Full Text
The Full Text of this article is available as a PDF (541.6 KB).
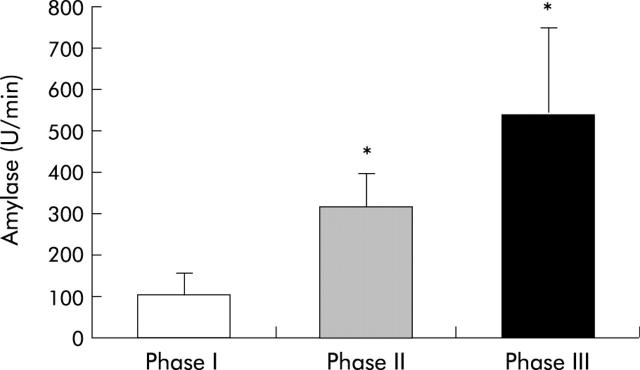
Figure 1.
Interdigestive amylase output in healthy volunteers during daytime. Enzyme output is cyclical: it is associated with intestinal motility and is higher during phases II and III compared with phase I (*p<0.05 v phase I).322
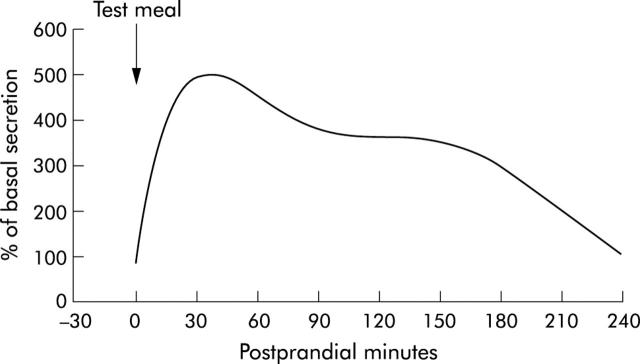
Figure 2.
Digestive pancreatic enzyme response to a regular meal. Enzyme delivery into the duodenum increases rapidly and reaches maximal values within the first postprandial hour or even within 20–30 minutes postprandially. Following peak output, enzyme secretion decreases to almost stable secretory rates at lower levels until about 3–4 hours postprandially depending on the size of the meal. The interdigestive range is reached again at the end of the digestive period.7–13
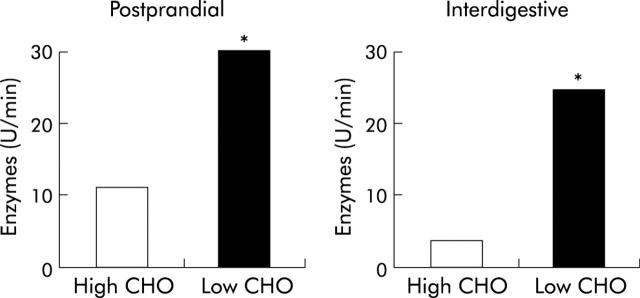
Figure 3.
Effect of chronic ingestion of diets with varying nutrient composition in healthy volunteers. Following ingestion of a diet low in carbohydrate content (10%) and high in fat content (40%) for four weeks, postprandial enzyme output was about twice as high as following a high carbohydrate (80%), low fat (10%) diet (geometric means, ANOVA: *p<0.05). Interdigestive pancreatic enzyme output was about four times higher with the low carbohydrate, high fat diet. Thus, chronic ingestion of a high fat diet is associated with higher enzyme outputs than a carbohydrate rich diet.24
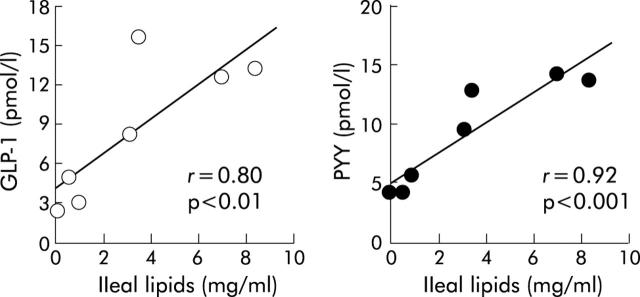
Figure 4.
Ileal lipid exposure dose dependently releases glucagon-like peptide-1(GLP-1) and peptide YY (PYY) in healthy humans.20
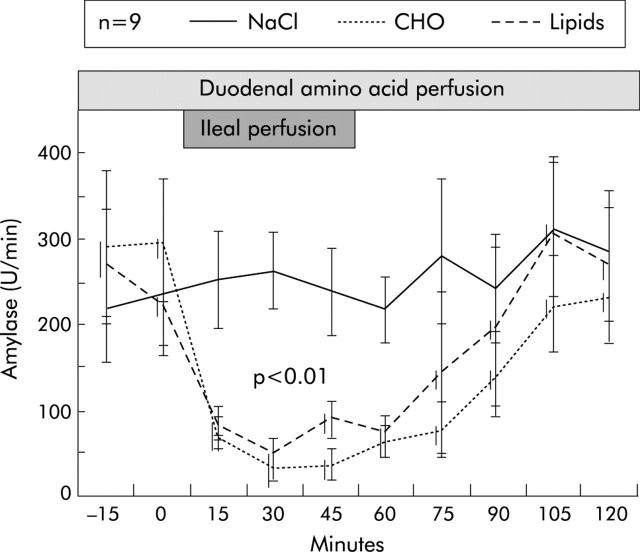
Figure 5.
Inhibition of endogenously stimulated pancreatic enzyme secretion by ileal perfusion of carbohydrates (CHO) and lipids in healthy humans.17
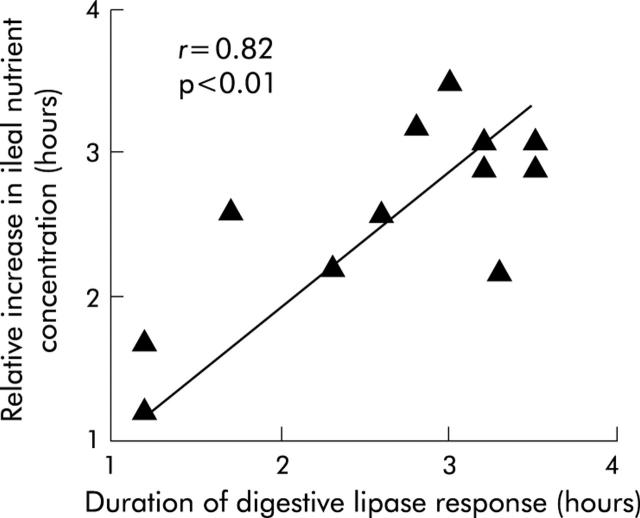
Figure 6.
Correlation between the duration of digestive lipase secretion and the time of the relative increase in ileal nutrient concentration in healthy humans.18
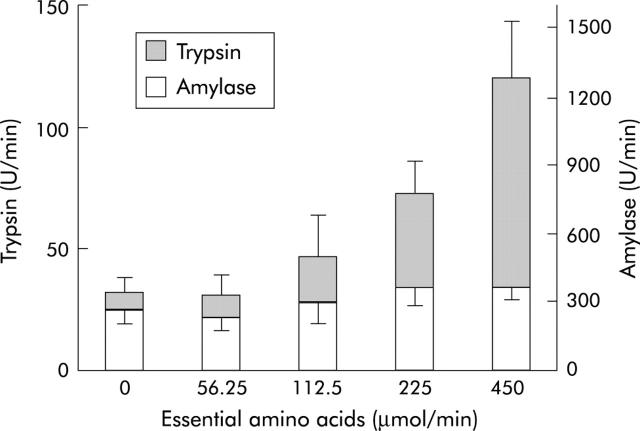
Figure 7.
Differential effects of graded duodenal nutrient perfusion on protease and amylase outputs in healthy humans. Essential amino acids at doses up to 450 µg/min dose dependently stimulated trypsin but had no effect on amylase output (*p<0.05).49
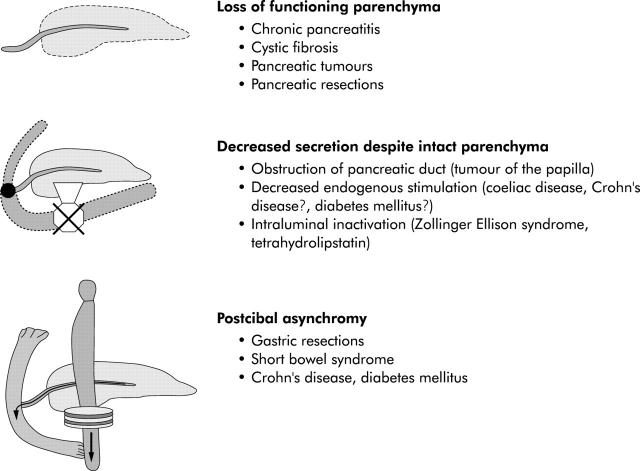
Figure 8.
Pathomechanisms causing intraluminal pancreatic enzyme deficiency.
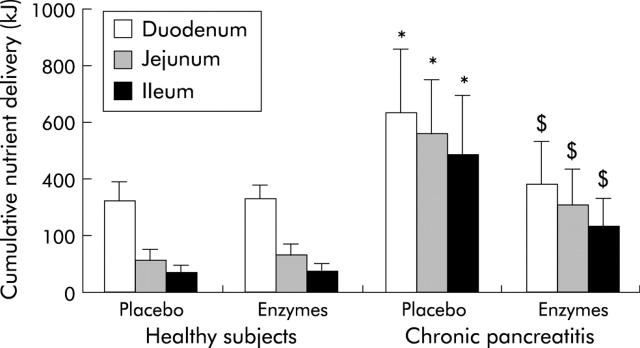
Figure 9.
Nutrient malabsorption with and without enzyme supplementation in healthy subjects (n = 14) and patients with chronic pancreatitis (n = 12).104
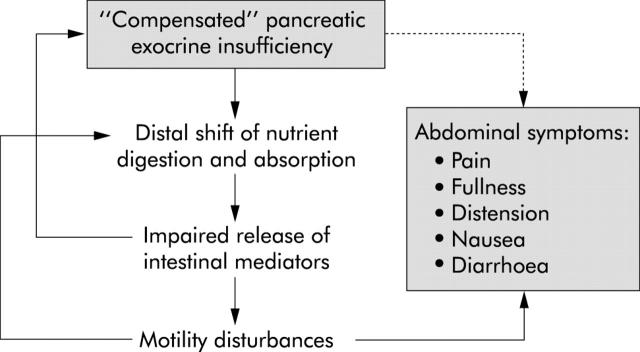
Figure 10.
Potential pathophysiological role of "compensated" pancreatic exocrine insufficiency for abdominal symptoms: in patients with mildly to moderately decreased exocrine function a distal shift of nutrient digestion and absorption may lead to impaired release of intestinal mediators. This, in turn may cause motility disturbances inducing abdominal symptoms and it may aggravate exocrine insufficiency. Accordingly, abdominal symptoms would not be a consequence of increased loss of nutrients but of a disturbance of the integrated regulation of gastrointestinal secretory and motor functions.
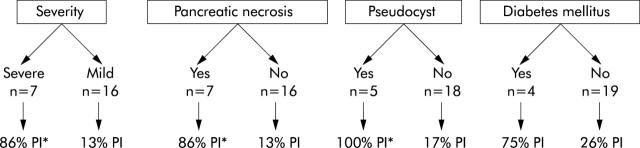
Figure 11.
Incidence of pancreatic exocrine insufficiency (PI) in 23 patients recovering from acute pancreatitis depending on severity of disease and presence of complications.169 *p = 0.002 mild v severe or absence v presence of symptoms.
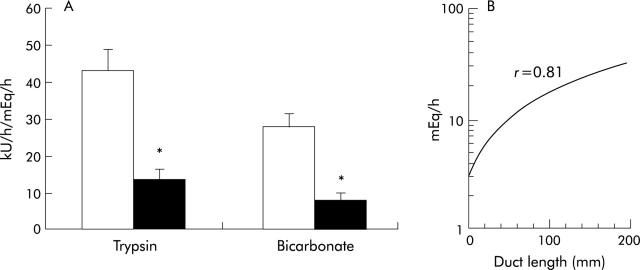
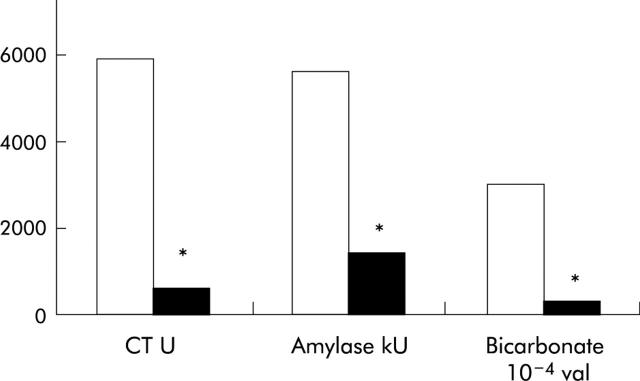
Figure 12.
(A) Trypsin and bicarbonate response to CCK are significantly decreased in patients with pancreatic cancer (n = 17, black bars) compared with controls (n = 17, open bars). (B) Bicarbonate output in response to CCK is significantly correlated with (remaining) length of pancreatic duct for combined pancreatic cancer and control groups (*p<0.05).171
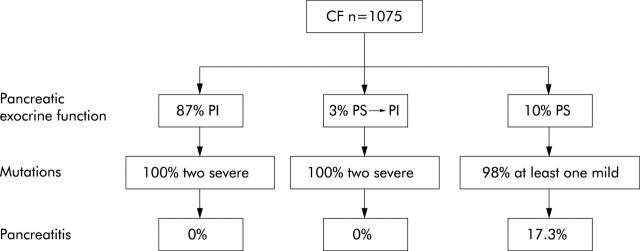
Figure 13.
About 10% of patients with cystic fibrosis remain pancreatic sufficient (PS), 3% develop pancreatic insufficiency during the course of the disease (PS → PI) and 87% show pancreatic exocrine insufficiency at diagnosis (PI). All patients with PI or PS → PI in whom complete genotype analysis could be performed showed severe CFTR gene mutations on both alleles. By contrast, almost all patients with PS had at least one mild mutation. Only patients with PS appear to be at risk of developing (recurrent) acute pancreatitis.183
Figure 14.
Median enzyme and bicarbonate output in patients before (open bars) and three months after (black bars) total gastrectomy due to gastric cancer. Preoperatively, all patients had normal pancreatic exocrine function. Median output of all parameters in response to direct stimulation by secretin and cerulein was markedly and significantly decreased postoperatively (*p<0.01).98
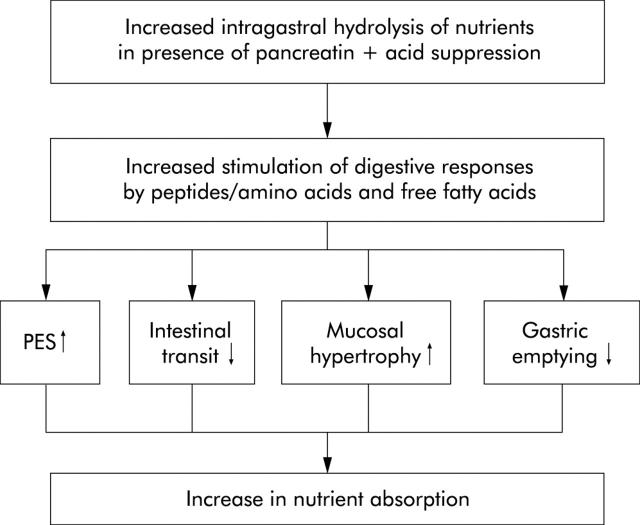
Figure 15.
Theoretical effect of pancreatic enzyme supplementation in short bowel syndrome (PES, pancreatic enzyme secretion). Application of unprotected pancreatic enzymes together with gastric acid suppression therapy should enable gastric digestion of nutrients with increased delivery of digestive products to the small bowel. Because of the superior stimulatory capacity of peptides, amino acids, and free fatty acids compared with macronutrients, conditions for nutrient absorption should be improved by increased endogenous pancreatic enzyme secretion, delayed gastric emptying, and small intestinal transit as well as mucosal hypertrophy.
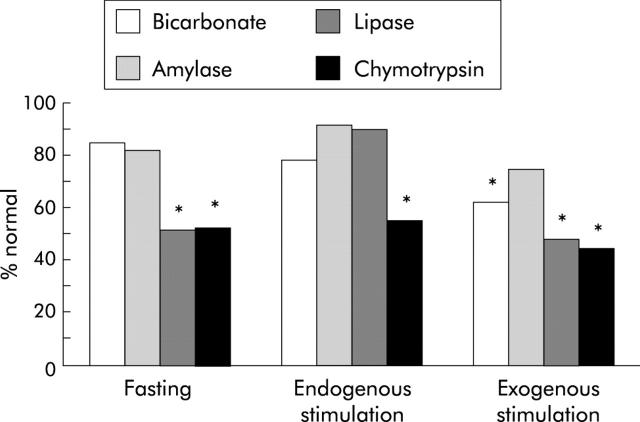
Figure 16.
Exocrine pancreatic insufficiency in type I diabetes mellitus: differential susceptibility of individual enzymes to endogenous and exogenous stimulation. Amylase output was normal in the interdigestive state, during moderate endogenous and maximal exogenous stimulation. Diminished fasting lipase output increased regularly in response to endogenous but not to exogenous stimulation. Chymotrypsin output was most susceptible and was decreased under all experimental conditions.262
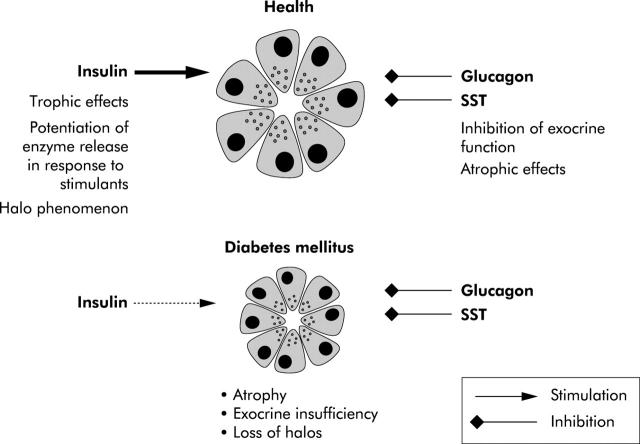
Figure 17.
Imbalance of stimulatory and inhibitory pancreatic islet hormones in diabetes mellitus.273
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams C. K., Hamosh M., Dutta S. K., Hubbard V. S., Hamosh P. Role of nonpancreatic lipolytic activity in exocrine pancreatic insufficiency. Gastroenterology. 1987 Jan;92(1):125–129. doi: 10.1016/0016-5085(87)90848-1. [DOI] [PubMed] [Google Scholar]
- Abrams C. K., Hamosh M., Hubbard V. S., Dutta S. K., Hamosh P. Lingual lipase in cystic fibrosis. Quantitation of enzyme activity in the upper small intestine of patients with exocrine pancreatic insufficiency. J Clin Invest. 1984 Feb;73(2):374–382. doi: 10.1172/JCI111222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achem-Karam S. R., Funakoshi A., Vinik A. I., Owyang C. Plasma motilin concentration and interdigestive migrating motor complex in diabetic gastroparesis: effect of metoclopramide. Gastroenterology. 1985 Feb;88(2):492–499. doi: 10.1016/0016-5085(85)90512-8. [DOI] [PubMed] [Google Scholar]
- Adler G., Hupp T., Kern H. F. Course and spontaneous regression of acute pancreatitis in the rat. Virchows Arch A Pathol Anat Histol. 1979 May 14;382(1):31–47. doi: 10.1007/BF01102739. [DOI] [PubMed] [Google Scholar]
- Adrian T. E., Ferri G. L., Bacarese-Hamilton A. J., Fuessl H. S., Polak J. M., Bloom S. R. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985 Nov;89(5):1070–1077. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- Alzaid A., Aideyan O., Nawaz S. The size of the pancreas in diabetes mellitus. Diabet Med. 1993 Oct;10(8):759–763. doi: 10.1111/j.1464-5491.1993.tb00160.x. [DOI] [PubMed] [Google Scholar]
- Anagnostides A., Chadwick V. S., Selden A. C., Maton P. N. Sham feeding and pancreatic secretion. Evidence for direct vagal stimulation of enzyme output. Gastroenterology. 1984 Jul;87(1):109–114. [PubMed] [Google Scholar]
- Andersen J. R., Bendtsen F., Ovesen L., Pedersen N. T., Rune S. J., Tage-Jensen U. Pancreatic insufficiency. Duodenal and jejunal pH, bile acid activity, and micellar lipid solubilization. Int J Pancreatol. 1990 Jun;6(4):263–270. [PubMed] [Google Scholar]
- Angelini G., Cavallini G., Bovo P., Brocco G., Castagnini A., Lavarini E., Merigo F., Tallon N., Scuro L. A. Pancreatic function in chronic inflammatory bowel disease. Int J Pancreatol. 1988 Mar;3(2-3):185–193. doi: 10.1007/BF02798930. [DOI] [PubMed] [Google Scholar]
- Armand M., Pasquier B., André M., Borel P., Senft M., Peyrot J., Salducci J., Portugal H., Jaussan V., Lairon D. Digestion and absorption of 2 fat emulsions with different droplet sizes in the human digestive tract. Am J Clin Nutr. 1999 Dec;70(6):1096–1106. doi: 10.1093/ajcn/70.6.1096. [DOI] [PubMed] [Google Scholar]
- Augarten A., Katznelson D., Dubenbaum L., Doolman R., Sela B. A., Lusky A., Szeinberg A., Kerem B. S., Paret G., Gazit E. Serum lipase levels pre and post Lundh meal: evaluation of exocrine pancreatic status in cystic fibrosis. Int J Clin Lab Res. 1998;28(4):226–229. doi: 10.1007/s005990050049. [DOI] [PubMed] [Google Scholar]
- Balasubramanian K., Zentler-Munro P. L., Batten J. C., Northfield T. C. Increased intragastric acid-resistant lipase activity and lipolysis in pancreatic steatorrhoea due to cystic fibrosis. Pancreas. 1992;7(3):305–310. doi: 10.1097/00006676-199205000-00006. [DOI] [PubMed] [Google Scholar]
- Bali A., Stableforth D. E., Asquith P. Prolonged small-intestinal transit time in cystic fibrosis. Br Med J (Clin Res Ed) 1983 Oct 8;287(6398):1011–1013. doi: 10.1136/bmj.287.6398.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beger H. G., Schlosser W., Friess H. M., Büchler M. W. Duodenum-preserving head resection in chronic pancreatitis changes the natural course of the disease: a single-center 26-year experience. Ann Surg. 1999 Oct;230(4):512–523. doi: 10.1097/00000658-199910000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beglinger C., Fried M., Whitehouse I., Jansen J. B., Lamers C. B., Gyr K. Pancreatic enzyme response to a liquid meal and to hormonal stimulation. Correlation with plasma secretin and cholecystokinin levels. J Clin Invest. 1985 May;75(5):1471–1476. doi: 10.1172/JCI111850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beglinger C., Taylor I. L., Grossman M. I., Solomon T. E. Pancreatic polypeptide inhibits exocrine pancreatic responses to six stimulants. Am J Physiol. 1984 Mar;246(3 Pt 1):G286–G291. doi: 10.1152/ajpgi.1984.246.3.G286. [DOI] [PubMed] [Google Scholar]
- Boivin M., Lanspa S. J., Zinsmeister A. R., Go V. L., DiMagno E. P. Are diets associated with different rates of human interdigestive and postprandial pancreatic enzyme secretion? Gastroenterology. 1990 Dec;99(6):1763–1771. doi: 10.1016/0016-5085(90)90485-j. [DOI] [PubMed] [Google Scholar]
- Bond J. H., Jr, Levitt M. D. Fate of soluble carbohydrate in the colon of rats and man. J Clin Invest. 1976 May;57(5):1158–1164. doi: 10.1172/JCI108383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boreham B., Ammori B. J. A prospective evaluation of pancreatic exocrine function in patients with acute pancreatitis: correlation with extent of necrosis and pancreatic endocrine insufficiency. Pancreatology. 2003;3(4):303–308. doi: 10.1159/000071768. [DOI] [PubMed] [Google Scholar]
- Bouquet J., Sinaasappel M., Neijens H. J. Malabsorption in cystic fibrosis: mechanisms and treatment. J Pediatr Gastroenterol Nutr. 1988;7 (Suppl 1):S30–S35. doi: 10.1097/00005176-198811001-00007. [DOI] [PubMed] [Google Scholar]
- Bovo P., Cataudella G., Di Francesco V., Vaona B., Filippini M., Marcori M., Montesi G., Rigo L., Frulloni L., Brunori M. P. Intraluminal gastric pH in chronic pancreatitis. Gut. 1995 Feb;36(2):294–298. doi: 10.1136/gut.36.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt T., Adler G., Koop I., Arnold R. Effect of atropine on intestinal phase of pancreatic secretion in man. Digestion. 1988;41(2):108–115. doi: 10.1159/000199739. [DOI] [PubMed] [Google Scholar]
- Bozkurt T., Maroske D., Adler G. Exocrine pancreatic function after recovery from necrotizing pancreatitis. Hepatogastroenterology. 1995 Feb;42(1):55–58. [PubMed] [Google Scholar]
- Braganza J. M., Herman K., Hine P., Kay G., Sandle G. I. Pancreatic enzymes in human duodenal juice--a comparison of responses in secretin pancreozymin and Lundh Borgström tests. Gut. 1978 May;19(5):358–366. doi: 10.1136/gut.19.5.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge W. R., Burke C. A., Brand D. L., Chey W. Y. Increased interdigestive pancreatic trypsin secretion in alcoholic pancreatic disease. Dig Dis Sci. 1985 May;30(5):431–439. doi: 10.1007/BF01318175. [DOI] [PubMed] [Google Scholar]
- Brunner H., Northfield T. C., Hofmann A. F., Go V. L., Summerskill W. H. Gastric emptying and secretion of bile acids, cholesterol, and pancreatic enzymes during digestion. Duodenal perfusion studies in healthy subjects. Mayo Clin Proc. 1974 Nov;49(11):851–860. [PubMed] [Google Scholar]
- Bruno M. J., Rauws E. A., Hoek F. J., Tytgat G. N. Comparative effects of adjuvant cimetidine and omeprazole during pancreatic enzyme replacement therapy. Dig Dis Sci. 1994 May;39(5):988–992. doi: 10.1007/BF02087549. [DOI] [PubMed] [Google Scholar]
- Bytzer P., Talley N. J., Leemon M., Young L. J., Jones M. P., Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001 Sep 10;161(16):1989–1996. doi: 10.1001/archinte.161.16.1989. [DOI] [PubMed] [Google Scholar]
- CHAPIN L. E., SCUDAMORE H. H., BAGGENSTOSS A. H., BARGEN J. A. Regional enteritis: associated visceral changes. Gastroenterology. 1956 Mar;30(3):404–415. [PubMed] [Google Scholar]
- CHEY W. Y., SHAY H., SHUMAN C. R. EXTERNAL PANCREATIC SECRETION IN DIABETES MELLITUS. Ann Intern Med. 1963 Dec;59:812–821. doi: 10.7326/0003-4819-59-6-812. [DOI] [PubMed] [Google Scholar]
- Cantor P., Mortensen P. E., Myhre J., Gjorup I., Worning H., Stahl E., Survill T. T. The effect of the cholecystokinin receptor antagonist MK-329 on meal-stimulated pancreaticobiliary output in humans. Gastroenterology. 1992 May;102(5):1742–1751. doi: 10.1016/0016-5085(92)91738-p. [DOI] [PubMed] [Google Scholar]
- Carriere F., Barrowman J. A., Verger R., Laugier R. Secretion and contribution to lipolysis of gastric and pancreatic lipases during a test meal in humans. Gastroenterology. 1993 Sep;105(3):876–888. doi: 10.1016/0016-5085(93)90908-u. [DOI] [PubMed] [Google Scholar]
- Carrière F., Renou C., Lopez V., De Caro J., Ferrato F., Lengsfeld H., De Caro A., Laugier R., Verger R. The specific activities of human digestive lipases measured from the in vivo and in vitro lipolysis of test meals. Gastroenterology. 2000 Oct;119(4):949–960. doi: 10.1053/gast.2000.18140. [DOI] [PubMed] [Google Scholar]
- Carroccio A., Iacono G., Lerro P., Cavataio F., Malorgio E., Soresi M., Baldassarre M., Notarbartolo A., Ansaldi N., Montalto G. Role of pancreatic impairment in growth recovery during gluten-free diet in childhood celiac disease. Gastroenterology. 1997 Jun;112(6):1839–1844. doi: 10.1053/gast.1997.v112.pm9178674. [DOI] [PubMed] [Google Scholar]
- Carroccio A., Iacono G., Montalto G., Cavataio F., Di Marco C., Balsamo V., Notarbartolo A. Exocrine pancreatic function in children with coeliac disease before and after a gluten free diet. Gut. 1991 Jul;32(7):796–799. doi: 10.1136/gut.32.7.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroccio A., Iacono G., Montalto G., Cavataio F., Lorello D., Greco L., Soresi M., Notarbartolo A. Pancreatic enzyme therapy in childhood celiac disease. A double-blind prospective randomized study. Dig Dis Sci. 1995 Dec;40(12):2555–2560. doi: 10.1007/BF02220441. [DOI] [PubMed] [Google Scholar]
- Carroccio A., Iacono G., Montalto G., Cavataio F., Lorello D., Soresi M., Di Martino D., Notarbartolo A. Pancreatic insufficiency in celiac disease is not dependent on nutritional status. Dig Dis Sci. 1994 Oct;39(10):2235–2242. doi: 10.1007/BF02090377. [DOI] [PubMed] [Google Scholar]
- Cleghorn G., Benjamin L., Corey M., Forstner G., Dati F., Durie P. Serum immunoreactive pancreatic lipase and cationic trypsinogen for the assessment of exocrine pancreatic function in older patients with cystic fibrosis. Pediatrics. 1986 Mar;77(3):301–306. [PubMed] [Google Scholar]
- Collins B. J., Bell P. M., Boyd S., Kerr J., Buchanan K. D., Love A. H. Endocrine and exocrine pancreatic function in treated coeliac disease. Pancreas. 1986;1(2):143–147. doi: 10.1097/00006676-198603000-00006. [DOI] [PubMed] [Google Scholar]
- Conwell Darwin L., Zuccaro Gregory, Jr, Vargo John J., Morrow J. Brad, Obuchowski Nancy, Dumot John A., Trolli Patricia A., Burton Allison, O'laughlin Cathy, Van Lente Frederick. An endoscopic pancreatic function test with cholecystokinin-octapeptide for the diagnosis of chronic pancreatitis. Clin Gastroenterol Hepatol. 2003 May;1(3):189–194. doi: 10.1053/cgh.2003.50028. [DOI] [PubMed] [Google Scholar]
- Conwell Darwin L., Zuccaro Gregory, Morrow J. Brad, Van Lente Frederick, O'Laughlin Cathy, Vargo John J., Dumot John A. Analysis of duodenal drainage fluid after cholecystokinin (CCK) stimulation in healthy volunteers. Pancreas. 2002 Nov;25(4):350–354. doi: 10.1097/00006676-200211000-00005. [DOI] [PubMed] [Google Scholar]
- Conwell Darwin L., Zuccaro Gregory, Morrow J. Brad, Van Lente Frederick, Obuchowski Nancy, Vargo John J., Dumot John A., Trolli Patricia, Shay Steven S. Cholecystokinin-stimulated peak lipase concentration in duodenal drainage fluid: a new pancreatic function test. Am J Gastroenterol. 2002 Jun;97(6):1392–1397. doi: 10.1111/j.1572-0241.2002.05675.x. [DOI] [PubMed] [Google Scholar]
- Czakó L., Hajnal F., Németh J., Takács T., Lonovics J. Effect of a liquid meal given as a bolus into the jejunum on human pancreatic secretion. Pancreas. 1999 Mar;18(2):197–202. doi: 10.1097/00006676-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Czakó L., Yamamoto M., Otsuki M. Exocrine pancreatic function in rats after acute pancreatitis. Pancreas. 1997 Jul;15(1):83–90. doi: 10.1097/00006676-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Czakó L., Yamamoto M., Otsuki M. Pancreatic fluid hypersecretion in rats after acute pancreatitis. Dig Dis Sci. 1997 Feb;42(2):265–272. doi: 10.1023/a:1018893230319. [DOI] [PubMed] [Google Scholar]
- DOOLEY R. R., GUILMETTE F., LEUBNER H., PATTERSON P. R., SHWACHMAN H., WEIL C. Cystic fibrosis of the pancreas with varying degrees of pancreatic insufficiency. AMA J Dis Child. 1956 Oct;92(4):347–368. doi: 10.1001/archpedi.1956.02060030341004. [DOI] [PubMed] [Google Scholar]
- DREILING D. A., Sr Studies in pancreatic function. V. The use of the secretin test in the diagnosis of pancreatitis and in the demonstration of pancreatic insufficiencies in gastrointestinal disorders. Gastroenterology. 1953 Aug;24(4):540–555. [PubMed] [Google Scholar]
- Dagorn J. C., Michel R., Morisset J. Nonparallel courses of intrapancreatic levels of exportable enzymes after a fatty meal. Proc Soc Exp Biol Med. 1976 Mar;151(3):608–610. doi: 10.3181/00379727-151-39270. [DOI] [PubMed] [Google Scholar]
- Dalzell A. M., Freestone N. S., Billington D., Heaf D. P. Small intestinal permeability and orocaecal transit time in cystic fibrosis. Arch Dis Child. 1990 Jun;65(6):585–588. doi: 10.1136/adc.65.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMagno E. P., Go V. L., Summerskill W. H. Relations between pancreatic enzyme outputs and malabsorption in severe pancreatic insufficiency. N Engl J Med. 1973 Apr 19;288(16):813–815. doi: 10.1056/NEJM197304192881603. [DOI] [PubMed] [Google Scholar]
- DiMagno E. P., Go W. L., Summerskill W. H. Impaired cholecystokinin-pancreozymin secretion, intraluminal dilution, and maldigestion of fat in sprue. Gastroenterology. 1972 Jul;63(1):25–32. [PubMed] [Google Scholar]
- DiMagno E. P., Malagelada J. R., Go V. L., Moertel C. G. Fate of orally ingested enzymes in pancreatic insufficiency. Comparison of two dosage schedules. N Engl J Med. 1977 Jun 9;296(23):1318–1322. doi: 10.1056/NEJM197706092962304. [DOI] [PubMed] [Google Scholar]
- DiMagno E. P., Malagelada J. R., Go V. L. Relationship between alcoholism and pancreatic insufficiency. Ann N Y Acad Sci. 1975 Apr 25;252:200–207. doi: 10.1111/j.1749-6632.1975.tb19157.x. [DOI] [PubMed] [Google Scholar]
- DiMagno E. P., Malagelada J. R., Go V. L. The relationships between pancreatic ductal obstruction and pancreatic secretion in man. Mayo Clin Proc. 1979 Mar;54(3):157–162. [PubMed] [Google Scholar]
- Domínguez-Muñoz J. E., Pieramico O., Büchler M., Malfertheiner P. Exocrine pancreatic function in the early phase of human acute pancreatitis. Scand J Gastroenterol. 1995 Feb;30(2):186–191. doi: 10.3109/00365529509093260. [DOI] [PubMed] [Google Scholar]
- Dooley C. P., Valenzuela J. E. Duodenal volume and osmoreceptors in the stimulation of human pancreatic secretion. Gastroenterology. 1984 Jan;86(1):23–27. [PubMed] [Google Scholar]
- Dukehart M. R., Dutta S. K., Vaeth J. Dietary fiber supplementation: effect on exocrine pancreatic secretion in man. Am J Clin Nutr. 1989 Nov;50(5):1023–1028. doi: 10.1093/ajcn/50.5.1023. [DOI] [PubMed] [Google Scholar]
- Dunger D. B., Burns C., Ghale G. K., Muller D. P., Spitz L., Grant D. B. Pancreatic exocrine and endocrine function after subtotal pancreatectomy for nesidioblastosis. J Pediatr Surg. 1988 Feb;23(2):112–115. doi: 10.1016/s0022-3468(88)80136-2. [DOI] [PubMed] [Google Scholar]
- Durie P. R. The pathophysiology of the pancreatic defect in cystic fibrosis. Acta Paediatr Scand Suppl. 1989;363:41–44. doi: 10.1111/apa.1989.78.s363.41. [DOI] [PubMed] [Google Scholar]
- Durno Carol, Corey Mary, Zielenski Julian, Tullis Elizabeth, Tsui Lap-Chee, Durie Peter. Genotype and phenotype correlations in patients with cystic fibrosis and pancreatitis. Gastroenterology. 2002 Dec;123(6):1857–1864. doi: 10.1053/gast.2002.37042. [DOI] [PubMed] [Google Scholar]
- Dyck W. P., Texter E. C., Jr, Lasater J. M., Hightower N. C., Jr Influence of glucagon on pancreatic exocrine secretion in man. Gastroenterology. 1970 Apr;58(4):532–539. [PubMed] [Google Scholar]
- Eddes E. H., Masclee A. A., Lamers C. B., Gooszen H. G. Duodenum preserving resection of the head of the pancreas in painful chronic pancreatitis. Eur J Surg. 1996 Jul;162(7):545–549. [PubMed] [Google Scholar]
- Ekelund K., Johansson C. Output of bile and pancreatic enzymes after test meals with different fat content. Influence of body weight on pancreatic enzyme composition. Acta Physiol Scand. 1980 Oct;110(2):161–165. doi: 10.1111/j.1748-1716.1980.tb06646.x. [DOI] [PubMed] [Google Scholar]
- Ekelund K., Johansson C. Output of bilirubin and pancreatic enzymes in response to different liquid test meals in man. Scand J Gastroenterol. 1975;10(5):507–511. [PubMed] [Google Scholar]
- Emoto T., Miyata M., Izukura M., Yumiba T., Mizutani S., Sakamoto T., Matsuda H. Simultaneous observation of endocrine and exocrine functions of the pancreas responding to somatostatin in man. Regul Pept. 1997 Jan 15;68(1):1–8. doi: 10.1016/s0167-0115(96)00125-5. [DOI] [PubMed] [Google Scholar]
- Ertan A., Arimura A., Akdamar K., Shibata T., Groot K., Luciano M., Mather F. J., Degertekin H., Agrawal N., Ryan J. Pancreatic immunoreactive somatostatin and diabetes mellitus. Dig Dis Sci. 1984 Jul;29(7):625–630. doi: 10.1007/BF01347295. [DOI] [PubMed] [Google Scholar]
- Escobar H., Perdomo M., Vasconez F., Camarero C., del Olmo M. T., Suárez L. Intestinal permeability to 51Cr-EDTA and orocecal transit time in cystic fibrosis. J Pediatr Gastroenterol Nutr. 1992 Feb;14(2):204–207. doi: 10.1097/00005176-199202000-00015. [DOI] [PubMed] [Google Scholar]
- Evander A., Hederström E., Hultberg B., Ihse I. Exocrine pancreatic secretion in acute experimental pancreatitis. Digestion. 1982;24(3):159–167. doi: 10.1159/000198792. [DOI] [PubMed] [Google Scholar]
- Evenepoel P., Claus D., Geypens B., Hiele M., Geboes K., Rutgeerts P., Ghoos Y. Amount and fate of egg protein escaping assimilation in the small intestine of humans. Am J Physiol. 1999 Nov;277(5 Pt 1):G935–G943. doi: 10.1152/ajpgi.1999.277.5.G935. [DOI] [PubMed] [Google Scholar]
- Evenepoel P., Claus D., Geypens B., Maes B., Hiele M., Rutgeerts P., Ghoos Y. Evidence for impaired assimilation and increased colonic fermentation of protein, related to gastric acid suppression therapy. Aliment Pharmacol Ther. 1998 Oct;12(10):1011–1019. doi: 10.1046/j.1365-2036.1998.00377.x. [DOI] [PubMed] [Google Scholar]
- Fine K. D., Meyer R. L., Lee E. L. The prevalence and causes of chronic diarrhea in patients with celiac sprue treated with a gluten-free diet. Gastroenterology. 1997 Jun;112(6):1830–1838. doi: 10.1053/gast.1997.v112.pm9178673. [DOI] [PubMed] [Google Scholar]
- Frey C. F., Child C. G., Fry W. Pancreatectomy for chronic pancreatitis. Ann Surg. 1976 Oct;184(4):403–413. doi: 10.1097/00000658-197610000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Abramson S., Meyer J. H. Passage of salivary amylase through the stomach in humans. Dig Dis Sci. 1987 Oct;32(10):1097–1103. doi: 10.1007/BF01300195. [DOI] [PubMed] [Google Scholar]
- Fried M., Erlacher U., Schwizer W., Löchner C., Koerfer J., Beglinger C., Jansen J. B., Lamers C. B., Harder F., Bischof-Delaloye A. Role of cholecystokinin in the regulation of gastric emptying and pancreatic enzyme secretion in humans. Studies with the cholecystokinin-receptor antagonist loxiglumide. Gastroenterology. 1991 Aug;101(2):503–511. doi: 10.1016/0016-5085(91)90031-f. [DOI] [PubMed] [Google Scholar]
- Fried M., Jansen J. B., Harpole T., Taylor I. L., Lamers C. B., Reedy T., Elashoff J., Meyer J. H. Pancreatobiliary responses to an intragastric amino acid meal: comparison to albumin, dextrose, and a maximal cholecystokinin stimulus. Gastroenterology. 1989 Dec;97(6):1544–1549. doi: 10.1016/0016-5085(89)90401-0. [DOI] [PubMed] [Google Scholar]
- Fried M., Mayer E. A., Jansen J. B., Lamers C. B., Taylor I. L., Bloom S. R., Meyer J. H. Temporal relationships of cholecystokinin release, pancreatobiliary secretion, and gastric emptying of a mixed meal. Gastroenterology. 1988 Nov;95(5):1344–1350. doi: 10.1016/0016-5085(88)90371-x. [DOI] [PubMed] [Google Scholar]
- Frier B. M., Saunders J. H., Wormsley K. G., Bouchier I. A. Exocrine pancreatic function in juvenile-onset diabetes mellitus. Gut. 1976 Sep;17(9):685–691. doi: 10.1136/gut.17.9.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friess H., Böhm J., Müller M. W., Glasbrenner B., Riepl R. L., Malfertheiner P., Büchler M. W. Maldigestion after total gastrectomy is associated with pancreatic insufficiency. Am J Gastroenterol. 1996 Feb;91(2):341–347. [PubMed] [Google Scholar]
- Funakoshi A., Tateishi K., Shinozaki H., Miyasaka K., Ito T., Wakasugi H. Plasma pancreastatin responses after intrajejunal infusion of liquid meal in patients with chronic pancreatitis. Dig Dis Sci. 1990 Jun;35(6):721–725. doi: 10.1007/BF01540174. [DOI] [PubMed] [Google Scholar]
- Gaia E., Andriulli A., Tappero G., Piantino P., Jayme A., Rocca G., Musorrofiti A. Influence of sex and body size on pancreatic bicarbonate and enzyme output. J Clin Gastroenterol. 1984 Oct;6(5):429–433. doi: 10.1097/00004836-198410000-00007. [DOI] [PubMed] [Google Scholar]
- Gan K. H., Geus W. P., Lamers C. B., Heijerman H. G. Effect of omeprazole 40 mg once daily on intraduodenal and intragastric pH in H. pylori-negative healthy subjects. Dig Dis Sci. 1997 Nov;42(11):2304–2309. doi: 10.1023/a:1018827003641. [DOI] [PubMed] [Google Scholar]
- Garnacho Montero J., Aznar Martín A., Corrochano Dalí M. D., González García J., Aznar Martín T., De la Vega Vázquez J. M., Holgado Silva C. Evolución de la función exocrina del páncreas tras la pancreatitis aguda. Factores pronósticos. Rev Esp Enferm Apar Dig. 1989 Jul;76(1):19–24. [PubMed] [Google Scholar]
- Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965 Oct;14(10):619–633. doi: 10.2337/diab.14.10.619. [DOI] [PubMed] [Google Scholar]
- Geus W. P., Eddes E. H., Gielkens H. A., Gan K. H., Lamers C. B., Masclee A. A. Post-prandial intragastric and duodenal acidity are increased in patients with chronic pancreatitis. Aliment Pharmacol Ther. 1999 Jul;13(7):937–943. doi: 10.1046/j.1365-2036.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- Gilbeau J. P., Poncelet V., Libon E., Derue G., Heller F. R. The density, contour, and thickness of the pancreas in diabetics: CT findings in 57 patients. AJR Am J Roentgenol. 1992 Sep;159(3):527–531. doi: 10.2214/ajr.159.3.1503017. [DOI] [PubMed] [Google Scholar]
- Gisolfi C. V., Summers R. W., Lambert G. P., Xia T. Effect of beverage osmolality on intestinal fluid absorption during exercise. J Appl Physiol (1985) 1998 Nov;85(5):1941–1948. doi: 10.1152/jappl.1998.85.5.1941. [DOI] [PubMed] [Google Scholar]
- Glasbrenner B., Bruckel J., Gritzmann R., Adler G. Cephalic phase of pancreatic polypeptide release: a valid test of autonomic neuropathy in diabetics? Diabetes Res Clin Pract. 1995 Nov;30(2):117–123. doi: 10.1016/0168-8227(95)01153-6. [DOI] [PubMed] [Google Scholar]
- Glasbrenner B., Dominguez-Munoz E., Riepl R. L., Vetsi A., Malfertheiner P. Cholecystokinin and pancreatic polypeptide release in diabetic patients with and without autonomic neuropathy. Dig Dis Sci. 1995 Feb;40(2):406–411. doi: 10.1007/BF02065429. [DOI] [PubMed] [Google Scholar]
- Go V. L., Hofmann A. F., Summerskill W. H. Pancreozymin bioassay in man based on pancreatic enzyme secretion: potency of specific amino acids and other digestive products. J Clin Invest. 1970 Aug;49(8):1558–1564. doi: 10.1172/JCI106373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda K., Sasaki E., Nagata K., Fukai M., Ohsawa N., Hahafusa T. Pancreatic volume in type 1 and type 2 diabetes mellitus. Acta Diabetol. 2001;38(3):145–149. doi: 10.1007/s005920170012. [DOI] [PubMed] [Google Scholar]
- Gregory P. C. Gastrointestinal pH, motility/transit and permeability in cystic fibrosis. J Pediatr Gastroenterol Nutr. 1996 Dec;23(5):513–523. doi: 10.1097/00005176-199612000-00001. [DOI] [PubMed] [Google Scholar]
- Guimbaud R., Moreau J. A., Bouisson M., Durand S., Escourrou J., Vaysse N., Frexinos J. Intraduodenal free fatty acids rather than triglycerides are responsible for the release of CCK in humans. Pancreas. 1997 Jan;14(1):76–82. doi: 10.1097/00006676-199701000-00012. [DOI] [PubMed] [Google Scholar]
- Gullo L., Costa P. L., Fontana G., Labò G. Investigation of exocrine pancreatic function by continuous infusion of caerulein and secretin in normal subjects and in chronic pancreatitis. Digestion. 1976;14(2):97–107. doi: 10.1159/000197914. [DOI] [PubMed] [Google Scholar]
- Gullo L., Costa P. L., Ventrucci M., Mattioli S., Viti G., Labò G. Exocrine pancreatic function after total gastrectomy. Scand J Gastroenterol. 1979;14(4):401–407. [PubMed] [Google Scholar]
- HARRIS R., NORMAN A. P., PAYNE W. W. The effect of pancreatin therapy on fat absorption and nitrogen retention in children with fibrocystic disease of the pancreas. Arch Dis Child. 1955 Oct;30(153):424–427. doi: 10.1136/adc.30.153.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halm U., Löser C., Löhr M., Katschinski M., Mössner J. A double-blind, randomized, multicentre, crossover study to prove equivalence of pancreatin minimicrospheres versus microspheres in exocrine pancreatic insufficiency. Aliment Pharmacol Ther. 1999 Jul;13(7):951–957. doi: 10.1046/j.1365-2036.1999.00566.x. [DOI] [PubMed] [Google Scholar]
- Harada H., Hayashi T., Ono A., Yamamoto N., Ikubo I., Shundo T., Negron A., Mishima K., Kimura I. Analysis of human pure pancreatic juice in chronic pancreatitis and cancer of the pancreas. Gastroenterol Jpn. 1978;13(6):461–467. doi: 10.1007/BF02774912. [DOI] [PubMed] [Google Scholar]
- Hardt P. D., Krauss A., Bretz L., Porsch-Ozcürümez M., Schnell-Kretschmer H., Mäser E., Bretzel R. G., Zekhorn T., Klör H. U. Pancreatic exocrine function in patients with type 1 and type 2 diabetes mellitus. Acta Diabetol. 2000;37(3):105–110. doi: 10.1007/s005920070011. [DOI] [PubMed] [Google Scholar]
- Hardt Philip D., Hauenschild Annette, Nalop Jens, Marzeion Axel M., Jaeger Clemens, Teichmann Joachim, Bretzel Reinhard G., Hollenhorst Manfred, Kloer Hans U., S2453112/S2453113 Study Group High prevalence of exocrine pancreatic insufficiency in diabetes mellitus. A multicenter study screening fecal elastase 1 concentrations in 1,021 diabetic patients. Pancreatology. 2003 Sep 24;3(5):395–402. doi: 10.1159/000073655. [DOI] [PubMed] [Google Scholar]
- Hegnhøj J., Hansen C. P., Rannem T., Søbirk H., Andersen L. B., Andersen J. R. Pancreatic function in Crohn's disease. Gut. 1990 Sep;31(9):1076–1079. doi: 10.1136/gut.31.9.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijerman H. G., Lamers C. B., Bakker W., Dijkman J. H. Improvement of fecal fat excretion after addition of omeprazole to pancrease in cystic fibrosis is related to residual exocrine function of the pancreas. Dig Dis Sci. 1993 Jan;38(1):1–6. doi: 10.1007/BF01296765. [DOI] [PubMed] [Google Scholar]
- Heijerman H. G., Lamers C. B., Bakker W. Omeprazole enhances the efficacy of pancreatin (pancrease) in cystic fibrosis. Ann Intern Med. 1991 Feb 1;114(3):200–201. doi: 10.7326/0003-4819-114-3-200. [DOI] [PubMed] [Google Scholar]
- Heikius B., Niemelä S., Lehtola J., Karttunen T., Lähde S. Pancreatic duct abnormalities and pancreatic function in patients with chronic inflammatory bowel disease. Scand J Gastroenterol. 1996 May;31(5):517–523. doi: 10.3109/00365529609006775. [DOI] [PubMed] [Google Scholar]
- Heptner G., Domschke S., Domschke W. Exocrine pancreatic function after gastrectomy. Specificity of indirect tests. Gastroenterology. 1989 Jul;97(1):147–153. doi: 10.1016/0016-5085(89)91428-5. [DOI] [PubMed] [Google Scholar]
- Herrlinger K. R., Stange E. F. The pancreas and inflammatory bowel diseases. Int J Pancreatol. 2000 Jun;27(3):171–179. doi: 10.1385/IJGC:27:3:171. [DOI] [PubMed] [Google Scholar]
- Hiele M., Ghoos Y., Rutgeerts P., Vantrappen G. Starch digestion in normal subjects and patients with pancreatic disease, using a 13CO2 breath test. Gastroenterology. 1989 Feb;96(2 Pt 1):503–509. doi: 10.1016/0016-5085(89)91577-1. [DOI] [PubMed] [Google Scholar]
- Hildebrand P., Beglinger C., Gyr K., Jansen J. B., Rovati L. C., Zuercher M., Lamers C. B., Setnikar I., Stalder G. A. Effects of a cholecystokinin receptor antagonist on intestinal phase of pancreatic and biliary responses in man. J Clin Invest. 1990 Mar;85(3):640–646. doi: 10.1172/JCI114486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand P., Petrig C., Burckhardt B., Ketterer S., Lengsfeld H., Fleury A., Hadváry P., Beglinger C. Hydrolysis of dietary fat by pancreatic lipase stimulates cholecystokinin release. Gastroenterology. 1998 Jan;114(1):123–129. doi: 10.1016/s0016-5085(98)70640-7. [DOI] [PubMed] [Google Scholar]
- Hirota M., Hashimoto M., Hiratsuka M., Ohboshi C., Yoshimoto S., Yano M., Mizuno A., Shima K. Alterations of plasma immunoreactive glucagon-like peptide-1 behavior in non-insulin-dependent diabetics. Diabetes Res Clin Pract. 1990 May-Jun;9(2):179–185. doi: 10.1016/0168-8227(90)90110-f. [DOI] [PubMed] [Google Scholar]
- Hoeden R., Mesa A. V., Delcourt A. Functional exploration of chronic pancreatitis by duodenal intubation. Comparative study of the Lundh meal and duodenal hormones based on a survey of literature. Secretin-caerulein test carried out by the authors. Prospective conclusions. Acta Gastroenterol Belg. 1976 Nov-Dec;39(11-12):509–521. [PubMed] [Google Scholar]
- Holgate A. M., Read N. W. Effect of ileal infusion of intralipid on gastrointestinal transit, ileal flow rate, and carbohydrate absorption in humans after ingestion of a liquid meal. Gastroenterology. 1985 Apr;88(4):1005–1011. doi: 10.1016/s0016-5085(85)80021-4. [DOI] [PubMed] [Google Scholar]
- Holtmann G., Kelly D. G., DiMagno E. P. Nutrients and cyclical interdigestive pancreatic enzyme secretion in humans. Gut. 1996 Jun;38(6):920–924. doi: 10.1136/gut.38.6.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmann G., Kelly D. G., Sternby B., DiMagno E. P. Survival of human pancreatic enzymes during small bowel transit: effect of nutrients, bile acids, and enzymes. Am J Physiol. 1997 Aug;273(2 Pt 1):G553–G558. doi: 10.1152/ajpgi.1997.273.2.G553. [DOI] [PubMed] [Google Scholar]
- Horowitz M., Edelbroek M. A., Wishart J. M., Straathof J. W. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia. 1993 Sep;36(9):857–862. doi: 10.1007/BF00400362. [DOI] [PubMed] [Google Scholar]
- Hotz J., Goberna R., Clodi P. H. Reserve capacity of the exocrine pancreas. Enzyme secretion and fecal fat assimilation in the 95 percent pancreatectomized rat. Digestion. 1973 Oct;9(3):212–223. doi: 10.1159/000197448. [DOI] [PubMed] [Google Scholar]
- Icks A., Haastert B., Giani G., Rathmann W. Low fecal elastase-1 in type I diabetes mellitus. Z Gastroenterol. 2001 Oct;39(10):823–830. doi: 10.1055/s-2001-17867. [DOI] [PubMed] [Google Scholar]
- Ihse I., Arnesjö B., Kugelberg C., Lilja P. Intestinal activities of trypsin, lipase, and phospholipase after a test meal. An evaluation of 474 examinations. Scand J Gastroenterol. 1977;12(6):663–668. doi: 10.3109/00365527709181700. [DOI] [PubMed] [Google Scholar]
- Ihse I., Lilja P., Evander A., Skude G. Time-related enzyme concentrations in duodenal aspirates after ingestion of a test meal. Scand J Gastroenterol. 1977;12(5):629–635. doi: 10.3109/00365527709181345. [DOI] [PubMed] [Google Scholar]
- Ikenaga H., Katoh H., Motohara T., Okushiba S., Shimozawa E., Kanaya S., Takahashi T. Duodenum-preserving resection of the head of the pancreas--modified procedures and long-term results-. Hepatogastroenterology. 1995 Sep-Oct;42(5):706–710. [PubMed] [Google Scholar]
- Imagawa A., Hanafusa T., Miyagawa J., Matsuzawa Y. A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies. Osaka IDDM Study Group. N Engl J Med. 2000 Feb 3;342(5):301–307. doi: 10.1056/NEJM200002033420501. [DOI] [PubMed] [Google Scholar]
- Imagawa A., Hanafusa T., Miyagawa J., Matsuzawa Y. A proposal of three distinct subtypes of type 1 diabetes mellitus based on clinical and pathological evidence. Ann Med. 2000 Nov;32(8):539–543. doi: 10.3109/07853890008998833. [DOI] [PubMed] [Google Scholar]
- Imagawa A., Hanafusa T., Tamura S., Moriwaki M., Itoh N., Yamamoto K., Iwahashi H., Yamagata K., Waguri M., Nanmo T. Pancreatic biopsy as a procedure for detecting in situ autoimmune phenomena in type 1 diabetes: close correlation between serological markers and histological evidence of cellular autoimmunity. Diabetes. 2001 Jun;50(6):1269–1273. doi: 10.2337/diabetes.50.6.1269. [DOI] [PubMed] [Google Scholar]
- Isaksson G., Ihse I. Pain reduction by an oral pancreatic enzyme preparation in chronic pancreatitis. Dig Dis Sci. 1983 Feb;28(2):97–102. doi: 10.1007/BF01315137. [DOI] [PubMed] [Google Scholar]
- Isaksson G., Lundquist I., Akesson B., Ihse I. Effects of pectin and wheat bran on intraluminal pancreatic enzyme activities and on fat absorption as examined with the triolein breath test in patients with pancreatic insufficiency. Scand J Gastroenterol. 1984 Jun;19(4):467–472. [PubMed] [Google Scholar]
- Isaksson G., Lundquist I., Ihse I. Effect of dietary fiber on pancreatic enzyme activity in vitro. Gastroenterology. 1982 May;82(5 Pt 1):918–924. [PubMed] [Google Scholar]
- Ishibashi T., Matsumoto S., Harada H., Ochi K., Tanaka J., Seno T., Oka H., Miyake H., Kimura I. [Aging and exocrine pancreatic function evaluated by the recently standardized secretin test]. Nihon Ronen Igakkai Zasshi. 1991 Sep;28(5):599–605. doi: 10.3143/geriatrics.28.599. [DOI] [PubMed] [Google Scholar]
- Izbicki J. R., Bloechle C., Knoefel W. T., Kuechler T., Binmoeller K. F., Broelsch C. E. Duodenum-preserving resection of the head of the pancreas in chronic pancreatitis. A prospective, randomized trial. Ann Surg. 1995 Apr;221(4):350–358. doi: 10.1097/00000658-199504000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N. K., Boivin M., Zinsmeister A. R., Brown M. L., Malagelada J. R., DiMagno E. P. Effect of ileal perfusion of carbohydrates and amylase inhibitor on gastrointestinal hormones and emptying. Gastroenterology. 1989 Feb;96(2 Pt 1):377–387. doi: 10.1016/0016-5085(89)91561-8. [DOI] [PubMed] [Google Scholar]
- Jalleh R. P., Williamson R. C. Pancreatic exocrine and endocrine function after operations for chronic pancreatitis. Ann Surg. 1992 Dec;216(6):656–662. doi: 10.1097/00000658-199212000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P. B., Mortensen P. B. Colonic digestion and absorption of energy from carbohydrates and medium-chain fat in small bowel failure. JPEN J Parenter Enteral Nutr. 1999 Sep-Oct;23(5 Suppl):S101–S105. doi: 10.1177/014860719902300525. [DOI] [PubMed] [Google Scholar]
- Johansen H. K., Nir M., Høiby N., Koch C., Schwartz M. Severity of cystic fibrosis in patients homozygous and heterozygous for delta F508 mutation. Lancet. 1991 Mar 16;337(8742):631–634. doi: 10.1016/0140-6736(91)92449-c. [DOI] [PubMed] [Google Scholar]
- Kanno T., Saito A. The potentiating influences of insulin on pancreozymin-induced hyperpolarization and amylase release in the pancreatic acinar cell. J Physiol. 1976 Oct;261(3):505–521. doi: 10.1113/jphysiol.1976.sp011571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsborg M., Bang la Cour B., Worning H. Pancreatic enzyme secretion and pancreatic pseudocysts in patients with chronic pancreatitis. Int J Pancreatol. 1997 Jun;21(3):199–203. doi: 10.1007/BF02821605. [DOI] [PubMed] [Google Scholar]
- Kato H., Nakao A., Kishimoto W., Nonami T., Harada A., Hayakawa T., Takagi H. 13C-labeled trioctanoin breath test for exocrine pancreatic function test in patients after pancreatoduodenectomy. Am J Gastroenterol. 1993 Jan;88(1):64–69. [PubMed] [Google Scholar]
- Katschinski M., Dippel C., Reinshagen M., Schirra J., Arnold R., Nustede R., Beglinger C., Adler G. Induction of the fed pattern of human exocrine pancreatic secretion by nutrients: role of cholecystokinin and neurotensin. Clin Investig. 1992 Oct;70(10):902–908. doi: 10.1007/BF00180436. [DOI] [PubMed] [Google Scholar]
- Keller J., Gröger G., Cherian L., Günther B., Layer P. Circadian coupling between pancreatic secretion and intestinal motility in humans. Am J Physiol Gastrointest Liver Physiol. 2001 Feb;280(2):G273–G278. doi: 10.1152/ajpgi.2001.280.2.G273. [DOI] [PubMed] [Google Scholar]
- Keller J., Rünzi M., Goebell H., Layer P. Duodenal and ileal nutrient deliveries regulate human intestinal motor and pancreatic responses to a meal. Am J Physiol. 1997 Mar;272(3 Pt 1):G632–G637. doi: 10.1152/ajpgi.1997.272.3.G632. [DOI] [PubMed] [Google Scholar]
- Keller Jutta, Layer Peter. Circadian pancreatic enzyme pattern and relationship between secretory and motor activity in fasting humans. J Appl Physiol (1985) 2002 Aug;93(2):592–600. doi: 10.1152/japplphysiol.00807.2001. [DOI] [PubMed] [Google Scholar]
- Keller Jutta, Layer Peter. Pancreatic Enzyme Supplementation Therapy. Curr Treat Options Gastroenterol. 2003 Oct;6(5):369–374. doi: 10.1007/s11938-003-0039-0. [DOI] [PubMed] [Google Scholar]
- Kingham J. G., Levison D. A., Fairclough P. D. Diarrhoea and reversible enteropathy in Zollinger-Ellison syndrome. Lancet. 1981 Sep 19;2(8247):610–612. doi: 10.1016/s0140-6736(81)92746-x. [DOI] [PubMed] [Google Scholar]
- Kitagawa M., Naruse S., Ishiguro H., Nakae Y., Kondo T., Hayakawa T. Evaluating exocrine function tests for diagnosing chronic pancreatitis. Pancreas. 1997 Nov;15(4):402–408. doi: 10.1097/00006676-199711000-00011. [DOI] [PubMed] [Google Scholar]
- Klass H. J., Sandle G. I., Kay P. M., Davies P., Braganza J. M. Quantitation of tryptic responses to endogenous and exogenous stimulation in chronic pancreatitis. Digestion. 1986;35(2):95–101. doi: 10.1159/000199352. [DOI] [PubMed] [Google Scholar]
- Klöppel G., Clemens A. Atiologie und Pathogenese des Insulin-abhängigen Diabetes mellitus. Verh Dtsch Ges Pathol. 1996;80:93–103. [PubMed] [Google Scholar]
- Klöppel G., Clemens A. Insulinabhängiger Diabetes mellitus. Aktuelle Aspekte zur Morphologie, Atiologie und Pathogenese. Pathologe. 1996 Jul;17(4):269–275. doi: 10.1007/s002920050165. [DOI] [PubMed] [Google Scholar]
- Kodama M., Tanaka T. Residual function of exocrine pancreas after operation for chronic pancreatitis by N-benzoyl-L-tyrosyl-p-aminobenzoic acid test (NBT-PABA test). Digestion. 1984;30(1):41–46. doi: 10.1159/000199089. [DOI] [PubMed] [Google Scholar]
- Kogire M., Inoue K., Doi R., Sumi S., Takaori K., Yun M., Hosotani R., Tobe T. Pancreatic exocrine deficiency after partial pancreatectomy in conscious dogs. Eur J Surg. 1993 May;159(5):283–286. [PubMed] [Google Scholar]
- Konturek S. J., Tasler J., Obtulowicz W. Characteristics of inhibition of pancreatic secretion by glucagon. Digestion. 1974;10(2):138–149. doi: 10.1159/000197533. [DOI] [PubMed] [Google Scholar]
- Koop I., Bozkurt T., Adler G., Schafmayer A., Becker H. D., Arnold R. Plasma cholecystokinin and pancreatic enzyme secretion in patients with coeliac sprue. Z Gastroenterol. 1987 Feb;25(2):124–129. [PubMed] [Google Scholar]
- Kopelman H., Durie P., Gaskin K., Weizman Z., Forstner G. Pancreatic fluid secretion and protein hyperconcentration in cystic fibrosis. N Engl J Med. 1985 Feb 7;312(6):329–334. doi: 10.1056/NEJM198502073120601. [DOI] [PubMed] [Google Scholar]
- Kramer M. F., Tan H. T. The peri-insular acini of the pancreas of the rat. Z Zellforsch Mikrosk Anat. 1968;86(2):163–170. doi: 10.1007/BF00348522. [DOI] [PubMed] [Google Scholar]
- Kristidis P., Bozon D., Corey M., Markiewicz D., Rommens J., Tsui L. C., Durie P. Genetic determination of exocrine pancreatic function in cystic fibrosis. Am J Hum Genet. 1992 Jun;50(6):1178–1184. [PMC free article] [PubMed] [Google Scholar]
- Kuhel D. G., Zheng S., Tso P., Hui D. Y. Adenovirus-mediated human pancreatic lipase gene transfer to rat bile: gene therapy of fat malabsorption. Am J Physiol Gastrointest Liver Physiol. 2000 Nov;279(5):G1031–G1036. doi: 10.1152/ajpgi.2000.279.5.G1031. [DOI] [PubMed] [Google Scholar]
- LAZARUS S. S., VOLK B. W. The effect of protracted glucagon administration on blood glucose and on pancreatic morphology. Endocrinology. 1958 Sep;63(3):359–371. doi: 10.1210/endo-63-3-359. [DOI] [PubMed] [Google Scholar]
- Ladas S. D., Giorgiotis K., Raptis S. A. Complex carbohydrate malabsorption in exocrine pancreatic insufficiency. Gut. 1993 Jul;34(7):984–987. doi: 10.1136/gut.34.7.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankisch P. G., Fuchs K., Schmidt H., Peiper H. J., Creutzfeldt W. Ergebnisse der operativen Behandlung der chronischen Pankreatitis mit besonderer Berücksichtigung der exokrinen und endokrinen Funktion. Dtsch Med Wochenschr. 1975 May 9;100(19):1048-50, 1059-60. doi: 10.1055/s-0028-1106333. [DOI] [PubMed] [Google Scholar]
- Lankisch P. G., Manthey G., Otto J., Koop H., Talaulicar M., Willms B., Creutzfeldt W. Exocrine pancreatic function in insulin-dependent diabetes mellitus. Digestion. 1982;25(3):211–216. doi: 10.1159/000198833. [DOI] [PubMed] [Google Scholar]
- Lankisch P. G., Schmidt I. Fecal elastase 1 is not the indirect pancreatic function test we have been waiting for. Dig Dis Sci. 2000 Jan;45(1):166–167. doi: 10.1023/a:1005486032702. [DOI] [PubMed] [Google Scholar]
- Lankisch P. G., Seidensticker F., Otto J., Lubbers H., Mahlke R., Stockmann F., Folsch U. R., Creutzfeldt W. Secretin-pancreozymin test (SPT) and endoscopic retrograde cholangiopancreatography (ERCP): both are necessary for diagnosing or excluding chronic pancreatitis. Pancreas. 1996 Mar;12(2):149–152. doi: 10.1097/00006676-199603000-00007. [DOI] [PubMed] [Google Scholar]
- Laugier R., Bernard J. P., Berthezene P., Dupuy P. Changes in pancreatic exocrine secretion with age: pancreatic exocrine secretion does decrease in the elderly. Digestion. 1991;50(3-4):202–211. doi: 10.1159/000200762. [DOI] [PubMed] [Google Scholar]
- Layer P. H., DiMagno E. P. Natural histories of alcoholic and idiopathic chronic pancreatitis. Pancreas. 1996 Apr;12(3):318–320. doi: 10.1097/00006676-199604000-00020. [DOI] [PubMed] [Google Scholar]
- Layer P., Carlson G. L., DiMagno E. P. Partially purified white bean amylase inhibitor reduces starch digestion in vitro and inactivates intraduodenal amylase in humans. Gastroenterology. 1985 Jun;88(6):1895–1902. doi: 10.1016/0016-5085(85)90016-2. [DOI] [PubMed] [Google Scholar]
- Layer P., Chan A. T., Go V. L., DiMagno E. P. Human pancreatic secretion during phase II antral motility of the interdigestive cycle. Am J Physiol. 1988 Feb;254(2 Pt 1):G249–G253. doi: 10.1152/ajpgi.1988.254.2.G249. [DOI] [PubMed] [Google Scholar]
- Layer P., Go V. L., DiMagno E. P. Fate of pancreatic enzymes during small intestinal aboral transit in humans. Am J Physiol. 1986 Oct;251(4 Pt 1):G475–G480. doi: 10.1152/ajpgi.1986.251.4.G475. [DOI] [PubMed] [Google Scholar]
- Layer P., Gröger G., Grandt D., Cherian L. Das terminale Ileum als Koregulator der zyklischen interdigestiven Pankreassekretion beim Menschen. Med Klin (Munich) 1993 Feb 15;88 (Suppl 1):15–17. [PubMed] [Google Scholar]
- Layer P., Holst J. J., Grandt D., Goebell H. Ileal release of glucagon-like peptide-1 (GLP-1). Association with inhibition of gastric acid secretion in humans. Dig Dis Sci. 1995 May;40(5):1074–1082. doi: 10.1007/BF02064202. [DOI] [PubMed] [Google Scholar]
- Layer P., Hotz J., Goebell H. Stimulatory effect of hypercalcemia on pancreatic secretion is prevented by pretreatment with cholecystokinin and cholinergic agonists. Pancreas. 1986;1(6):478–482. doi: 10.1097/00006676-198611000-00002. [DOI] [PubMed] [Google Scholar]
- Layer P., Jansen J. B., Cherian L., Lamers C. B., Goebell H. Feedback regulation of human pancreatic secretion. Effects of protease inhibition on duodenal delivery and small intestinal transit of pancreatic enzymes. Gastroenterology. 1990 May;98(5 Pt 1):1311–1319. [PubMed] [Google Scholar]
- Layer P., Keller J. Pancreatic enzymes: secretion and luminal nutrient digestion in health and disease. J Clin Gastroenterol. 1999 Jan;28(1):3–10. doi: 10.1097/00004836-199901000-00002. [DOI] [PubMed] [Google Scholar]
- Layer P., Peschel S., Schlesinger T., Goebell H. Human pancreatic secretion and intestinal motility: effects of ileal nutrient perfusion. Am J Physiol. 1990 Feb;258(2 Pt 1):G196–G201. doi: 10.1152/ajpgi.1990.258.2.G196. [DOI] [PubMed] [Google Scholar]
- Layer P., Schlesinger T., Gröger G., Goebell H. Modulation of human periodic interdigestive gastrointestinal motor and pancreatic function by the ileum. Pancreas. 1993 Jul;8(4):426–432. doi: 10.1097/00006676-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Layer P., Yamamoto H., Kalthoff L., Clain J. E., Bakken L. J., DiMagno E. P. The different courses of early- and late-onset idiopathic and alcoholic chronic pancreatitis. Gastroenterology. 1994 Nov;107(5):1481–1487. doi: 10.1016/0016-5085(94)90553-3. [DOI] [PubMed] [Google Scholar]
- Layer P., Zinsmeister A. R., DiMagno E. P. Effects of decreasing intraluminal amylase activity on starch digestion and postprandial gastrointestinal function in humans. Gastroenterology. 1986 Jul;91(1):41–48. doi: 10.1016/0016-5085(86)90436-1. [DOI] [PubMed] [Google Scholar]
- Layer P., vd Ohe M., Müller M. K., Beglinger C. Effects of somatostatin on the exocrine pancreas. Scand J Gastroenterol. 1991 Feb;26(2):129–136. doi: 10.3109/00365529109025022. [DOI] [PubMed] [Google Scholar]
- Layer P., von der Ohe M. R., Holst J. J., Jansen J. B., Grandt D., Holtmann G., Goebell H. Altered postprandial motility in chronic pancreatitis: role of malabsorption. Gastroenterology. 1997 May;112(5):1624–1634. doi: 10.1016/s0016-5085(97)70045-3. [DOI] [PubMed] [Google Scholar]
- Layer Peter, Keller Jutta. Gastric lipase and pancreatic exocrine insufficiency. Clin Gastroenterol Hepatol. 2005 Jan;3(1):25–27. doi: 10.1016/s1542-3565(04)00607-x. [DOI] [PubMed] [Google Scholar]
- Layer Peter, Keller Jutta. Lipase supplementation therapy: standards, alternatives, and perspectives. Pancreas. 2003 Jan;26(1):1–7. doi: 10.1097/00006676-200301000-00001. [DOI] [PubMed] [Google Scholar]
- Levitt M. D., Hirsh P., Fetzer C. A., Sheahan M., Levine A. S. H2 excretion after ingestion of complex carbohydrates. Gastroenterology. 1987 Feb;92(2):383–389. doi: 10.1016/0016-5085(87)90132-6. [DOI] [PubMed] [Google Scholar]
- Lipp R. W., Schnedl W. J., Hammer H. F., Kotanko P., Leb G., Krejs G. J. Effects of postprandial walking on delayed gastric emptying and intragastric meal distribution in longstanding diabetics. Am J Gastroenterol. 2000 Feb;95(2):419–424. doi: 10.1111/j.1572-0241.2000.01761.x. [DOI] [PubMed] [Google Scholar]
- Littlewood J. M., Wolfe S. P. Control of malabsorption in cystic fibrosis. Paediatr Drugs. 2000 May-Jun;2(3):205–222. doi: 10.2165/00128072-200002030-00005. [DOI] [PubMed] [Google Scholar]
- Loba J. M., Saryusz-Wolska M., Czupryniak L., Kukulski K. Pancreatic polypeptide secretion in diabetic patients with delayed gastric emptying and autonomic neuropathy. J Diabetes Complications. 1997 Nov-Dec;11(6):328–333. doi: 10.1016/s1056-8727(96)00103-1. [DOI] [PubMed] [Google Scholar]
- Logsdon C. D. Stimulation of pancreatic acinar cell growth by CCK, epidermal growth factor, and insulin in vitro. Am J Physiol. 1986 Oct;251(4 Pt 1):G487–G494. doi: 10.1152/ajpgi.1986.251.4.G487. [DOI] [PubMed] [Google Scholar]
- Löhr-Happe A., Peiper M., Lankisch P. G. Natural course of operated pseudocysts in chronic pancreatitis. Gut. 1994 Oct;35(10):1479–1482. doi: 10.1136/gut.35.10.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löhr M., Klöppel G. Pathologie des Pankreas beim chronischen Typ 1 Diabetes mellitus: B-Zell-Gehalt, exokrine Atrophie und Angiopathie. Verh Dtsch Ges Pathol. 1987;71:114–119. [PubMed] [Google Scholar]
- MacGregor I. L., Deveney C., Way L. W., Meyer J. H. The effect of acute hyperglycemia on meal-stimulated gastric, biliary, and pancreatic secretion, and serum gastrin. Gastroenterology. 1976 Feb;70(2):197–202. [PubMed] [Google Scholar]
- Maeda H., Danel C., Crystal R. G. Adenovirus-mediated transfer of human lipase complementary DNA to the gallbladder. Gastroenterology. 1994 Jun;106(6):1638–1644. doi: 10.1016/0016-5085(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Malagelada J. R., Go V. L., DiMagno E. P., Summerskill W. H. Interactions between intraluminal bile acids and digestive products on pancreatic and gallbladder function. J Clin Invest. 1973 Sep;52(9):2160–2165. doi: 10.1172/JCI107400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagelada J. R., Go V. L., Summerskill W. H. Altered pancreatic and biliary function after vagotomy and pyloroplasty. Gastroenterology. 1974 Jan;66(1):22–27. [PubMed] [Google Scholar]
- Malagelada J. R., Go V. L., Summerskill W. H. Different gastric, pancreatic, and biliary responses to solid-liquid or homogenized meals. Dig Dis Sci. 1979 Feb;24(2):101–110. doi: 10.1007/BF01324736. [DOI] [PubMed] [Google Scholar]
- Mally M. I., Cirulli V., Hayek A., Otonkoski T. ICA69 is expressed equally in the human endocrine and exocrine pancreas. Diabetologia. 1996 Apr;39(4):474–480. doi: 10.1007/BF00400680. [DOI] [PubMed] [Google Scholar]
- Maouyo D., Guan D., Rivard N., Morisset J. Modulation of the relationship between amylase and chymotrypsinogen secretion in atropine- and MK329-infused rats. Pancreas. 1995 Nov;11(4):330–340. doi: 10.1097/00006676-199511000-00003. [DOI] [PubMed] [Google Scholar]
- Maouyo D., Morisset J. Amazing pancreas: specific regulation of pancreatic secretion of individual digestive enzymes in rats. Am J Physiol. 1995 Feb;268(2 Pt 1):E349–E359. doi: 10.1152/ajpendo.1995.268.2.E349. [DOI] [PubMed] [Google Scholar]
- Maouyo D., Morisset J. Modulation of pancreatic secretion of individual digestive enzymes in octreotide (SMS 201-995)-infused rats. Pancreas. 1997 Jan;14(1):47–57. doi: 10.1097/00006676-199701000-00008. [DOI] [PubMed] [Google Scholar]
- Maton P. N., Selden A. C., Fitzpatrick M. L., Chadwick V. S. Defective gallbladder emptying and cholecystokinin release in celiac disease. Reversal by gluten-free diet. Gastroenterology. 1985 Feb;88(2):391–396. doi: 10.1016/0016-5085(85)90497-4. [DOI] [PubMed] [Google Scholar]
- Matsushita K., Okabayashi Y., Koide M., Hasegawa H., Otsuki M., Kasuga M. Potentiating effect of insulin on exocrine secretory function in isolated rat pancreatic acini. Gastroenterology. 1994 Jan;106(1):200–206. doi: 10.1016/s0016-5085(94)95395-3. [DOI] [PubMed] [Google Scholar]
- McArthur K. E., Feldman M. Gastric acid secretion, gastrin release, and gastric emptying in humans as affected by liquid meal temperature. Am J Clin Nutr. 1989 Jan;49(1):51–54. doi: 10.1093/ajcn/49.1.51. [DOI] [PubMed] [Google Scholar]
- Meyer J. H., Lake R. Mismatch of duodenal deliveries of dietary fat and pancreatin from enterically coated microspheres. Pancreas. 1997 Oct;15(3):226–235. doi: 10.1097/00006676-199710000-00003. [DOI] [PubMed] [Google Scholar]
- Miller L. J., Clain J. E., Malagelada J. R., Go V. L. Control of human postprandial pancreatic exocrine secretion: a function of the gastroduodenal region. Dig Dis Sci. 1979 Feb;24(2):150–154. doi: 10.1007/BF01324743. [DOI] [PubMed] [Google Scholar]
- Mitchell C. J., Playforth M. J., Kelleher J., McMahon M. J. Functional recovery of the exocrine pancreas after acute pancreatitis. Scand J Gastroenterol. 1983 Jan;18(1):5–8. doi: 10.3109/00365528309181549. [DOI] [PubMed] [Google Scholar]
- Mizuno R., Hayakawa T., Noda A. Elastase secretion in pancreatic disease. Am J Gastroenterol. 1985 Feb;80(2):113–117. [PubMed] [Google Scholar]
- Morales M., Galván E., Mery C. M., Castro G., Uscanga L. F., Robles-Díaz G. Exocrine pancreatic insufficiency in tropical sprue. Digestion. 2001;63(1):30–34. doi: 10.1159/000051869. [DOI] [PubMed] [Google Scholar]
- Moreau H., Bernadac A., Gargouri Y., Benkouka F., Laugier R., Verger R. Immunocytolocalization of human gastric lipase in chief cells of the fundic mucosa. Histochemistry. 1989;91(5):419–423. doi: 10.1007/BF00493829. [DOI] [PubMed] [Google Scholar]
- Moreau H., Laugier R., Gargouri Y., Ferrato F., Verger R. Human preduodenal lipase is entirely of gastric fundic origin. Gastroenterology. 1988 Nov;95(5):1221–1226. doi: 10.1016/0016-5085(88)90354-x. [DOI] [PubMed] [Google Scholar]
- Moreau J., Bouisson M., Balas D., Ravaud A., Stupnik S., Buscail L., Vaysse N., Ribet A. Gastric lipase in alcoholic pancreatitis. Comparison of secretive profiles following pentagastrin stimulation in normal adults and patients with pancreatic insufficiency. Gastroenterology. 1990 Jul;99(1):175–180. doi: 10.1016/0016-5085(90)91245-2. [DOI] [PubMed] [Google Scholar]
- Murayama K. M., Drew J. B., Nahrwold D. L., Joehl R. J. Acute edematous pancreatitis impairs pancreatic secretion in rats. Surgery. 1990 Mar;107(3):302–310. [PubMed] [Google Scholar]
- Murphy M. S., Brunetto A. L., Pearson A. D., Ghatei M. A., Nelson R., Eastham E. J., Bloom S. R., Green A. A. Gut hormones and gastrointestinal motility in children with cystic fibrosis. Dig Dis Sci. 1992 Feb;37(2):187–192. doi: 10.1007/BF01308170. [DOI] [PubMed] [Google Scholar]
- Mäkelä A., Kuusi T., Seppälä K., Schöder T. Duodenal secretion of phospholipase A2, amylase, and bicarbonate in chronic pancreatitis. J Gastroenterol. 1998 Apr;33(2):260–266. doi: 10.1007/s005350050080. [DOI] [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Aguilar-Parada E., Unger R. H. Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med. 1970 Jul 16;283(3):109–115. doi: 10.1056/NEJM197007162830301. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Takeuchi T. Pancreatic steatorrhea, malabsorption, and nutrition biochemistry: a comparison of Japanese, European, and American patients with chronic pancreatitis. Pancreas. 1997 May;14(4):323–333. doi: 10.1097/00006676-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Niederau C., Niederau M., Lüthen R., Strohmeyer G., Ferrell L. D., Grendell J. H. Pancreatic exocrine secretion in acute experimental pancreatitis. Gastroenterology. 1990 Oct;99(4):1120–1127. doi: 10.1016/0016-5085(90)90633-c. [DOI] [PubMed] [Google Scholar]
- Nilsson H., Bergström B., Lilja B., Juul-Möller S., Carlsson J., Sundkvist G. Prospective study of autonomic nerve function in type 1 and type 2 diabetic patients: 24 hour heart rate variation and plasma motilin levels disturbed in parasympathetic neuropathy. Diabet Med. 1995 Nov;12(11):1015–1021. doi: 10.1111/j.1464-5491.1995.tb00415.x. [DOI] [PubMed] [Google Scholar]
- Nordgaard I., Mortensen P. B. Digestive processes in the human colon. Nutrition. 1995 Jan-Feb;11(1):37–45. [PubMed] [Google Scholar]
- Nouri-Sorkhabi M. H., Chapman B. E., Kuchel P. W., Gruca M. A., Gaskin K. J. Parallel secretion of pancreatic phospholipase A(2), phospholipase A(1), lipase, and colipase in children with exocrine pancreatic dysfunction. Pediatr Res. 2000 Dec;48(6):735–740. doi: 10.1203/00006450-200012000-00006. [DOI] [PubMed] [Google Scholar]
- Nousia-Arvanitakis S., Karagiozoglou-Lamboudes T., Aggouridaki C., Malaka-Lambrellis E., Galli-Tsinopoulou A., Xefteri M. Influence of jejunal morphology changes on exocrine pancreatic function in celiac disease. J Pediatr Gastroenterol Nutr. 1999 Jul;29(1):81–85. doi: 10.1097/00005176-199907000-00019. [DOI] [PubMed] [Google Scholar]
- O'Keefe S. J., Cariem A. K., Levy M. The exacerbation of pancreatic endocrine dysfunction by potent pancreatic exocrine supplements in patients with chronic pancreatitis. J Clin Gastroenterol. 2001 Apr;32(4):319–323. doi: 10.1097/00004836-200104000-00008. [DOI] [PubMed] [Google Scholar]
- O'Keefe Stephen J. D., Lee Ronzo B., Anderson Frank P., Gennings Chris, Abou-Assi Souheil, Clore John, Heuman Douglas, Chey William. Physiological effects of enteral and parenteral feeding on pancreaticobiliary secretion in humans. Am J Physiol Gastrointest Liver Physiol. 2003 Jan;284(1):G27–G36. doi: 10.1152/ajpgi.00155.2002. [DOI] [PubMed] [Google Scholar]
- Ogden J. M., O'Keefe S. J., Louw J. A., Adams G., Marks I. N. Duodenal juice total protein and pancreatic enzyme synthesis, turnover, and secretion in patients after acute pancreatitis. Gut. 1993 Sep;34(9):1261–1266. doi: 10.1136/gut.34.9.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka T., Yamaguchi K., Chijiiwa K., Tanaka M. Postoperative pancreatic exocrine function influences body weight maintenance after pylorus-preserving pancreatoduodenectomy. Am J Surg. 2001 Nov;182(5):524–529. doi: 10.1016/s0002-9610(01)00745-0. [DOI] [PubMed] [Google Scholar]
- Olsen O., Schaffalitzky de Muckadell O. B., Cantor P. Fat and pancreatic secretion. Scand J Gastroenterol. 1989 Jan;24(1):74–80. doi: 10.3109/00365528909092242. [DOI] [PubMed] [Google Scholar]
- Osawa Saori, Kataoka Keisho, Sakagami Junichi, Sogame Yoshio, Kawasaki Chizu, Takaoka Kyojiro, Yasuda Hiroaki, Takatera Ami. Relation between morphologic changes in the main pancreatic duct and exocrine pancreatic function after a secretin test. Pancreas. 2002 Jul;25(1):12–19. doi: 10.1097/00006676-200207000-00005. [DOI] [PubMed] [Google Scholar]
- Otte M., Thurmayr G. R., Dageförde J., Thurmayr R., Forell M. M. Pankreassekretion bei einheimischer Sprue. Dtsch Med Wochenschr. 1985 Feb 15;110(7):259–264. doi: 10.1055/s-2008-1068809. [DOI] [PubMed] [Google Scholar]
- Owyang C., Louie D. S., Tatum D. Feedback regulation of pancreatic enzyme secretion. Suppression of cholecystokinin release by trypsin. J Clin Invest. 1986 Jun;77(6):2042–2047. doi: 10.1172/JCI112534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicot L., Mas E., Thivolet C., Lombardo D. Circulating antibodies against an exocrine pancreatic enzyme in type 1 diabetes. Diabetes. 1999 Dec;48(12):2316–2323. doi: 10.2337/diabetes.48.12.2316. [DOI] [PubMed] [Google Scholar]
- Perez M. M., Newcomer A. D., Moertel C. G., Go V. L., Dimagno E. P. Assessment of weight loss, food intake, fat metabolism, malabsorption, and treatment of pancreatic insufficiency in pancreatic cancer. Cancer. 1983 Jul 15;52(2):346–352. doi: 10.1002/1097-0142(19830715)52:2<346::aid-cncr2820520228>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Perri F., Pastore M., Festa V., Clemente R., Quitadamo M., D'Altilia M. R., Niro G., Paolucci P., Andriulli A. Intraduodenal lipase activity in celiac disease assessed by means of 13C mixed-triglyceride breath test. J Pediatr Gastroenterol Nutr. 1998 Oct;27(4):407–410. doi: 10.1097/00005176-199810000-00008. [DOI] [PubMed] [Google Scholar]
- Pieramico O., Dominguez-Muñoz J. E., Nelson D. K., Böck W., Büchler M., Malfertheiner P. Interdigestive cycling in chronic pancreatitis: altered coordination among pancreatic secretion, motility, and hormones. Gastroenterology. 1995 Jul;109(1):224–230. doi: 10.1016/0016-5085(95)90288-0. [DOI] [PubMed] [Google Scholar]
- Proesmans Marijke, De Boeck Kris. Omeprazole, a proton pump inhibitor, improves residual steatorrhoea in cystic fibrosis patients treated with high dose pancreatic enzymes. Eur J Pediatr. 2003 Sep 17;162(11):760–763. doi: 10.1007/s00431-003-1309-5. [DOI] [PubMed] [Google Scholar]
- Raimondo M., DiMagno E. P. Lipolytic activity of bacterial lipase survives better than that of porcine lipase in human gastric and duodenal content. Gastroenterology. 1994 Jul;107(1):231–235. doi: 10.1016/0016-5085(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Rathmann W., Haastert B., Icks A., Giani G., Hennings S., Mitchell J., Curran S., Wareham N. J. Low faecal elastase 1 concentrations in type 2 diabetes mellitus. Scand J Gastroenterol. 2001 Oct;36(10):1056–1061. doi: 10.1080/003655201750422657. [DOI] [PubMed] [Google Scholar]
- Read N. W., McFarlane A., Kinsman R. I., Bates T. E., Blackhall N. W., Farrar G. B., Hall J. C., Moss G., Morris A. P., O'Neill B. Effect of infusion of nutrient solutions into the ileum on gastrointestinal transit and plasma levels of neurotensin and enteroglucagon. Gastroenterology. 1984 Feb;86(2):274–280. [PubMed] [Google Scholar]
- Regan P. T., DiMagno E. P. Exocrine pancreatic insufficiency in celiac sprue: a cause of treatment failure. Gastroenterology. 1980 Mar;78(3):484–487. [PubMed] [Google Scholar]
- Regan P. T., Malagelada J. R., DiMagno E. P., Glanzman S. L., Go V. L. Comparative effects of antacids, cimetidine and enteric coating on the therapeutic response to oral enzymes in severe pancreatic insufficiency. N Engl J Med. 1977 Oct 20;297(16):854–858. doi: 10.1056/NEJM197710202971603. [DOI] [PubMed] [Google Scholar]
- Regan P. T., Malagelada J. R., Dimagno E. P., Go V. L. Rationale for the use of cimetidine in pancreatic insufficiency. Mayo Clin Proc. 1978 Feb;53(2):79–83. [PubMed] [Google Scholar]
- Regan P. T., Malagelada J. R., Go V. L., Wolf A. M., DiMagno E. P. A prospective study of the antisecretory and therapeutic effects of cimetidine and glucagon in human acute pancreatitis. Mayo Clin Proc. 1981 Aug;56(8):499–503. [PubMed] [Google Scholar]
- Renou C., Carrière F., Ville E., Grandval P., Joubert-Collin M., Laugier R. Effects of lansoprazole on human gastric lipase secretion and intragastric lipolysis in healthy human volunteers. Digestion. 2001;63(4):207–213. doi: 10.1159/000051891. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Rogos R., Appelt G., Möller T., Wegner D. Der Sekretin-Pankreozymin-Test mit Volumenkorrektur zur Funktionsdiagnostik der pankreatobiliären Sekretion. Dtsch Z Verdau Stoffwechselkr. 1987;47(5):242–251. [PubMed] [Google Scholar]
- Rushakoff R. A., Goldfine I. D., Beccaria L. J., Mathur A., Brand R. J., Liddle R. A. Reduced postprandial cholecystokinin (CCK) secretion in patients with noninsulin-dependent diabetes mellitus: evidence for a role for CCK in regulating postprandial hyperglycemia. J Clin Endocrinol Metab. 1993 Feb;76(2):489–493. doi: 10.1210/jcem.76.2.8432795. [DOI] [PubMed] [Google Scholar]
- Sarles H., Augustine P., Laugier R., Mathew S., Dupuy P. Pancreatic lesions and modifications of pancreatic juice in tropical chronic pancreatitis (tropical calcific diabetes). Dig Dis Sci. 1994 Jun;39(6):1337–1344. doi: 10.1007/BF02093802. [DOI] [PubMed] [Google Scholar]
- Sarles J., Moreau H., Verger R. Human gastric lipase: ontogeny and variations in children. Acta Paediatr. 1992 Jun-Jul;81(6-7):511–513. doi: 10.1111/j.1651-2227.1992.tb12284.x. [DOI] [PubMed] [Google Scholar]
- Sato N., Yamaguchi K., Yokohata K., Shimizu S., Morisaki T., Chijiiwa K., Tanaka M. Short-term and long-term pancreatic exocrine and endocrine functions after pancreatectomy. Dig Dis Sci. 1998 Dec;43(12):2616–2621. doi: 10.1023/a:1026686824173. [DOI] [PubMed] [Google Scholar]
- Savage A. P., Adrian T. E., Carolan G., Chatterjee V. K., Bloom S. R. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut. 1987 Feb;28(2):166–170. doi: 10.1136/gut.28.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele G. A., Fukuoka S. I., Kern H. F., Freedman S. D. Pancreatic dysfunction in cystic fibrosis occurs as a result of impairments in luminal pH, apical trafficking of zymogen granule membranes, and solubilization of secretory enzymes. Pancreas. 1996 Jan;12(1):1–9. doi: 10.1097/00006676-199601000-00001. [DOI] [PubMed] [Google Scholar]
- Schmidt W. E., Creutzfeldt W., Schleser A., Choudhury A. R., Nustede R., Höcker M., Nitsche R., Sostmann H., Rovati L. C., Fölsch U. R. Role of CCK in regulation of pancreaticobiliary functions and GI motility in humans: effects of loxiglumide. Am J Physiol. 1991 Feb;260(2 Pt 1):G197–G206. doi: 10.1152/ajpgi.1991.260.2.G197. [DOI] [PubMed] [Google Scholar]
- Schwarz A., Büchler M., Usinger K., Rieger H., Glasbrenner B., Friess H., Kunz R., Beger H. G. Importance of the duodenal passage and pouch volume after total gastrectomy and reconstruction with the Ulm pouch: prospective randomized clinical study. World J Surg. 1996 Jan;20(1):60–67. doi: 10.1007/s002689900011. [DOI] [PubMed] [Google Scholar]
- Schäppi M. G., Smith V. V., Cubitt D., Milla P. J., Lindley K. J. Faecal elastase 1 concentration is a marker of duodenal enteropathy. Arch Dis Child. 2002 Jan;86(1):50–53. doi: 10.1136/adc.86.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibold F., Mörk H., Tanza S., Müller A., Holzhüter C., Weber P., Scheurlen M. Pancreatic autoantibodies in Crohn's disease: a family study. Gut. 1997 Apr;40(4):481–484. doi: 10.1136/gut.40.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidensticker F., Otto J., Lankisch P. G. Recovery of the pancreas after acute pancreatitis is not necessarily complete. Int J Pancreatol. 1995 Jun;17(3):225–229. doi: 10.1007/BF02785818. [DOI] [PubMed] [Google Scholar]
- Semakula C., Vandewalle C. L., Van Schravendijk C. F., Sodoyez J. C., Schuit F. C., Foriers A., Falorni A., Craen M., Decraene P., Pipeleers D. G. Abnormal circulating pancreatic enzyme activities in more than twenty-five percent of recent-onset insulin-dependent diabetic patients: association of hyperlipasemia with high-titer islet cell antibodies. Belgian Diabetes Registry. Pancreas. 1996 May;12(4):321–333. doi: 10.1097/00006676-199605000-00001. [DOI] [PubMed] [Google Scholar]
- Silva M. E., Vezozzo D. P., Ursich M. J., Rocha D. M., Cerri G. G., Wajchenberg B. L. Ultrasonographic abnormalities of the pancreas in IDDM and NIDDM patients. Diabetes Care. 1993 Sep;16(9):1296–1297. doi: 10.2337/diacare.16.9.1296. [DOI] [PubMed] [Google Scholar]
- Singh J., Adeghate E., Salido G. M., Pariente J. A., Yago M. D., Juma L. O. Interaction of islet hormones with cholecystokinin octapeptide-evoked secretory responses in the isolated pancreas of normal and diabetic rats. Exp Physiol. 1999 Mar;84(2):299–318. doi: 10.1111/j.1469-445x.1999.01733.x. [DOI] [PubMed] [Google Scholar]
- Singh J. Mechanism of action of insulin on acetylcholine-evoked amylase secretion in the mouse pancreas. J Physiol. 1985 Jan;358:469–482. doi: 10.1113/jphysiol.1985.sp015562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skude G., Ihse I. Salivary amylase in duodenal aspirates. Scand J Gastroenterol. 1976;11(1):17–20. [PubMed] [Google Scholar]
- Slaff J., Jacobson D., Tillman C. R., Curington C., Toskes P. Protease-specific suppression of pancreatic exocrine secretion. Gastroenterology. 1984 Jul;87(1):44–52. [PubMed] [Google Scholar]
- Sommer H. Functional recovery of the exocrine pancreas in rats after partial resection. Eur Surg Res. 1987;19(5):318–322. doi: 10.1159/000128716. [DOI] [PubMed] [Google Scholar]
- Soper N. J., Chapman N. J., Kelly K. A., Brown M. L., Phillips S. F., Go V. L. The 'ileal brake' after ileal pouch-anal anastomosis. Gastroenterology. 1990 Jan;98(1):111–116. doi: 10.1016/0016-5085(90)91298-k. [DOI] [PubMed] [Google Scholar]
- Spiller R. C., Trotman I. F., Adrian T. E., Bloom S. R., Misiewicz J. J., Silk D. B. Further characterisation of the 'ileal brake' reflex in man--effect of ileal infusion of partial digests of fat, protein, and starch on jejunal motility and release of neurotensin, enteroglucagon, and peptide YY. Gut. 1988 Aug;29(8):1042–1051. doi: 10.1136/gut.29.8.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen A. M., Haddad A. C., Phillips S. F. Passage of carbohydrate into the colon. Direct measurements in humans. Gastroenterology. 1983 Sep;85(3):589–595. [PubMed] [Google Scholar]
- Stöcker W., Otte M., Ulrich S., Normann D., Finkbeiner H., Stöcker K., Jantschek G., Scriba P. C. Autoimmunity to pancreatic juice in Crohn's disease. Results of an autoantibody screening in patients with chronic inflammatory bowel disease. Scand J Gastroenterol Suppl. 1987;139:41–52. doi: 10.3109/00365528709089774. [DOI] [PubMed] [Google Scholar]
- Sun W. M., Houghton L. A., Read N. W., Grundy D. G., Johnson A. G. Effect of meal temperature on gastric emptying of liquids in man. Gut. 1988 Mar;29(3):302–305. doi: 10.1136/gut.29.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W. M., Penagini R., Hebbard G., Malbert C., Jones K. L., Emery S., Dent J., Horowitz M. Effect of drink temperature on antropyloroduodenal motility and gastric electrical activity in humans. Gut. 1995 Sep;37(3):329–334. doi: 10.1136/gut.37.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Mizumoto A., Rerknimitr R., Sarr M. G., DiMango E. P. Effect of bacterial or porcine lipase with low- or high-fat diets on nutrient absorption in pancreatic-insufficient dogs. Gastroenterology. 1999 Feb;116(2):431–437. doi: 10.1016/s0016-5085(99)70141-1. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Mizumoto A., Sarr M. G., DiMagno E. P. Bacterial lipase and high-fat diets in canine exocrine pancreatic insufficiency: a new therapy of steatorrhea? Gastroenterology. 1997 Jun;112(6):2048–2055. doi: 10.1053/gast.1997.v112.pm9178698. [DOI] [PubMed] [Google Scholar]
- Symersky T., Vu M. K., Frölich M., Biemond I., Masclee A. A. M. The effect of equicaloric medium-chain and long-chain triglycerides on pancreas enzyme secretion. Clin Physiol Funct Imaging. 2002 Sep;22(5):307–311. doi: 10.1046/j.1475-097x.2002.00435.x. [DOI] [PubMed] [Google Scholar]
- Symonds Erin L., Omari Taher I., Webster Judy M., Davidson Geoffrey P., Butler Ross N. Relation between pancreatic lipase activity and gastric emptying rate in children with cystic fibrosis. J Pediatr. 2003 Dec;143(6):772–775. doi: 10.1067/S0022-3476(03)00581-X. [DOI] [PubMed] [Google Scholar]
- Talley S. J., Bytzer P., Hammer J., Young L., Jones M., Horowitz M. Psychological distress is linked to gastrointestinal symptoms in diabetes mellitus. Am J Gastroenterol. 2001 Apr;96(4):1033–1038. doi: 10.1111/j.1572-0241.2001.03605.x. [DOI] [PubMed] [Google Scholar]
- Taylor C. J., Hillel P. G., Ghosal S., Frier M., Senior S., Tindale W. B., Read N. Gastric emptying and intestinal transit of pancreatic enzyme supplements in cystic fibrosis. Arch Dis Child. 1999 Feb;80(2):149–152. doi: 10.1136/adc.80.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimister P. W., Hopman W. P., Loualidi A., Rosenbusch G., Willems H. L., Trijbels F. J., Jansen J. B. Cholestyramine influences meal-stimulated pancreaticobiliary function and plasma cholecystokinin independent of gastric emptying and food digestion. Scand J Gastroenterol. 1997 Aug;32(8):778–784. doi: 10.3109/00365529708996534. [DOI] [PubMed] [Google Scholar]
- Thimister P. W., Hopman W. P., Sloots C. E., Rosenbusch G., Willems H. L., Trijbels F. J., Jansen J. B. Role of intraduodenal proteases in plasma cholecystokinin and pancreaticobiliary responses to protein and amino acids. Gastroenterology. 1996 Feb;110(2):567–575. doi: 10.1053/gast.1996.v110.pm8566605. [DOI] [PubMed] [Google Scholar]
- Thimister P. W., Hopman W. P., van Roermund R. F., Willems H. L., Rosenbusch G., Woestenborghs R., Jansen J. B. Inhibition of pancreaticobiliary secretion by loperamide in humans. Hepatology. 1997 Aug;26(2):256–261. doi: 10.1002/hep.510260201. [DOI] [PubMed] [Google Scholar]
- Thiruvengadam R., DiMagno E. P. Inactivation of human lipase by proteases. Am J Physiol. 1988 Oct;255(4 Pt 1):G476–G481. doi: 10.1152/ajpgi.1988.255.4.G476. [DOI] [PubMed] [Google Scholar]
- Tiscornia O. M., Cresta M. A., de Lehmann E. S., Celener D., Dreiling D. A. Effects of sex and age on pancreatic secretion. Int J Pancreatol. 1986 Jul;1(2):95–118. doi: 10.1007/BF02788443. [DOI] [PubMed] [Google Scholar]
- Tiscornia O. M. Human exocrine pancreatic response with different types of secretin. Influence of sex, age, and previous intraduodenal sorbitol infusion. Am J Gastroenterol. 1978 Feb;69(2):166–175. [PubMed] [Google Scholar]
- Unger R. H., Aguilar-Parada E., Müller W. A., Eisentraut A. M. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970 Apr;49(4):837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VACCA J. B., HENKE W. J., KNIGHT W. A., Jr THE EXOCRINE PANCREAS IN DIABETES MELLITUS. Ann Intern Med. 1964 Aug;61:242–247. doi: 10.7326/0003-4819-61-2-242. [DOI] [PubMed] [Google Scholar]
- Vellas B., Balas D., Moreau J., Bouisson M., Senegas-Balas F., Guidet M., Ribet A. Exocrine pancreatic secretion in the elderly. Int J Pancreatol. 1988 Dec;3(6):497–502. doi: 10.1007/BF02788208. [DOI] [PubMed] [Google Scholar]
- Vidon N., Pfeiffer A., Franchisseur C., Bovet M., Rongier M., Bernier J. J. Effect of different caloric loads in human jejunum on meal-stimulated and nonstimulated biliopancreatic secretion. Am J Clin Nutr. 1988 Mar;47(3):400–405. doi: 10.1093/ajcn/47.3.400. [DOI] [PubMed] [Google Scholar]
- Walkowiak J., Cichy W. K., Herzig K. H. Comparison of fecal elastase-1 determination with the secretin-cholecystokinin test in patients with cystic fibrosis. Scand J Gastroenterol. 1999 Feb;34(2):202–207. doi: 10.1080/00365529950173104. [DOI] [PubMed] [Google Scholar]
- Walkowiak J., Herzig K. H. Fecal elastase-1 is decreased in villous atrophy regardless of the underlying disease. Eur J Clin Invest. 2001 May;31(5):425–430. doi: 10.1046/j.1365-2362.2001.00822.x. [DOI] [PubMed] [Google Scholar]
- Walkowiak J., Herzig K. H., Witt M., Pogorzelski A., Piotrowski R., Barra E., Sobczynska-Tomaszewska A., Trawinska-Bartnicka M., Strzykala K., Cichy W. Analysis of exocrine pancreatic function in cystic fibrosis: one mild CFTR mutation does not exclude pancreatic insufficiency. Eur J Clin Invest. 2001 Sep;31(9):796–801. doi: 10.1046/j.1365-2362.2001.00876.x. [DOI] [PubMed] [Google Scholar]
- Walkowiak Jaroslaw, Nousia-Arvanitakis Sanda, Agguridaki Christina, Fotoulaki Maria, Strzykala Krystyna, Balassopoulou Angeliki, Witt Michal, Herzig Karl-Heinz. Longitudinal follow-up of exocrine pancreatic function in pancreatic sufficient cystic fibrosis patients using the fecal elastase-1 test. J Pediatr Gastroenterol Nutr. 2003 Apr;36(4):474–478. doi: 10.1097/00005176-200304000-00010. [DOI] [PubMed] [Google Scholar]
- Warshaw A. L., Rattner D. W., Fernández-del Castillo C., Z'graggen K. Middle segment pancreatectomy: a novel technique for conserving pancreatic tissue. Arch Surg. 1998 Mar;133(3):327–331. doi: 10.1001/archsurg.133.3.327. [DOI] [PubMed] [Google Scholar]
- Weizman Z., Ben-Zion Y. Z., Binsztok M., Maor E., Porath A. Correlation of clinical characteristics and small bowel histopathology in celiac disease. J Pediatr Gastroenterol Nutr. 1997 May;24(5):555–558. doi: 10.1097/00005176-199705000-00012. [DOI] [PubMed] [Google Scholar]
- Weizman Z., Forstner G. G., Gaskin K. J., Kopelman H., Wong S., Durie P. R. Bentiromide test for assessing pancreatic dysfunction using analysis of para-aminobenzoic acid in plasma and urine. Studies in cystic fibrosis and Shwachman's syndrome. Gastroenterology. 1985 Sep;89(3):596–604. doi: 10.1016/0016-5085(85)90456-1. [DOI] [PubMed] [Google Scholar]
- Welch I. M., Cunningham K. M., Read N. W. Regulation of gastric emptying by ileal nutrients in humans. Gastroenterology. 1988 Feb;94(2):401–404. doi: 10.1016/0016-5085(88)90428-3. [DOI] [PubMed] [Google Scholar]
- Wettergren A., Schjoldager B., Mortensen P. E., Myhre J., Christiansen J., Holst J. J. Truncated GLP-1 (proglucagon 78-107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci. 1993 Apr;38(4):665–673. doi: 10.1007/BF01316798. [DOI] [PubMed] [Google Scholar]
- Wisén O., Hellström P. M., Johansson C. Meal energy density as a determinant of postprandial gastrointestinal adaptation in man. Scand J Gastroenterol. 1993 Aug;28(8):737–743. doi: 10.3109/00365529309098283. [DOI] [PubMed] [Google Scholar]
- Wormsley K. G. The effect of vagotomy on the human pancreatic response to direct and indirect stimulation. Scand J Gastroenterol. 1972;7(1):85–91. doi: 10.3109/00365527209180742. [DOI] [PubMed] [Google Scholar]
- Wøjdemann M., Olsen O., Nørregaard P., Sternby B., Rehfeld J. F. Gastric lipase secretion after sham feeding and cholinergic blockade. Dig Dis Sci. 1997 May;42(5):1070–1075. doi: 10.1023/a:1018805623669. [DOI] [PubMed] [Google Scholar]
- Wøjdemann M., Sternby B., Larsen S., Olsen O. Cephalic phase of lipolysis is impaired in pancreatic insufficiency: role of gastric lipase. Scand J Gastroenterol. 2000 Feb;35(2):204–211. doi: 10.1080/003655200750024407. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Yokohata K., Nakano K., Ohtani K., Ogawa Y., Chijiiwa K., Tanaka M. Which is a less invasive pancreatic head resection: PD, PPPD, or DPPHR? Dig Dis Sci. 2001 Feb;46(2):282–288. doi: 10.1023/a:1005644614104. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Yokohata K., Ohkido M., Watanabe M., Ogawa Y., Chijiiwa K., Tanaka M. Which is less invasive--distal pancreatectomy or segmental resection? Int Surg. 2000 Oct-Dec;85(4):297–302. [PubMed] [Google Scholar]
- Yasuda H., Takada T., Toyota N., Amano H., Yoshida M., Takada Y., Takada K., Hijikata H. Limited pancreatectomy: significance of postoperative maintenance of pancreatic exocrine function. J Hepatobiliary Pancreat Surg. 2000;7(5):466–472. doi: 10.1007/s005340070016. [DOI] [PubMed] [Google Scholar]
- el Newihi H., Dooley C. P., Saad C., Staples J., Zeidler A., Valenzuela J. E. Impaired exocrine pancreatic function in diabetics with diarrhea and peripheral neuropathy. Dig Dis Sci. 1988 Jun;33(6):705–710. doi: 10.1007/BF01540434. [DOI] [PubMed] [Google Scholar]
- von der Ohe M., Layer P., Wollny C., Ensinck J. W., Peeters T. L., Beglinger C., Goebell H. Somatostatin 28 and coupling of human interdigestive intestinal motility and pancreatic secretion. Gastroenterology. 1992 Sep;103(3):974–981. doi: 10.1016/0016-5085(92)90031-s. [DOI] [PubMed] [Google Scholar]