Abstract
Objective:
To evaluate the survival of patients with pseudomyxoma peritonei (PMP) treated by cytoreductive surgery and intraoperative hyperthermic intraperitoneal chemotherapy (HIPEC), and to identify factors with prognostic value.
Summary Background Data:
PMP is a clinical syndrome characterized by progressive intraperitoneal accumulation of mucous and mucinous implants, usually derived from a ruptured mucinous neoplasm of the appendix. Survival is dominated by pathology.
Methods:
A total of 103 patients (34 men and 69 women) treated at The Netherlands Cancer Institute between 1996 and 2004 were identified. Survival was calculated from date of initial treatment and corrected for a second procedure. PMP was pathologically categorized into disseminated peritoneal adenomucinosis (DPAM), peritoneal mucinous carcinomatosis (PMCA), and an intermediate subtype (PMCA-I). Clinical and pathologic factors were analyzed to identify their prognostic value for survival.
Results:
Median follow-up was 51.5 months (range, 0.1–99.5 months). Recurrence developed in 44%. A second procedure for recurrence was performed in 11 patients. The median disease-free interval was 25.6 months (95% confidence interval [CI], 14.8–43.6 months). The 3-year and 5-year disease-free survival probability was 43.6% (95% CI, 34.4%–55.2%) and 37.4% (95% CI, 28.2%–49.5%), respectively. The disease-specific 3-year and 5-year survival probability was 70.9% (95% CI, 62.0%–81.2%) and 59.5% (95% CI 48.7%–72.5%), respectively. Factors associated with survival were pathological subtype, completeness of cytoreduction, and degree and location of tumor load (P < 0.05). The main prognostic factor, independently associated with survival, was the pathologic subtype (P < 0.01).
Conclusion:
Cytoreductive surgery in combination with intraoperative HIPEC is a feasible treatment strategy for PMP in terms of survival. The pathologic subtype remains the dominant factor in survival. Patients should be centralized to improve survival by a combination of surgical experience and adequate patient selection.
The 5-year disease-specific survival probability of pseudomyxoma peritonei patients after treatment by cytoreductive surgery and intraoperative hyperthermic intraperitoneal chemotherapy reaches 60%. Patients with pseudomyxoma peritonei pathologically categorized as adenomucinosis benefit most from this treatment strategy with a 5-year disease-specific survival probability of 72%.
Pseudomyxoma peritonei (PMP) is a rare disease with an incidence of approximately 1 per million a year.1 It is characterized by intraperitoneal disseminated mucous produced by adenomucinous tumor cells in implants on peritoneal surfaces. These implants are the final stage of a distribution process following the rupture of an intraperitoneal located mucinous neoplasm.2 PMP is reported to originate from mucinous (cyst)adenoma, mucoceles, and mucinous (cyst)adenocarcinoma of mostly appendix (or ovary).1,3–6 Incidental PMP has unusual origins.7,8
Clinically, PMP is a slowly progressive disease. It mainly expresses as a so-called “jelly belly,” caused by the abundant intraperitoneal mucous. Sometimes patients present themselves with symptoms arising from the primary tumor, mimicking appendicitis. In other cases, they present with an inguinal herniated sac or an ovarian mass.9–11 As PMP progresses, the excessive mucous accumulation causes compression of the intestines. Gastrointestinal function is compromised and eventually obstruction is imminent. Consequently, PMP always results in the death of the patient unless radically treated.5
At The Netherlands Cancer Institute, aggressive cytoreductive surgery in combination with intraoperative hyperthermic intraperitoneal chemotherapy (HIPEC) is used as the treatment of PMP since 1995. The aim of this study is to evaluate this treatment strategy in terms of survival, and to identify clinical and pathologic prognostic factors for survival.
METHODS
Patient Population
Patients were diagnosed PMP based on clinical symptoms, excessive abdominal mucous with characteristic distribution on computed tomography (CT) scans, and by pathologic assessment. Both primary and recurrent PMP was included in the study. Patients with evidence of liver or lung metastases on CT scan were excluded from treatment.
Treatment Schedule
The principles of cytoreductive surgery and HIPEC have been described in detail elsewhere.12 Briefly, this treatment modality consists of resecting (potentially) all macroscopic tumor deposits on parietal and/or visceral peritoneal surfaces, combined if necessary with resection of involved viscera. This is followed by intraoperative HIPEC with mitomycin C 35 mg/m2 for 90 minutes at 40°C to 41°C to erase microscopic residual tumor. At the end of the procedure, the necessary anastomoses are made. A specialized surgical team performs the treatment.
At surgery, tumor load is recorded in 7 distinctive abdominal regions: pelvis, ileocecum, omentum/transverse colon, small bowel/mesentery, subhepatic region, and subphrenic left and right regions. Maximum diameter of tumor deposits are graded into 0 cm, 1 to 5 cm, and >5 cm. Completeness of cytoreduction is recorded at the end of surgery: no residual macroscopic tumor (R1), residual macroscopic tumor smaller than 2.5 mm in any region (R2a), and tumor deposits larger than 2.5 mm in any region (R2b).
Additional Treatment and Follow-up
PMP patients with pathologic evidence of malignant features at initial treatment were in addition treated with systemic chemotherapy conform hospital protocol. The hospital protocol consisted of 6 months 5-fluorouracil (400 mg/m2) and leucovorin (80 mg/m2) weekly. Patients had to be in a good condition. Long-term follow-up was performed by physical examination and assessment of tumor markers CEA and CA 19.9 every 3 months. A CT scan was performed every 6 months. Recurrence or progression was diagnosed in case of a profound raise of tumor marker(s) and/or evidence of relapse on CT scan, or during relaparotomy for any other cause.
Data Collection and Statistical Analysis
Data were obtained from a prospective database of clinical records, surgical reports, reports of CT scans, laboratory and pathology reports, and contact with the patient's general practitioner. PMP was classified retrospectively into 3 pathologic subtypes according to Ronnett's criteria: disseminated peritoneal adenomucinosis (DPAM), peritoneal mucinous carcinomatosis (PMCA), and an intermediate group (PMCA-I).4
The analyses were performed on patients treated for PMP between 1996 and 2004. In some patients, a second procedure was performed after recurrence or progression. A Cox proportional hazard model was used to analyze the effect of several clinical and pathologic factors on disease-specific survival (DSS) and disease-free survival (DFS). Possible clinical and pathologic prognostic factors for recurrence or death of disease that were investigated include age, gender, previous laparotomy, tumor load and involved regions, result of cytoreduction, pathology, and additional systemic chemotherapy. The choice for independent factors in the multivariate model was based on the univariate results.
The survival time was calculated from the date of first cytoreduction and HIPEC. An event was defined as a recurrence at any site or disease related death. All analyses were stratified for the number of procedures of cytoreduction and HIPEC. Patients with missing data were excluded. Significance was defined as a P value less than 0.05. Data were analyzed using the Statistical Package for Social Sciences (SPSS Inc., Chicago, IL), version 11.5, and SAS version 8.2.
RESULTS
Clinical and Pathologic Features
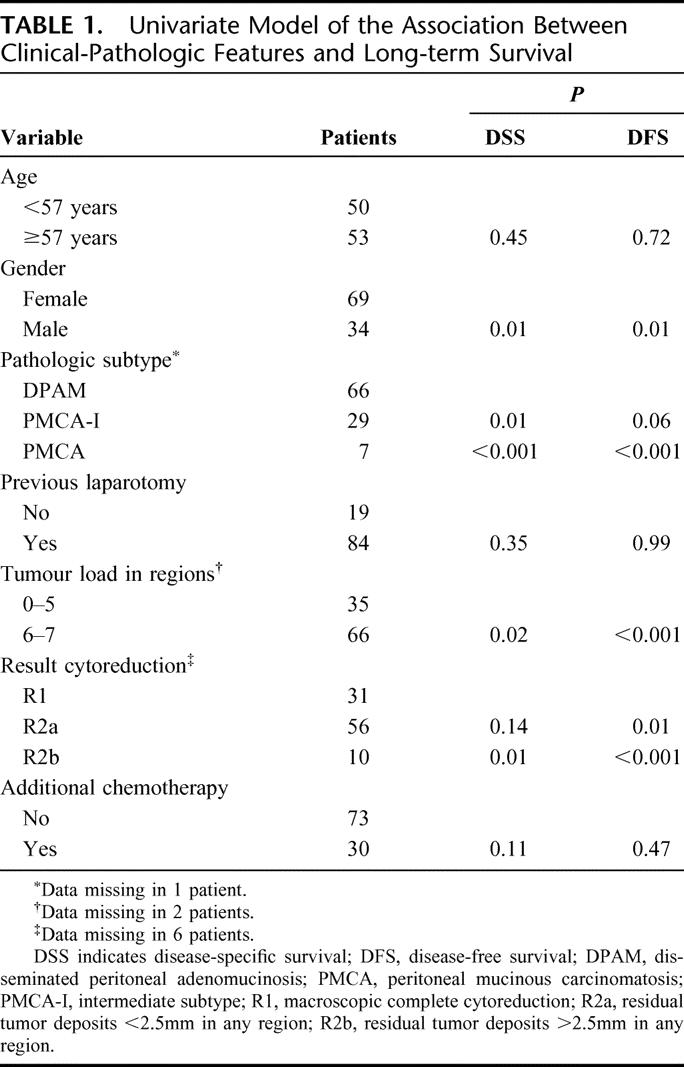
Clinical and pathologic features of 103 PMP patients are shown in Table 1. The median age was 57 years (range, 30–77 years). Previous abdominal surgery was performed once in 59 patients (57%) and twice or more in 25 patients (24%). Twelve patients (12%) had received systemic chemotherapy prior to cytoreduction and HIPEC. PMP was of appendiceal origin in 92 patients (91%). Other origins included urachus (n = 2), ovary (n = 3), colon (n = 3), and pancreas (n = 1). The origin was unknown in 2 patients. In comparison to male patients, female patients showed more DPAM pathology (68% vs. 59%), limited extent of disease (0–5 regions in 40% vs. 23%), and complete cytoreduction (39% vs. 19%).
TABLE 1. Univariate Model of the Association Between Clinical-Pathologic Features and Long-term Survival
Treatment
A total of 114 procedures were performed in 103 patients. The ability to achieve complete cytoreduction was correlated with the tumor load (P < 0.001, χ2). In case of tumor load in 6 to 7 regions, macroscopic complete cytoreduction (R1) after first treatment was accomplished in 4 of 66 patients (6%). When PMP involved 5 or fewer regions, an R1 cytoreduction after first treatment could be achieved in 27 of 35 patients (77%). Accomplishing complete cytoreduction necessitated aggressive surgery, leading to mainly surgical complications in 62 of 114 procedures (54%) within 60 patients. Most important complications consisted of (small) bowel leakages and fistula. Three patients died within 30 days after treatment.
Systemic chemotherapy was given after cytoreduction and HIPEC in 30 of 103 patients (29%). Two patients were not eligible because of an unfavorable clinical condition. In 2 patients, chemotherapy had to be stopped because of toxicity. Seven patients died of complications before systemic chemotherapy was started. Because of the applied retrospective pathologic classification in subtypes, some patients crossed over from PMP with malignant features to DPAM, and the other way around. Thus, some patients with DPAM had received systemic chemotherapy and some patients with PMCA-I had not. None of the patients with DPAM, PMCA, or PMCA-I that received systemic chemotherapy showed any objective response.
Survival Analysis
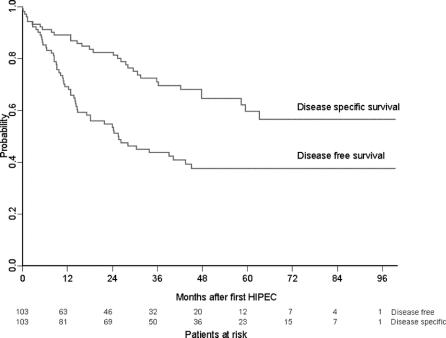
Median follow-up was 51.5 months (range, 0.1–99.5 months). The 3-year and 5-year DSS probability was 70.9% (95% confidence interval [CI], 62.0%–81.2%) and 59.5% (95% CI, 48.7%–72.5%), respectively. Recurrence of PMP after initial cytoreduction and HIPEC developed in 45 of 103 patients (44%) with a median disease-free interval of 25.6 months (95% CI, 14.8–43.6 months). The 3-year and 5-year DFS probability was 43.6% (95% CI, 34.4%–55.2%) and 37.4% (95% CI, 28.2%–49.5%), respectively. The survival probability is shown in Figure 1.
FIGURE 1. Disease-specific and disease-free survival probability.
At end of follow-up, 68 of 103 (66%) patients were alive. Sixty-three patients (61%), of whom 15 had died, had no evidence of disease at end of follow-up. Twenty patients (19%) had died of recurrence or progression, which was mostly located in multiple regions (n = 9) or in the subhepatic region (n = 5). There was 1 case of extraperitoneal metastasis at time of recurrence, in a patient with the PMCA subtype.
Eleven patients underwent a second procedure of cytoreduction and HIPEC. Seven patients had only a second debulking laparotomy for recurrent disease. The 3-year DSS survival probability calculated from date of second cytoreduction and HIPEC was 77.8% (95% CI, 54.9%–100%). The 3-year DFS probability of these patients after the second procedure was 33.3% (95% CI, 13.2%–84.0%).
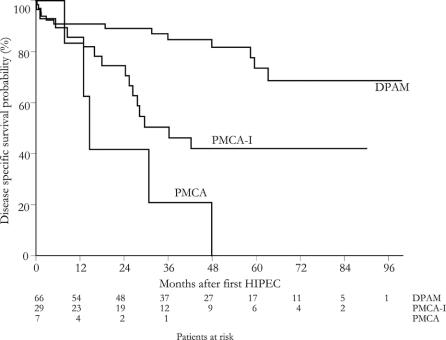
Table 1 shows a univariate model and Table 2 shows a multivariate model of the association between clinical-pathologic factors and survival. Survival was dominated by DPAM pathology, as shown in Figure 2. In univariate analysis, affected regions associated with a decreased DSS probability were the subdiaphragmatic left and subhepatic region (P = 0.02). Small bowel involvement was nearly significant (P = 0.06). In case of tumor load >5 cm involving small bowel, diaphragm, or liver, the DSS probability decreased significantly (P < 0.04).
TABLE 2. Multivariate Model of the Association Between Clinical-Pathologic Features and Long-term Survival
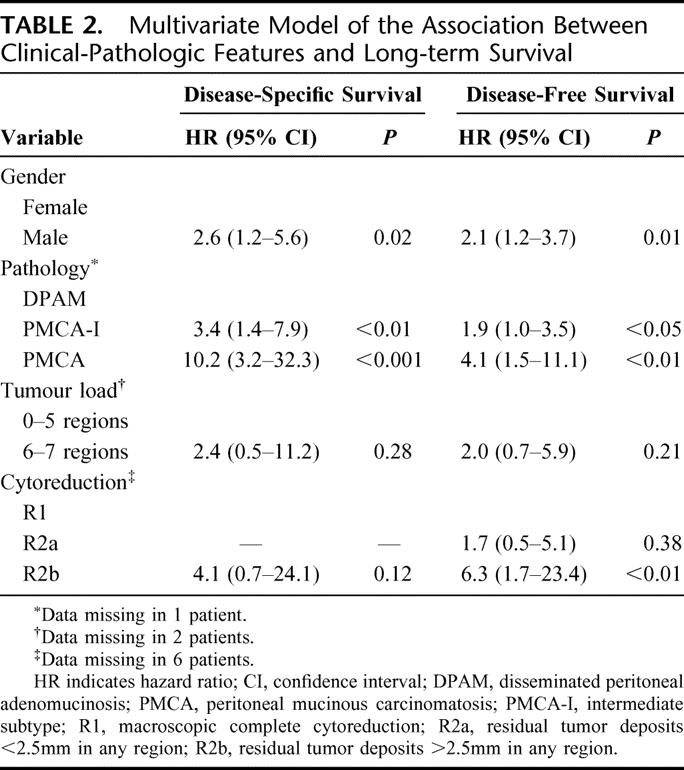
FIGURE 2. Disease-specific survival probability for pathologic subtype.
DISCUSSION
Pseudomyxoma peritonei is an indolent disease, and long-term survival up to 20 years has been described.14 Cytoreductive surgery is the key to successful treatment in PMP. International consensus regarding the most beneficial treatment strategy, however, is lacking, and there are 3 known approaches. The traditional approach consists of serial debulking surgery without the intention to be complete. The result of this strategy is excellent on a short term. Morbidity is low and patients recover to a normal life. However, almost all will recur in a few years time. A second debulking is usually more difficult and less successful. The second symptom-free interval is shorter and followed by a third operation and a fourth. Eventually, either the tumor becomes inoperable and the patient dies of cachexia, or the patient dies of complications of the operation. Gough et al published a series of 26 patients treated this way with an estimated 5-year survival of 53%.15 At end of follow-up, only 3% were free of disease.
Recently, an analysis of 97 PMP patients treated at the Memorial Sloan-Kettering Cancer Center was presented by Miner et al.16 An average of 2.2 debulking operations was performed, and intraperitoneal chemotherapy was applied in 31%. They managed to reach complete cytoreduction in 53 patients (55%). Eventually, their treatment strategy resulted in a 10-year actuarial survival of 21%, calculated from date of diagnosis. The disease-free rate at end of follow-up was 12%.
Sugarbaker introduced an approach including extensive cytoreductive surgery with HIPEC.17 The surgical approach is based on extraperitoneal dissection, which allows the complete removal of peritoneal masses while dissecting in a tumor-free plain. These peritonectomy procedures can be very helpful, especially in the pelvis and on the subdiaphragmatic areas.18 The idea of HIPEC is based on exposure of residual tumor cells on the peritoneal surface to very high doses of cytotoxic drugs potentiated by hyperthermia.19 A local effect is hereby created that can never be reached by systemic exposure, due to general toxicity. In this way, it may be assumed that at least the reseeding of surgical surfaces by free tumor cells, which are so widely present during these operations, can be prevented. Whether HIPEC also succeeds to sterilize tumor cells in remaining tumor deposits is uncertain. In several studies reporting on this aggressive treatment combination, a large variation in survival rates is given, as shown in Table 3.20–25 This wide range can be explained by the use of different treatment protocols and different inclusion criteria influencing survival.
TABLE 3. Comparison of International Survival Results After Treatment of Pseudomyxoma Peritonei
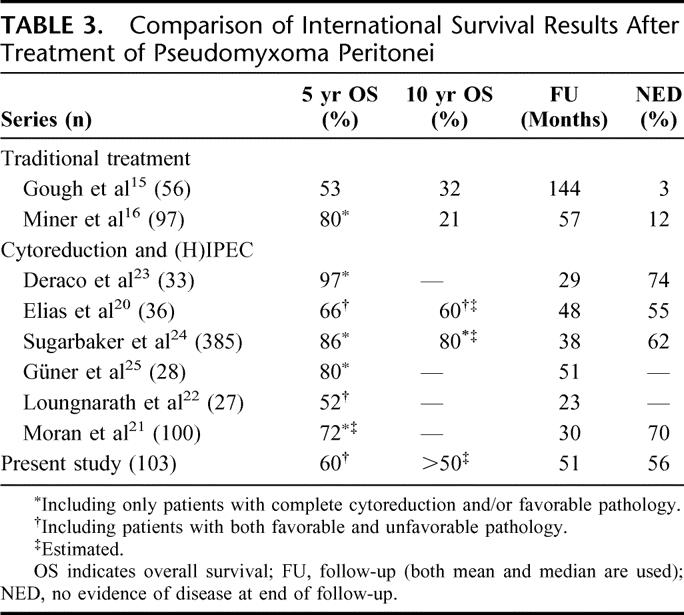
In our series, we report our results from the date of cytoreductive surgery and HIPEC. We think that this represents more accurately the impact of treatment on the course of disease. Although the median follow-up was only 51 months, the Kaplan-Meier survival curve (including patients with a poor prognosis) reached a plateau of 60% after 5 years. It seems therefore likely that the actuarial 10-year survival will be in the same range. At end of follow-up, 56% of patients were free of disease. Although 5-year survival for patients with DPAM/low-grade pathology seems comparable, the 10-year survival and DFS compare favorably to the Memorial Sloan Kettering Cancer Center data. These values support the effectiveness of HIPEC in PMP, as shown in Table 3, although it is clear that comparing these data produces some problems as differences exist in both the applied pathologic classification and the inclusion of pathology and completeness of cytoreduction in the survival analysis.
An important prognostic factor for long-term survival in PMP is pathology. Ronnett et al4 first described a pathologic classification that divides PMP into diagnostic categories with different prognosis: DPAM, PMCA, and an intermediate/hybrid group. It is a useful classification as PMP pathology shows a fluid transition from DPAM to PMCA. However, PMCA seems to behave like peritoneal carcinomatosis of ordinary colorectal adenocarcinoma origin. It has a very poor prognosis, shown in the Sugarbaker series and confirmed by our results.24 We agree with Sugarbaker and colleagues that PMCA should not be classified as PMP but treated as peritoneal disseminated colorectal cancer as described by Verwaal et al.26
Completeness of cytoreduction is strongly associated with the extent of disease, and has prognostic value for survival as well.20,27 Our data show that in case of limited disease (0–5 regions) a 5-year survival up to 82% is a realistic aim. Patients with extensive PMP are prone to incomplete cytoreduction and a complicated recovery. In addition, this series demonstrates that a large tumor load involving small bowel, diaphragm, or subhepatic region indicates a worse prognosis. We agree with Miner et al16 that in these extensive PMP cases a planned 2-step approach toward complete cytoreduction is preferable. We now resect the ileocecum, the omentum, and if necessary the ovaries in the first operation. In a second stage after 3 months, when the patient has recovered, the cytoreduction is completed and intraoperative HIPEC is given.
Our series presents some other interesting observations. Women had a better prognosis, even after correction for pathology and extent of disease in the multivariate analysis. Maybe the dominance of ovarian involvement alters the behavior of PMP.
Another observation of some significance is that none of the patients that received systemic 5-fluorouracil-based chemotherapy showed an objective response. This supports strongly the value of an aggressive locoregional surgical approach in these cases.
Cytoreduction and HIPEC have been quite a toxic treatment modality in our hands. Our data show, however convincingly, that a learning curve exists, with a reduced toxicity and mortality in the last 50 cases.28
CONCLUSION
Cytoreductive surgery with intraoperative HIPEC is a treatment strategy with encouraging survival results for selected PMP patients. The pathologic subtype remains the dominant factor for survival. Improvement of survival can be achieved by a combination of surgical experience and adequate patient selection. We that that only by centralization of PMP patients the necessary expertise can be built up to improve the outcome of this fascinating, but difficult to treat, patient group.
Footnotes
Reprints: Robert M. Smeenk, MD, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, the Netherlands. E-mail: r.smeenk@nki.nl.
REFERENCES
- 1.Mukherjee A, Parvaiz A, Cecil TD, et al. Pseudomyxoma peritonei usually originates from the appendix: a review of the evidence. Eur J Gynaecol Oncol. 2004;25:411–414. [PubMed] [Google Scholar]
- 2.Sugarbaker PH. Pseudomyxoma peritonei: a cancer whose biology is characterized by a redistribution phenomenon. Ann Surg. 1994;219:109–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinson FL, Ambrose NS. Pseudomyxoma peritonei. Br J Surg. 1998;85:1332–1339. [DOI] [PubMed] [Google Scholar]
- 4.Ronnett BM, Zahn CM, Kurman RJ, et al. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis: a clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to ‘pseudomyxoma peritonei’. Am J Surg Pathol. 1995;19:1390–1408. [DOI] [PubMed] [Google Scholar]
- 5.Sugarbaker PH, Ronnett BM, Archer A, et al. Pseudomyxoma peritonei syndrome. Adv Surg. 1996;30:233–280. [PubMed] [Google Scholar]
- 6.Wertheim I, Fleischhacker D, McLachlin CM, et al. Pseudomyxoma peritonei: a review of 23 cases. Obstet Gynecol. 1994;84:17–21. [PubMed] [Google Scholar]
- 7.Chejfec G, Rieker WJ, Jablokow VR, et al. Pseudomyxoma peritonei associated with colloid carcinoma of the pancreas. Gastroenterology. 1986;90:202–205. [DOI] [PubMed] [Google Scholar]
- 8.de Bree E, Witkamp A, Van De Vijver M, et al. Unusual origins of Pseudomyxoma peritonei. J Surg Oncol. 2000;75:270–274. [DOI] [PubMed] [Google Scholar]
- 9.Baker WC, Goldman LB, DeVere White RW. Pseudomyxoma peritonei presenting as a scrotal mass. J Urol. 1988;139:821–822. [DOI] [PubMed] [Google Scholar]
- 10.Esquivel J, Sugarbaker PH. Pseudomyxoma peritonei in a hernia sac: analysis of 20 patients in whom mucoid fluid was found during a hernia repair. Eur J Surg Oncol. 2001;27:54–58. [DOI] [PubMed] [Google Scholar]
- 11.Ronnett BM, Kurman RJ, Zahn CM, et al. Pseudomyxoma peritonei in women: a clinicopathologic analysis of 30 cases with emphasis on site of origin, prognosis, and relationship to ovarian mucinous tumors of low malignant potential. Hum Pathol. 1995;26:509–524. [DOI] [PubMed] [Google Scholar]
- 12.Verwaal VJ, Van Tinteren H, Ruth SV, et al. Toxicity of cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. J Surg Oncol. 2004;85:61–67. [DOI] [PubMed] [Google Scholar]
- 13.Deleted in proof.
- 14.Lang H, Jahne J, Flemming P, et al. Pseudomyxoma peritonei of appendiceal origin: a report of seven cases and a review of published reports. Eur J Surg. 1995;161:355–360. [PubMed] [Google Scholar]
- 15.Gough DB, Donohue JH, Schutt AJ, et al. Pseudomyxoma peritonei: long-term patient survival with an aggressive regional approach. Ann Surg. 1994;219:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miner TJ, Shia J, Jaques DP, et al. Long-term survival following treatment of pseudomyxoma peritonei: an analysis of surgical therapy. Ann Surg. 2005;241:300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugarbaker PH. Surgical treatment of peritoneal carcinomatosis: 1988 Du Pont lecture. Can J Surg. 1989;32:164–170. [PubMed] [Google Scholar]
- 18.Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witkamp AJ, de Bree E, Van Goethem R, et al. Rationale and techniques of intra-operative hyperthermic intraperitoneal chemotherapy. Cancer Treat Rev. 2001;27:365–374. [DOI] [PubMed] [Google Scholar]
- 20.Elias D, Laurent S, Antoun S, et al. Pseudomyxoma peritonei treated with complete resection and immediate intraperitoneal chemotherapy. Gastroenterol Clin Biol. 2003;27:407–412. [PubMed] [Google Scholar]
- 21.Moran BJ, Mukherjee A, Sexton R. Operability and early outcome in 100 consecutive laparotomies for peritoneal malignancy. Br J Surg. 2006;93:100–104. [DOI] [PubMed] [Google Scholar]
- 22.Loungnarath R, Causeret S, Bossard N, et al. Cytoreductive surgery with intraperitoneal chemohyperthermia for the treatment of pseudomyxoma peritonei: a prospective study. Dis Colon Rectum. 2005;48:1372–1379. [DOI] [PubMed] [Google Scholar]
- 23.Deraco M, Baratti D, Inglese MG, et al. Peritonectomy and intraperitoneal hyperthermic perfusion (IPHP): a strategy that has confirmed its efficacy in patients with pseudomyxoma peritonei. Ann Surg Oncol. 2004;11:393–398. [DOI] [PubMed] [Google Scholar]
- 24.Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol. 1999;6:727–731. [DOI] [PubMed] [Google Scholar]
- 25.Guner Z, Schmidt U, Dahlke MH, et al. Cytoreductive surgery and intraperitoneal chemotherapy for pseudomyxoma peritonei. Int J Colorectal Dis. 2005;20:155–160. [DOI] [PubMed] [Google Scholar]
- 26.Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–3743. [DOI] [PubMed] [Google Scholar]
- 27.Sugarbaker PH. Cytoreductive surgery and peri-operative intraperitoneal chemotherapy as a curative approach to pseudomyxoma peritonei syndrome. Eur J Surg Oncol. 2001;27:239–243. [DOI] [PubMed] [Google Scholar]
- 28.Smeenk RM, Verwaal VJ, Zoetmulder FA. Toxicity and mortality of cytoreduction and intraoperative hyperthermic intraperitoneal chemotherapy in pseudomyxoma peritonei-a report of 103 procedures. Eur J Surg Oncol. 2006;32:186–190. [DOI] [PubMed] [Google Scholar]