Abstract
Objective:
The aims of this study were to use a comprehensive whole-mount pathologic analysis to characterize microscopic patterns of residual disease, as well as circumferential and distal resection margins, in rectal cancer treated with preoperative CMT; and to identify clinicopathologic factors associated with residual disease.
Summary Background Data:
Recent studies have shown that preoperative combined modality therapy (CMT) for rectal cancer enhances rates of sphincter preservation. However, the efficacy of preoperative CMT in conjunction with a total mesorectal excision (TME)-based resection, in terms of resection margins using whole-mount sections, has not been reported. Furthermore, since patterns of residual disease and extent of distal spread following preoperative CMT are largely unknown, intraoperative determination of distal rectal transection remains a surgical challenge.
Methods:
We prospectively accrued 109 patients with endorectal ultrasound (ERUS)-staged, locally advanced rectal cancer (T2–T4 and/or N1), located a median distance of 7 cm from the anal verge, requiring preoperative CMT, and undergoing a TME-based resection. Comprehensive whole-mount pathologic analysis was performed, with particular emphasis on extent of residual disease, margin status, and intramural tumor extension. Clinicopathologic factors associated with residual disease were identified.
Results:
A sphincter-preserving resection was feasible in 87 patients (80%), and in all 109 patients, distal margins were negative (median, 2.1 cm; range, 0.4–10 cm). Intramural extension beyond the gross mucosal edge of residual tumor was observed in only 2 patients (1.8%), both ≤0.95 cm. There were no positive circumferential margins (median, 10 mm; range, 1–28 mm), although 6 were less than or equal to 1 mm. On multivariate analysis, residual disease was observed more frequently in distally located tumors (distance from anal verge <5 cm) (P = 0.03).
Conclusion:
Our comprehensive pathologic analysis suggests that, following preoperative CMT and a TME-based resection, distal margins of 1 cm may provide for complete removal of locally advanced rectal cancer. Although residual cancer following preoperative CMT was more likely in the setting of distally located tumors, occult tumor beneath the mucosal edge was rare and, when present, limited to less than 1 cm. Our results extend the indications for sphincter preservation, as distal resection margins of only 1 cm may be acceptable for rectal cancer treated with preoperative CMT.
This prospective study involved 109 patients with locally advanced primary rectal adenocarcinoma treated at a specialty cancer center. Using whole-mount pathologic analysis of surgical specimens following combined modality therapy, we attempted to characterize microscopic patterns of residual disease, evaluate resection margins, and identify clinicopathologic factors associated with residual disease.
One of the major determinants of disease recurrence following resection of a rectal cancer is a positive circumferential and/or distal resection margin.1–3 Therefore, a major objective in the treatment of rectal cancer patients is procurement of negative gross and histologic resection margins while performing a sphincter-preserving resection. However, since it is often difficult to intraoperatively determine the exact extent of tumor extension on any given patient, and therefore to determine the least possible distal margin of resection, a surgeon pursuing a sphincter-preserving resection must rely on established guidelines based upon detailed pathologic studies of resected specimens. Because distal intramural tumor extension below the mucosa is noted in up to 40% of patients, with extension of more than 1 cm in 4% to 6% of cases, a distal resection margin of 2 cm has traditionally been advocated in nonirradiated patients to optimize oncologic outcome.4–6
Preoperative combined modality therapy (CMT) has been shown to improve local control and sphincter preservation rates in patients with locally advanced rectal cancer [endorectal ultrasound (ERUS) T3–T4 and/or N1 or clinically bulky].7 We recently reported that sphincter preservation rates of over 70% may be achieved in rectal cancer patients treated with preoperative CMT and a total mesorectal excision (TME)-based resection.8 It is possible that newly developed techniques, such as intersphincteric resection, may further increase sphincter preservation rates.9 However, because of the lack of detailed whole-mount pathologic analysis following preoperative CMT and the surgeon’s inability to accurately determine the extent of residual disease intraoperatively,10 patient selection for sphincter preservation following preoperative CMT remains a unique challenge. Currently, the length of grossly normal bowel distal to a rectal cancer that is required to obtain negative microscopic circumferential and distal resection margins remains largely unknown in rectal cancer patients treated with preoperative CMT and TME.
To our knowledge, there is no published prospective data using a comprehensive whole-mount pathologic analysis to evaluate resection margin status in rectal cancers treated with preoperative CMT and TME. Therefore, our aims were: 1) to use a comprehensive whole-mount pathologic analysis to characterize microscopic patterns of residual disease, as well as circumferential and distal resection margins, in rectal cancer treated with preoperative CMT; and 2) to identify clinicopathologic factors associated with residual disease.
METHODS
Patient Population
Our study group consisted of 109 prospectively accrued patients with locally advanced (ERUS T2–T4 and/or N1 or clinically bulky) primary rectal adenocarcinomas who were treated with preoperative CMT at Memorial Sloan Kettering Cancer Center, from February 2000 to August 2004. During this time period, a total of 507 patients received preoperative CMT at Memorial Sloan Kettering Cancer Center. Of these, 109 were enrolled in a prospective study designed to compare the ability of fluorodeoxyglucose positron emission tomography (FDG-PET) with that of computed tomography (CT) scanning to assess the response of rectal cancer to preoperative CMT, and to evaluate the efficacy of whole-mount pathologic analysis for determining patterns of residual disease as well as circumferential and distal margins of resection. Inclusion criteria for this study included biopsy-confirmed primary rectal adenocarcinoma, pre- and post-CMT PET and CT scans, and formal rectal cancer resection. Patients with distant disease were included only if their distant disease was considered amenable to complete surgical resection. Those with distant disease not deemed resectable were usually offered treatment with systemic chemotherapy, and were excluded from this study.
Endorectal Ultrasound Assessment
ERUS was performed with a Bruel and Kjaer 2102 Hawk ultrasound machine (Naerum, Denmark) equipped with a rotating endosonic probe and a 10-mHz transducer. ERUS stage was determined according to previously published techniques.11 Circular or oval structures measuring >3 mm were considered to be malignant lymph nodes. Lymph nodes measuring <3 mm with central hyperechogenicity were considered benign. A total of 103 patients (94%) were staged with pre-CMT ERUS, while 6 patients (6%) were technically unable to undergo ERUS due to the bulky, near-obstructing nature of their tumors. The pre-CMT ERUS stages of this study population are summarized in Table 1.
TABLE 1. Precombined Modality Therapy (CMT) Endorectal Ultrasound (ERUS) Stages of the Study Population
Preoperative Combined Modality Therapy
All patients received preoperative 5-fluorouracil-based chemotherapy [(bolus infusion, n = 49 (43%); continuous infusion, n = 60 (52%)]. The most common protocol for bolus infusion chemotherapy was 5-fluorouracil (325 mg/m2 per day) with leucovorin (20 mg/m2 per day) given for 2 cycles of 5 consecutive days on week 1 (days 1–5) and 5 (days 29–33) of radiation therapy. The most common protocol for continuous infusion chemotherapy was 5-fluorouracil (225 mg/m2 per day) for a 6-week continuous cycle.
External beam radiation therapy (median dose, 5040 cGy; range, 4860–5400 cGy) was delivered according to previously published techniques.12 Using 15 Mv photons, a 3- or 4-field technique was used. The perineum was blocked as much as possible in the lateral fields. The most common regimen was 180 cGy per day for 26 days, followed by a 360 cGy boost to the primary tumor.
The median time from completion of radiation to surgery was 48 days (range, 19–155 days). For a variety of medical and nonmedical reasons, 4 patients in our study population had an interval of 70 days or longer between completion of radiation therapy and surgery.
Technique of Total Mesorectal Excision
Surgical resection was performed using the principles of TME. Surgical dissection was performed along an embryologic plane comprising avascular areolar tissue between the mesorectal fascia and the fascia of the pelvic sidewall, as previously described.13,14 In patients with low and midrectal cancers (0–10 cm from the anal verge), a total mesorectal excision was performed. In patients with upper rectal cancers (10–13 cm from the anal verge), the mesorectum was divided at a right angle to the bowel wall, 5 cm distal to the mucosal edge of the tumor.
Basis for Sphincter Preservation
For the majority of patients in our cohort, the decision to pursue sphincter-preserving surgery was made before commencement of preoperative CMT. This determination was based on digital rectal and proctoscopic examinations, as well as ERUS imaging, showing at least a 1 cm margin of clearance between the tumor’s distal-most location and the upper-most portion of the anorectal ring. This determination was made with the patient in a conscious state. For distal rectal cancer cases that abutted the anorectal ring but did not involve the sphincters and went on to achieve a very good response to preoperative CMT, an intersphincteric resection was pursued to obtain a 1-cm margin of clearance. This determination was made intraoperatively. The decision to proceed with abdominoperineal resection was made based on the following criteria: 1) clear involvement of the internal/external sphincters by tumor; and 2) preoperative determination of limited anorectal function, which both surgeon and patient understood as likely to result in poor postoperative function and poor quality of life.
Pathologic Evaluation
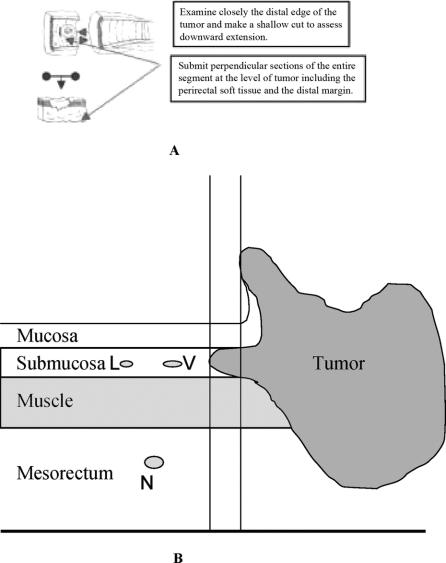
Detailed comprehensive pathologic analysis was performed by 2 dedicated pathologists (J.S. and A.S.), using standardized whole-mount sections via the method described by Quirke et al.1 Circumferential resection margin (CRM) was defined as the closest distance between viable tumor cells and the lateral edge of the resected specimen. When viable tumor was present in a lymph node, or present as a mesorectal deposit with a smooth contour not contiguous with the main mass, it was regarded as a lymph node metastasis (N+) and was not included in evaluation of the CRM. When viable tumor cells were present in the mesorectal soft tissue as dispersed tumor deposits without a nodular contour, and when these cells were closest to the lateral edge of the resected specimen, the distance between such cells and the lateral resection margin was documented as the CRM. The distal resection margin was measured from the distal mucosal edge of tumor to the resection edge on the gross specimen. In addition, distal extension of viable tumor cells in the bowel wall below the mucosa was documented, as depicted in Figure 1.
FIGURE 1. A, Examination of rectal specimen for distal mural spread beneath mucosal edge of the tumor. B, Schematic diagram representing potential sites of viable tumor extension beneath distal-most mucosal edge of the tumor. Sites include the submucosa, muscle, mesorectum, lymphatic vessel, blood vessel, and nerve. L, lymphatic vessel; V, blood vessel; N, nerve.
The pathologic response of the rectal cancer was determined by comparing the viable tumor cells remaining in the resected specimen, following preoperative CMT, to areas that demonstrated treatment effect but no viable tumor. Treatment response was expressed as a percentage (0%–100%) characterized by the replacement of neoplastic glands with loosely collagenized fibrous tissue and scattered chronic inflammatory cells, as previously reported by our institution.15,16 Downstaging by CMT was evaluated by comparing pathologic tumor stage (according to the AJCC TNM Rectal Cancer Staging System17) to preoperative ERUS stage.
Statistical Analysis
Proportions were compared using a Fisher exact test, and means were compared using a 2-tailed t test. Multivariate analysis was conducted using a logistic regression model. Statistical analysis was performed using the SPSS Software system (version 11.0, Chicago, IL). A P value of less than or equal to 0.05 was considered statistically significant. The study was reviewed and approved by the Memorial Sloan-Kettering Cancer Center Institutional Review Board. Written informed consent was obtained from each study participant.
RESULTS
Patient Population
A total of 109 patients were prospectively enrolled from the years 2000 to 2004. The median age was 60 years (range, 26–80 years). There were 41 females (38%) and 68 males (62%). Tumors were located a median distance of 7 cm from the anal verge (range, 0–13 cm). Sphincter preservation was achieved with a low anterior resection (LAR) in 87 patients (80%). The remaining 22 patients (20%) underwent an abdominoperineal resection (APR) (Table 2).
TABLE 2. Characteristics of the Study Population
Pathologic Response of Tumor to Preoperative Combined Modality Therapy
Eighteen patients (16%) had a pathologic complete response (pCR) (ypT0N0). On univariate analysis, a number of variables were examined: distance from the anal verge (0–5 cm vs. 6–13 cm); ERUS positive lymph nodes; pathology positive lymph nodes; tumor differentiation; and extent into the perirectal fat (≤3 mm or >3 mm). Of these, distance from the anal verge and pathology-positive lymph nodes were significant variables on both univariate and multivariate analysis. One of 33 patients (3%) with rectal cancer located 0 to 5 cm from the anal verge achieved a pCR, compared with 14 of 70 patients (20%) with tumor located 6 to 10 cm from the anal verge, and 3 of 6 patients (50%) with tumor located 11 to 13 cm from the anal verge.
On multivariate analysis, pCR was significantly less likely for distally located tumors (0–5 cm from the anal verge) than for tumors located more than 5 cm from the anal verge (P = 0.03) (Table 3). Of note, there was no difference in pre-CMT ERUS stage between tumors located 0 to 5 cm from the anal verge and those located more than 5 cm from the anal verge (P = 0.16).
TABLE 3. Relationship Between Pathologic Complete Response (pCR) Rate and Distance of the Rectal Cancer From the Anal Verge
Relative to ERUS, pathologic stage following preoperative CMT decreased in 65 patients (60%), remained unchanged in 28 patients (26%), and increased in 9 patients (8%). ERUS data was not available on 6 patients (6%).
Pathologic Evaluation of Resection Margins
In cases where residual tumor was present, the median CRM was 10 mm (range, 1–28 mm). The median CRM was similar after an LAR and APR [10 mm (range, <1–28 mm)]. Although there were no positive CRMs, 2 patients (1.8%) had a CRM of less than 1 mm (one following an LAR, the other following an APR) (Table 4).
TABLE 4. Distal and Circumferential Margins Following Preoperative Combined Modality Therapy (CMT) and Rectal Resection With Total Mesorectal Excision (TME)
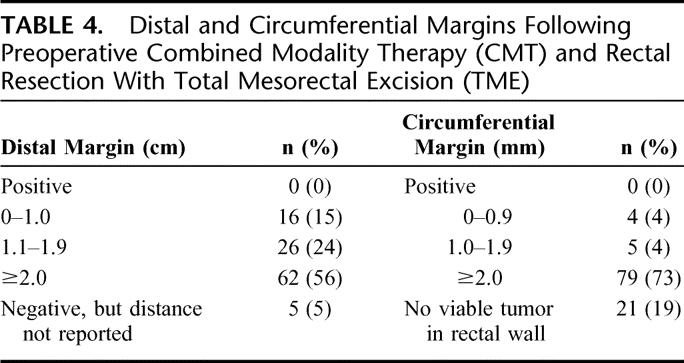
The median distal margin for the entire study population was 2.1 cm (range, 0.4–10 cm). The median distal margin after LAR was 1.9 cm (range, 0.4–10 cm), and after APR 2.5 cm (range, 1.2–8.2 cm). There were no positive distal margins (Table 4).
Distal extension of residual tumor beyond the distal-most mucosal edge was noted in only 2 patients (1.8%). In 1 case (pretherapy ERUS T3N1), there was continuous extension of tumor within the submucosa for a distance of 9.5 mm. In the other case (pretherapy ERUS T3N1), tumor extended within the muscularis propria for 3.0 mm.
In 7 of 87 patients (8%) undergoing LAR, concern about the adequacy of the distal margin led the surgeon to obtain intraoperative frozen section. Tumor was not detected at the resection margin in any of these 7 cases.
DISCUSSION
This report is, to our knowledge, the first comprehensive whole-mount pathologic analysis of circumferential and distal margins, as well as distal intramural tumor extension, in patients with locally advanced rectal cancer treated with preoperative CMT. Following preoperative CMT and TME, we did not identify any true-positive CRMs, in contrast to reported positive CRM rates of up to 25% when preoperative CMT is not used.2 Although based on a historical control group, this observation underscores a major advantage of preoperative CMT, namely, its ability to reduce CRM positive rates. We also found that occult tumor extension beneath the mucosal edge was rare (<2% of cases) and, when present, limited to less than 1 cm. We anticipate that our results, based on this comprehensive whole-mount pathologic approach, may extend the indications for sphincter preservation of locally advanced rectal cancer following preoperative CMT.
Since preoperative CMT can lead to a complete or near complete (>95%) response in 21% of rectal cancer cases,8 and since it is difficult for the operating surgeon to determine extent of response or residual disease,10 intraoperative determination of where to transect the rectum to assure a negative distal margin has been based on an extrapolation of data from nonirradiated specimens.4–6 In general, a 1- to 2-cm distal margin of resection has been recommended even after preoperative CMT.18–20 Our data demonstrate that, following preoperative CMT, a 1-cm margin of distal clearance beyond the mucosal edge should assure a negative distal margin in the majority of rectal cancer cases. This knowledge is likely to increase the indications for sphincter-saving resections, including those incorporating an intersphincteric approach.
Our comprehensive pathologic analysis suggests that negative CRM can be achieved in the majority of rectal cancer patients treated with preoperative CMT and TME. This finding has major prognostic significance, as a positive CRM has been associated with worse long-term oncologic outcome when compared with a negative CRM.1,21,22 In studies examining the prognostic significance of surgical margins in nonirradiated rectal specimens, a positive CRM (defined as ≤1 mm) was documented in 7% to 22% of cases, and was associated with local failure rates of up to 78% and distant metastases rates of up to 40%.2,3,22,23 In our study, 2 patients (1.8%) had a CRM measuring less than 1 mm. Recent evidence suggests that a CRM greater than 2 mm is necessary for optimal local control after rectal cancer resection without preoperative CMT. Our data suggest that up to 94% of patients treated with preoperative CMT, followed by rectal cancer resection with TME, can expect a CRM of 2 mm or greater. For the approximately 6% of patients with a negative CRM of less than 2 mm following preoperative CMT and TME, more aggressive postoperative therapy may be indicated. However, long-term follow-up of this patient population will be required to confirm this recommendation.
Our data suggest that rectal cancers located less than 5 cm from the anal verge are significantly less likely to have pCR following preoperative CMT than tumors located more proximally. Since pre-CMT ERUS suggests similar staging between distal and proximal lesions in our study population, the noted difference in response may be due to biologic differences between proximal and distal lesions and/or to limited effectiveness of preoperative CMT on distal rectal cancer located within the confined space of a bony, muscular pelvis. Follow-up of these patients will determine if this differential response translates into improved local control, disease-free survival, and overall survival.
CONCLUSION
Our comprehensive, prospectively acquired pathologic data suggests that, following preoperative CMT and radical rectal cancer resection, distal margins of 1 cm will assure removal of all local disease in the majority of cases. Although distally located tumors were more likely to be associated with residual cancer in the resected specimen, occult tumor beneath the mucosal edge was rare and, when present, was limited to less than 1 cm. Our results indicate that distal resection margins of only 1 cm may be acceptable for rectal cancer treated with preoperative CMT. In cases where distal clearance is uncertain, an intraoperative frozen section of the distal-most margin is encouraged. It is, however, important to recognize that, although these exciting observations may extend the indications for sphincter preservation, long-term oncologic follow-up of these results will be necessary.
Footnotes
Supported in part by the National Cancer Institute R01 82534-01 (to J.G.G.).
Reprints: Jose G. Guillem, MD, MPH, Colorectal Service, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, Room C-1077, New York, NY 10021. E-mail: guillemj@mskcc.org.
REFERENCES
- 1.Quirke P, Durdey P, Dixon MF, et al. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection: histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;8514:996–999. [DOI] [PubMed] [Google Scholar]
- 2.Adam IJ, Mohamdee MO, Martin IG, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344:707–711. [DOI] [PubMed] [Google Scholar]
- 3.Wibe A, Rendedal PR, Svensson E, et al. Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer. Br J Surg. 2002;89:327–334. [DOI] [PubMed] [Google Scholar]
- 4.Andreola S, Leo E, Belli F, et al. Distal intramural spread in adenocarcinoma of the lower third of the rectum treated with total rectal resection and coloanal anastomosis. Dis Colon Rectum. 1997;40:25–29. [DOI] [PubMed] [Google Scholar]
- 5.Shirouzu K, Isomoto H, Kakegawa T. Distal spread of rectal cancer and optimal distal margin of resection for sphincter-preserving surgery. Cancer. 1995;76:388–392. [DOI] [PubMed] [Google Scholar]
- 6.Kwok SP, Lau WY, Leung KL, et al. Prospective analysis of the distal margin of clearance in anterior resection for rectal carcinoma. Br J Surg. 1996;83:969–972. [DOI] [PubMed] [Google Scholar]
- 7.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. [DOI] [PubMed] [Google Scholar]
- 8.Guillem JG, Chessin DB, Cohen AM, et al. Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg. 2005;241:829–836;discussion 836–828. [DOI] [PMC free article] [PubMed]
- 9.Rullier E, Laurent C, Bretagnol F, et al. Sphincter-saving resection for all rectal carcinomas: the end of the 2-cm distal rule. Ann Surg. 2005;241:465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guillem JG, Chessin DB, Shia J, et al. Clinical examination following preoperative chemoradiation for rectal cancer is not a reliable surrogate end point. J Clin Oncol. 2005;23:3475–3479. [DOI] [PubMed] [Google Scholar]
- 11.Beynon J, Roe AM, Foy DM, et al. Preoperative staging of local invasion in rectal cancer using endoluminal ultrasound. J R Soc Med. 1987;80:23–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skibber JM HP, Minsky BD. Cancer of the Rectum, 6th ed. Philadelphia: Lippincott Williams and Wilkins, 2001. [Google Scholar]
- 13.Heald RJ, Moran BJ, Ryall RD, et al. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978–1997. Arch Surg. 1998;133:894–899. [DOI] [PubMed] [Google Scholar]
- 14.Enker WE, Thaler HT, Cranor ML, et al. Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg. 1995;181:335–346. [PubMed] [Google Scholar]
- 15.Ruo L, Tickoo S, Klimstra DS, et al. Long-term prognostic significance of extent of rectal cancer response to preoperative radiation and chemotherapy. Ann Surg. 2002;236:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guillem JG, Puig-La Calle J Jr, Akhurst T, et al. Prospective assessment of primary rectal cancer response to preoperative radiation and chemotherapy using 18-fluorodeoxyglucose positron emission tomography. Dis Colon Rectum. 2000;43:18–24. [DOI] [PubMed] [Google Scholar]
- 17.AJCC. AJCC Cancer Staging Manual, 6th ed. New York: Springer-Verlag, 2002. [Google Scholar]
- 18.Kuvshinoff B, Maghfoor I, Miedema B, et al. Distal margin requirements after preoperative chemoradiotherapy for distal rectal carcinomas: are < or = 1 cm distal margins sufficient? Ann Surg Oncol. 2001;8:163–169. [DOI] [PubMed] [Google Scholar]
- 19.Moore HG, Riedel E, Minsky BD, et al. Adequacy of 1-cm distal margin after restorative rectal cancer resection with sharp mesorectal excision and preoperative combined-modality therapy. Ann Surg Oncol. 2003;10:80–85. [DOI] [PubMed] [Google Scholar]
- 20.Andreola S, Leo E, Belli F, et al. Adenocarcinoma of the lower third of the rectum surgically treated with a <10-MM distal clearance: preliminary results in 35 N0 patients. Ann Surg Oncol. 2001;8:611–615. [DOI] [PubMed] [Google Scholar]
- 21.de Haas-Kock DF, Baeten CG, Jager JJ, et al. Prognostic significance of radial margins of clearance in rectal cancer. Br J Surg. 1996;83:781–785. [DOI] [PubMed] [Google Scholar]
- 22.Hall NR, Finan PJ, al-Jaberi T, et al. Circumferential margin involvement after mesorectal excision of rectal cancer with curative intent: predictor of survival but not local recurrence? Dis Colon Rectum. 1998;41:979–983. [DOI] [PubMed] [Google Scholar]
- 23.Wibe A, Syse A, Andersen E, et al. Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: anterior vs. abdominoperineal resection. Dis Colon Rectum. 2004;47:48–58. [DOI] [PubMed] [Google Scholar]