Abstract
Objective:
To compare the efficacy and safety of partial hepatectomy aiming grossly at a narrow (1 cm) and a wide (2 cm) resection margin in patients with macroscopically solitary hepatocellular carcinoma (HCC).
Summary Background Data:
For HCC treated with partial hepatectomy, the extent of the margin of liver resection remains controversial despite extensive studies.
Methods:
We conducted a prospective randomized trial in patients with solitary HCC. From January 1999 to February 2003, 169 patients with solitary HCC were stratified according to tumor size and randomized to undergo partial hepatectomy aiming grossly at either a narrow (1 cm) (n = 84) or a wide resection margin (2 cm) (n = 85). Analyses were done on an intention-to-treat basis.
Results:
The demographic and pathologic data were similar in the 2 groups. The mean ± SD for the final resection margin of the narrow and the wide margin groups were 0.7 ± 0.4 cm and 1.9 ± 0.6 cm, respectively. There was no significant difference in the morbidity and in-hospital mortality between the 2 groups of patients. The 1-, 2-, 3-, and 5-year overall survival rates for the narrow and the wide margin groups were 92.9%, 83.3%, 70.9%, and 49.1% and 96.5%, 91.8%, 86.9%, and 74.9%, respectively. The difference was significant (stratified log-rank test, P = 0.008). Multivariate analysis identified the presence of micrometastases and the treatment allocation were independent risk factors for tumor-related death. At the time of censor, 75 (44.4%) patients had developed tumor recurrence. All recurrences at the margins of liver resection were observed in the narrow margin group. Multiple tumor recurrence was also significantly higher in the narrow margin group (χ2 test, P = 0.018). Survival after tumor recurrence was significantly better in the wide margin group than the narrow margin group (log-rank test, P = 0.017).
Conclusion:
For macroscopically solitary HCC, a resection margin aiming grossly at 2 cm efficaciously and safely decreased postoperative recurrence rate and improved survival outcomes when compared with a gross resection margin aiming at 1 cm, especially for HCC ≤2 cm.
A prospective randomized trial conducted on patients with macroscopically solitary HCC showed that a gross resection margin aiming at 2 cm efficaciously and safely improved survival outcomes when compared with a resection margin aiming at 1 cm, especially for HCC ≤2 cm.
Hepatocellular carcinoma (HCC) is one of the most common malignancies in Asia and Africa, and its incidence is increasing in the Western world.1 Partial hepatectomy is still considered as the treatment of choice, and it gives a potential of a cure for HCC. Unfortunately, long-term survival after partial hepatectomy is still unsatisfactory because of the high incidence of tumor recurrence, and intrahepatic recurrence is the commonest form of recurrence.2 The aims of partial hepatectomy for HCC are to resect the tumor with an adequate margin to prevent future recurrence and to preserve enough functioning liver parenchyma to allow the patient to survive the operation. This is especially important in HCC because the majority of patients have underlying cirrhosis,3 and preservation of enough nontumorous liver parenchyma is critical for the success of the operation.4 Despite extensive studies, the optimal liver resection margin is still controversial. Furthermore, Ko et al hypothesized that unnecessary and excessive sacrifice of liver parenchyma leads to increased hepatocyte regeneration and might enhance hepatocarcinogenesis in the liver remnant.5
Most published reports on the resection margin for HCC are retrospective studies, and the results are controversial probably because of the difference in the selection of patients. Our previous study on macroscopically solitary HCC without vascular invasion suggested that a wide resection margin might improve the chance of clearance of micrometastasis.6 We therefore hypothesized that with a strict criteria used for selection of patients, the optimal liver resection margin can be determined in that selected group of patients. A prospective randomized study was performed to determine whether a wide resection margin aiming at 2 cm had survival benefit over a narrow resection margin aiming at 1 cm.
We followed the criteria used by us and by other authors: macroscopically solitary HCC meant HCC with a single nodule, and without gross tumor satellite deposits and vascular invasion.6 Micrometastasis was defined as tumor satellite or tumor thrombus no larger than 0.2 cm.7 Resection margin was defined as the shortest measured distance from the edge of tumor to the plane of liver transection.6,8
PATIENTS AND METHODS
This study was approved by the Ethical Committee of the Cancer Center of the Sun Yat-Sen University and it followed the ethical guidelines of the 1975 Declaration of Helsinki. Written informed consent was obtained from every patient before surgery. The study was designed according to the CONSORT statement.
Protocol
Criteria of Adequate Resection Margin
After exploration and mobilization of the liver, we determined the plane of hepatic parenchymal transection with the use of an ultrasound. For wide resection margin, we aimed at a minimum margin of 2 cm in the shortest distance from the edge of the tumor to the plane of liver transection. For narrow resection margin, we aimed at a margin of 1 cm. The final resection margin was subsequently determined microscopically on the resected specimen.
Selection of Patients
From January 1999 to February 2003, all patients who had a preoperative diagnosis of HCC and underwent hepatectomy at the Cancer Center of the Sun Yat-sen University were considered to be included into the study. Routine preoperative investigations of the patients included blood biochemistry, alpha-fetoprotein (AFP), chest radiography, abdominal ultrasonography, computed tomography of the abdomen, and indocyanine green clearance test. The preoperative diagnosis of HCC was based on the diagnostic criteria for HCC used by the European Association for the Study of the Liver (EASL).9 HCC was diagnosed by at least 2 radiologic imaging showing characteristic features of HCC; or one radiologic imaging showing characteristic features of HCC associated with AFP >400; or cytologic/histologic evidence. Hepatectomies were performed by experienced surgeons. Only patients who met the inclusion criteria and agreed to participate in the trial were enrolled. The inclusion criteria were: 1) a preoperative diagnosis of HCC with no previous treatment; 2) compensated cirrhosis with Child-Pugh class A, or no cirrhosis; 3) solitary HCC on preoperative investigations, and on intraoperative ultrasound and gross examination of the liver during the surgery; 4) on exploration and intraoperative ultrasound the tumor could safely be resected with a wide margin as defined by the aforementioned criteria, and the patient was judged to have adequate liver functional reserve to survive the operation.
Hepatectomy
Partial hepatectomy was performed following the techniques described previously.10 Anatomic resection, in the form of segmentectomy and/or subsegmentectomy as described by Makuuchi et al,11 was our preferred surgical method in liver resection. In segmentectomy, the hepatic parenchyma was transected at the intersegmental plane as described by Couinaud. If the hepatic parenchymal transection plane needed to go beyond the intersegmental plane to achieve the desired extent of resection margin, the small portal branches supplying the liver parenchyma up to the aimed transection plane were punctured under ultrasound guidance and injected with dye, and then liver subsegmentectomy was performed either alone or in combination with segmentectomy along the plane of demarcation as delineated by the injected dye. We always resected the whole liver segment(s) which contained the tumor in anatomic resection. In nonanatomic resection (or wedge resection), the resection was done with no regard to segmental or subsegmental plane. Wedge resection was only performed for a superficial tumor situated at the border of more than one liver segment.
Pathologic Examination
After liver resection, the specimen was prefixed and then serially sliced into 5 to 10 mm thick slices. Large histopathologic sections were prepared and the shrinkage rates after formalin fixation were calculated as we reported previously.6 Two pathologists examined each large section independently under microscopy. The final resection margin was determined microscopically. The shortest distance from the microscopic edge of the tumor to the liver transection plane was measured. As the shrinkage rate after fixation was measured, the final resection margin, which was the extent of the resection margin on the unfixed specimen, could be calculated.
Follow-up
After discharge from hospital, all patients were followed up at an interval of 3 months. At each follow-up visit, serum AFP, abdominal ultrasonography and chest radiography were done. When tumor recurrence or metastases were suspected, further investigations consisting of computed tomography and hepatic angiography were done. Biopsies were done when necessary. The diagnosis of tumor recurrence was based on cytologic/histologic evidence, or on the noninvasive diagnostic criteria for HCC used by the EASL as mentioned previously.9 The number and the location of recurrent tumors were recorded when the diagnosis of recurrence was first established. Recurrence at the liver transection margin was defined as intrahepatic recurrence located less than 2 cm from the resection margin, regardless of whether there was any simultaneous recurrence in the distant liver remnant or extrahepatically. Patients with recurrence were treated aggressively with surgery, local ablative therapy, regional therapy, or systemic therapy if technically possible.12 This study was censored on September 1, 2005.
Sample Size
Sample size was computed using the overall survival as the main endpoint.13 For patients who underwent hepatectomy for solitary HCC, the 5-year survival was estimated to be approximately 45%, based on previously published studies.10,14 For patients who underwent hepatectomy with a wide margin for solitary HCC, the 5-year survival was estimated to be approximately 70%, based on our previous study.6 Using a 2-sided test with 90% power at a significance level of 5%, the minimal sample size needed to detect a significant difference was calculated to be 84 patients in each treatment group. We randomized 173 patients into this study.
Statistical Methods
All statistical analyses were done on an intent-to-treat basis unless indicated otherwise. We set primary and secondary endpoints to be overall survival and recurrence-free survival, respectively. For patients who developed tumor recurrence and received potentially curative treatment subsequently, only the first recurrence was used for analysis. The data were presented as mean ± SD, or as median and range for continuous variable. For comparison of groups, the t test, the χ2 test, and the Fisher exact test were used as appropriate. The overall survival rates and disease-free survival rates after operation were evaluated by the Kaplan-Meier method and compared by the stratified log-rank test. For univariate analysis of risk factors for recurrence, patients with recurrences were compared with patients who did not develop recurrence. This was similarly done for univariate analysis of risk factors for death. For multivariate analysis, the Cox stepwise regression model was performed. A difference of 0.05 was taken as significant. The SPSS Statistical Software (SPSS Inc., Chicago, IL) was used for statistical calculation.
Assignment
Randomization
Randomization was performed with stratification according to tumor size (≤2.0 cm, 2.1–5.0 cm, >5 cm). After laparotomy and intraoperative ultrasound, patients were assigned via computer-generated allocation to one of the 2 groups: 1) narrow margin group: the tumor was resected with a gross resection margin aiming at 1 cm; and 2) wide margin group: the tumor was resected with a gross margin aiming at 2 cm.
Blinding Procedures
Patients were kept blind to the treatment allocation. Double blind and double-dummy techniques were not used because they were not feasible given the nature of the treatment.
RESULTS
Patients
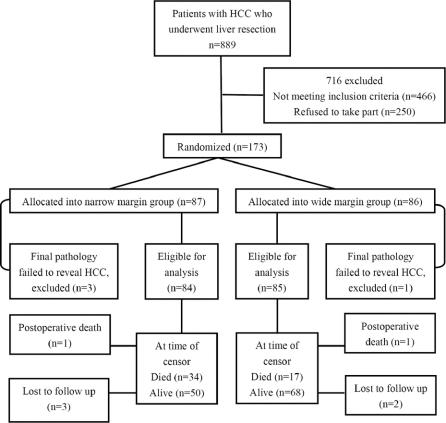
During the study period from January 1999 to February 2003, 889 patients with HCC underwent partial hepatectomy at the Hepatobiliary Department of the Cancer Center of the Sun Yat-sen University. A total of 466 patients were excluded because they did not fit into the inclusion criteria, and 250 patients refused to take part in the study. Finally, we randomized 173 patients: 86 in the wide margin group and 87 in the narrow margin group (Fig. 1). These patients represented 40.9% of the 423 patients with solitary HCC who were eligible for the study.
FIGURE 1. Flow chart of the study.
Of the 173 patients randomized, 4 (2.3%) were subsequently excluded because the final pathology showed cirrhotic regenerative nodules in 3 patients and focal nodular hyperplasia in 1 patient. A total of 169 patients were left for final analysis, with 84 in the narrow margin group and 85 in the wide margin group.
After partial hepatectomy, the specimen was examined and measured according to the procedures reported previously.6 In the wide margin group, no specimens showed microscopically involved resection margin by tumor. In the narrow margin group, 4 (4.8%) specimens showed microscopically involved margin. These patients were included in the final analysis. Three patients in the narrow margin group and 2 patients in the wide margin group were lost to follow-up, and their data were censored at the time of their last follow-up visit, which were 24, 36, 31, 25, and 41months after surgery, respectively.
Baseline Characteristics of Patients
Of the 169 patients in the final analysis, 155 (91.7%) were men and 14 (8.3%) were women. The mean age ± SD was 49.7 ± 11.5 years (range, 14–81 years). Demographic data were well matched between the 2 groups (Table 1). The final resection margin usually differed only slightly from the aimed resection margin except in 3 patients in whom the difference was marked. One patient in the narrow margin group had a final parenchymal resection margin of 2 cm, and 2 patients in the wide margin group had a final parenchymal resection margin of 1 cm, and 1 cm, respectively. All these 3 patients were included in the respective randomized groups in the final intention-to-treat analysis.
TABLE 1. Demographic Data
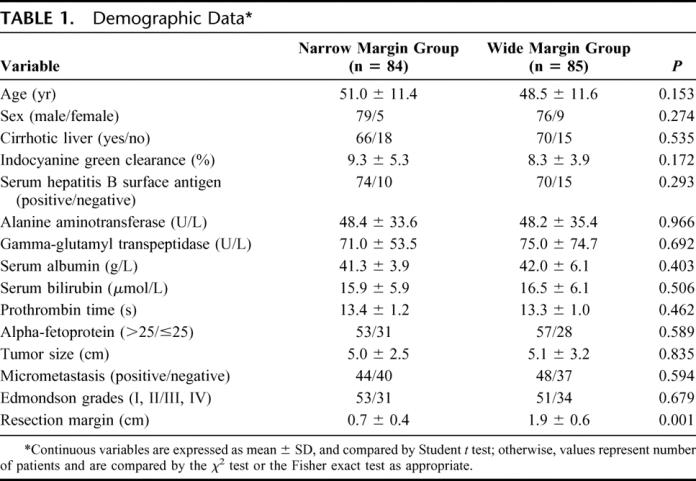
Operative Variables and Perioperative Outcomes
Table 2 shows the operative variables and perioperative outcomes in the 2 groups of patients, and there was no significant difference between the 2 groups. A total of 88 (wide/narrow, 45/43) complications occurred in 64 (wide/narrow, 31/33) patients, resulting in an overall operative morbidity rate of 37.9%. In the narrow margin group, morbidity included moderate/severe ascites (n = 15), pleural effusion (n = 13), transient jaundice (n = 8), bronchopneumonia (n = 3), biliary fistula (n = 1), wound dehiscence (n = 1), liver failure (n = 1), and renal failure (n = 1). One patient died of liver failure and renal failure 7 days after surgery. In the wide margin group, morbidity included moderate/severe ascites (n = 17), transient jaundice (n = 14), pleural effusion (n = 5), wound dehiscence (n = 3), bronchopneumonia (n = 2), gastrointestinal bleeding (n = 2), subphrenic abscess (n = 1), and liver failure (n = 1). One patient died of liver failure 42 days after surgery.
TABLE 2. Operative Variables and Perioperative Outcomes
Survival
On follow-up, 49 more patients (29.0%) had died. In the narrow margin group, 32 patients died of tumor progression and 1 died of advanced cirrhosis. In the wide margin group, 14 patients died of tumor progression, 1 died of advanced cirrhosis, and 1 died of brain hemorrhage. The mean ± SD for the follow-up periods for the narrow and the wide margin groups were 39.6 ± 17.0 months and 43.7± 14.9 months, respectively.
The 1-, 2-, 3-, and 5-year overall survival rates and recurrence-free survival rates for all the patients were 94.7%, 87.6%, 79.0%, 61.3% and 78.1%, 68.1%, 57.5%, 47.0%, respectively. The 1-, 2-, 3-, and 5-year overall survival rates for the narrow and the wide margin groups were 92.9%, 83.3%, 70.9%, 49.1% and 96.5%, 91.8%, 86.9%, 74.9%, respectively (Fig. 2). The difference in the overall survival rates in the 2 groups was significant (stratified log-rank test, P = 0.008).
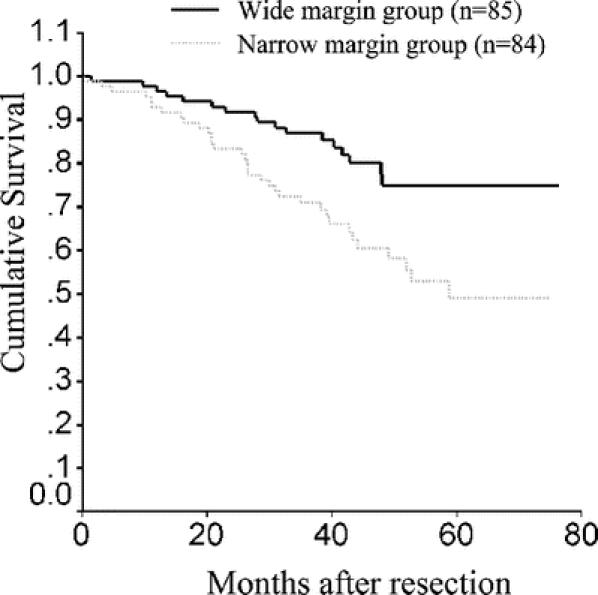
FIGURE 2. Overall survival curves for narrow and wide resection margin groups.
In patients with HCC ≤2 cm, the 1-, 2-, 3-, and 5-year overall survival rates for the narrow and the wide margin groups were 100.0%, 90.0%, 60.0%, 60.0% and 100.0%, 100.0%, 100.0%, 100.0%, respectively. The difference was significant (log-rank test, P = 0.017). In patients with HCC 2.1 to 5.0 cm, the 1-, 2-, 3-, and 5-year overall survival rates for the narrow and the wide margin groups were 97.5%, 85.0%, 79.3%, 56.8%, and 100.0%, 97.6%, 90.2%, 76.8%, respectively. The difference was not significant (log-rank test, P = 0.17). In patients with HCC >5.0 cm, the 1-, 2-, 3-, and 5-year overall survival rates for the narrow and the wide margin groups were 85.3%, 79.4%, 64.6%, 38.3%, and 90.6%, 81.3%, 77.9%, 63.1%, respectively. The difference was not significant (log-rank test, P = 0.12).
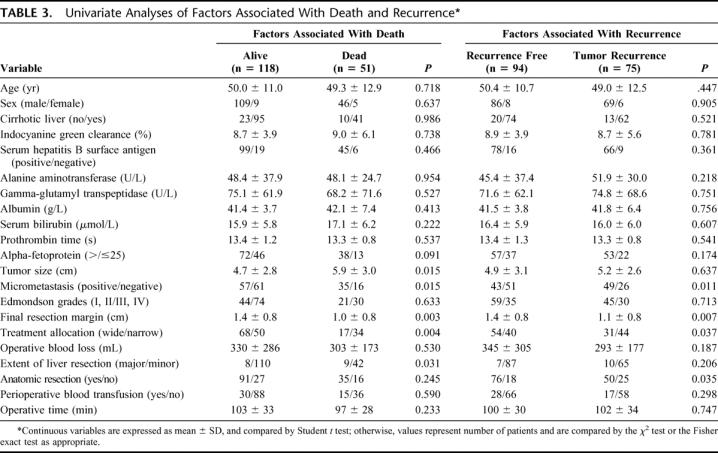
On univariate analysis, 5 factors were significantly associated with death: extent of liver resection (major/minor) (χ2 test, P = 0.031), extent of final resection margin (Student t test, P = 0.003), presence of micrometastasis (χ2 test, P = 0.015), treatment allocation (χ2 test, P = 0.004), and tumor size (Student t test, P = 0.015) (Table 3). When variables listed in Table 3 were included in the Cox regression multivariate analysis, 2 variables showed independent prognostic value for death: presence of micrometastases (odds ratio, 2.361; 95% confidence interval, 1.299–4.292, P = 0.005), and treatment allocation (odds ratio, 0.410; 95% confidence interval, 0.229–0.736, P = 0.003).
TABLE 3. Univariate Analyses of Factors Associated With Death and Recurrence
The 1-, 2-, 3-, and 5-year recurrence-free survival rates for the narrow and the wide margin groups were 71.4%, 58.3%, 49.2%, 40.9% and 84.7%, 77.7%, 65.5%, 52.7%, respectively (Fig. 3). The difference was significant (stratified log-rank test, P = 0.046).
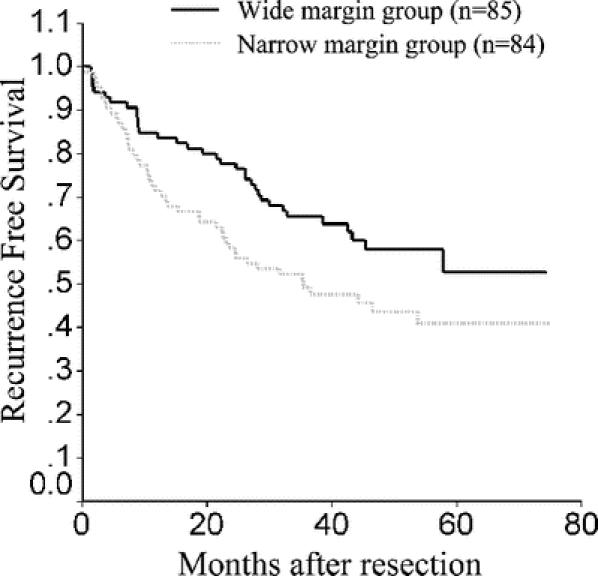
FIGURE 3. Recurrence-free survival curves for narrow and wide resection margin groups.
In patients with HCC ≤2 cm, the 1-, 2-, 3-, and 5-year recurrence free survival rates for the narrow and the wide margin groups were 80.0%, 70.0%, 60.0%, 60.0% and 100.0%, 91.7%, 74.1%, 61.7%, respectively. The difference was not significant (log-rank test, P = 0.56). In patients with HCC 2.1 to 5.0 cm, the 1-, 2-, 3-, and 5-year recurrence free survival rates for the narrow and the wide margin groups were 77.5%, 60.0%, 51.4%, 39.3%, and 87.8%, 78.1%, 68.1%, 63.9%, respectively. The difference was not significant (log-rank test, P = 0.08). In patients with HCC >5.0 cm, the 1-, 2-, 3-, and 5-year recurrence-free survival rates for the narrow and the wide margin groups were 61.8%, 52.9%, 43.7%, 35.1%, and 75.0%, 71.9%, 58.9%, 42.2%, respectively. The difference was not significant (log-rank test, P = 0.32).
Univariate analysis showed that 4 factors were significantly associated with recurrence: anatomic resection (χ2 test, P = 0.035), final resection margin (Student t test, P = 0.007), presence of micrometastasis (χ2 test, P = 0.011), and treatment allocation (χ2 test, P = 0.037) (Table 3). When variables listed in Table 3 were included in the Cox regression multivariate analysis, 2 variables showed independent prognostic value for recurrence: presence of micrometastasis (odds ratio, 2.109; 95% confidence interval, 1.307–3.403, P = 0.002), and final resection margin (odds ratio, 0.598; 95% confidence interval, 0.423–0.845, P = 0.004).
Recurrence
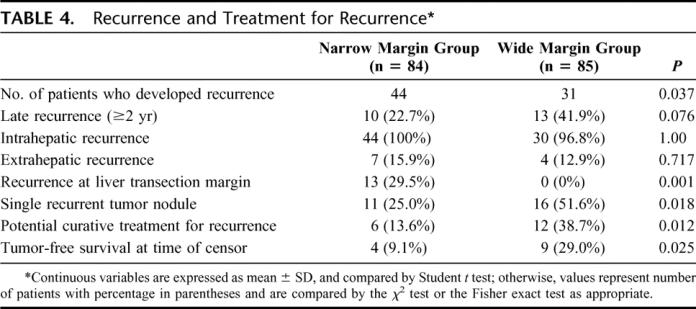
At the time of censor, 75 (44.4%) patients had developed recurrence. The diagnosis of recurrence was based on cytologic/histologic evidence in 41 (54.7%) patients, and on noninvasive diagnostic criteria in 34 patients (45.3%).9 Table 4 shows the pattern of recurrence and the treatment in the 2 groups of patients. All 13 recurrences at the liver transection margin happened in the narrow margin group, including 4 patients (30.8%) who developed recurrence at the liver transection margin alone and 9 (69.2%) associated with recurrence in the distant liver remnant. Potential curative treatment was performed in 17 patients (22.7%) with recurrent tumor. Treatment included hepatic reresection (n = 14), percutaneous radiofrequency ablation (n = 2), and salvage liver transplantation (n = 1).
TABLE 4. Recurrence and Treatment for Recurrence
For the 75 patients who developed recurrence, the mean ± SD for the follow-up periods after recurrence for the narrow and the wide margin groups were 16.9 ± 13.3 months and 21.6 ± 12.6 months, respectively. The 1-, 2-year survival rates after recurrence for the narrow and the wide margin groups were 58.2%, 32.7% and 83.0%, 59.5%, respectively (Fig. 4). The difference was significant (log-rank test, P = 0.017).
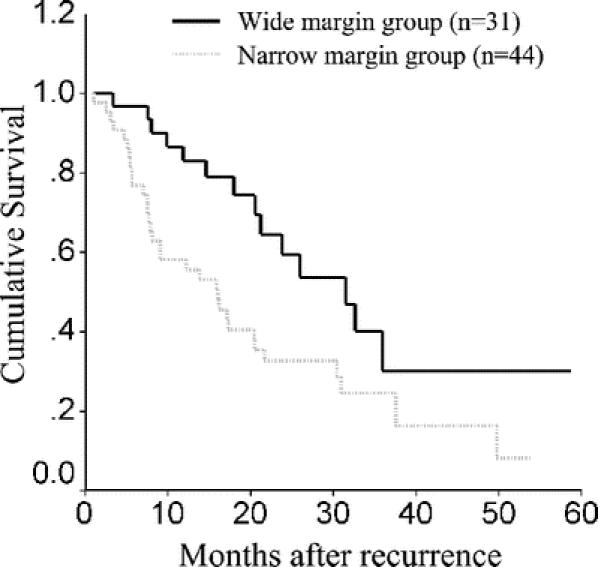
FIGURE 4. Overall survival curves after recurrence for narrow and wide resection margin groups.
DISCUSSION
Since micrometastases disseminate via portal venous branches, anatomic resection is preferred over nonanatomic resection in liver resection carried out with curative intent.15,16 However, our previous study showed that, even at an early T stage when the tumor was solitary and small, micrometastases spread along as well as against the direction of the portal venous flow, and the incidence of micrometastases was closely related to the distance from the primary HCC.6 Thus, an anatomic liver resection with a wider resection margin theoretically gives a higher potential for a cure. However, preserving nontumorous liver parenchyma is also an important consideration, especially in cirrhotic liver resection. The advantages of preserving as much liver parenchyma as possible include not only decreasing the incidence of postoperative liver failure but also improving the chance of performing multimodality treatment and repeat resections in case of tumor recurrence. The optimal liver resection margin is still controversial. Most published studies looking at the long-term survival for patients with HCC treated with partial hepatectomy are retrospective studies. Some of these studies identified the extent of liver resection margin to be an independent prognostic factor,2,17–20 while others showed no correlation between the extent of the resection margin and the incidence of recurrence.21–25 Furthermore, while some studies revealed anatomic resection to have a beneficial effect on recurrence-free survival after partial hepatectomy for HCC,15,16 other studies found that anatomic or nonanatomic resection, and major or minor liver resection, had no significant impact on the risk of tumor recurrence.26–29 It is interesting to note that one study showed the recurrence-free survival after major resection was significantly lower than after minor liver resection.5
Such controversial results could well be because of the difference in the selection of patients in these studies. Lai et al8 surveyed a series of surgical specimens from patients with HCC, most of them presented with multiple tumor nodules or macroscopic vascular invasion. They showed that micrometastases commonly spread extensively from the primary tumor. It is not surprising that for patients with a relatively advanced T stage HCC, partial hepatectomy, even with a wide resection margin or in the form of anatomic resection fails to provide cure to a significant proportion of patients.9 However, in our previous study, we showed that micrometastases were only found beyond 1 cm from the primary tumor in very few patients with macroscopically solitary tumor without vascular invasion. Accordingly, a 1-cm resection margin might be adequate for the majority of patients, but a 2-cm margin might help to reduce tumor recurrence if the patient can survive the larger liver resection.
Accordingly, we designed this study to compare the outcome of 2 groups of patients who underwent partial hepatectomies with a gross margin aiming at 1 cm or 2 cm. It should be noted that the aimed resection margin is different from the final resection margin. The former is determined by intraoperative ultrasound carried out before the liver parenchymal transection. The latter was measured on the resected specimens under microscopy. In addition to the resection margin aimed by the surgeon, it is also affected by the following factors: 1) the microscopic infiltration of the tumor beyond the edge of the tumor as shown on intraoperative ultrasound; 2) the rotating, lifting, and stretching of the liver parenchyma around the tumor as the plane of liver transaction was determined; and 3) the deviation from the planned parenchymal transection plane during the liver resection.
From the results of this study, the incidences of the overall morbidity and in-hospital mortality in the 2 groups were not significantly different. This might partly be due to patient selection. In this study, many of the tumors were small or they were situated peripherally. Therefore, even a 2-cm resection margin did not result in too excessive a sacrifice of the liver parenchyma. However, the overall and recurrence-free survivals were significantly higher in the wide margin group than in the narrow margin group. As multivariate analysis showed that treatment allocation was a variable independently related to overall survival, and the final resection margin was a variable independently related to recurrence-free survival, partial hepatectomy with a 2-cm margin is recommended for patients with a macroscopically solitary HCC without vascular invasion, provided anatomic factors and the severity of the underlying cirrhosis allow such a resection to be carried out.
From our results, the survival outcomes of the wide margin group were better than the narrow margin group in each tumor size stratum, but the differences had not reached statistical significance for strata 2.1 to 5 cm, and >5 cm. This could well be because of the small sample size in these strata. However, in patients with HCC ≤2 cm, the overall survivals were significantly better in the wide margin group than the narrow margin group despite a very small sample size (wide/narrow, 12/10), implying that the benefit of a wide margin is more prominent for small HCC.
Several studies revealed that recurrent tumors commonly occurred in the liver remnant at a segment distant from the resection margin, or at multiple liver segments.21,22,30,31 These observations are consistent with the results of this study. In this study, multiple tumor recurrences were significantly higher in the narrow margin group than in the wide margin group, and the rate of potentially curative treatment of tumor recurrence and survival after recurrence were significantly higher in the wide margin group than the narrow margin group. These observations suggest that the extent of the resection margin affects the pattern of recurrence. Micrometastases occur commonly around the primary tumor even for small and solitary HCC (mainly in the form of micro tumor thrombi inside vascular lumen).6 We postulate that, during hepatic resection with a narrow margin, some of these micro tumor thrombi at the liver transection plane may be pushed into the larger vessels in the liver remnant and disseminate, leading to recurrence at a distance from the resection margin.
CONCLUSION
For macroscopically solitary HCC, this study provided evidence that a gross resection margin aiming at 2 cm provided better survival outcome than a narrow resection margin aiming at 1 cm, especially for HCC ≤2 cm. A narrow resection margin should only be contemplated when a 2-cm resection margin is not technically feasible, either because of anatomic reasons or because of the severity of the underlying cirrhosis.
Footnotes
Supported in part by the Guangzhou Science and Technique Foundation (Grant No. 2005Z3-E0111) and the Guangdong Provincial Health Department Medical Science Foundation (Grant No. B2005051).
Reprints: Jin-Qing Li, MD, Department of Hepatobiliary Oncology, Cancer Center, Sun Yat-sen University, Guangzhou, 510060, P.R. China. E-mail: shiming@mail.sysu.edu.cn.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, et al. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. [DOI] [PubMed] [Google Scholar]
- 2.Nagasue N, Uchida M, Makino Y, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology. 1993;105:488–494. [DOI] [PubMed] [Google Scholar]
- 3.Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(suppl):35–50. [DOI] [PubMed] [Google Scholar]
- 4.Mullin EJ, Metcalfe MS, Maddern GJ. How much liver resection is too much? Am J Surg. 2005;190:87–97. [DOI] [PubMed] [Google Scholar]
- 5.Ko S, Nakajima Y, Kanehiro H, et al. Significant influence of accompanying chronic hepatitis status on recurrence of hepatocellular carcinoma after hepatectomy: result of multivariate analysis. Ann Surg. 1996;224:591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi M, Zhang CQ, Zhang YQ, et al. Micrometastases of solitary hepatocellular carcinoma and appropriate resection margin. World J Surg. 2004;28:376–381. [DOI] [PubMed] [Google Scholar]
- 7.Hermanek P, Henson D, Hutter R, et al. International Union Against Cancer [TNM Supplement 1993]: A Commentary on Uniform Use. Berlin: Springer, 1993:7, 14. [Google Scholar]
- 8.Lai EC, You KT, Ng IO, et al. The pathological basis of resection margin for hepatocellular carcinoma. World J Surg. 1993;17:786–790; discussion 791. [DOI] [PubMed]
- 9.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma: conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. [DOI] [PubMed] [Google Scholar]
- 10.Zhou XD, Tang ZY, Yang BH, et al. Experience of 1000 patients who underwent hepatectomy for small hepatocellular carcinoma. Cancer. 2001;91:1479–1486. [DOI] [PubMed] [Google Scholar]
- 11.Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet. 1985;161:346–350. [PubMed] [Google Scholar]
- 12.Poon RT, Fan ST, Lo CM, et al. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999;229:216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedman LS. Tables of the number of patients required in clinical trials using the logrank test. Stat Med. 1982;1:121–129. [DOI] [PubMed] [Google Scholar]
- 14.Liver Cancer Study Group of Japan. Primary liver cancer in Japan: clinicopathologic features and results of surgical treatment. Ann Surg. 1990;211:277–287. [PMC free article] [PubMed] [Google Scholar]
- 15.Regimbeau JM, Kianmanesh R, Farges O, et al. Extent of liver resection influences the outcome in patients with cirrhosis and small hepatocellular carcinoma. Surgery. 2002;131:311–317. [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa K, Kokudo N, Imamura H, et al. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005;242:252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lise M, Bacchetti S, Da Pian P, et al. Prognostic factors affecting long term outcome after liver resection for hepatocellular carcinoma: results in a series of 100 Italian patients. Cancer. 1998;82:1028–1036. [DOI] [PubMed] [Google Scholar]
- 18.Liver Cancer Study Group of Japan. Predictive factors for long term prognosis after partial hepatectomy for patients with hepatocellular carcinoma in Japan. Cancer. 1994;74:2772–2780. [DOI] [PubMed] [Google Scholar]
- 19.Ikai I, Arii S, Kojiro M, et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer. 2004;101:796–802. [DOI] [PubMed] [Google Scholar]
- 20.Ng IO, Lai EC, Fan ST, et al. Prognostic significance of pathologic features of hepatocellular carcinoma: a multivariate analysis of 278 patients. Cancer. 1995;76:2443–2448. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida Y, Kanematsu T, Matsumata T, et al. Surgical margin and recurrence after resection of hepatocellular carcinoma in patients with cirrhosis: further evaluation of limited hepatic resection. Ann Surg. 1989;209:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poon RT, Fan ST, Ng IO, et al. Significance of resection margin in hepatectomy for hepatocellular carcinoma: a critical reappraisal. Ann Surg. 2000;231:544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamanaka N, Okamoto E, Toyosaka A, et al. Prognostic factors after hepatectomy for hepatocellular carcinomas: a univariate and multivariate analysis. Cancer. 1990;65:1104–1110. [DOI] [PubMed] [Google Scholar]
- 24.Jwo SC, Chiu JH, Chau GY, et al. Risk factors linked to tumor recurrence of human hepatocellular carcinoma after hepatic resection. Hepatology. 1992;16:1367–1371. [DOI] [PubMed] [Google Scholar]
- 25.Izumi R, Shimizu K, Ii T, et al. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology. 1994;106:720–727. [DOI] [PubMed] [Google Scholar]
- 26.Lau H, Fan ST, Ng IO, et al. Long term prognosis after hepatectomy for hepatocellular carcinoma: a survival analysis of 204 consecutive patients. Cancer. 1998;83:2302–2311. [PubMed] [Google Scholar]
- 27.Okada S, Shimada K, Yamamoto J, et al. Predictive factors for postoperative recurrence of hepatocellular carcinoma. Gastroenterology. 1994;106:1618–1624. [DOI] [PubMed] [Google Scholar]
- 28.Fuster J, Garcia-Valdecasas JC, Grande L, et al. Hepatocellular carcinoma and cirrhosis: results of surgical treatment in a European series. Ann Surg. 1996;223:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adachi E, Maeda T, Matsumata T, et al. Risk factors for intrahepatic recurrence in human small hepatocellular carcinoma. Gastroenterology. 1995;108:768–775. [DOI] [PubMed] [Google Scholar]
- 30.Matsumata T, Kanematsu T, Takenaka K, et al. Patterns of intrahepatic recurrence after curative resection of hepatocellular carcinoma. Hepatology. 1989;9:457–460. [DOI] [PubMed] [Google Scholar]
- 31.Belghiti J, Panis Y, Farges O, et al. Intrahepatic recurrence after resection of hepatocellular carcinoma complicating cirrhosis. Ann Surg. 1991;214:114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]