Abstract
Background:
Laparoscopic surgery for gastric cancer is technically feasible, but it is not widely accepted because it has not been evaluated from the standpoint of oncologic outcome. We conducted a retrospective, multicenter study of a large series of patients in Japan to evaluate the short- and long-term outcomes of laparoscopic gastrectomy for early gastric cancer (EGC).
Methods:
The study group comprised 1294 patients who underwent laparoscopic gastrectomy during the period April 1994 through December 2003 in 16 participating surgical units (Japanese Laparoscopic Surgery Study Group). The short- and long-term outcomes of these patients were examined.
Results:
Distal gastrectomy was performed in 1185 patients (91.5%), proximal gastrectomy in 54 (4.2%), and total gastrectomy in 55 (4.3%); all were performed laparoscopically. The morbidity and mortality rates associated with these operations were 14.8% and 0%, respectively. Histologically, 1212 patients (93.7%) had stage IA disease, 75 (5.8%) had stage IB disease, and 7 (0.5%) had stage II disease (the UICC staging). Cancer recurred in only 6 (0.6%) of 1294 patients treated curatively (median follow-up, 36 months; range, 13–113 months). The 5-year disease-free survival rate was 99.8% for stage IA disease, 98.7% for stage IB disease, and 85.7% for stage II disease.
Conclusions:
Although our findings may be considered preliminary, our data indicate that laparoscopic surgery for EGC yields good short- and long-term oncologic outcomes.
This retrospective, multicenter study of a large series of patients in Japan evaluated the short- and long-term outcomes of laparoscopic gastrectomy for early gastric cancer (EGC). This study demonstrated that laparoscopic surgery for EGC yielded good short- and long-term oncologic outcomes.
In Japan, the incidence of early gastric cancer has increased to more than 50% of the overall incidence of gastric cancer because of the development of diagnostic instruments and increased use of mass and individual screenings.1,2 For the management of patients with early gastric cancer (EGC), minimally invasive therapies, such as endoscopic and laparoscopic procedures, have been available since the 1980s.3,4 Since the first report of laparoscopy-assisted distal gastrectomy (LADG) in 1994, LADG has been widely adopted for EGC and the number of patients undergoing LADG has been increasing in Japan.5 Laparoscopy-assisted gastrectomy (LAG) is now performed not only as distal gastrectomy but also as proximal gastrectomy and total gastrectomy.6–8
Several small retrospective studies analyzing the short-term outcome of LAG showed that patients who underwent LAG had less pain, earlier recovery to active daily life, a shorter hospital stay, and better quality of life than patients who underwent conventional open surgery.9–11 However, LAG for the treatment of malignancies remains controversial because of the lack of large-scale study data on the short-term and long-term outcomes.
To clarify the short- and long-term outcomes of LAG for EGC, we examined the clinical data obtained by 16 surgical departments that are members of the Japanese Laparoscopic Surgery Study Group.
MATERIALS AND METHODS
The study included 1294 patients with EGC who underwent LAG in one of the 16 participating departments during the period 1994 through 2003. The patients who underwent LAG in each institution for that period were all registered for the present study. All tumors were adenocarcinomas that were shown by preoperative gastric endoscopy and barium meal study to be present only in the mucosal or submucosal layer of the stomach and were not candidates for endoscopic mucosal resection. Patients with cancer in another organ or with previous upper abdominal laparotomy or with cardiac, pulmonary, or hepatic insufficiency were not included. The exclusion criteria in insufficiency of the organs were 1) operative cardiovascular risk greater than New York Heart Association II, 2) operative pulmonary risk greater than Hugh-Jones II, and 3) severe liver disease (Child classes B and C). All participating surgeons were personally responsible for obtaining the written informed consent of their patients. According to the location of the tumor, LADG, laparoscopy-assisted proximal gastrectomy (LAPG), or laparoscopy-assisted total gastrectomy (LATG) was performed.
As described previously,5,6,8 LAG consisted of the following procedures: 1) laparoscopic dissection of the lesser and greater omentum, ligation and division of the main vessels to mobilize the stomach under pneumoperitoneum, 2) laparoscopic D1+α, D1+β, or D2 lymph node dissection, based on the Guidelines of the Japan Gastric Cancer Association, and 3) resection of the distal two thirds (LADG), proximal third (LAPG), or total stomach (LATG), depending on the location of the tumor, followed by reconstruction by the Billroth-I, esophagogastrostomy, or Roux-en-Y method through a 5- to 7-cm-long minilaparotomy incision. To establish techniques of LAG as an oncologic surgery, the laparoscopic procedures for lymph node dissection in each institution had been reviewed by video examination in the group conferences.
Data obtained for each patient included the following: age, sex, body mass index, previous laparotomy, surgical procedure, operation time, conversion to open surgery, postoperative complications, postoperative oncologic outcome, histologic type of tumor, depth of tumor invasion, lymph node metastasis, and clinical stage according to the UICC staging and the WHO classification of tumors.12,13
All patients were monitored postoperatively by physical examination, and blood tests including a test for serum carcinoembryonic antigen at least every 3 months for the first year, every 6 months for the next 2 years, and every year for 5 years, and thereafter by abdominal ultrasonography, computed tomography, chest radiography, and gastroscopy at least once each year.
Data were compared between the three types of laparoscopic surgeries (LADG, LAPG, and LATG). Differences in categorical variables such as postoperative complications, tumor recurrences, and other clinicopathologic factors were analyzed by χ2 test, and differences in continuous variables were analyzed by Student t test. Survival rates were calculated by the Kaplan-Meier method. A P value of <0.05 was considered significant.
RESULTS
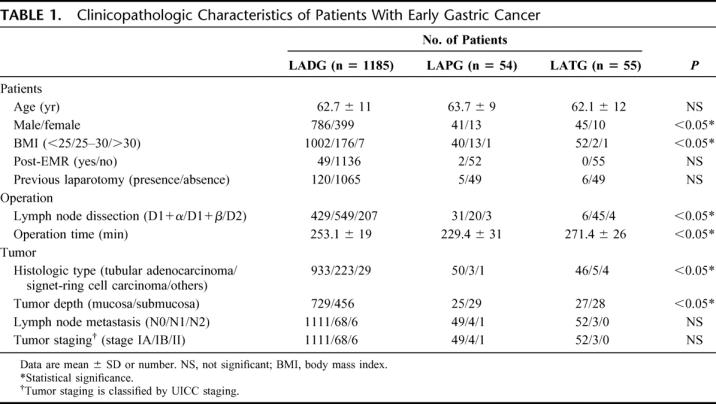
Laparoscopic procedures consisted of 1185 (91.5%) LADGs, 54 (4.2%) LAPGs, and 55 (4.3%) LATGs, and the total patient group comprised 872 men and 422 women. The clinicopathologic characteristics of the study patients are shown in Table 1. The percentages of female patients and of mildly obese patients were greater in the LADG group than in the other groups. D1+β and D2 lymph node dissection were performed frequently in the LADG group because of the high frequency of signet-ring cells carcinoma. The operation time of LATG was longer than that of LADG or LAPG. There were no other differences between groups in patient characteristics or pathologic characteristics of tumors. According to UICC staging, there were 1212 (93.7%) stage IA tumors, 75 (5.8%) stage IB tumors, and 7 (0.5%) stage II tumors.
TABLE 1. Clinicopathologic Characteristics of Patients With Early Gastric Cancer
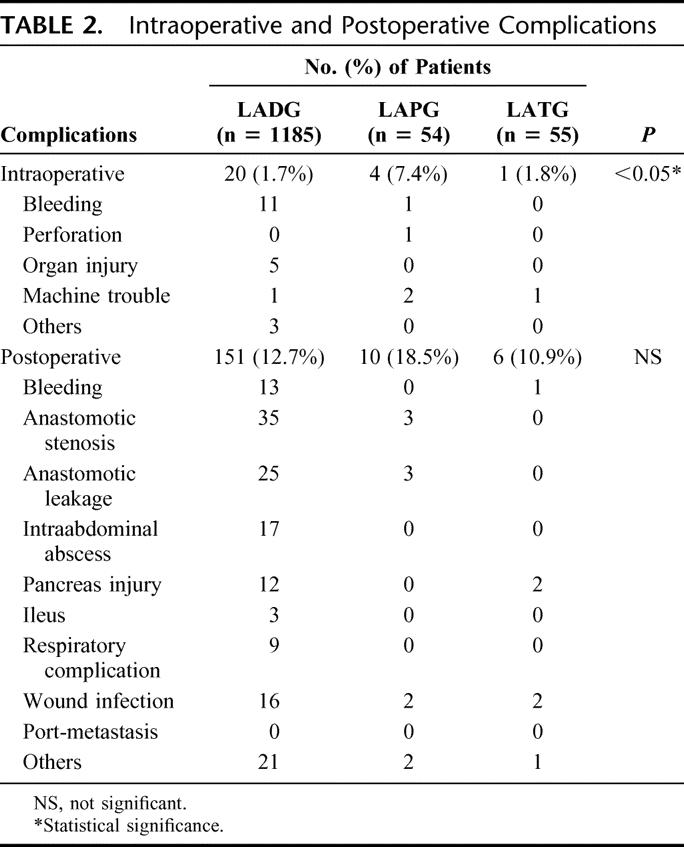
Intraoperative and postoperative complications occurred in 25 (1.9%) of the 1294 patients and 167 patients (12.9%), respectively (Table 2). Conversion to open surgery was required in only 14 cases (1.1%) because of intraoperative complications: bleeding in 9 cases, mechanical trouble in 3, and others in 2. Bleeding was the most frequent intraoperative complication, and it resulted mainly from the injury to the branches of the left gastric artery, short gastric vein, or spleen. Intraoperative complications occurred more frequently during LAPG than during other laparoscopic procedures (P < 0.05). The most frequent postoperative complications were anastomotic stenosis, anastomotic leakage, and wound infection, and there was no significant difference in the incidence of postoperative complications between laparoscopic procedures. Intraoperative and postoperative complications were not associated with any of the factors studied, including sex, age, body mass index, history of laparotomy and tumor stage.
TABLE 2. Intraoperative and Postoperative Complications
There were only 6 cancer recurrences, 1 local recurrence, 1 lymph node recurrence, 2 peritoneal disseminations, 1 liver metastasis, and 1 skin metastasis at the abdominal wall different from the port-site, during the median follow-up period of 36 months (range, 13–113 months). The cancer in all 6 recurrent cases invaded to the deeper submucosal layer. In 3 of 6 cases, lymph node metastasis (N2) was detected histologically, and the tumors were classified as stage II tumors. Recurrence was not associated with any surgical procedure, complications, or conversion to open gastrectomy. The 5-year disease-free survival rate was 99.8% for stage IA disease, 98.7% for stage IB disease, and 85.7% for stage II (Fig. 1). The 5-year disease-free survival rate was 99.4% for patients who underwent LADG, 98.7% for those who underwent LAPG, and 93.7% for those who underwent LATG (Fig. 2).
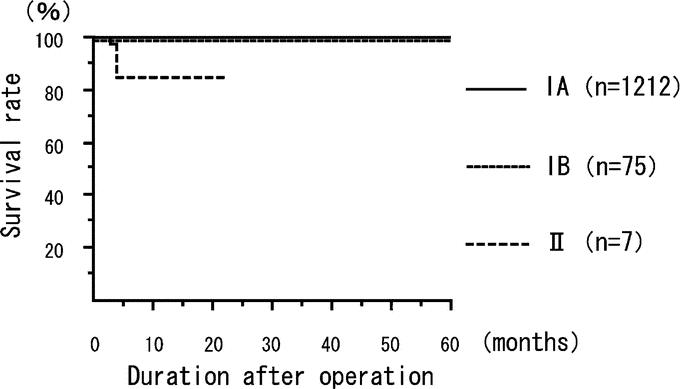
FIGURE 1. The disease-free survival rate in 1294 treated patients with early gastric cancer. The 5-year disease-free survival rate was 99.8% for stage IA, 98.7% for stage IB, and 85.7% for stage II. Tumor staging system is used with classification by the UICC staging.
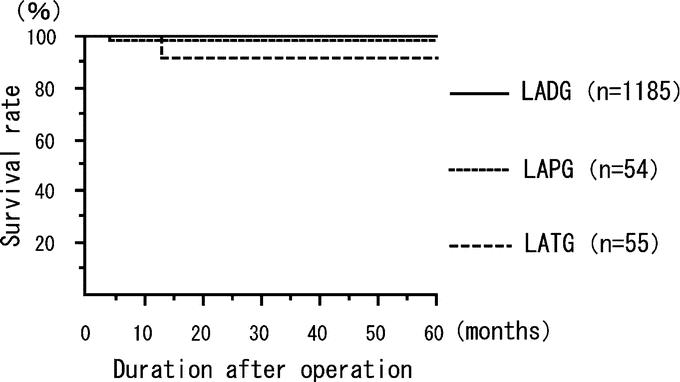
FIGURE 2. The disease-free survival rate according to operation. The 5-year disease-free survival rate was 99.4% for LADG, 98.7% for LAPG, and 93.2% for LATG.
DISCUSSION
This retrospective multicenter study is the first investigation of short- and long-term outcomes of LAG for EGC in a large series of patients in Japan. Both the mortality rate and the morbidity rate associated with LAG were shown to be as low as those of conventional open gastrectomy,14 and the 5-year survival rate of patients who underwent LAG for EGC was as good as that of patients who underwent conventional open surgery for EGC.15,16
Since LADG for EGC was first reported in 1994,5 several laparoscopic procedures for EGC have been developed and have been performed by a limited number of surgeons.6–8 Over the last decade, the number of LAGs for early cancer has rapidly increased, and the indication for LAG has extended to advanced cancer.17 Several studies of the short-term outcome of LAG in comparison to open gastrectomy showed the several advantages of LAG, including less invasiveness, less pain, earlier recovery of bowel movement, and shorter hospital stay.9–11 We have reported additional advantages of LADG, including less impaired respiratory function, better preservation of postoperative TH1 cell-mediated immune function, and better postoperative quality of life.18 Some studies, however, indicated technical difficulties and limitations in lymph node dissection performed during LAG.19 Therefore, we performed a retrospective multicenter study to clarify the technical feasibility and oncologic outcome of LAG for EGC in Japan.
The prognosis of patients with EGC is known to be excellent, with 5-year survival rates of 90% or more.15,16 Multivariate analysis has shown that lymph node metastasis is the only significant predictive factor for recurrence of EGC.20 Several recent studies showed that the extent of lymph node metastasis in patient with EGC was associated with tumor size and depth of invasion.21 However, the extent of lymph node dissection for EGC remains controversial.22 In the patients included in the present study, the lymph node dissection was performed laparoscopically according to the Guidelines of the Japanese Gastric Cancer Association. Several studies have evaluated laparoscopic lymph node dissection. Adachi et al, in a retrospective study of 96 patients with EGC, showed that the number of lymph nodes dissected laparoscopically was no different from that of lymph node dissected during open surgery.9 Yano et al also conducted a retrospective study of patients with EGC and reported that the number of resected lymph nodes in D1+α lymph node dissection did not differ between LAG and open gastrectomy.23 On the contrary, Miura et al showed less number of dissected lymph nodes along major curvature and the celiac and splenic arteries in LAG than open gastrectomy.24 In the present retrospective study, which covered a quite long time period, the number of resected lymph nodes could not be evaluated because data of the number of resected lymph nodes in several institutions were incomplete. To establish techniques of LAG as an oncologic surgery, the laparoscopic procedures for lymph node dissection in each institution had been reviewed by video examination in the group conferences.
There are few studies on the long-term outcome of LAG for EGC. Huscher et al25 recently showed, on the basis of the first prospective randomized trial in small series of 59 patients with EGC or advanced gastric cancer comparing the 5-year results of subtotal gastrectomy against those of with laparoscopic and open approaches, that LAG is a safe oncologic procedure; ie, the oncologic outcome matches that of conventional open surgery.25 Our preliminary prospective randomized trial with a mean follow-up period of 21.5 months showed no difference in curability between laparoscopic and open procedures for EGC.26 Weber et al also did not observe a difference in the 18-month survival rate between patients with gastric cancer who underwent LAG and those who underwent open gastrectomy.27 Although the present multicenter study of a large patient series was an uncontrolled study and the follow-up period was short, the survival rate of patients with EGC who underwent LAG was shown to be good. These data suggest that LAG is feasible for EGC from the standpoint of oncologic outcome.
Several studies have investigated mortality and morbidity associated with LAG. Huscher et al reported LAG-associated mortality and morbidity rates of 3.3% and 26.7%, respectively, in a randomized trial, and these rates were the same as those of open gastrectomy.25 Adachi et al reported, on the basis of a retrospective study comparing 49 LAGs and 53 open gastrectomies, that there was no difference in the incidence of operative complications.9 Tanimura et al, in a retrospective study of 160 LAGs, showed that major complications such as anastomotic leakage and pancreatic injury occurred in only 6 cases (3.8%).8 Shimizu et al reported the mortality and morbidity rates in 85 patients who underwent initial LAG were 0% and 11.8%, respectively.28 In the present study, the mortality and morbidity rates were 0% and 14.8%, respectively, and the rate of conversion to open surgery was 1.1%. The conversion to open surgery in LAG for EGC was not associated with worse short- and long-term outcome in the present study. As laparoscopic surgeries for gastrointestinal disease have been considered as technically complex procedures with longer operation time, the significance of learning curve has been emphasized to perform them safely.29,30 Although, in the present study, it seemed to take more 30 to 60 minutes to perform LAG than open gastrectomy, the incidence of operative complications was as low for LAG as it was for open surgery. These findings suggest that LAG with longer operation time is safe for EGC.
CONCLUSION
Our multicenter study of a large patient series showed that LAG is safe for EGC, with an oncologic outcome as good as that of conventional open surgery. Results of this retrospective nonrandomized clinical analysis should be confirmed by large-scale prospective randomized trials.
ACKNOWLEDGMENTS
The following centers and surgeons participated in the multicenter study initiated by the Japanese Laparoscopic Surgery Study Group (JLSSG): Seigo Kitano, Norio Shiraishi, Masafumi Inomata, Kazuhiro Yasuda, Oita University Faculty of Medicine (Oita); Ichiro Uyama, Masahiro Ochiai, Fujita Health University Hospital (Aichi); Kenichi Sugihara, Kazuyuki Kojima, Masayuki Enomoto, Masamichi Yasuno, Tokyo Medical and Dental University (Tokyo); Nobuhiko Tanigawa, Osaka Medical University (Osaka); Hitoshi Katai, National Cancer Center Hospital (Tokyo); Shinei Kudo, Showa University Northern Yokohama Hospital (Yokohama); Shinichi Sakuramoto, Kitasato University, School of Medicine (Kanagawa); Shuji Takiguchi, Morito Monden, Osaka University (Osaka); Shinya Tanimura, Masayuki Higashino, Yosuke Fukunaga, Osaka City General Hospital (Osaka); Yugo Nagai, Izumi Otsu Municipal Hospital (Osaka), Hirokazu Noshiro, Kyusyu University Graduate School of Medicine (Fukuoka), Ken Hayashi, Showa Inan General Hospital, Center on Endoscopic Surgery (Nagano); Hideki Hayashi, Takenori Ochiai, Graduate School of Medicine, Chiba University (Chiba); Tetsu Fukunaga, The Cancer Institute Hospital of Japanese Foundation for Cancer Research (JFCR) (Tokyo); Masaki Fukunaga, Juntendo Urayasu Hospital, Juntendo University School of Medicine (Chiba); Minoru Matsuda, Tomokazu Hoshi, Shinichi Kasai, Surugadai Nihon University Hospital (Tokyo); Tatsuo Yamakawa, Nobuo Murata, Teikyo University, Mizonokuchi Hospital (Kanagawa); Katsuhiko Yanaga, Jikei University School of Medicine (Tokyo).
Footnotes
Supported in part by a Grant-in-Aid for Cancer Research from the Japanese Ministry of Health, Labor, and Welfare (No. 13-17).
Reprints: Seigo Kitano, MD, Department of Surgery I, Oita University Faculty of Medicine, 1-1 Idaigaoka, Yufu, Oita 879-5593, Japan. E-mail: geka1@med.oita-u.ac.jp.
REFERENCES
- 1.Matsukuma A, Furusawa M, Tomoda H, et al. A clinicopathological study of asymptomatic gastric cancer. Br J Cancer. 1996;74:1647–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adachi Y, Mori M, Maehara Y, et al. Prognostic factors of node-negative gastric carcinoma: univariate and multivariate analyses. J Am Coll Surg. 1997;184:373–377. [PubMed] [Google Scholar]
- 3.Tada M, Murakami A, Karita M, et al. Endoscopic resection of early gastric cancer. Endoscopy. 1993;25:445–450. [DOI] [PubMed] [Google Scholar]
- 4.Kitano S, Shimoda K, Miyahara M, et al. Laparoscopic approaches in the management of patients with early gastric carcinoma. Surg Laparosc Endosc. 1995;5:359–362. [PubMed] [Google Scholar]
- 5.Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146–148. [PubMed] [Google Scholar]
- 6.Uyama I, Sugioka A, Matsui H, et al. Laparoscopic side-to-side esophagogastrostomy using a linear stapler after proximal gastrectomy. Gastric Cancer. 200;4:98–102. [DOI] [PubMed]
- 7.Mochiki E, Kamimura H, Haga N, et al. The technique of laparoscopically assisted total gastrectomy with jejunal interposition for early gastric cancer. Surg Laparosc. 2002;16:540–544. [DOI] [PubMed] [Google Scholar]
- 8.Tanimura S, Higashino M, Fukunaga Y, et al. Laparoscopic with regional lymph node dissection for upper gastric cancer. Gastric Cancer. 2003;6:64–68. [DOI] [PubMed] [Google Scholar]
- 9.Adachi Y, Shiraishi N, Shiromizu A, et al. Laparoscopy-assisted Billroth I gastrectomy compared with conventional open gastrectomy. Arch Surg. 2000;135:806–810. [DOI] [PubMed] [Google Scholar]
- 10.Adachi Y, Suematsu T, Shiraishi N, et al. Quality of life after laparoscopy-assisted Billroth I gastrectomy. Ann Surg. 1999;229:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mochiki E, Nakabayashi T, Kamimura H, et al. Gastrointestinal recover and outcome after laparoscopy-assisted versus conventional open distal gastrectomy for early gastric cancer. World J Surg. 2002;26:1145–1149. [DOI] [PubMed] [Google Scholar]
- 12.Sobin LH, Wittekind CH. TNM Classification of Malignant Tumors, 6th ed. Heidelberg: Springer-Verlag, 2002. [Google Scholar]
- 13.Hamilton S, Aaltonen L. Pathology and Genetics of Tumors of the Digestive System. New York: WHO Publications, 2000. [Google Scholar]
- 14.Sasako M. Risk factors for surgical treatment in the Dutch Gastric Cancer Trial. Br J Surg. 1997;84:1567–1571. [DOI] [PubMed] [Google Scholar]
- 15.Maruyama K, Sasako M, Kinoshita T, et al. Surgical treatment for gastric cancer: the Japanese approach. Semin Oncol. 1996;23:360–368. [PubMed] [Google Scholar]
- 16.Siewert JR, Sendler A. The current management of gastric cancer. Adv Surg. 1999;33:69–93. [PubMed] [Google Scholar]
- 17.Kitano S, Bandoh T, Kawano K. Endoscopic surgery in Japan. Minim Invasive Ther Allied Technol. 2001;10:215–219. [DOI] [PubMed] [Google Scholar]
- 18.Fujii K, Sonoda K, Izumi K, et al. T lymphocyte subsets and Th1/Th2 balance after laparoscopy-assisted distal gastrectomy. Surg Endosc. 2003;17:1440–1444. [DOI] [PubMed] [Google Scholar]
- 19.Fujiwara M, Kodera Y, Kasai Y, et al. Laparoscopy-assisted distal gastrectomy with systemic lymph node dissection for early gastric carcinoma: a review of 43 cases. J Am Coll Surg. 2003;196:75–81. [DOI] [PubMed] [Google Scholar]
- 20.Isozaki H, Tanaka N, Okajima K. General and specific prognostic factor of early gastric carcinoma treated with curative surgery. Hepatogastroenterology. 1999;46:1800–1808. [PubMed] [Google Scholar]
- 21.Yasuda K, Shiraishi N, Suematsu T, et al. Rate of detection of lymph node metastasis is correlated with the depth of submucosal invasion in early stage gastric carcinoma. Cancer. 1999;85:2119–2123. [DOI] [PubMed] [Google Scholar]
- 22.Hioki K, Nakane Y, Yamamoto M. Surgical strategy for early gastric cancer. Br J Surg. 1990;77:1330–1334. [DOI] [PubMed] [Google Scholar]
- 23.Yano H, Monden T, Kinuta M, et al. The usefulness of laparoscopy-assisted distal gastrectomy in comparison with that open distal gastrectomy for early gastric cancer. Gastric Cancer. 2001;4:93–97. [DOI] [PubMed] [Google Scholar]
- 24.Miura S, Kodera Y, Fujiwara M, et al. Laparoscopy-assisted distal gastrectomy with lymph node dissection: a critical reappraisal from the viewpoint of lymph node retrieval. J Am Coll Surg. 2004;198:933–938. [DOI] [PubMed] [Google Scholar]
- 25.Huscher CGS, Mingoli A, Sgarzini G, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitano S, Shiraishi N, Fujii K, et al. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery. 2002;131(suppl):306–311. [DOI] [PubMed] [Google Scholar]
- 27.Weber KJ, Reyes CD, Gagner M, et al. Comparison of laparoscopic and open gastrectomy for malignant disease. Surg Endosc. 2003;17:968–971. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu S, Noshiro H, Nagai E, et al. Laparoscopic gastric surgery in a Japanese institute: analysis of the initial 100 procedures. J Am Coll Surg. 2003;197:372–378. [DOI] [PubMed] [Google Scholar]
- 29.Rege RV, Joehl RJ. A learning curve for laparoscopic splenectomy at an academic institution. J Surg Res. 1999;81:27–32. [DOI] [PubMed] [Google Scholar]
- 30.Schlachta CM, Mamazza J, Seshadri PA, et al. Defining a learning curve for laparoscopic colorectal resections. Dis Colon Rectum. 2001;44:217–222. [DOI] [PubMed] [Google Scholar]


