Abstract
Objective:
The purpose of this study is to measure abdominal wall myopathic histologic and mechanical changes during incisional herniation and its effect on incisional hernia repairs.
Summary Background Data:
Unloaded skeletal muscles undergo characteristic atrophic changes, including change in fiber type composition, decreased cross-sectional area, and pathologic fibrosis. We hypothesize that these atrophic changes decrease muscle elastic properties and may contribute to the high laparotomy wound failure rate observed following incisional hernia repair.
Methods:
A rat model of chronic incisional hernia formation was used. Failing midline laparotomy incisions developed into incisional hernias. Controls were uninjured and sham laparotomy (healed) groups. Internal oblique muscles were harvested for fiber typing, measurement of cross-sectional area, collagen deposition, and mechanical analysis. Mesh hernia repairs were performed on a second group of rats with chronic incisional hernias or acute anterior abdominal wall myofascial defects.
Results:
The hernia group developed lateral abdominal wall shortening and oblique muscle atrophy. This was associated with a change in the distribution of oblique muscle fiber types, decreased cross-sectional area, and pathologic fibrosis consistent with myopathic disuse atrophy. These muscles exhibited significant decreased extensibility and increased stiffness. The healed (sham) laparotomy group expressed an intermediate phenotype between the uninjured and hernia groups. Recurrent hernia formation was most frequent in the chronic hernia model, and hernia repairs mechanically disrupted at a lower force compared with nonherniated abdominal walls.
Conclusions:
The internal oblique muscles of the abdominal wall express a pattern of changes consistent with those seen in chronically unloaded skeletal muscles. The internal oblique muscles become fibrotic during herniation, reducing abdominal wall compliance and increasing the transfer of load forces to the midline wound at the time of hernia repair.
The abdominal wall is unloaded during the development of midline incisional hernias, leading to myopathic disuse atrophy. Adaptive muscle responses result in decreased abdominal wall compliance and increased transfer of load forces to subsequent abdominal wall repairs, which may contribute to the high rate of recurrent incisional hernia formation.
Incisional hernia formation is one of the most common complications after laparotomy.1 Prospective studies report that 11% of all midline abdominal wall closures will fail to completely heal and form incisional hernias.2–4 Approximately 200,000 incisional hernia repairs are performed each year in the United States,5 making incisional hernia formation the most common indication for reoperation following abdominal operations. Randomized controlled trials find an alarmingly high hernia recurrence rate following mesh (24%) and suture (43%) repairs.6,7 It is suggested that the introduction of mesh implants has not reduced the incidence of recurrent incisional hernia formation but only increased the interval of time until the recurrence is detected.8
Incisional hernia formation requires laparotomy wound failure, and the mechanism of laparotomy wound failure is multifactorial. Limited studies focus on wound collagen deposition and the local wound healing environment.9 The abdominal wall is, however, a muscular structure under a dynamic equilibrium of load forces that are disturbed following laparotomy and during incisional hernia formation. When a midline incisional hernia develops, the normal force across the composite myofascial structure is lost, functionally resulting in passive unloading of the lateral abdominal wall. Although the adjacent rectus muscles maintain their origin and insertion, the insertion of lateral oblique muscles is lost following midline laparotomy and incisional hernia formation. The linea alba of the abdominal wall is anatomically a tendon that, when severed, should induce pathologic abdominal wall muscle changes similar to those observed in the soleus and gastrocnemius muscles when the Achilles tendon is divided.
Studies of rodent hind limb unloading,10 spaceflight travel,11 and tenotomy12 all document profound changes in the phenotype of the affected muscles. Specifically, the unloaded muscles develop a disuse atrophy characterized by a change in fiber composition, a decrease in fiber cross-sectional area, and a pathologic deposition of intramuscular collagen.10–12 The purpose of this study was to measure abdominal wall myopathic histologic and mechanical changes during incisional herniation. We tested the hypothesis that midline abdominal wall incisional hernia formation unloads the abdominal wall oblique muscles, resulting in disuse atrophy that reduces abdominal wall compliance and significantly impairs the fidelity of incisional hernia repair.
MATERIALS AND METHODS
Incisional Hernia Model
An established animal model of incisional hernias was used.13,14 Male Sprague-Dawley rats (Harlan Inc., Indianapolis, IN) weighing 250 to 300 g were acclimated and housed under standard conditions. All animal care and operative procedures were performed in accordance with the United States Public Health Service Guide for the Care of Laboratory Animals (NIH Publication No. 85-23, 1985) and were performed with the prior approval of the Ann Arbor Veterans Affairs Medical Center Institutional Animal Care and Use Committee. Briefly, a 6 × 3 cm ventral full-thickness skin flap was raised through the avascular prefascial plane, and a 5-cm full-thickness laparotomy incision was made through the linea alba. The skin flap was replaced and closed with an absorbable suture. Two control groups were used: an uninjured (normal) abdominal wall group (n = 7) and a healed laparotomy (sham) group (n = 7). In the uninjured abdominal wall control group, a skin flap was raised and closed without laparotomy. In the sham group, the skin flap was raised, and a 5-cm laparotomy incision was made and closed with a running, permanent, 4-0 Prolene suture. The hernia group developed large midline abdominal wall defects (Fig. 1).
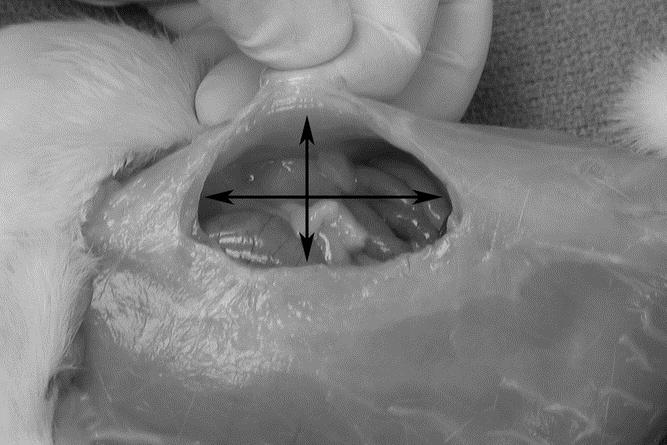
FIGURE 1. Abdominal wall defect in the hernia group. Dimensions were measured as craniocaudal length (long arrow) and transverse width.
After 35 days, the rats were killed and weighed. The skin was dissected free circumferentially and the hernia defects were measured for craniocaudal length and transverse width. Abdominal wall width was measured at the level of the umbilical process and defined as the distance between the posterior spinous process and the linea alba (control group), midline laparotomy wound (sham group), or edge of hernia ring (hernia group). The external oblique and rectus muscles (EOM and RM) were then dissected free from the internal abdominal wall. The interoblique plane was developed by dissecting between the ribs and the cephalad abdominal wall overlying the ribs. The internal oblique muscle (IOM) inserts onto the costal margins whereas the EOM inserts onto the anterior surface of ribs 4 to 12. This plane is then developed medially and is continuous with the posterior rectus sheath, the paraspinous muscles, the pubis bone, and the inguinal ligament (Fig. 2).

FIGURE 2. Lateral oblique muscle dissection in the interoblique plane.
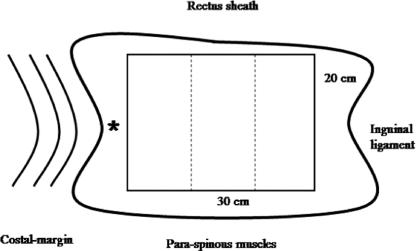
The IOM and transverse abdominis muscle (TM) bilayer was cut parallel to the length of the IOM fibers into strips approximately 12-mm wide, frozen in isopentane at −160°C and stored at −70°C for subsequent histologic treatment. The left IOM sheet was dissected free and trimmed to a rectangular sheet measuring 30 mm in length (cranio-caudally) and 20 mm in width (transversely). This rectangular sheet was weighed and then trisected into 10 × 20 mm strips for tensiometric analysis (Fig. 3). Additional IOM biopsies (2× 2 mm) were snap frozen in liquid nitrogen for biochemical analysis.
FIGURE 3. Illustration of IOM harvest. The 30 × 20 mm specimen was weighed and sectioned into three 10 × 20 mm strips for tensiometric analysis. *Position of adjacent muscle biopsy for collagen analysis.
Histochemistry
Frozen muscle samples were sectioned (12-μm thick) in a cryostat (Microm GmbH, HM 500, Walldorf, Germany) at −28°C. The fiber architecture of the IOM is nonspanning15; therefore, for uniformity, cross sections were cut in the same location for each sample and evaluated at ×100 magnification. The IOM transverse sections were stained with hematoxylin and eosin by the Mayer method.16,17 Additional muscle sections were incubated for the presence of calcium activated myosin-type adenosine triphosphatase (mATPase) (E.C.3.6.1.3) activity, as described.18 A representative sample of IOM fibers (at least 250 fibers from each muscle) were classified as type I, type IIA, or type IIB based on the mATPase activity scheme.17–20 Muscle fiber cross-sectional area was quantified using planimetry software (BioQuant-R&M Biometrics, Inc., Nashville, TN). At least 50 IOM fibers of each fiber type were quantified for each muscle section. Muscle sections were also stained for collagen content using a modified Gomori trichrome histochemical staining procedure.21
Tensiometric Analysis
Mechanical testing of abdominal wall strips was performed within 6 hours of necropsy. Sample width and thickness were measured with Digimatic calipers (Mitutoyo American Corp., Chicago, IL). Force extension curves were generated using an Instron Tensiometer (model 5542, Instron Corporation, Canton, MA) equipped with a 50 Newton (N) static load cell set at a crosshead speed of 10 mm per minute. Samples were mounted into the load frame using pneumatic graspers, preloaded to 0.1 N, and the gauge length measured between the grips. The load frame applied tensile loads to the IOM strips perpendicular to the normal linea alba (control group), midline laparotomy wound (sham group), or hernia defect (hernia group) until mechanical tissue disruption occurred. Data analysis was performed using the Merlin materials testing software package (Instron Corporation).
Data from the stretch loading were used to determine the following clinically important biomechanical properties: breaking strength, the maximum load force (Fmax) at mechanical failure (N); tensile strength, the maximum stress developed in the specimen per unit area, calculated as Fmax/cross-sectional area (N/mm2); toughness, the energy absorbed by the specimen under tension, calculated as the entire area under the force-extension curve from the origin to mechanical rupture (Joules); elongation, the increase in length of the tissue under a load, defined as the length of the specimen at mechanical disruption minus the original length (mm); and stiffness, the slope of the linear elastic region of the force-extension curve (N/mm).
Collagen Assay
Tissue collagen levels were measured using the Sircol collagen assay method as described by the manufacturer (Accurate Chemical and Scientific Corp., Westbury, NY). Results were standardized to total muscle protein and recorded as percentage of collagen/total protein.
Incisional Hernia Repair Model
A second group of rats underwent abdominal wall repair (n = 32). Sixteen animals (n = 16) first developed incisional hernias for 35 days, as described above (hernia-repair group). In the control-repair group (n = 16), normal abdominal walls were tested. Full-thickness, myofascial defects created in the control-repair group animals were the same dimensions as the incisional hernia defects that developed in the hernia-repair group. All abdominal wall defects in both groups were repaired with PROLITE polypropylene mesh (Atrium Medical Corporation, Norwalk, CT). The mesh was fashioned to the shape of the abdominal wall defect allowing for a 3- to 4-mm underlay. The mesh was sutured to the peritoneal surface of the abdominal wall muscles (underlay) using transmuscular, 5-0 Prolene sutures spaced 1 cm apart. The skin flap was then replaced and sutured closed with a running 4-0 Vicryl suture.
Animals were killed 28 days following hernia-repair or control-repair with an overdose of Nembutal (100 mg/kg i.p.). The entire ventral abdominal wall was excised, and the skin was separated from the musculofascial layer. The wound healing interface was closely examined for evidence of recurrent incisional hernia formation, defined as a wound-mesh defect greater than 2 mm. Fixation sutures were then removed. Abdominal wall strips in the shape of the uppercase letter “I” were taken perpendicular to the wound healing interface.14 A cutting template was used to mark the abdominal wall to minimize size variability between specimens. Abdominal wall strips were labeled and stored in phosphate-buffered saline until tensiometric mechanical analysis was performed as described above.
Statistical Analysis
Data were analyzed using SigmaStat software (Jandel Scientific, Corte Madera, CA). Multiple group analysis was performed with ANOVA to determine differences in group characteristics, anatomic measurements, fiber cross-sectional area, and tensiometric measurements. Directed group-to-group comparison was performed with t test. Multiple group fiber type analysis was performed with the Kruskal-Wallis equality of populations test while directed group-to-group comparison was performed with Wilcoxon rank sum test. The Fisher exact test was used to determine differences in the incidence of recurrent incisional hernias. Values were reported as the mean ± standard deviation of measurement. P values of <0.05 were considered significant.
RESULTS
Incisional Hernia Model
All rats in the hernia group developed large, abdominal wall defects. The mean hernia size was 49.5 ± 6.2 mm craniocaudal by 34.3 ± 2.6 mm transverse. No rats died during the experiments. All groups gained weight on the average, although the sham (healed) group gained 12.5% less weight (P < 0.05; Table 1). Internal oblique muscle atrophy was grossly evident following sham laparotomy and hernia formation, although hernia formation was associated with more muscle atrophy (P < 0.05; Table 1). The composite lateral abdominal wall retracted significantly following midline incisional hernia formation. The abdominal wall length of the hernia group (spinous process to the edge of the hernia ring) was 20% less than the uninjured group (spinous process to the linea alba) (P < 0.01; Table 1). Interestingly, the abdominal wall length of the sham (healed) group (spinous process to the healed laparotomy wound) was 10% less than the uninjured group.
TABLE 1. Baseline Group Characteristics and Gross Anatomic Measurements
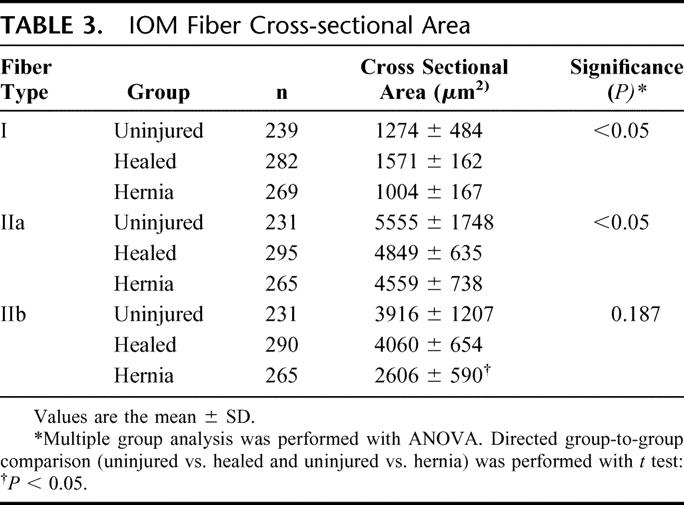
Nonspecific staining of the IOM fibers (hematoxylin and eosin) showed no significant microscopic differences between groups. Myofibrillar ATPase staining, however, demonstrated a shift in muscle fiber composition in both the sham and hernia groups (Fig. 4; Table 2). Uninjured abdominal wall IOM fiber type distribution is a mixture of type I and type II fibers with a 78% to 22% predominance of type II fibers relative to type I fibers. This distribution is similar to that found in the gracilis muscle of the rat and would be commonly known as a red muscle.22 A healing laparotomy and midline incisional hernia both induced similar changes in fiber type composition, but the absolute difference was most pronounced in the hernia group. The relative number of type I fibers decreased from 21.9% in the uninjured control group, to 20.5% in the healed group, to 18.0% in the hernia group (P= 0.01). There were reciprocal increases in the relative levels of type IIa fibers from a baseline of 29.5% in the uninjured control group, compared with 34.9% in the healed group and 39.4% in the hernia group (P= 0.01). There was a significant decrease in the type IIb fibers from 48.5% in the uninjured control group, to 44.5% in the healed group, to 42.5% in the hernia group (P < 0.05). Cross-sectional analysis of the specific fiber types revealed selective type IIb fiber atrophy in the hernia group (Fig. 4C, Table 3). The cross-sectional area of uninjured group IIb fibers compared with hernia group IIb fibers was 3916 ± 1207 μm2 versus 2606 ± 590 μm2 (P < 0.05).
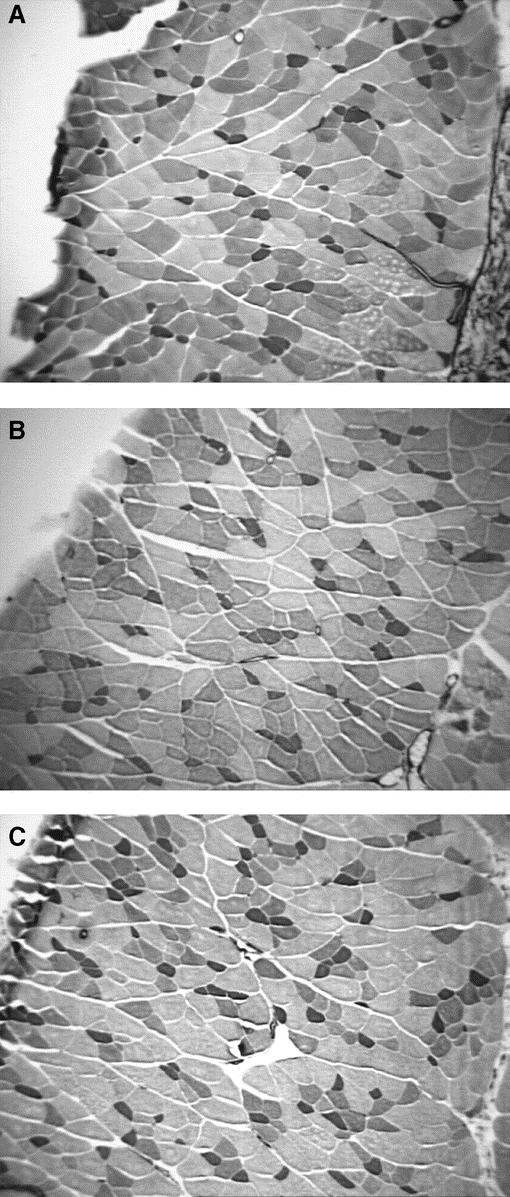
FIGURE 4. Photomicrographs of IOM myofibrillar ATPase stain groups: (A) uninjured, (B) sham (healed), and (C) hernia group. Note increasing proportion of type IIa (larger pale staining) fibers from uninjured, to healed, and hernia groups. There is evidence of selective type IIb atrophy (larger, darker staining fibers) in the hernia group. Original magnifications ×100.
TABLE 2. Abdominal Wall IOM Fiber Type Composition
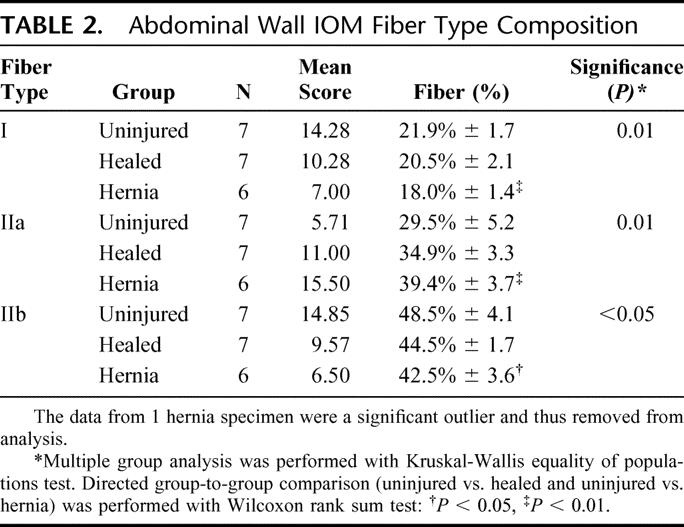
TABLE 3. IOM Fiber Cross-sectional Area
The internal oblique muscles harvested from both the healed and hernia groups demonstrated evidence of pathologic fibrosis compared with the uninjured control group. Trichrome staining revealed excessive collagen deposition in the perimysium (Fig. 5). Biochemical analysis suggested a nonsignificant trend toward increasing total muscular collagen in the uninjured compared with the healed compared with the hernia groups (8.3% ± 0.37% vs. 9.6% ± 2.8% vs. 10.8%± 0.63%, P = 0.1). Directed hypothesis driven t test comparing uninjured and hernia group total muscular collagen revealed significance (8.3% ± 0.37% vs. 10.8% ± 0.63%, P= 0.01).
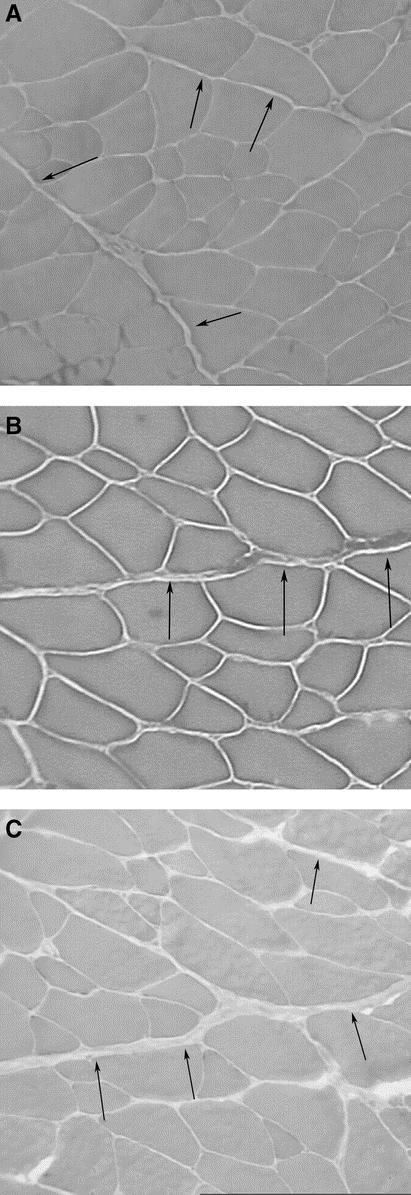
FIGURE 5. Representative IOM trichrome stains for collagen deposition: (A) uninjured group, (B) sham group, and (C) hernia group. There is increased collagen deposition in the perimysium (arrows) comparing uninjured, sham, and hernia groups. Original magnifications ×100.
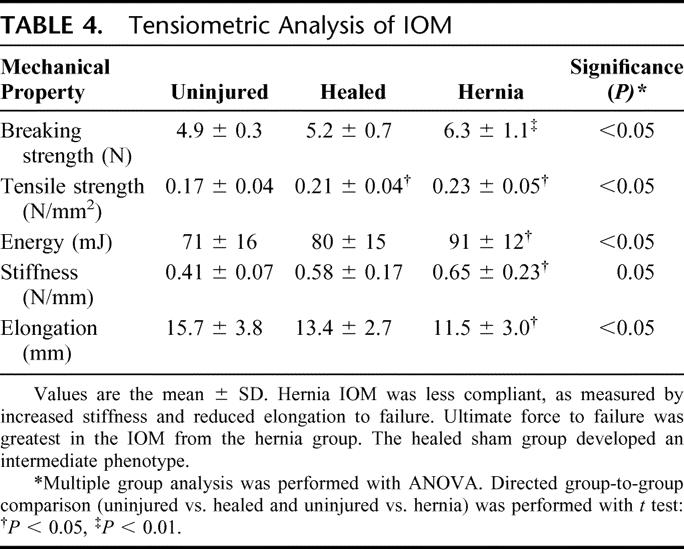
Tensiometric analysis was performed on uniform IOM strips with the line of tissue deformation directly perpendicular to the midline abdominal wall (uninjured and sham groups) or the hernia defect (hernia group). Muscle strips were therefore loaded nearly parallel to the course of the muscle fibers and along the known lines of maximal tension of the abdominal wall in situ. Tensiometric measurements found significant differences in IOM mechanical properties between the experimental groups. The greatest force was required to mechanically disrupt IOM in the hernia group (Table 4). A similar pattern of differences was observed in material toughness, as the hernia group IOM absorbed more energy prior to mechanical disruption. In contrast, the hernia group IOM demonstrated significantly reduced mechanical compliance and elongation, and increased tissue stiffness. The healed sham group again exhibited intermediate measures of elongation and stiffness compared with the hernia and uninjured groups. The fibrotic IOM harvested from the hernia group expressed the greatest ultimate tissue strength but the least mechanical compliance.
TABLE 4. Tensiometric Analysis of IOM
Incisional Hernia Repair Model
There was a significant increase in recurrent incisional hernia formation in the hernia-repair group compared with zero recurrences in the control-repair group (6 of 16 vs. 0 of 16, P = 0.02). The wound repair breaking strength was significantly decreased in the hernia repair group compared with the control-repair group (5.7 ± 0.9 vs. 14.2 ± 4.0 N, P< 0.001).
DISCUSSION
This study demonstrates that the IOM from herniated abdominal walls develop a pattern of changes similar with those seen in mechanically unloaded muscles.10,23 Internal oblique muscles in herniated abdominal walls developed pathologic fibrosis, disuse atrophy, and changes in muscle fiber type composition. Histologic staining found that perimysial collagen deposition is a mixture of type I and III collagen.24 Notably, there are no significant changes in the collagen content of the epimysial layers, which converge in the abdominal wall to form the midline linea alba. This is consistent with a mechanical mechanism remote from the laparotomy wound. Biochemical testing revealed that total IOM collagen content increased approximately 25% in this study, similar to values reported in the literature following extremity muscle unloading in the gastrocnemius (24%), a type II fiber predominant muscle, but less than that reported in the soleus (39%), a type I fiber predominant muscle. The increased collagen protein per total muscle protein is likely a function of both decreased muscle structural proteins and a relative increase in the collagen deposition.25,26
Myopathic disuse atrophic changes significantly altered the phenotype of the herniated anterior abdominal wall. The composite abdominal wall retracted more than 20% on each side resulting in a transverse defect that was about one fourth of the animal’s abdominal circumference. This large defect developed without the excision of any abdominal wall muscle, suggesting that hernia defects do not enlarge simply by repetitive evisceration of peritoneal contents dilating a fascial defect. Rather, the lateral muscular components of the abdominal wall retract away from the midline fascial defect. The concept of abdominal wall retraction has therapeutic implications: primary, autologous (myofascia to myofascia) sutured hernia repairs will result in excessive wound tension, a potential mechanism for the high recurrence rate reported following this type of repair.6,7 Prostheses were then designed to bridge or buttress the fascial gap and facilitate “tension-free” abdominal wall reconstruction. Alloplastic incisional hernia repair resulted in approximately 50% clinical reduction in recurrent incisional hernia formation6,7 but is associated with increased infection rate, intestinal fistulization, and foreign body reaction.27 Interestingly, abdominal wall length in the sham laparotomy group was approximately 10% less than that of the uninjured controls, suggesting that abdominal wall shortening occurs following routine laparotomy.
Material testing of the herniated abdominal wall IOM found increased tensile strength and mechanical toughness. In contrast, elastic properties were significantly reduced, similar to that reported in immobilized extremity muscle studies.28 Muscle stiffness was increased nearly 60% in herniated specimens and extensibility was decreased by 27% compared with uninjured controls. Altered mechanical properties may be due increased collagen content in the atrophied muscles. Increased collagen content has been demonstrated to be associated with increased biomaterial stiffness and reduced elastic properties.29 Alternatively, the change in the mechanical properties seen in herniated abdominal walls may be a direct manifestation of the individual myofibril atrophic changes. The number of sarcomeres arranged in series is known to decrease in chronically shortened muscles and manifests in decreased muscular compliance.30
An unexpected finding in this study was that the histologic and mechanical properties associated with a healed midline laparotomy incision exhibit an intermediate phenotype between uninjured controls and herniated specimens, suggesting that the changes observed in hernia specimens are not simply a function of decreased abdominal wall loading. The pathologic changes measured in the lateral abdominal wall appear to be a complex manifestation of biomechanical changes for which load is one variable. Alternatively, it is possible that the pain and decreased mobility associated with a major laparotomy decrease postoperative activity and thus normal abdominal wall loading, which may collectively contribute to the intermediate phenotype observed in the healed group.
This study finds that biomechanical changes in the function of abdominal wall muscles may contribute to the high rate of recurrent incisional hernias following repair. Following midline laparotomy wound failure and unloading of the abdominal wall, the oblique muscles undergo pathologic changes consistent with disuse muscular atrophy resulting in changes in fiber type composition, decreased cross-sectional area, and pathologic fibrosis. The disuse atrophic changes alter the material properties of the anterior abdominal wall effectively reducing abdominal wall compliance. Incisional hernia repair in the setting of a noncompliant abdominal wall results in mechanical impedance mismatch that increases the transfer of load forces to the wound healing interface. This manifests in mechanical wound failure at decreased abdominal wall loads and increased recurrent incisional hernia formation. Improved approaches to laparotomy and incisional hernia repair should account for the dynamic equilibrium of load forces across the abdominal wall. Implications exist for the design of new material implants and operative techniques like component separation.
Footnotes
Supported by a Department of Veterans Affairs Merit Review Grant (to M.G.F.).
Reprints: Michael G. Franz, MD, University of Michigan Health System, Division of Gastrointestinal Surgery, 1500 E. Medical Center Drive, 2922 H Taubman Center, Ann Arbor, MI 48109-0331. E-mail: mfranz@umich.edu.
REFERENCES
- 1.Duepree HJ, Senagore AJ, Delaney CP, et al. Does means of access affect the incidence of small bowel obstruction and ventral hernia after bowel resection? Laparoscopy versus laparotomy. J Am Coll Surgeons. 2003;197:177–181. [DOI] [PubMed] [Google Scholar]
- 2.Santora TA, Roslyn JJ. Incisional hernia. Surg Clin North Am. 1993;73:557–570. [DOI] [PubMed] [Google Scholar]
- 3.Mudge M, Hughes LE. Incisional hernia: a 10 year prospective study of incidence and attitudes. Br J Surg. 1985;72:70–71. [DOI] [PubMed] [Google Scholar]
- 4.Cassar K, Munro A. Surgical treatment of incisional hernia. Br J Surg. 2002;89:534–545. [DOI] [PubMed] [Google Scholar]
- 5.National Center for Health Statistics. Detailed Diagnoses and Procedures: National Hospital Discharge Survey. Washington, DC: National Center for Health Statistics, 1995;13:122. [Google Scholar]
- 6.Luijendijk RW, Hop WCJ, van den Tol P, et al. A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med. 2000;343:392–398. [DOI] [PubMed] [Google Scholar]
- 7.Burger JW, Luijendijk RW, Hop WC, et al. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg. 2004;240:578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flum DR, Horvath K, Koepsell T. Have outcomes of incisional hernia repair improved with time? A population based analysis. Ann Surg. 2003;237:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubay DA, Franz MG. Acute wound healing: the biology of acute wound failure. Surg Clin North Am. 2003;83:463–481. [DOI] [PubMed] [Google Scholar]
- 10.Talmadge RJ, Roy RR, Bodine-Fowler SC, et al. Adaptations in myosin heavy chain profile in chronically unloaded muscles. Basic Appl Myol. 1995;5:117–137. [PubMed] [Google Scholar]
- 11.Riley DA, Thompson JL, Krippendorf BB, et al. Review of spaceflight and hindlimb suspension unloading induced sarcomere damage and repair. Basic Appl Myol. 1995;5:139–145. [PubMed] [Google Scholar]
- 12.Jozsa L, Kannus P, Thoring J, et al. The effect of tenotomy and immobilization on intramuscular connective tissue: a morphometric and microscopic study in rat calf muscles. J Bone Joint Surg Br. 1990;72:293–297. [DOI] [PubMed] [Google Scholar]
- 13.Franz MG, Smith PD, Wachtel TL, et al. Fascial incisions heal faster than skin: a new model of abdominal wall repair. Surgery. 2001;129:203–208. [DOI] [PubMed] [Google Scholar]
- 14.Dubay DA, Wang X, Adamson B, et al. Progressive fascial wound failure impairs subsequent abdominal wall repairs: a new animal model of incisional hernia formation. Surgery. 2005;137:463–471. [DOI] [PubMed] [Google Scholar]
- 15.Boriek AM, Ortize J, Zhu DS. Fiber architecture of canine abdominal muscles. J Appl Physiol. 2002;92:725–735. [DOI] [PubMed] [Google Scholar]
- 16.Luna LG. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology, 3rd ed. New York: McGraw-Hill, 1960. [Google Scholar]
- 17.Dubowitz V, Sewry CA, Fitzsimons RB. Muscle Biopsy: A Practical Approach, 2nd ed. London: Bailliere Tindall, 1985. [Google Scholar]
- 18.Brooke MH, Kaiser KK. Three myosin adenosine triphosphatase systems: nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem. 1970;18:670–672. [DOI] [PubMed] [Google Scholar]
- 19.Brooke MH, Kaiser KK. Muscle fiber types: how many and what kind. Arch Neurol. 1970;23:369–379. [DOI] [PubMed] [Google Scholar]
- 20.Stoward PJ, Pearse AGE. Histochemistry: Theoretical and Applied, 4thed. Edinburgh: Churchill Livingstone, 1991:489–514. [Google Scholar]
- 21.Macniven I. Stable trichrome for the demonstration of the intermyofibrillary network in muscle biopsies. J Histotechnol. 1994;17:59–61. [Google Scholar]
- 22.Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. Am J Anat. 1984;171:259–272. [DOI] [PubMed] [Google Scholar]
- 23.Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol. 1990;68:1–12. [DOI] [PubMed] [Google Scholar]
- 24.Salonen V, Lehto M, Kalimo M, et al. Changes in intramuscular collagen and fibronectin in denervation atrophy. Muscle Nerve. 1985;8:125–131. [DOI] [PubMed] [Google Scholar]
- 25.Sawai H. Collagen metabolism of skeletal muscles following injuries of the peripheral nerves. Nippon Seikeigeka Gakkai Zasshi. 1982;56:753–764. [PubMed] [Google Scholar]
- 26.Williams PE, Goldspink G. Connective tissue changes in immobilized muscle. J Anat. 1984;138:343–350. [PMC free article] [PubMed] [Google Scholar]
- 27.Korenkov M, Sauerland S, Arndt M, et al. Randomized clinical trial of suture repair, polypropylene mesh or autodermal hernioplasty for incisional hernia. Br J Surg. 2002;89:50–56. [DOI] [PubMed] [Google Scholar]
- 28.Jarvinen TAH, Jozsa L, Kannus P, et al. Organization and distribution of intramuscular connective tissue in normal and immobilized skeletal muscles: an immunohistochemical, polarization and scanning electron microscopic study. J Muscle Res Cell Motil. 2002;23:245–254. [DOI] [PubMed] [Google Scholar]
- 29.Naimark WA, Lee JM, Limeback H, et al. Correlation of structure and viscoelastic properties in the pericardia of four mammalian species. Am J Physiol. 1992;263:H1095–H1106. [DOI] [PubMed] [Google Scholar]
- 30.Spector SA, Simard CP, Fournier M, et al. Architectural alterations of rat hind-limb skeletal muscles immobilized at different lengths. Exp Neurol. 1982;76:94–110. [DOI] [PubMed] [Google Scholar]