Abstract
Objective:
To compare primary resection and anastomosis (PRA) with and without defunctioning stoma to Hartmann's procedure (HP) as the optimal operative strategy for patients presenting with Hinchey stage III-IV, perforated diverticulitis.
Summary Background Data:
The choice of operation for perforated diverticulitis lies between HP and PRA. Postoperative mortality and morbidity can be high, and the long-term consequences life-altering, with no established criteria guiding clinicians towards selecting a particular procedure.
Methods:
Probability estimates for 6879 patients with Hinchey III-IV perforated diverticulitis were obtained from two databases (n = 204), supplemented by expert opinion and summary data from 12 studies (n = 6675) published between 1980 and 2005. The primary outcome was quality-adjusted life-years (QALYs) gained from each strategy. Factors considered were the risk of permanent stoma, morbidity, and mortality from the primary or reversal operations. Decision analysis from the patient's perspective was used to calculate the optimal operative strategy and sensitivity analysis performed.
Results:
A total of 135 PRA, 126 primary anastomoses with defunctioning stoma (PADS), and 6619 Hartmann's procedures (HP) were considered. The probability of morbidity and mortality was 55% and 30% for PRA, 40% and 25% for PADS, and 35% and 20% for HP, respectively. Stomas remained permanent in 27% of HP and in 8% of PADS. Analysis revealed the optimal strategy to be PADS with 9.98 QALYs, compared with 9.44 QALYs after HP and 9.02 QALYs after PRA. Complications after PRA reduced patients QALYs to a baseline of 2.713. Patients with postoperative complications during both primary and reversal operations for PADS and HP had QALYs of 0.366 and 0.325, respectively. HP became the optimal strategy only when risk of complications after PRA and PADS reached 50% and 44%, respectively.
Conclusion:
Primary anastomosis with defunctioning stoma may be the optimal strategy for selected patients with diverticular peritonitis as may represent a good compromise between postoperative adverse events, long-term quality of life and risk of permanent stoma. HP may be reserved for patients with risk of complications >40% to 50% after consideration of long-term implications.
A decision analytic study utilizing raw and published data is described, comparing primary resection and anastomosis to Hartmann's procedure for diverticular peritonitis when short- and long-term outcomes are considered. Primary anastomosis with defunctioning stoma was the overall optimal procedure until risk of morbidity exceeded 44% where Hartmann's procedure became the procedure of choice.
Diverticular disease of the colon is common in the Western world, with a prevalence of approximately 33% in patients over 60 years of age.1 Perforation associated with diverticular disease has concurrently increased in prevalence from 2.4 cases per 100,000 in 1986 to 3.8 cases per 100,000 in 2000.2 Surgical treatment has evolved from a three-stage procedure to a two-stage procedure of primary resection of the perforated segment and end colostomy (Hartmann's procedure [HP]) with subsequent restoration of intestinal continuity, after convincing evidence by two randomized trials.3,4
HP has been accepted as the “gold standard” for perforated diverticular disease by the great majority of colorectal surgeons in the United States and the United Kingdom.1 However, there is a growing body of evidence reporting on high complication rates with end stoma reversal procedures.3,5,6 Specifically, anastomotic leak rates of 2% to 30%7–9 and major complication rates of 5% to 25%10–13 have been reported. In addition, 20% to 50% of patients undergoing a Hartmann's procedure are never reversed.9,14,15
Primary resection and anastomosis (PRA) has been proposed as an alternative to Hartmann's procedure in the setting of peritonitis secondary to diverticular disease. Several published studies have reported on comparable morbidity and mortality rates after PRA when compared with HP for perforated diverticular disease.11,12,16 A systematic review by Salem and Flum has shown an overall mortality rate for PRA of 9.9% compared with 18.8% for HP.9 Furthermore, wound infection rate was reported as 9.6% for PRA versus 24.2% for HP. Overall anastomotic leak rate for PRA was 13.9%, whereas stoma-related complication rate for HP was 10.3%. Primary anastomosis with a proximal defunctioning stoma (PADS) was shown to result in even better outcomes in terms of anastomotic leak and wound infection compared with simple PRA. Limited conclusions can, however, be drawn from the existing studies due to significant selection bias in their results.
Determining the optimal operative strategy for patients with perforated diverticular disease involves a balance of postoperative morbidity and mortality after the primary operation, morbidity and mortality from the stoma reversal operation, the risk of permanent stoma, and the quality of life associated with each variable. A randomized controlled trial on the subject is unlikely to be achieved due to difficulties in patient recruitment in the emergency setting and institutional preferences. Decision analysis is particularly useful for comparisons of such competing management strategies in the setting of multiple endpoints and high levels of clinical complexity.17 The aim of the present study was to use decision analytic techniques to determine the optimal operative strategy for a hypothetical 65-year-old patient with peritonitis secondary to perforated diverticulitis.
METHODS
Decision analysis is a quantitative method for estimating the effectiveness of alternative management strategies under conditions of uncertainty.18 Three competing strategies were evaluated: 1) PRA, 2) primary anastomosis with defunctioning stoma (PADS), and 3) HP. The Hinchey classification19 was used to define the degree of peritoneal contamination as follows: (a) stage I, paracolic abscess or phlegmon; (b) stage II, pelvic abscess; (c) stage III, generalized purulent peritonitis; and (d) stage IV, generalized fecal peritonitis. Only stages III and IV were considered in the present study. The 3 strategies were firstly evaluated in the setting of the primary operation only and were then analyzed using a more complex model considering the long-term risks. Decision analysis was performed according to published guidelines.20–24
Base-Case Patient
This was a 65-year-old patient who presented with clinical symptoms suggestive of generalized peritonitis. There was no prior history of colorectal malignancy or benign colonic disease. It was assumed that emergency CT immediately after presentation demonstrated features of generalized peritonitis with widespread diverticular disease/diverticulitis. The patient comorbid conditions were classed as ASA II to III. Emergency operation was carried out with intraoperative findings confirming Hinchey stage III to IV disease secondary to perforated diverticular disease.
Data Sources
A Medline search was conducted of articles published from 1980 to October 2005 to identify English language publications reporting on HP,3,4,6,8,9,11–13,15,16, 25,56 HP reversal3,4,8–13,15,16,28,31,37,46,50,57–69 and PRA with and without a defunctioning stoma.2,6,8,11,13,16,27–29,40,41,43–45,47,48,51,53–56,63,70–82 Data were extracted only from comparative studies between PRA/PADS and HP with groups that were matched for preoperative risk factors and peritonitis severity. Critical appraisal of each study was performed by 2 authors (V.A.C. and P.P.T.) and studies were selected on the basis of recommendations regarding inclusion of studies in decision analytic models.22
Exclusion Criteria
(1) Studies with less than 10 operations or where indications for operations were indistinguishable between cancer and diverticular disease were excluded. (2) Studies that did not adequately distinguish between simple PRA and PADS and studies that excluded fecal peritonitis patients. (3) Studies with “outlier” outcomes compared with the majority of the studies as per published recommendations.22
Summary data were supplemented by data from 2 databases: (1) the Association of Coloproctology of Great Britain and Ireland (ACPGBI) diverticular disease database,83 a prospective database collected over 1 year (2003–2004) from 42 hospitals in the United Kingdom, comprised 539 patients; (2) the Cleveland Clinic Foundation (CCF) diverticular disease database84 of 1069 patients collected over 22 years (1981–2003). Expert opinion was also obtained from 5 specialist colorectal surgeons regarding the probabilities for outcomes. A weighted mean was obtained for each variable and used as the baseline estimate, taking into account the number of patients contributed to each outcome by each data source. The range obtained from the experts was used to guide the sensitivity analysis as it was felt that this was the most reliable source of information. In cases where probabilities were unobtainable from primary studies and databases, a systematic review9 on the subject was considered as the data source together with the expert opinion. Supplementation of published data with raw data from the two databases and expert opinion may account for some of the potential biases and disproportionate representation of groups created by elimination of studies using the exclusion criteria.
Utilities
A “utility” is a measure of the patient's or surgeon's relative preference for each individual outcome and is expressed as a single value between 0 and 1.85 Utility measures provide summary scores that aggregate the positive and negative aspects of quality of life and can incorporate attitudes toward risk and length of life.22 In the present study, utilities were used as weights to calculate quality-adjusted life expectancy. A utility of 1 was assigned for a patient that underwent primary resectional surgery and remained well thereon without the need for reoperation or a permanent stoma. A utility of 0 was assigned to any health state with the outcome of “death.” As there are no available published utilities specifically for diverticular disease in the literature, utilities used in the model were based on the few available studies of utility estimates for related colorectal diseases as reference guidelines. Postoperative complications were given a utility of 0.15 based on a previously published assessment85 and used by other decision analysts.86 Patients with a colostomy were assumed to have a utility of 0.8 based on a previously published assessment made by colostomy patients.87 Patients who underwent a second reversal operation were assigned utilities of 0.9 for defunctioning stoma reversal and 0.8 for Hartmann's reversal. This was based on expert judgment from the colorectal specialists who, after explanation of the utility concept, agreed unanimously to give a lower utility score for HP reversal, reflecting the increased technical difficulty and length of hospital stay, as well as the increased probability of operative complications after this procedure. The uncertainty for this utility was determined by one published study.88
Study Design and Decision Models
The analysis was designed from the patient perspective, using quality-adjusted life-years (QALYs) as the overall outcome measure. QALYs were calculated by subtracting the time period spent in hospital from the initial life expectancy and multiplying the residual value with the utilities assigned for each health state in the model pathway.22 This methods of calculating QALYs is recommended when outcomes consist of combinations of several different health states.89 Health states were divided into short-term states (days to weeks) and long-term states, with temporary hospitalizations for operative procedures considered as short-term states. Postoperative complications and permanent stoma were considered as long-term health states. Length of hospital stay after the primary operation was assumed to be 10 days for all 3 procedures and 5 or 7 days for PADS reversal and HP reversal, respectively. These values reflected the weighted mean length of stay as calculated from the ACPGBI and CCF databases.
Single-Operation Model
A decision tree that considered only the primary operation was initially designed. After each operation a patient may or may not suffer a postoperative complication. In the case of a postoperative complication, a patient may either be discharged home or die. Postoperative complications considered in the model were only those that may be life-threatening and/or have long-term impact in a patient's quality of life as follows: wound infection/dehiscence, anastomotic leak, stoma complications (retraction, stenosis, necrosis), organ failure, intraabdominal abscess, bowel obstruction, major bleeding, chest infection, acute coronary syndrome, pulmonary embolism, and acute neurologic events. The purpose of this initial model was to establish if the optimal operative technique would change when the need for stoma reversal and risk of permanent stoma were integrated in the analysis.
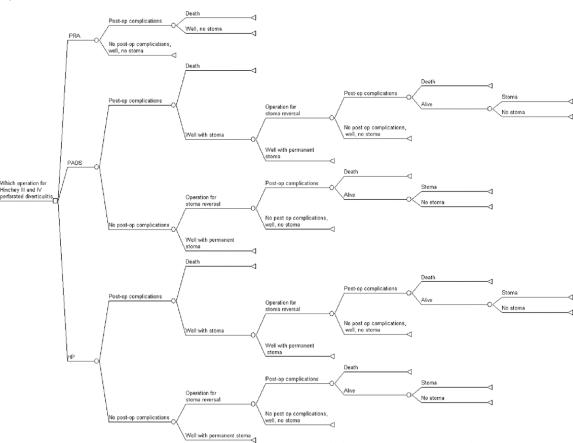
Multiple-Operation Model
This is an extension of the single operation model and is depicted in Figure 1. Patients undergoing PADS and HP were considered candidates for a second operation for stoma reversal, with similar outcome consideration as the primary operations.
FIGURE 1. Multiple-operation decision tree.
Model Assumptions
Assumptions used in this model were: 1) The diagnosis of peritonitis secondary to diverticular disease was assumed to be a definite one. 2) Length of stay for the primary operation was assumed to be the same for all 3 operations. 3) The utility of a defunctioning stoma was assumed to be the same as for colostomy. 4) Only Hinchey stages III and IV were considered. 5) Life expectancy for the base-case patients was assumed to be 18.11 years as determined by actuarial life expectancy tables. 6) Defunctioning stomas were assumed to be colostomies or ileostomies proximal to the anastomosis.
Sensitivity Analysis
Sensitivity analysis is the process of repeatedly analyzing the decision tree using different values for the outcome of interest.23 One-way sensitivity analysis was performed for all variables in the decision model to determine the impact of uncertainty in the estimates of the probabilities, utilities and QALYs. The purpose of the sensitivity analysis was to investigate the robustness of the “base-case” estimates and to identify factors that lead to a shift in optimal operative technique. If the model results did not change through the range of the variable being manipulated, the model was considered not to be sensitive to this variable (ie, there was no value in the plausible range that led to a change in the optimal operative strategy) and no threshold was identified for that variable. However, if the optimal operative strategy changed when a variable was manipulated through its plausible range, the model was considered to be sensitive to this variable and the value at which the optimal strategy changed was considered to be the “threshold value” for the variable. Two-way and three-way sensitivity analyses were subsequently performed for combinations for all influential variables. Threshold values were calculated for variables that would lead to a change in the preferred strategy when traversed. Sensitivity analysis was not performed for the “single operation model” as its sole purpose was to establish a possible change in the base-case estimate of an optimal operative strategy, when long-term outcomes were considered. The decision tree analysis and sensitivity analysis was performed using the software TreeAge Pro (TreeAge Software Inc.).
RESULTS
Review of Included Data Sources
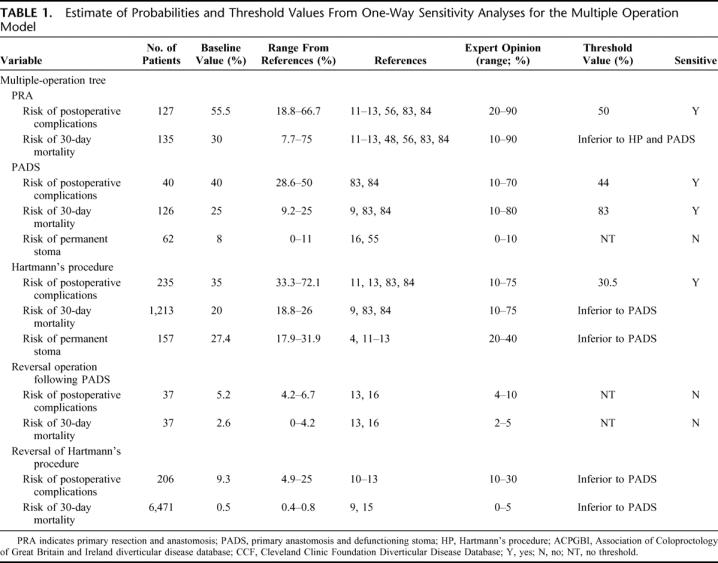
Sixty-seven relevant published studies were identified in the literature,3,6,8,9,11–13,15,16,25–82 of which 12 satisfied the inclusion criteria: one randomized controlled trial;4 three prospective nonrandomized studies;11,12,48 seven retrospective studies;10,13,15,16,55,56,69 and one systematic review.9 A total of 6879 patients were assigned to the three groups as follows: 135 (2.0%) PRA, 125 (1.8%) PADS, and 6619 (96.2%) HP or reversal operations, considered in differing combinations for each outcome. The results of the review of published articles, expert opinion, and the ACPGBI and CCF databases together with the range of uncertainties are summarized in Table 1. 4,9–13,16,48,55,56,83,84 There was a great degree of overlap between the individual outcome ranges for each strategy and expert opinion also varied significantly between specialists, reflecting the high degree of uncertainty regarding each operative strategy.
TABLE 1. Estimate of Probabilities and Threshold Values From One-Way Sensitivity Analyses for the Multiple Operation Model
Base-Case Analysis
Using the “single-operation model,” the quality adjusted life expectancy was 9.18 years for PRA, 11.66 years for PADS, and 12.51 years for HP. Therefore, HP offered a benefit of 0.85 QALYs, which corresponds to 10.2 quality-adjusted life months. For the “multiple-operation model” (Fig. 1), the quality adjusted life expectancy was 9.02 years for PRA, 9.44 years for HP, and 9.98 years for PADS. PADS was found to offer a benefit of 0.54 QALYs (equivalent to 6.5 quality-adjusted life months). In this model, a 1% to 3% probability of leak requiring reoperation and establishment of another stoma were also considered but did not amount to any difference in the overall results and were therefore omitted for the purposes of simplicity.
Sensitivity Analysis
The results of one-way sensitivity analyses are shown in Tables 1 to 3.4,9–13,16,48,55,56,83,84,85,87,88 The “multiple-operation model” was sensitive to analysis of values for postoperative complications. At a probability of complications higher than 44%, PADS was no longer the dominant strategy (Fig. 2, top). HP was the optimal operation only if complications after the procedure remained below 30.5%. Furthermore, sensitivity analysis revealed a change in operative strategy when the risk of operative mortality after PADS exceeded 83%. This value was, however, outside the plausible range and the model was not considered sensitive to this variable (Fig. 2, bottom).
TABLE 2. Estimate of Utilities and Threshold Values From One-Way Sensitivity Analyses
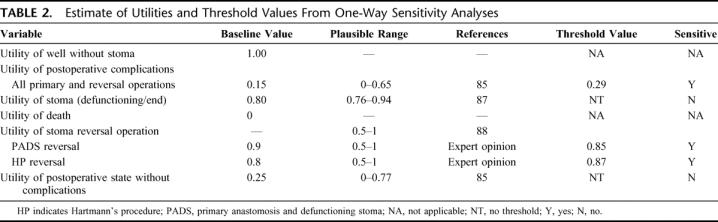
TABLE 3. Quality-Adjusted Life-Years (QALYs) for Individual Outcome Health States
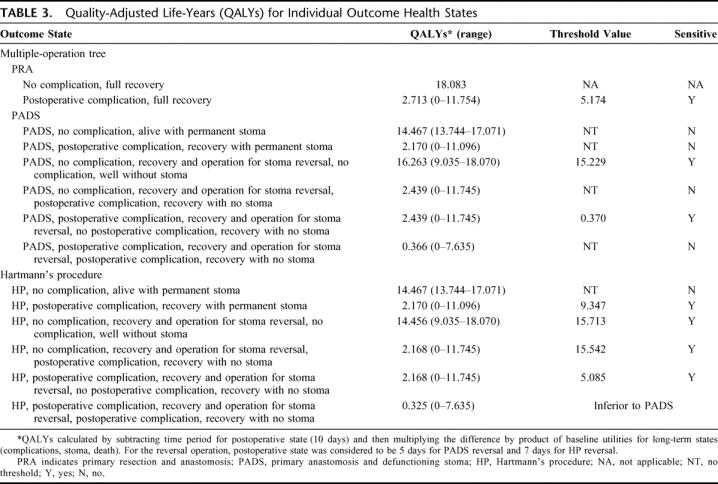
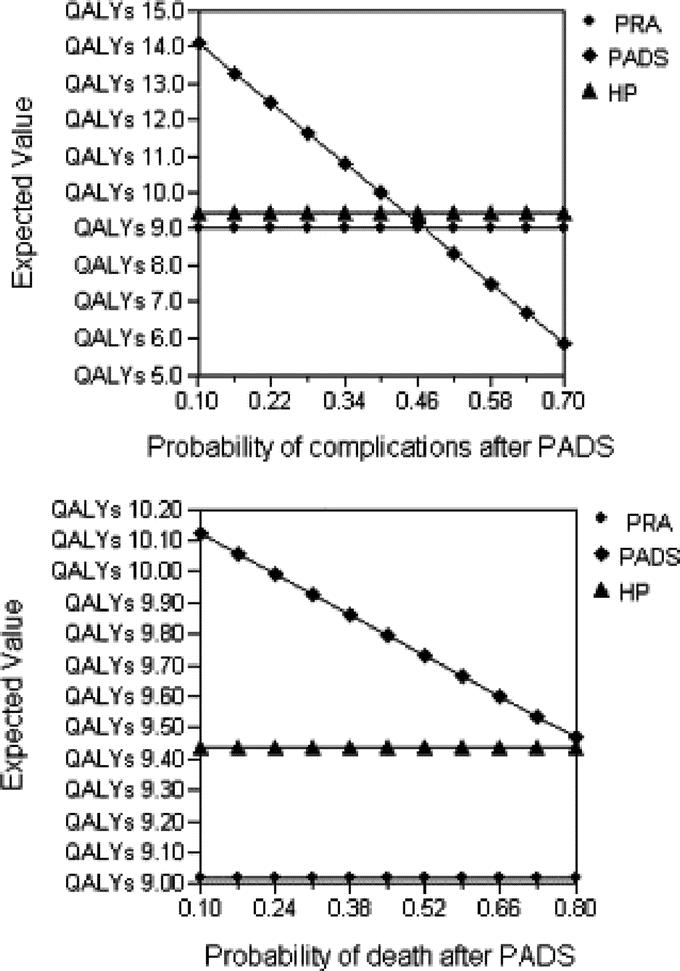
FIGURE 2. One-way sensitivity analysis for probability of complications and mortality after PADS for the multiple-operation model. PRA, primary resection and anastomosis; PADS, primary anastomosis with defunctioning stoma; HP, Hartmann's procedure; QALY, quality-adjusted life-years. Vertical dotted line represents the “threshold value” for complications of HP after which a change in operative strategy is indicated.
Three-way sensitivity analyses varying all the probabilities of complications after PRA, PADS, and HP is shown in Figure 3. At the lower limit of the plausible range for postoperative complications after HP (ie, 10%, Fig. 3, top), the optimal strategy when postoperative complications after PRA and PADS were kept at their baseline value, was HP. At a low probability of complications (<25%), simple PRA was the optimal operative procedure. At 25% to 35% probability of complications after PRA, PADS became the optimal strategy. At a probability of complications of >35% after simple PRA and >22% after PADS, HP became the optimal operative strategy. The middle graph of Figure 3 represents sensitivity analysis for a 30% probability of HP related complications. Under these circumstances, PADS became the operation of choice when the values of PRA and PADS-related complications were kept at the baseline. At this point, HP was the procedure of choice only if the probability of complications after PADS exceeded 40%. At a value of 75% for HP-related complications (Fig. 3, bottom), the choice of operation was only between simple PRA and PADS.
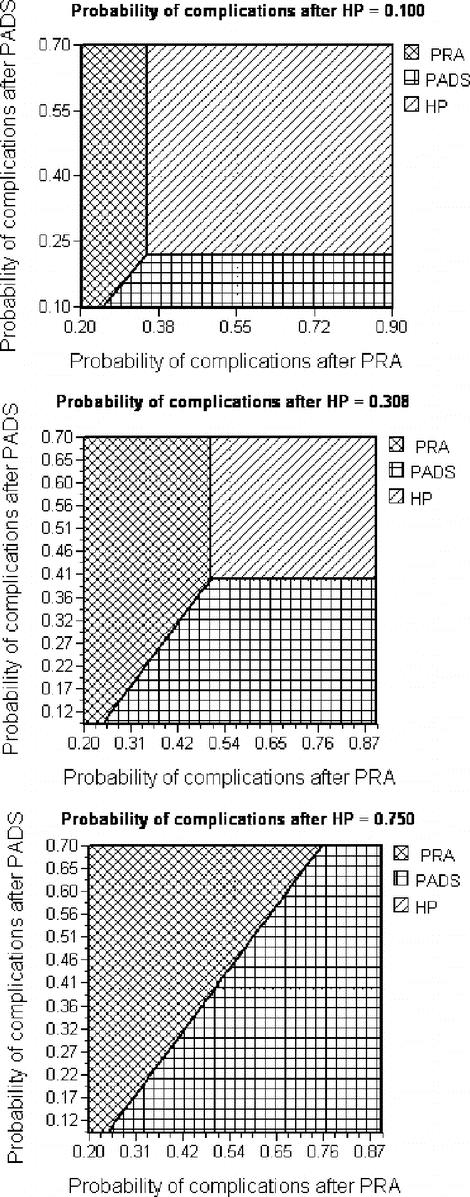
FIGURE 3. Three-way sensitivity analysis for probability of postoperative complications. PRA, primary resection and anastomosis; PADS, primary anastomosis with defunctioning stoma; HP, Hartmann's procedure. Vertical dotted line represents the baseline estimate of probability of complications after PRA. Horizontal dotted line represents the baseline estimate of probability of complications after PADS. The point of intersection between the 2 lines represents the optimal strategy at the given probability of HP-related complications.
Sensitivity analyses of utility values and QALYs for individual outcomes are shown in Tables 2 and 3, respectively. These analyses demonstrated that the model was sensitive to changes in two utility variables: the utility of postoperative complications and the utility of the stoma reversal operation. If the utility of postoperative complications was 0.14 higher (ie, 0.29 rather than 0.15), then simple PRA became the operation of choice. If the utility of PADS reversal was 0.05 lower (ie, 0.85 rather than 0.9) or if the utility of HP reversal was 0.07 higher (ie, 0.87 rather than 0.8), then HP became the operation of choice. The sensitivity of the model to these utilities was reflected in the outcomes that involved postoperative complications and/or operations for stoma reversals (Table 3). To illustrate these changes in the optimal strategy, a three-way sensitivity analysis was carried out, analyzing QALYs for PADS and HP primary and reversal operations without postoperative complications and QALYs for simple PRA after postoperative complications. This effectively reflected the sensitivity of the model to both utility for complications and stoma reversal operations. At the lower range of QALYs for HP primary and reversal procedures, the optimal strategy was PADS. This was, however, in turn sensitive to QALYs after PRA with complications, with 5.17 QALYs being the threshold value (Table 3). At 15.7 QALYs for HP primary and reversal operations, HP became the optimal strategy. This value is well within the range dictated by the published utility uncertainties. Finally, when QALYs for HP primary and reversal operations were at the maximum value (ie, 18.070, implying that the utility for reversal operation was 1), then HP became the procedure of choice.
Interpretation of Main Results
There is a shift of optimal operative strategy from HP to PADS when long-term outcomes are incorporated into the model. Patients undergoing PADS have been shown to have 6.5 additional months of life with acceptable quality (as defined by the assigned utilities) compared with HP. This was shown to be due to the increased risk of a permanent stoma after HP (27.4%) compared with PADS (8%), as well as due to the increased risk of operative complications after HP reversal compared with PADS reversal (9.3% vs. 5.2%). More importantly, the results of this study suggest that PADS should be considered the operation of choice, only if the risk of postoperative complications arising from this procedure was below 44%. At a complication rate above this level, the risks of PADS were found to outweigh the benefits and HP would be the operation of choice, provided that the complication rate from this procedure does not exceed 30%. The optimal operative strategy was also found to be dependent to the individual attitudes of the surgeons and patients (in terms of utilities) to the risk of postoperative complications and the need for the stoma reversal operation. This implies that the risk of complications should be weighed against the need for a reversal operation when choosing an operative procedure, this in turn being influenced by factors such as the experience and specialization of the surgeon, the hospital setting, and the relative acceptance of individual patients regarding further hospitalizations and repeat operative procedures.
DISCUSSION
The present study demonstrated a benefit of 6.5 quality-adjusted life months of PADS over HP when long-term outcomes were considered. This was in contrast to the superiority of HP by 10.2 quality-adjusted life months when only the primary operation was considered. In the published literature, gains in QALYs over 6 months are likely to be clinically significant, provided the model is robust to sensitivity analyses.23 The model was, however, sensitive to several variables, introducing a high degree of uncertainty around the baseline estimate. Sensitivity analyses were useful in quantifying the degree of uncertainty and offered directions regarding decision making and future research. In the present study, sensitivity analysis revealed that HP was the operation of choice, only if the risk of postoperative complications after PADS was more than 44%. The attitude of individual patients toward complications and the need for reversal operation were found to be the determinant factors in deciding on operative strategy.
The results of the present study are in line with a systematic review of 569 diverticular peritonitis patients with primary anastomosis, which reported a mortality rate of 9.9% compared with 18.8% for HP.9 Patients undergoing PADS had a mortality rate of 9.2%. One study reported on PADS and HP groups that were matched for comorbidity and degree of peritonitis.16 Mortality rates were 16% for PADS and 21% for HP, a difference that was not statistically significant. Furthermore, a study by Schilling et al11 evaluated matched groups of simple PRA versus HP in the setting of Hinchey III and IV disease and found comparable outcomes. Quality evidence exists for stoma reversal after HP in the form of a randomized controlled trial.4 A reversal rate of almost 70% was found, in agreement with several other published estimates,12,13 and was used as the baseline estimate. Risk of permanent stoma after PADS is recognizably less than that of HP at 8%16,55 with fewer complication rates.13,16 Interestingly, risk of death from PADS reversal was found to be higher than that of HP reversal, an assumption that may result from skewing of the data from strict application of the exclusion criteria. However, analysis of the model considering similar mortalities after the two operations, did not amount to any difference in the results.
A potential limitation is the degree of variation in study quality, raw data, and expert opinion used for derivation of probabilities for postoperative complications. Two studies were prospective nonrandomized in nature with groups matched for several preoperative variables.11,12 Both were consistent in reporting a complication rate close to 45% for PRA. One study used intracolonic lavage12 that may influence complication rates and both studies included very small number of patients. The remaining studies were all retrospective with unmatched groups. Selection bias was also present in the choice of operation for the ACPGBI83 and CCF,69,84 diverticular disease databases. All of these factors may have significant impact in determining complication rates and, in turn, bias the baseline estimate. In the analysis, we have attempted to accommodate for this by obtaining expert opinion as well as using a very wide range of uncertainty for carrying out the sensitivity analysis. In selecting the most appropriate evidence to use in this analysis, we have limited our sample size of PRA and PADS to only 3.8% of the overall sample size, with HP and HP reversal accounting for the rest. Using a very wide range of uncertainty of plausible values for all health states and supplementing the published data with expert opinion may account for the potential bias resulting from the disproportionate representation of each operative strategy in terms of sample size.
With regards to utility estimates for postoperative complications and for stoma reversal operations, no published studies exist specifically for diverticular disease. The utilities used for postoperative complications were derived from one study that used validated methods of derivation.85 There is a wide variation of patient perceptions regarding complications and a wide range was used for sensitivity analysis. Furthermore, utility estimates for the stoma reversal operations were obtained by expert opinion as no published estimates exist specifically for HP or PADS. The uncertainty of these estimates was determined using a published study that determined quality of life in patients with locally recurrent rectal cancers.88 The utilities in this study were derived using “standard gamble” interviews, a technique that is widely accepted as being very reliable in the setting of decision analysis.85 Our baseline estimates are subject to possible error as utilities based upon locally recurrent cancer may not be applicable to benign disease. Again, a wide range was used for sensitivity analysis to incorporate this shortcoming.
This is the first study to our knowledge that uses decision analysis to evaluate operative procedures for diverticular peritonitis. The fundamental clinical dilemma in our model reflected the possible higher incidence of postoperative morbidity and mortality after primary anastomotic procedures versus the higher incidence of reversal-related complications and permanent stoma after HP. Given the state of imperfect evidence and the high degree of uncertainty and sensitivity of the model, a firm conclusion cannot be made. The strengths of this study lie in the fact that all available evidence (published, existing databases, expert opinion) was gathered and incorporated into a model that highlighted two central issues regarding the optimal operative strategy. First, the risk of postoperative complications needs to be taken into account and evaluated using validated models preoperatively before a decision is made regarding operative strategy. Mortality and morbidity prediction models exist and have been validated in the setting of colorectal surgery.91 Second, the attitudes of individual patients toward operative complications and the need for a stoma reversal procedure must be assessed and incorporated into a decision toward a particular operative strategy. Furthermore, this form of decision analysis is ideally suited to resolve issues where randomized controlled studies are impractical and are unlikely to be undertaken.
Limitations of the present study include the following:
The significant healthcare costs involved in the issue could not be incorporated into the decision as there are no cost estimates available in the United Kingdom regarding the specific issue.
Stoma adaptation issues that may result in an improvement of quality of life with time, in patients with permanent stoma were not taken into consideration. The model however, was not sensitive to utility of permanent stoma even when this approached the value of 1; therefore, adaptation issues are unlikely to influence the results.
Derivation of utility estimates from patients distinct from the base-case patient in the current analysis.
CONCLUSION
PADS may be the procedure of choice for perforated diverticular disease when long-term outcomes are considered and adjusted for quality of life. Central to the operative strategy decision is accurate calculation of risk of complications using validated prediction models, as well as determination of patient attitudes towards complications and reversal operations. Based upon the best available data, it is our recommendation that PADS may be performed when the risk of morbidity and mortality is not excessive to obviate the need for a complicated reversal operation and a high risk of permanent stoma. HP should be reserved for patients with a high risk of complications after appropriate counseling regarding the long-term implications.
Footnotes
Reprints: Paris P. Tekkis, MD, FRCS, Imperial College London, Department of Biosurgery and Surgical Technology, St. Mary's Hospital, 10th Floor QEQM Building, Praed Street, London W2 1NY, United Kingdom. E-mail: p.tekkis@imperial.ac.uk.
REFERENCES
- 1.Wong WD, Wexner SD, Lowry A, et al. Practice parameters for the treatment of sigmoid diverticulitis-supporting documentation: the Standards Task Force. The American Society of Colon and Rectal Surgeons. Dis Colon Rectum. 2000;43:290–297. [DOI] [PubMed] [Google Scholar]
- 2.Makela J, Kiviniemi H, Laitinen S. Prevalence of perforated sigmoid diverticulitis is increasing. Dis Colon Rectum. 2002;45:955–961. [DOI] [PubMed] [Google Scholar]
- 3.Zeitoun G, Laurent A, Rouffet F, et al. Multicentre, randomized clinical trial of primary versus secondary sigmoid resection in generalized peritonitis complicating sigmoid diverticulitis. Br J Surg. 2000;87:1366–1374. [DOI] [PubMed] [Google Scholar]
- 4.Kronborg O. Treatment of perforated sigmoid diverticulitis: a prospective randomized trial. Br J Surg. 1993;80:505–507. [DOI] [PubMed] [Google Scholar]
- 5.Rodkey GV, Welch CE. Changing patterns in the surgical treatment of diverticular disease. Ann Surg. 1984;200:466–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagorney DM, Adson MA, Pemberton JH. Sigmoid diverticulitis with perforation and generalized peritonitis. Dis Colon Rectum. 1985;28:71–75. [DOI] [PubMed] [Google Scholar]
- 7.Wigmore SJ, Duthie GS, Young IE, et al. Restoration of intestinal continuity following Hartmann's procedure: the Lothian experience 1987–1992. Br J Surg. 1995;82:27–30. [DOI] [PubMed] [Google Scholar]
- 8.Belmonte C, Klas JV, Perez JJ, et al. The Hartmann procedure: first choice or last resort in diverticular disease? Arch Surg. 1996;131:612–615; discussion 616–617. [DOI] [PubMed]
- 9.Salem L, Flum DR. Primary anastomosis or Hartmann's procedure for patients with diverticular peritonitis? A systematic review Dis Colon Rectum. 2004;47:1953–1964. [DOI] [PubMed] [Google Scholar]
- 10.Elliott TB, Yego S, Irvin TT. Five-year audit of the acute complications of diverticular disease. Br J Surg. 1997;84:535–539. [PubMed] [Google Scholar]
- 11.Schilling MK, Maurer CA, Kollmar O, et al. Primary vs. secondary anastomosis after sigmoid colon resection for perforated diverticulitis (Hinchey Stage III and IV): a prospective outcome and cost analysis. Dis Colon Rectum. 2001;44:699–703; discussion 703–705. [DOI] [PubMed]
- 12.Regenet N, Pessaux P, Hennekinne S, et al. Primary anastomosis after intraoperative colonic lavage vs. Hartmann's procedure in generalized peritonitis complicating diverticular disease of the colon. Int J Colorectal Dis. 2003;18:503–507. [DOI] [PubMed] [Google Scholar]
- 13.Hold M, Denck H, Bull P. Surgical management of perforating diverticular disease in Austria. Int J Colorectal Dis. 1990;5:195–199. [DOI] [PubMed] [Google Scholar]
- 14.Auguste LJ, Wise L. Surgical management of perforated diverticulitis. Am J Surg. 1981;141:122–127. [DOI] [PubMed] [Google Scholar]
- 15.Salem L, Anaya DA, Roberts KE, et al. Hartmann's colectomy and reversal in diverticulitis: a population-level assessment. Dis Colon Rectum. 2005;48:988–995. [DOI] [PubMed] [Google Scholar]
- 16.Gooszen AW, Gooszen HG, Veerman W, et al. Operative treatment of acute complications of diverticular disease: primary or secondary anastomosis after sigmoid resection. Eur J Surg. 2001;167:35–39. [DOI] [PubMed] [Google Scholar]
- 17.Pettiti D. Meta-Analysis, Decision Analysis and Cost-Effectiveness Analysis: Methods of Quantitative Synthesis in Medicine. New York: Oxford University Press, 2000. [Google Scholar]
- 18.Richardson WS, Detsky AS. Users' guides to the medical literature. VII. How to use a clinical decision analysis. A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA. 1995;273:1292–1295. [DOI] [PubMed] [Google Scholar]
- 19.Hinchey EJ, Schaal PG, Richards GK. Treatment of perforated diverticular disease of the colon. Adv Surg. 1978;12:85–109. [PubMed] [Google Scholar]
- 20.Detsky AS, Naglie G, Krahn MD, et al. Primer on medical decision analysis: Part 1. Getting started. Med Decis Making. 1997;17:123–125. [DOI] [PubMed] [Google Scholar]
- 21.Detsky AS, Naglie G, Krahn MD, et al. Primer on medical decision analysis: Part 2. Building a tree. Med Decis Making. 1997;17:126–135. [DOI] [PubMed] [Google Scholar]
- 22.Naglie G, Krahn MD, Naimark D, et al. Primer on medical decision analysis: Part 3. Estimating probabilities and utilities. Med Decis Making. 1997;17:136–141. [DOI] [PubMed] [Google Scholar]
- 23.Krahn MD, Naglie G, Naimark D, et al. Primer on medical decision analysis: Part 4. Analyzing the model and interpreting the results. Med Decis Making. 1997;17:142–151. [DOI] [PubMed] [Google Scholar]
- 24.Naimark D, Krahn MD, Naglie G, et al. Primer on medical decision analysis: Part 5. Working with Markov processes. Med Decis Making. 1997;17:152–159. [DOI] [PubMed] [Google Scholar]
- 25.Edelmann G. Surgical treatment of colonic diverticulitis: a report of 205 cases. Int Surg. 1981;66:119–124. [PubMed] [Google Scholar]
- 26.Sakai L, Daake J, Kaminski DL. Acute perforation of sigmoid diverticula. Am J Surg. 1981;142:712–716. [DOI] [PubMed] [Google Scholar]
- 27.Killingback M. Management of perforative diverticulitis. Surg Clin North Am. 1983;63:97–115. [DOI] [PubMed] [Google Scholar]
- 28.Underwood JW, Marks CG. The septic complications of sigmoid diverticular disease. Br J Surg. 1984;71:209–211. [DOI] [PubMed] [Google Scholar]
- 29.Drumm J, Clain A. The management of acute colonic diverticulitis with suppurative peritonitis. Ann R Coll Surg Engl. 1984;66:90–91. [PMC free article] [PubMed] [Google Scholar]
- 30.Auguste L, Borrero E, Wise L. Surgical management of perforated colonic diverticulitis. Arch Surg. 1985;120:450–452. [DOI] [PubMed] [Google Scholar]
- 31.Shephard AA, Keighley MR. Audit on complicated diverticular disease. Ann R Coll Surg Engl. 1986;68:8–10. [PMC free article] [PubMed] [Google Scholar]
- 32.Hulkko OA, Laitinen ST, Haukipuro KA, et al. The Hartmann procedure for the treatment of colorectal emergencies. Acta Chir Scand. 1986;152:531–535. [PubMed] [Google Scholar]
- 33.Finlay IG, Carter DC. A comparison of emergency resection and staged management in perforated diverticular disease. Dis Colon Rectum. 1987;30:929–933. [DOI] [PubMed] [Google Scholar]
- 34.Marien B. The Hartmann procedure. Can J Surg. 1987;30:30–31. [PubMed] [Google Scholar]
- 35.Mealy K, Salman A, Arthur G. Definitive one-stage emergency large bowel surgery. Br J Surg. 1988;75:1216–1219. [DOI] [PubMed] [Google Scholar]
- 36.Christie PM, Shaw JH. Diverticular disease in Auckland. Aust NZ J Surg. 1988;58:795–799. [DOI] [PubMed] [Google Scholar]
- 37.Kourtesis GJ, Williams RA, Wilson SE. Surgical options in acute diverticulitis: value of sigmoid resection in dealing with the septic focus. Aust NZ J Surg. 1988;58:955–959. [DOI] [PubMed] [Google Scholar]
- 38.Schein M, Decker G. The Hartmann procedure: extended indications in severe intra-abdominal infection. Dis Colon Rectum. 1988;31:126–129. [DOI] [PubMed] [Google Scholar]
- 39.Berry AR, Turner WH, Mortensen NJ, et al. Emergency surgery for complicated diverticular disease: a five-year experience. Dis Colon Rectum. 1989;32:849–854. [DOI] [PubMed] [Google Scholar]
- 40.Peoples JB, Vilk DR, Maguire JP, et al. Reassessment of primary resection of the perforated segment for severe colonic diverticulitis. Am J Surg. 1990;159:291–293; discussion 294. [DOI] [PubMed]
- 41.Medina VA, Papanicolaou GK, Tadros RR, et al. Acute perforated diverticulitis: primary resection and anastomosis? Conn Med. 1991;55:258–261. [PubMed] [Google Scholar]
- 42.Smirniotis V, Tsoutsos D, Fotopoulos A, et al. Perforated diverticulitis: a surgical dilemma. Int Surg. 1992;77:44–47. [PubMed] [Google Scholar]
- 43.Sarin S, Boulos PB. Evaluation of current surgical management of acute inflammatory diverticular disease. Ann R Coll Surg Engl. 1991;73:278–282. [PMC free article] [PubMed] [Google Scholar]
- 44.Binda GA, Saccomani G, Gramegna A. Emergency surgery of complicated colonic diverticulitis. Acta Chir Belg. 1993;93:253–257. [PubMed] [Google Scholar]
- 45.Nespoli A, Ravizzini C, Trivella M, et al. The choice of surgical procedure for peritonitis due to colonic perforation. Arch Surg. 1993;128:814–818. [DOI] [PubMed] [Google Scholar]
- 46.Totte E, Creve U, Hubens A. The Hartmann procedure revisited. Acta Chir Belg. 1993;93:159–163. [PubMed] [Google Scholar]
- 47.Kriwanek S, Armbruster C, Beckerhinn P, et al. Prognostic factors for survival in colonic perforation. Int J Colorectal Dis. 1994;9:158–162. [DOI] [PubMed] [Google Scholar]
- 48.Tudor RG, Farmakis N, Keighley MR. National audit of complicated diverticular disease: analysis of index cases. Br J Surg. 1994;81:730–732. [DOI] [PubMed] [Google Scholar]
- 49.Kressner U, Antonsson J, Ejerblad S, et al. Intraoperative colonic lavage and primary anastomosis: an alternative to Hartmann procedure in emergency surgery of the left colon. Eur J Surg. 1994;160:287–292. [PubMed] [Google Scholar]
- 50.Desai DC, Brennan EJ Jr, Reilly JF, et al. The utility of the Hartmann procedure. Am J Surg. 1998;175:152–154. [DOI] [PubMed] [Google Scholar]
- 51.Isbister WH, Prasad J. Emergency large bowel surgery: a 15-year audit. Int J Colorectal Dis. 1997;12:285–290. [DOI] [PubMed] [Google Scholar]
- 52.Khan AL, Ah-See AK, Crofts TJ, et al. Surgical management of the septic complications of diverticular disease. Ann R Coll Surg Engl. 1995;77:16–20. [PMC free article] [PubMed] [Google Scholar]
- 53.Tucci G, Torquati A, Grande M, et al. Major acute inflammatory complications of diverticular disease of the colon: planning of surgical management. Hepatogastroenterology. 1996;43:839–845. [PubMed] [Google Scholar]
- 54.Faltyn J, Jungwirth J. Surgical treatment of the perforated colon with peritonitis. Ann Ital Chir. 1996;67:211–213. [PubMed] [Google Scholar]
- 55.Wedell J, Banzhaf G, Chaoui R, et al. Surgical management of complicated colonic diverticulitis. Br J Surg. 1997;84:380–383. [PubMed] [Google Scholar]
- 56.Zorcolo L, Covotta L, Carlomagno N, et al. Safety of primary anastomosis in emergency colorectal surgery. Colorectal Dis. 2003;5:262–269. [DOI] [PubMed] [Google Scholar]
- 57.Liebert CW Jr, DeWeese BM. Primary resection without anastomosis for perforation of acute diverticulitis. Surg Gynecol Obstet. 1981;152:30–32. [PubMed] [Google Scholar]
- 58.Bakker FC, Hoitsma HF, Den Otter G. The Hartmann procedure. Br J Surg. 1982;69:580–582. [DOI] [PubMed] [Google Scholar]
- 59.Bell GA, Panton ON. Hartmann resection for perforated sigmoid diverticulitis: a retrospective study of the Vancouver General Hospital experience. Dis Colon Rectum. 1984;27:253–256. [PubMed] [Google Scholar]
- 60.Ling L, Aberg T. Hartmann procedure. Acta Chir Scand. 1984;150:413–417. [PubMed] [Google Scholar]
- 61.Hackford AW, Schoetz DJ Jr, Coller JA, et al. Surgical management of complicated diverticulitis: the Lahey Clinic experience, 1967 to 1982. Dis Colon Rectum. 1985;28:317–321. [DOI] [PubMed] [Google Scholar]
- 62.Mallonga ET, Brummelkamp WH, Van Gulik TM, et al. The Hartmann procedure: its role in acute complicated diverticulitis. Neth J Surg. 1986;38:171–174. [PubMed] [Google Scholar]
- 63.Gregg RO. An ideal operation for diverticulitis of the colon. Am J Surg. 1987;153:285–290. [DOI] [PubMed] [Google Scholar]
- 64.Sweeney JL, Hoffmann DC. Restoration of continuity after Hartmann's procedure for the complications of diverticular disease. Aust NZ J Surg. 1987;57:823–825. [DOI] [PubMed] [Google Scholar]
- 65.Haas PA, Haas GP. A critical evaluation of the Hartmann's procedure. Am Surg. 1988;54:380–385. [PubMed] [Google Scholar]
- 66.Geoghegan JG, Rosenberg IL. Experience with early anastomosis after the Hartmann procedure. Ann R Coll Surg Engl. 1991;73:80–82. [PMC free article] [PubMed] [Google Scholar]
- 67.Eisenstat TE, Rubin RJ, Salvati EP. Surgical management of diverticulitis: the role of the Hartmann procedure. Dis Colon Rectum. 1983;26:429–432. [DOI] [PubMed] [Google Scholar]
- 68.Khan AL, Ah-See AK, Crofts TJ, et al. Reversal of Hartmann's colostomy. J R Coll Surg Edinb. 1994;39:239–242. [PubMed] [Google Scholar]
- 69.Aydin HN, Remzi FH, Tekkis PP, et al. Hartmann's reversal is associated with high postoperative adverse events. Dis Colon Rectum. 2005;48:2117–2126. [DOI] [PubMed] [Google Scholar]
- 70.Farkouh E, Hellou G, Allard M, et al. Resection and primary anastomosis for diverticulitis with perforation and peritonitis. Can J Surg. 1982;25:314–316. [PubMed] [Google Scholar]
- 71.Trillo C, Paris MF, Brennan JT. Primary anastomosis in the treatment of acute disease of the unprepared left colon. Am Surg. 1998;64:821–824; discussion 824–825. [PubMed]
- 72.Umbach TW, Dorazio RA. Primary resection and anastomosis for perforated left colon lesions. Am Surg. 1999;65:931–933. [PubMed] [Google Scholar]
- 73.Hoemke M, Treckmann J, Schmitz R, et al. Complicated diverticulitis of the sigmoid: a prospective study concerning primary resection with secure primary anastomosis. Dig Surg. 1999;16:420–424. [DOI] [PubMed] [Google Scholar]
- 74.Gooszen AW, Tollenaar RA, Geelkerken RH, et al. Prospective study of primary anastomosis following sigmoid resection for suspected acute complicated diverticular disease. Br J Surg. 2001;88:693–697. [DOI] [PubMed] [Google Scholar]
- 75.Blair NP, Germann E. Surgical management of acute sigmoid diverticulitis. Am J Surg. 2002;183:525–528. [DOI] [PubMed] [Google Scholar]
- 76.Thow GB. Emergency left colon resection with primary anastomosis. Dis Colon Rectum. 1980;23:17–24. [DOI] [PubMed] [Google Scholar]
- 77.Biondo S, Jaurrieta E, Marti Rague J, et al. Role of resection and primary anastomosis of the left colon in the presence of peritonitis. Br J Surg. 2000;87:1580–1584. [DOI] [PubMed] [Google Scholar]
- 78.Landen S, Nafteux P. Primary anastomosis and diverting colostomy in diffuse diverticular peritonitis. Acta Chir Belg. 2002;102:24–29. [DOI] [PubMed] [Google Scholar]
- 79.Koruth NM, Hunter DC, Krukowski ZH, et al. Immediate resection in emergency large bowel surgery: a 7 year audit. Br J Surg. 1985;72:703–707. [DOI] [PubMed] [Google Scholar]
- 80.Alanis A, Papanicolaou GK, Tadros RR, et al. Primary resection and anastomosis for treatment of acute diverticulitis. Dis Colon Rectum. 1989;32:933–939. [DOI] [PubMed] [Google Scholar]
- 81.Saccomani GE, Santi F, Gramegna A. Primary resection with and without anastomosis for perforation of acute diverticulitis. Acta Chir Belg. 1993;93:169–172. [PubMed] [Google Scholar]
- 82.Schwesinger WH, Page CP, Gaskill HV 3rd, et al. Operative management of diverticular emergencies: strategies and outcomes. Arch Surg. 2000;135:558–562; discussion 562–563. [DOI] [PubMed]
- 83.Constantinides VA, Tekkis PP, Senapati A. Comparison of POSSUM scoring systems and the Surgical Risk Scale in patients undergoing surgery for complicated diverticular disease. Dis Colon Rectum. 2006. (Epub ahead of print). [DOI] [PubMed]
- 84.Aydin HN, Remzi FH, Tekkis PP, et al. Evaluation of the surgical options for the treatment of diverticular disease: the Cleveland Clinic Diverticular Disease Propensity Score. Dis Colon Rectum. 2006;49:629–639. [DOI] [PubMed] [Google Scholar]
- 85.Torrance GW. Measurement of health state utilities for economic appraisal. J Health Econ. 1986;5:1–30. [DOI] [PubMed] [Google Scholar]
- 86.Kennedy ED, Urbach DR, Krahn MD, et al. Azathioprine or ileocolic resection for steroid-dependent terminal ileal Crohn's disease? A Markov analysis. Dis Colon Rectum. 2004;47:2120–2130. [DOI] [PubMed] [Google Scholar]
- 87.Boyd NF, Sutherland HJ, Heasman KZ, et al. Whose utilities for decision analysis? Med Decis Making. 1990;10:58–67. [DOI] [PubMed] [Google Scholar]
- 88.Miller AR, Cantor SB, Peoples GE, et al. Quality of life and cost effectiveness analysis of therapy for locally recurrent rectal cancer. Dis Colon Rectum. 2000;43:1695–1701; discussion 1701–1703. [DOI] [PubMed]
- 89.Froberg DG, Kane RL. Methodology for measuring health-state preferences: I. Measurement strategies. J Clin Epidemiol. 1989;42:345–354. [DOI] [PubMed] [Google Scholar]
- 90.Deleted in proof.
- 91.Tekkis PP, Prytherch DR, Kocher HM, et al. Development of a dedicated risk-adjustment scoring system for colorectal surgery (colorectal POSSUM). Br J Surg. 2004;91:1174–1182. [DOI] [PubMed] [Google Scholar]