Abstract
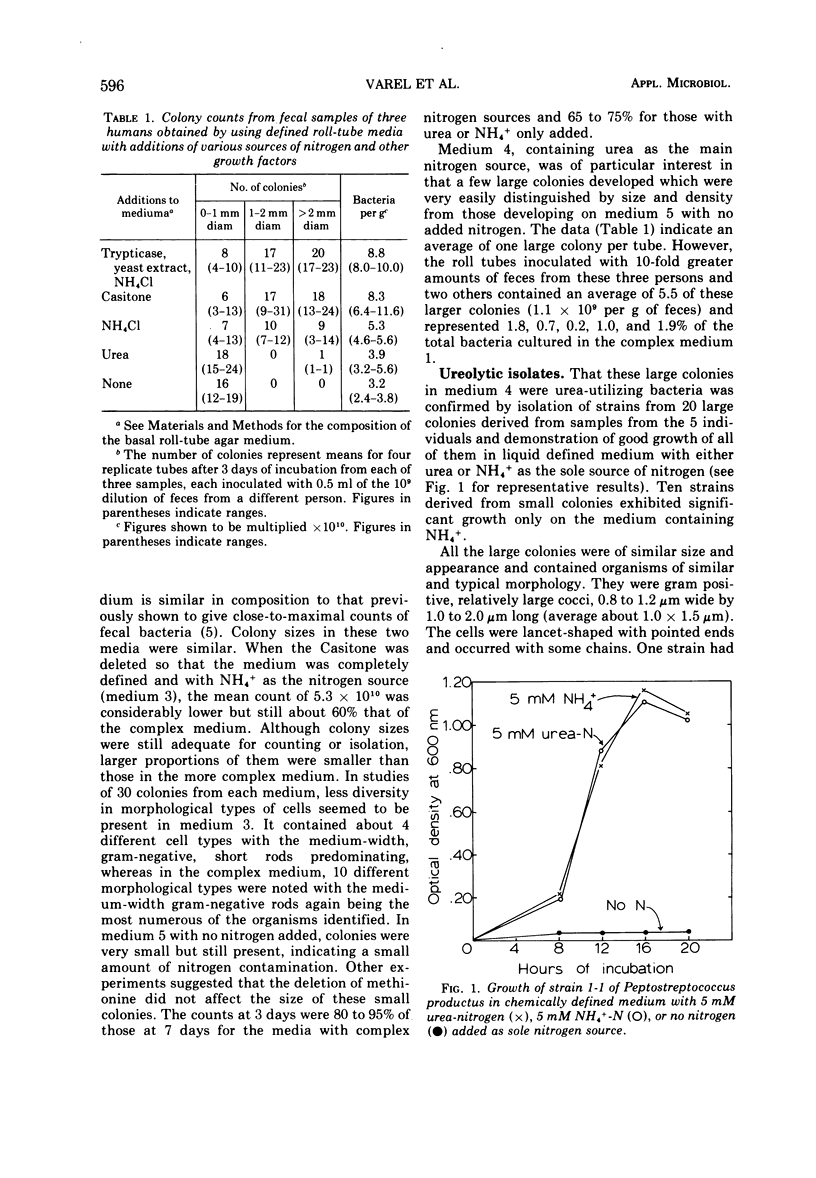
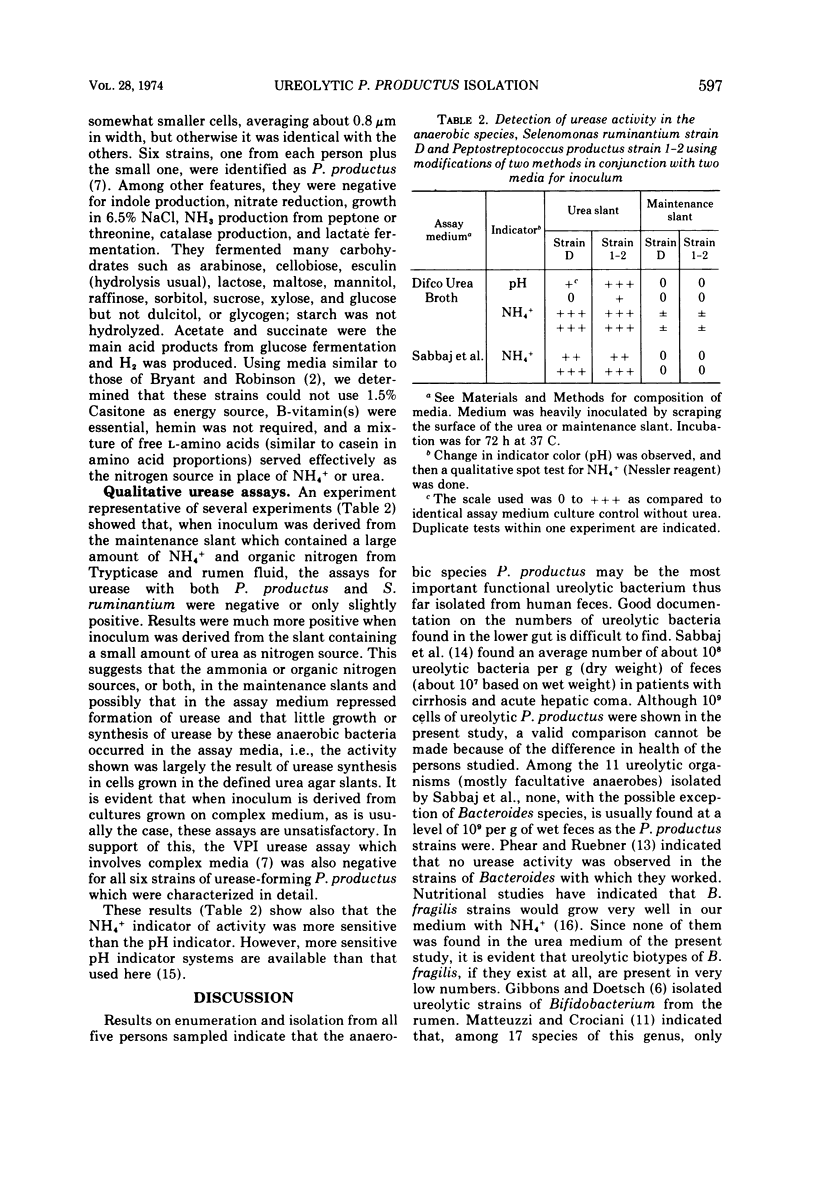
Colony counts of fecal samples from three persons, obtained by using a chemically defined anaerobic roll-tube medium (containing glucose, maltose, glycerol, minerals, hemin, B-vitamins, methionine, volatile fatty acids, sulfide, bicarbonate, agar, carbon dioxide (gas phase), and 1 mM NH4+ as main nitrogen source), averaged 60% of the 8.8 × 1010 bacteria per g obtained when 0.2% Trypticase and 0.05% yeast extract were added to the otherwise identical medium. When 0.2% vitamin-free Casitone replaced Trypticase and yeast extract, counts were 94% those of the more complex medium. When urea-nitrogen was added to the defined medium as the main nitrogen source in place of NH4+, counts of relatively large colonies averaged 1.0 × 109 per g of feces from five persons—1.1% of counts on the medium containing Trypticase and yeast extract. All of the organisms from the large colonies in the urea roll tubes were morphologically similar, and all six representative strains isolated were identified as urease-forming Peptostreptococcus productus, a species not previously known to produce urease. Ureolytic strains of Selenomonas ruminantium and P. productus were negative for urease activity in three assay media when inocula were from media containing complex nitrogen sources. The study documents that P. productus is the most numerous ureolytic species so far found in human feces and suggests that NH4+ and more complex organic nitrogen sources strongly repress its production of urease. The study also indicates the efficacy of chemically defined media for direct selective isolation of nutritional groups of bacteria from feces.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRYANT M. P., ROBINSON I. M. Some nutritional characteristics of predominant culturable ruminal bacteria. J Bacteriol. 1962 Oct;84:605–614. doi: 10.1128/jb.84.4.605-614.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller C., Crabill M. R., Bryant M. P. Anaerobic roll tube media for nonselective enumeration and isolation of bacteria in human feces. Appl Microbiol. 1971 Oct;22(4):522–529. doi: 10.1128/am.22.4.522-529.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS R. J., DOETSCH R. N. Physiological study of an obligately anaerobic ureolytic bacterium. J Bacteriol. 1959 Apr;77(4):417–428. doi: 10.1128/jb.77.4.417-428.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John A., Isaacson H. R., Bryant M. P. Isolation and characteristics of a ureolytic strain of Selenomonas ruminatium. J Dairy Sci. 1974 Sep;57(9):1003–1014. doi: 10.3168/jds.s0022-0302(74)85001-0. [DOI] [PubMed] [Google Scholar]
- Krämer J., Kaltwasser H., Schlegel H. G. Die Bedutung der Ureaserepression für die taxonomische Klassifizierung von Bakterine. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. 1967;121(4):414–423. [PubMed] [Google Scholar]
- Matteuzzi D., Crociani F. Urease production and DNA-homology in the species bifidobacterium suis. Arch Mikrobiol. 1973 Dec 4;94(1):93–95. doi: 10.1007/BF00414081. [DOI] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974 May;27(5):961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHEAR E. A., RUEBNER B. The in vitro production of ammonium and amines by intestinal bacteria in relation to nitrogen toxicity as a factor in hepatic coma. Br J Exp Pathol. 1956 Jun;37(3):253–262. [PMC free article] [PubMed] [Google Scholar]
- Sabbaj J., Sutter V. L., Finegold S. M. Urease and deaminase activities of fecal bacteria in hepatic coma. Antimicrob Agents Chemother (Bethesda) 1970;10:181–185. [PubMed] [Google Scholar]
- Stewart D. J. The urease activity of fluorescent pseudomonads. J Gen Microbiol. 1965 Nov;41(2):169–174. doi: 10.1099/00221287-41-2-169. [DOI] [PubMed] [Google Scholar]
- Varel V. H., Bryant M. P. Nutritional features of Bacteroides fragilis subsp. fragilis. Appl Microbiol. 1974 Aug;28(2):251–257. doi: 10.1128/am.28.2.251-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visek W. J. Effects of urea hydrolysis on cell life-span and metabolism. Fed Proc. 1972 May-Jun;31(3):1178–1193. [PubMed] [Google Scholar]



