar]
- Ketterer B. Protective role of glutathione and glutathione transferases in mutagenesis and carcinogenesis. Mutat Res. 1988 Dec;202(2):343–361. doi: 10.1016/0027-5107(88)90197-2. [DOI] [PubMed] [Google Scholar]
- Kim D. K., Lim S. W., Lee S., Sohn S. E., Kim S., Hahn C. G., Carroll B. J. Serotonin transporter gene polymorphism and antidepressant response. Neuroreport. 2000 Jan 17;11(1):215–219. doi: 10.1097/00001756-200001170-00042. [DOI] [PubMed] [Google Scholar]
- Kim R. B., Leake B. F., Choo E. F., Dresser G. K., Kubba S. V., Schwarz U. I., Taylor A., Xie H. G., McKinsey J., Zhou S. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther. 2001 Aug;70(2):189–199. doi: 10.1067/mcp.2001.117412. [DOI] [PubMed] [Google Scholar]
- Kohno M., Yokokawa K., Minami M., Kano H., Yasunari K., Hanehira T., Yoshikawa J. Association between angiotensin-converting enzyme gene polymorphisms and regression of left ventricular hypertrophy in patients treated with angiotensin-converting enzyme inhibitors. Am J Med. 1999 May;106(5):544–549. doi: 10.1016/s0002-9343(99)00067-4. [DOI] [PubMed] [Google Scholar]
- Krynetski E. Y., Evans W. E. Pharmacogenetics of cancer therapy: getting personal. Am J Hum Genet. 1998 Jul;63(1):11–16. doi: 10.1086/301941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krynetski E. Y., Evans W. E. Pharmacogenetics of cancer therapy: getting personal. Am J Hum Genet. 1998 Jul;63(1):11–16. doi: 10.1086/301941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krynetski E. Y., Schuetz J. D., Galpin A. J., Pui C. H., Relling M. V., Evans W. E. A single point mutation leading to loss of catalytic activity in human thiopurine S-methyltransferase. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):949–953. doi: 10.1073/pnas.92.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T., Chiba K. Frequencies of thiopurine S-methyltransferase mutant alleles (TPMT*2, *3A, *3B and *3C) in 151 healthy Japanese subjects and the inheritance of TPMT*3C in the family of a propositus. Br J Clin Pharmacol. 2001 May;51(5):475–477. doi: 10.1046/j.1365-2125.2001.01371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl P., Zhang J., Lin Y., Lamba J., Assem M., Schuetz J., Watkins P. B., Daly A., Wrighton S. A., Hall S. D. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001 Apr;27(4):383–391. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- Kuehl P., Zhang J., Lin Y., Lamba J., Assem M., Schuetz J., Watkins P. B., Daly A., Wrighton S. A., Hall S. D. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001 Apr;27(4):383–391. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- Kumagai K., Hiyama K., Ishioka S., Sato H., Yamanishi Y., McLeod H. L., Konishi F., Maeda H., Yamakido M. Allelotype frequency of the thiopurine methyltransferase (TPMT) gene in Japanese. Pharmacogenetics. 2001 Apr;11(3):275–278. doi: 10.1097/00008571-200104000-00012. [DOI] [PubMed] [Google Scholar]
- Lee J. T., Kroemer H. K., Silberstein D. J., Funck-Brentano C., Lineberry M. D., Wood A. J., Roden D. M., Woosley R. L. The role of genetically determined polymorphic drug metabolism in the beta-blockade produced by propafenone. N Engl J Med. 1990 Jun 21;322(25):1764–1768. doi: 10.1056/NEJM199006213222502. [DOI] [PubMed] [Google Scholar]
- Lennard L. Clinical implications of thiopurine methyltransferase--optimization of drug dosage and potential drug interactions. Ther Drug Monit. 1998 Oct;20(5):527–531. doi: 10.1097/00007691-199810000-00014. [DOI] [PubMed] [Google Scholar]
- Lennard L., Van Loon J. A., Lilleyman J. S., Weinshilboum R. M. Thiopurine pharmacogenetics in leukemia: correlation of erythrocyte thiopurine methyltransferase activity and 6-thioguanine nucleotide concentrations. Clin Pharmacol Ther. 1987 Jan;41(1):18–25. doi: 10.1038/clpt.1987.4. [DOI] [PubMed] [Google Scholar]
- Lennard M. S., Tucker G. T., Silas J. H., Freestone S., Ramsay L. E., Woods H. F. Differential stereoselective metabolism of metoprolol in extensive and poor debrisoquin metabolizers. Clin Pharmacol Ther. 1983 Dec;34(6):732–737. doi: 10.1038/clpt.1983.242. [DOI] [PubMed] [Google Scholar]
- Liggett S. B. beta(2)-adrenergic receptor pharmacogenetics. Am J Respir Crit Care Med. 2000 Mar;161(3 Pt 2):S197–S201. doi: 10.1164/ajrccm.161.supplement_2.a1q4-10. [DOI] [PubMed] [Google Scholar]
- Lima J. J., Thomason D. B., Mohamed M. H., Eberle L. V., Self T. H., Johnson J. A. Impact of genetic polymorphisms of the beta2-adrenergic receptor on albuterol bronchodilator pharmacodynamics. Clin Pharmacol Ther. 1999 May;65(5):519–525. doi: 10.1016/S0009-9236(99)70071-8. [DOI] [PubMed] [Google Scholar]
- Mahgoub A., Idle J. R., Dring L. G., Lancaster R., Smith R. L. Polymorphic hydroxylation of Debrisoquine in man. Lancet. 1977 Sep 17;2(8038):584–586. doi: 10.1016/s0140-6736(77)91430-1. [DOI] [PubMed] [Google Scholar]
- Marian A. J., Safavi F., Ferlic L., Dunn J. K., Gotto A. M., Ballantyne C. M. Interactions between angiotensin-I converting enzyme insertion/deletion polymorphism and response of plasma lipids and coronary atherosclerosis to treatment with fluvastatin: the lipoprotein and coronary atherosclerosis study. J Am Coll Cardiol. 2000 Jan;35(1):89–95. doi: 10.1016/s0735-1097(99)00535-5. [DOI] [PubMed] [Google Scholar]
- Martinez F. D., Graves P. E., Baldini M., Solomon S., Erickson R. Association between genetic polymorphisms of the beta2-adrenoceptor and response to albuterol in children with and without a history of wheezing. J Clin Invest. 1997 Dec 15;100(12):3184–3188. doi: 10.1172/JCI119874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masellis M., Basile V., Meltzer H. Y., Lieberman J. A., Sevy S., Macciardi F. M., Cola P., Howard A., Badri F., Nöthen M. M. Serotonin subtype 2 receptor genes and clinical response to clozapine in schizophrenia patients. Neuropsychopharmacology. 1998 Aug;19(2):123–132. doi: 10.1016/S0893-133X(98)00007-4. [DOI] [PubMed] [Google Scholar]
- McCarthy T. V., Quane K. A., Lynch P. J. Ryanodine receptor mutations in malignant hyperthermia and central core disease. Hum Mutat. 2000;15(5):410–417. doi: 10.1002/(SICI)1098-1004(200005)15:5<410::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- McLeod H. L., Evans W. E. Pharmacogenomics: unlocking the human genome for better drug therapy. Annu Rev Pharmacol Toxicol. 2001;41:101–121. doi: 10.1146/annurev.pharmtox.41.1.101. [DOI] [PubMed] [Google Scholar]
- McLeod H. L., Krynetski E. Y., Relling M. V., Evans W. E. Genetic polymorphism of thiopurine methyltransferase and its clinical relevance for childhood acute lymphoblastic leukemia. Leukemia. 2000 Apr;14(4):567–572. doi: 10.1038/sj.leu.2401723. [DOI] [PubMed] [Google Scholar]
- McLeod H. L. Pharmacokinetic differences between ethnic groups. Lancet. 2002 Jan 5;359(9300):78–78. doi: 10.1016/s0140-6736(02)07296-3. [DOI] [PubMed] [Google Scholar]
- McLeod H. L., Pritchard S. C., Githang'a J., Indalo A., Ameyaw M. M., Powrie R. H., Booth L., Collie-Duguid E. S. Ethnic differences in thiopurine methyltransferase pharmacogenetics: evidence for allele specificity in Caucasian and Kenyan individuals. Pharmacogenetics. 1999 Dec;9(6):773–776. doi: 10.1097/00008571-199912000-00012. [DOI] [PubMed] [Google Scholar]
- McLeod H. L., Relling M. V., Liu Q., Pui C. H., Evans W. E. Polymorphic thiopurine methyltransferase in erythrocytes is indicative of activity in leukemic blasts from children with acute lymphoblastic leukemia. Blood. 1995 Apr 1;85(7):1897–1902. [PubMed] [Google Scholar]
- Meyer U. A. Pharmacogenetics and adverse drug reactions. Lancet. 2000 Nov 11;356(9242):1667–1671. doi: 10.1016/S0140-6736(00)03167-6. [DOI] [PubMed] [Google Scholar]
- Meyer U. A. Pharmacogenetics: the slow, the rapid, and the ultrarapid. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):1983–1984. doi: 10.1073/pnas.91.6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H., Uetrecht J., Cribb A. E., Miller M. A., Zahid N., Hill J., Josephy P. D., Grant D. M., Spielberg S. P. In vitro formation, disposition and toxicity of N-acetoxy-sulfamethoxazole, a potential mediator of sulfamethoxazole toxicity. J Pharmacol Exp Ther. 1995 Sep;274(3):1099–1104. [PubMed] [Google Scholar]
- Ohmichi N., Iwai N., Uchida Y., Shichiri G., Nakamura Y., Kinoshita M. Relationship between the response to the angiotensin converting enzyme inhibitor imidapril and the angiotensin converting enzyme genotype. Am J Hypertens. 1997 Aug;10(8):951–955. doi: 10.1016/s0895-7061(97)00121-0. [DOI] [PubMed] [Google Scholar]
- Okamura A., Ohishi M., Rakugi H., Katsuya T., Yanagitani Y., Takiuchi S., Taniyama Y., Moriguchi K., Ito H., Higashino Y. Pharmacogenetic analysis of the effect of angiotensin-converting enzyme inhibitor on restenosis after percutaneous transluminal coronary angioplasty. Angiology. 1999 Oct;50(10):811–822. doi: 10.1177/000331979905001005. [DOI] [PubMed] [Google Scholar]
- Ongphiphadhanakul B., Chanprasertyothin S., Payatikul P., Tung S. S., Piaseu N., Chailurkit L., Chansirikarn S., Puavilai G., Rajatanavin R. Oestrogen-receptor-alpha gene polymorphism affects response in bone mineral density to oestrogen in post-menopausal women. Clin Endocrinol (Oxf) 2000 May;52(5):581–585. doi: 10.1046/j.1365-2265.2000.00979.x. [DOI] [PubMed] [Google Scholar]
- Otterness D., Szumlanski C., Lennard L., Klemetsdal B., Aarbakke J., Park-Hah J. O., Iven H., Schmiegelow K., Branum E., O'Brien J. Human thiopurine methyltransferase pharmacogenetics: gene sequence polymorphisms. Clin Pharmacol Ther. 1997 Jul;62(1):60–73. doi: 10.1016/S0009-9236(97)90152-1. [DOI] [PubMed] [Google Scholar]
- Penno G., Chaturvedi N., Talmud P. J., Cotroneo P., Manto A., Nannipieri M., Luong L. A., Fuller J. H. Effect of angiotensin-converting enzyme (ACE) gene polymorphism on progression of renal disease and the influence of ACE inhibition in IDDM patients: findings from the EUCLID Randomized Controlled Trial. EURODIAB Controlled Trial of Lisinopril in IDDM. Diabetes. 1998 Sep;47(9):1507–1511. doi: 10.2337/diabetes.47.9.1507. [DOI] [PubMed] [Google Scholar]
- Perna A., Ruggenenti P., Testa A., Spoto B., Benini R., Misefari V., Remuzzi G., Zoccali C. ACE genotype and ACE inhibitors induced renoprotection in chronic proteinuric nephropathies1. Kidney Int. 2000 Jan;57(1):274–281. doi: 10.1046/j.1523-1755.2000.00818.x. [DOI] [PubMed] [Google Scholar]
- Phillips K. A., Veenstra D. L., Oren E., Lee J. K., Sadee W. Potential role of pharmacogenomics in reducing adverse drug reactions: a systematic review. JAMA. 2001 Nov 14;286(18):2270–2279. doi: 10.1001/jama.286.18.2270. [DOI] [PubMed] [Google Scholar]
- Poulsen L., Brøsen K., Arendt-Nielsen L., Gram L. F., Elbaek K., Sindrup S. H. Codeine and morphine in extensive and poor metabolizers of sparteine: pharmacokinetics, analgesic effect and side effects. Eur J Clin Pharmacol. 1996;51(3-4):289–295. doi: 10.1007/s002280050200. [DOI] [PubMed] [Google Scholar]
- Prasad A., Narayanan S., Husain S., Padder F., Waclawiw M., Epstein N., Quyyumi A. A. Insertion-deletion polymorphism of the ACE gene modulates reversibility of endothelial dysfunction with ACE inhibition. Circulation. 2000 Jul 4;102(1):35–41. doi: 10.1161/01.cir.102.1.35. [DOI] [PubMed] [Google Scholar]
- Rao V. V., Dahlheimer J. L., Bardgett M. E., Snyder A. Z., Finch R. A., Sartorelli A. C., Piwnica-Worms D. Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drug-permeability barrier. Proc Natl Acad Sci U S A. 1999 Mar 30;96(7):3900–3905. doi: 10.1073/pnas.96.7.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbeck T. R., Jaffe J. M., Walker A. H., Wein A. J., Malkowicz S. B. Modification of clinical presentation of prostate tumors by a novel genetic variant in CYP3A4. J Natl Cancer Inst. 1998 Aug 19;90(16):1225–1229. doi: 10.1093/jnci/90.16.1225. [DOI] [PubMed] [Google Scholar]
- Relling M. V., Hancock M. L., Boyett J. M., Pui C. H., Evans W. E. Prognostic importance of 6-mercaptopurine dose intensity in acute lymphoblastic leukemia. Blood. 1999 May 1;93(9):2817–2823. [PubMed] [Google Scholar]
- Relling M. V., Hancock M. L., Rivera G. K., Sandlund J. T., Ribeiro R. C., Krynetski E. Y., Pui C. H., Evans W. E. Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J Natl Cancer Inst. 1999 Dec 1;91(23):2001–2008. doi: 10.1093/jnci/91.23.2001. [DOI] [PubMed] [Google Scholar]
- Relling M. V., Rubnitz J. E., Rivera G. K., Boyett J. M., Hancock M. L., Felix C. A., Kun L. E., Walter A. W., Evans W. E., Pui C. H. High incidence of secondary brain tumours after radiotherapy and antimetabolites. Lancet. 1999 Jul 3;354(9172):34–39. doi: 10.1016/S0140-6736(98)11079-6. [DOI] [PubMed] [Google Scholar]
- Roses A. D. Pharmacogenetics. Hum Mol Genet. 2001 Oct 1;10(20):2261–2267. doi: 10.1093/hmg/10.20.2261. [DOI] [PubMed] [Google Scholar]
- Sachidanandam R., Weissman D., Schmidt S. C., Kakol J. M., Stein L. D., Marth G., Sherry S., Mullikin J. C., Mortimore B. J., Willey D. L. A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms. Nature. 2001 Feb 15;409(6822):928–933. doi: 10.1038/35057149. [DOI] [PubMed] [Google Scholar]
- Sakaeda T., Nakamura T., Horinouchi M., Kakumoto M., Ohmoto N., Sakai T., Morita Y., Tamura T., Aoyama N., Hirai M. MDR1 genotype-related pharmacokinetics of digoxin after single oral administration in healthy Japanese subjects. Pharm Res. 2001 Oct;18(10):1400–1404. doi: 10.1023/a:1012244520615. [DOI] [PubMed] [Google Scholar]
- Sallee F. R., DeVane C. L., Ferrell R. E. Fluoxetine-related death in a child with cytochrome P-450 2D6 genetic deficiency. J Child Adolesc Psychopharmacol. 2000 Spring;10(1):27–34. doi: 10.1089/cap.2000.10.27. [DOI] [PubMed] [Google Scholar]
- Sasaki M., Oki T., Iuchi A., Tabata T., Yamada H., Manabe K., Fukuda K., Abe M., Ito S. Relationship between the angiotensin converting enzyme gene polymorphism and the effects of enalapril on left ventricular hypertrophy and impaired diastolic filling in essential hypertension: M-mode and pulsed Doppler echocardiographic studies. J Hypertens. 1996 Dec;14(12):1403–1408. doi: 10.1097/00004872-199612000-00003. [DOI] [PubMed] [Google Scholar]
- Sata F., Sapone A., Elizondo G., Stocker P., Miller V. P., Zheng W., Raunio H., Crespi C. L., Gonzalez F. J. CYP3A4 allelic variants with amino acid substitutions in exons 7 and 12: evidence for an allelic variant with altered catalytic activity. Clin Pharmacol Ther. 2000 Jan;67(1):48–56. doi: 10.1067/mcp.2000.104391. [DOI] [PubMed] [Google Scholar]
- Sata F., Sapone A., Elizondo G., Stocker P., Miller V. P., Zheng W., Raunio H., Crespi C. L., Gonzalez F. J. CYP3A4 allelic variants with amino acid substitutions in exons 7 and 12: evidence for an allelic variant with altered catalytic activity. Clin Pharmacol Ther. 2000 Jan;67(1):48–56. doi: 10.1067/mcp.2000.104391. [DOI] [PubMed] [Google Scholar]
- Schaeffeler E., Eichelbaum M., Brinkmann U., Penger A., Asante-Poku S., Zanger U. M., Schwab M. Frequency of C3435T polymorphism of MDR1 gene in African people. Lancet. 2001 Aug 4;358(9279):383–384. doi: 10.1016/S0140-6736(01)05579-9. [DOI] [PubMed] [Google Scholar]
- Scharfetter J., Chaudhry H. R., Hornik K., Fuchs K., Sieghart W., Kasper S., Aschauer H. N. Dopamine D3 receptor gene polymorphism and response to clozapine in schizophrenic Pakastani patients. Eur Neuropsychopharmacol. 1999 Dec;10(1):17–20. doi: 10.1016/s0924-977x(99)00044-9. [DOI] [PubMed] [Google Scholar]
- Schinkel A. H., Wagenaar E., Mol C. A., van Deemter L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996 Jun 1;97(11):2517–2524. doi: 10.1172/JCI118699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütz E., Gummert J., Mohr F., Oellerich M. Azathioprine-induced myelosuppression in thiopurine methyltransferase deficient heart transplant recipient. Lancet. 1993 Feb 13;341(8842):436–436. doi: 10.1016/0140-6736(93)93028-y. [DOI] [PubMed] [Google Scholar]
- Seidegård J., Ekström G. The role of human glutathione transferases and epoxide hydrolases in the metabolism of xenobiotics. Environ Health Perspect. 1997 Jun;105 (Suppl 4):791–799. doi: 10.1289/ehp.105-1470052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindrup S. H., Brøsen K. The pharmacogenetics of codeine hypoalgesia. Pharmacogenetics. 1995 Dec;5(6):335–346. doi: 10.1097/00008571-199512000-00001. [DOI] [PubMed] [Google Scholar]
- Sindrup S. H., Poulsen L., Brøsen K., Arendt-Nielsen L., Gram L. F. Are poor metabolisers of sparteine/debrisoquine less pain tolerant than extensive metabolisers? Pain. 1993 Jun;53(3):335–339. doi: 10.1016/0304-3959(93)90229-I. [DOI] [PubMed] [Google Scholar]
- Smeraldi E., Zanardi R., Benedetti F., Di Bella D., Perez J., Catalano M. Polymorphism within the promoter of the serotonin transporter gene and antidepressant efficacy of fluvoxamine. Mol Psychiatry. 1998 Nov;3(6):508–511. doi: 10.1038/sj.mp.4000425. [DOI] [PubMed] [Google Scholar]
- Spielberg S. P. N-acetyltransferases: pharmacogenetics and clinical consequences of polymorphic drug metabolism. J Pharmacokinet Biopharm. 1996 Oct;24(5):509–519. doi: 10.1007/BF02353477. [DOI] [PubMed] [Google Scholar]
- Stanulla M., Schrappe M., Brechlin A. M., Zimmermann M., Welte K. Polymorphisms within glutathione S-transferase genes (GSTM1, GSTT1, GSTP1) and risk of relapse in childhood B-cell precursor acute lymphoblastic leukemia: a case-control study. Blood. 2000 Feb 15;95(4):1222–1228. [PubMed] [Google Scholar]
- Stavroulakis G. A., Makris T. K., Krespi P. G., Hatzizacharias A. N., Gialeraki A. E., Anastasiadis G., Triposkiadis P., Kyriakidis M. Predicting response to chronic antihypertensive treatment with fosinopril: the role of angiotensin-converting enzyme gene polymorphism. Cardiovasc Drugs Ther. 2000 Aug;14(4):427–432. doi: 10.1023/a:1007820401377. [DOI] [PubMed] [Google Scholar]
- Steward D. J., Haining R. L., Henne K. R., Davis G., Rushmore T. H., Trager W. F., Rettie A. E. Genetic association between sensitivity to warfarin and expression of CYP2C9*3. Pharmacogenetics. 1997 Oct;7(5):361–367. doi: 10.1097/00008571-199710000-00004. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Mihara K., Kondo T., Tanaka O., Nagashima U., Otani K., Kaneko S. The relationship between dopamine D2 receptor polymorphism at the Taq1 A locus and therapeutic response to nemonapride, a selective dopamine antagonist, in schizophrenic patients. Pharmacogenetics. 2000 Jun;10(4):335–341. doi: 10.1097/00008571-200006000-00007. [DOI] [PubMed] [Google Scholar]
- Sweeney C., McClure G. Y., Fares M. Y., Stone A., Coles B. F., Thompson P. A., Korourian S., Hutchins L. F., Kadlubar F. F., Ambrosone C. B. Association between survival after treatment for breast cancer and glutathione S-transferase P1 Ile105Val polymorphism. Cancer Res. 2000 Oct 15;60(20):5621–5624. [PubMed] [Google Scholar]
- Tai H. L., Fessing M. Y., Bonten E. J., Yanishevsky Y., d'Azzo A., Krynetski E. Y., Evans W. E. Enhanced proteasomal degradation of mutant human thiopurine S-methyltransferase (TPMT) in mammalian cells: mechanism for TPMT protein deficiency inherited by TPMT*2, TPMT*3A, TPMT*3B or TPMT*3C. Pharmacogenetics. 1999 Oct;9(5):641–650. doi: 10.1097/01213011-199910000-00011. [DOI] [PubMed] [Google Scholar]
- Tai H. L., Krynetski E. Y., Schuetz E. G., Yanishevski Y., Evans W. E. Enhanced proteolysis of thiopurine S-methyltransferase (TPMT) encoded by mutant alleles in humans (TPMT*3A, TPMT*2): mechanisms for the genetic polymorphism of TPMT activity. Proc Natl Acad Sci U S A. 1997 Jun 10;94(12):6444–6449. doi: 10.1073/pnas.94.12.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai H. L., Krynetski E. Y., Yates C. R., Loennechen T., Fessing M. Y., Krynetskaia N. F., Evans W. E. Thiopurine S-methyltransferase deficiency: two nucleotide transitions define the most prevalent mutant allele associated with loss of catalytic activity in Caucasians. Am J Hum Genet. 1996 Apr;58(4):694–702. [PMC free article] [PubMed] [Google Scholar]
- Tan S., Hall I. P., Dewar J., Dow E., Lipworth B. Association between beta 2-adrenoceptor polymorphism and susceptibility to bronchodilator desensitisation in moderately severe stable asthmatics. Lancet. 1997 Oct 4;350(9083):995–999. doi: 10.1016/S0140-6736(97)03211-X. [DOI] [PubMed] [Google Scholar]
- Taube J., Halsall D., Baglin T. Influence of cytochrome P-450 CYP2C9 polymorphisms on warfarin sensitivity and risk of over-anticoagulation in patients on long-term treatment. Blood. 2000 Sep 1;96(5):1816–1819. [PubMed] [Google Scholar]
- Tew K. D. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994 Aug 15;54(16):4313–4320. [PubMed] [Google Scholar]
- Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., Willingham M. C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyndale R. F., Droll K. P., Sellers E. M. Genetically deficient CYP2D6 metabolism provides protection against oral opiate dependence. Pharmacogenetics. 1997 Oct;7(5):375–379. doi: 10.1097/00008571-199710000-00006. [DOI] [PubMed] [Google Scholar]
- Whale R., Quested D. J., Laver D., Harrison P. J., Cowen P. J. Serotonin transporter (5-HTT) promoter genotype may influence the prolactin response to clomipramine. Psychopharmacology (Berl) 2000 May;150(1):120–122. doi: 10.1007/s002130000432. [DOI] [PubMed] [Google Scholar]
- Wormhoudt L. W., Commandeur J. N., Vermeulen N. P. Genetic polymorphisms of human N-acetyltransferase, cytochrome P450, glutathione-S-transferase, and epoxide hydrolase enzymes: relevance to xenobiotic metabolism and toxicity. Crit Rev Toxicol. 1999 Jan;29(1):59–124. doi: 10.1080/10408449991349186. [DOI] [PubMed] [Google Scholar]
- Yates C. R., Krynetski E. Y., Loennechen T., Fessing M. Y., Tai H. L., Pui C. H., Relling M. V., Evans W. E. Molecular diagnosis of thiopurine S-methyltransferase deficiency: genetic basis for azathioprine and mercaptopurine intolerance. Ann Intern Med. 1997 Apr 15;126(8):608–614. doi: 10.7326/0003-4819-126-8-199704150-00003. [DOI] [PubMed] [Google Scholar]
- Yu Y. W., Tsai S. J., Lin C. H., Hsu C. P., Yang K. H., Hong C. J. Serotonin-6 receptor variant (C267T) and clinical response to clozapine. Neuroreport. 1999 Apr 26;10(6):1231–1233. doi: 10.1097/00001756-199904260-00014. [DOI] [PubMed] [Google Scholar]
- Zhou H. H., Koshakji R. P., Silberstein D. J., Wilkinson G. R., Wood A. J. Altered sensitivity to and clearance of propranolol in men of Chinese descent as compared with American whites. N Engl J Med. 1989 Mar 2;320(9):565–570. doi: 10.1056/NEJM198903023200905. [DOI] [PubMed] [Google Scholar]
- Zill P., Baghai T. C., Zwanzger P., Schüle C., Minov C., Riedel M., Neumeier K., Rupprecht R., Bondy B. Evidence for an association between a G-protein beta3-gene variant with depression and response to antidepressant treatment. Neuroreport. 2000 Jun 26;11(9):1893–1897. doi: 10.1097/00001756-200006260-00018. [DOI] [PubMed] [Google Scholar]