Abstract
Objective:
To describe unplanned procedures following colorectal cancer surgery that might be used as intermediate outcome measures, and to determine their association with mortality and length of stay.
Summary Background:
Variation in the quality of surgical care, especially for common illnesses like colorectal cancer, has received increasing attention. Nonfatal complications resulting in procedural interventions are likely to play a role in poor outcomes but have not been well explored.
Methods:
Cohort analysis of 26,638 stage I to III colorectal cancer patients in the 1992 to 1996 SEER-Medicare database. Independent variables: sociodemographics, tumor characteristics, comorbidity, and acuity. Primary outcome: postoperative procedural intervention. Analysis: Logistic regression identified patient characteristics predicting postoperative procedures and the adjusted risk of 30-day mortality and prolonged hospitalization among patients with postoperative procedures.
Results:
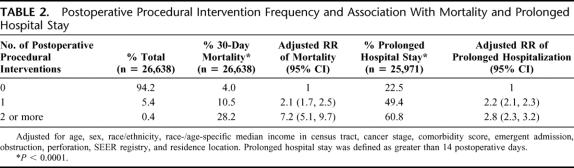
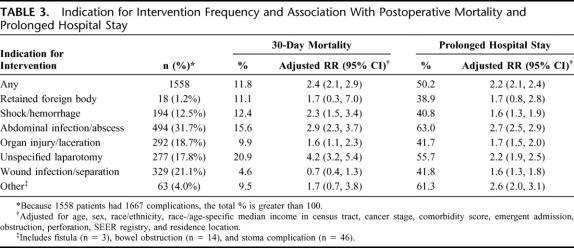
A total of 5.8% of patients required postoperative intervention. Patient characteristics had little impact on the frequency of postoperative procedures, except for acute medical conditions, including bowel perforation (relative risk [RR] = 3.0, 95% confidence interval [CI] = 2.5–3.6), obstruction (RR = 1.6; 95% CI = 1.4–1.8), and emergent admission (RR = 1.3; 95% CI = 1.1–1.4). After a postoperative procedure, patients were more likely to experience early mortality (RR = 2.4; 95% CI = 2.1–2.9) and prolonged hospitalization (RR = 2.2; 95% CI = 2.1–2.4). The most common interventions were performed for abdominal infection (31.7%; RR mortality = 2.9; 95% CI = 2.3–3.7), wound complications (21.1%; RR mortality = 0.7; 95% CI = 0.4–1.3), and organ injury (18.7%; RR mortality = 1.6; 95% CI = 1.1–2.3).
Conclusions:
Postoperative complications requiring additional procedures among colorectal cancer patients correlate with established measures of surgical quality. Prospective tracking of postoperative procedures as complication markers may facilitate outcome studies and quality improvement programs.
Nonfatal complications resulting in post-operative procedural interventions are likely to play a role in poor outcomes but have not been well explored. This study uses population-based data to describe unplanned procedures following colorectal cancer surgery that might be used as intermediate outcome measures, for research and quality improvement programs.
Interest in measuring surgical quality is growing rapidly. With increasing recognition that surgical outcomes vary widely, patients are seeking more detailed information about providers’ performance prior to undergoing treatment. Providers are interested in assessing their own performance for quality improvement purposes. Payers are looking for better data by which to steer selected populations of surgical patients to high-quality providers. To meet these various interests, policy makers and health services researchers have redoubled their efforts to develop and implement quality indicators germane to surgery.
Unfortunately, however, current measures of surgical quality have major flaws which limit their usefulness. Although simple and direct, operative mortality is too uncommon after most procedures to allow consistent and reliable measurement of surgical quality and too blunt to direct quality improvement efforts. Volume of care has been correlated with decreased mortality in a variety of settings and among groups of hospitals1–3 but has limited sensitivity and flexibility as a target for quality improvement since it is likely a proxy for resources or processes of care not yet identified. Nonfatal surgical complications that result in reoperation or other procedural intervention are likely to play a role in poor outcomes, including postoperative death,4 but have not been well explored. Such complications leading to early reoperation are often the result of intraoperative technical problems, such as anastomotic leaks and wound infections,5 and could provide a useful target for quality measurement and quality improvement.
We investigated complications of surgery in the context of colorectal cancer, a common and potentially fatal disease6 that is primarily treated with well-established surgical techniques.7 Our aim was to identify unplanned procedural interventions following colorectal cancer surgery that might be used as intermediate outcomes in quality improvement efforts and to explore their association with operative mortality and prolonged hospitalization.
METHODS
Data
We used files from the well-described SEER-Medicare linked database8,9 for patients diagnosed from 1992 to 1996. During the time of this study, the 13 SEER registries ascertained incident colorectal cancer cases in 6 states and 7 county-based areas in 4 other states representing approximately 14% of the U.S. population. The SEER program data include information on tumor location, stage of disease, demographics, and primary radiation therapy and surgical treatment.10,11 The Medicare claims data include all billed claims for services provided to colorectal cancer patients, including the original surgical resection as well as all postoperative procedural interventions in both the inpatient and outpatient settings.8
Patients
Patients 66 years of age and older with a diagnosis of American Joint Committee on Cancer (AJCC)12 stage I to III colorectal cancer between 1992 and 1996 (n = 26,638) were identified from the linked SEER-Medicare database (SEER cancer site codes: colon = 18.0, 18.2–18.9; rectosigmoid = 19.9; and rectal = 20.9). Patients were required to be enrolled in parts A and B of fee-for-service Medicare for the 12 months preceding diagnosis to ascertain comorbidity. Starting with the month of diagnosis, they were required to be enrolled for an additional 6 months or until death, whichever came first, to enable tracking of procedures. Patients were excluded from the study if the SEER database indicated that they had 1) nonadenocarcinoma histology (SEER histology codes 8140-47, 8210-11, 8220-21, 8260-63, 8480-81, 8490) (n = 675); 2) prior colorectal cancer (n = 997); 3) incomplete enrollment for the year preceding diagnosis (n = 8844); 4) incomplete enrollment after diagnosis as stipulated (n = 256); or 5) no resection within the 6 month period starting with month of diagnosis (n = 2466).
Sociodemographic and Environmental Variables
The SEER database contains information on patient date of birth, race/ethnicity, and gender. Residence location (urban, rural) based on Rural Urban Commuting Area codes was identified from the plurality ZIP codes on the Medicare claims in the diagnosis month.13,14 Geographic region was represented by SEER registry as detailed above. The median household income of race-/age-matched individuals within the census tract provided by the Patient Entitlement and Diagnosis Summary File served as a proxy for socioeconomic status.
Tumor Characteristics
We used SEER data to define AJCC stage of disease (I, II, and III).
Clinical Variables
To measure comorbidity, we adapted the Romano-Charlson comorbidity index, based on outpatient and inpatient diagnoses made during the 11 months prior to the month before colorectal cancer diagnosis.15,16 This index creates a weighted score using International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) diagnosis codes for 18 conditions, such as myocardial infarction, congestive heart failure, and chronic pulmonary disease.15,16 We classified individuals into 3 categories of comorbidity: an index score of 0, 1, and 2 or more. To allow adjustment for acuity of illness in multivariate analysis, we used ICD-9 diagnosis codes from the Medicare claims to identify individuals with obstructive colorectal cancer, (560.89 and 560.9) and perforated cancers (569.83). We also used the claims data to identify patients admitted to the hospital under emergent conditions (MedPAR variable 118).
Outcome Variables
The primary outcome measure was performance of a postoperative procedural intervention between one and 30 days after the index operation. All procedures were identified by the appropriate ICD-9 procedure codes and Current Procedural Terminology (CPT) codes from the Medicare files. A previous study of ICD-9-CM procedure and diagnosis codes for Medicare patients compared with chart review demonstrated 89% agreement for surgical cases;17 moreover, procedures reported on physician claims, especially for acute complications, are considered highly valid.18 Postoperative procedural interventions were categorized by surgical complications that such an intervention would aim to correct. For example, patients undergoing open drainage of intra-abdominal abscess, percutaneous drainage of intra-abdominal abscess, or drainage of a subphrenic abscess were classified as having undergone management of intra-abdominal infection in the postoperative period.
To avoid overcounting, each complication category was assigned no more than once to an individual patient. For example, if a patient had more than one procedure indicating treatment of a wound infection, wound infection was counted as a complication only once. Similarly, only one complication was counted per day. For dates on which more than one procedure was coded, we applied the following rules: 1) prioritize a technical or system error above others, and 2) combine procedures that represent one error. For example, because retained foreign body is clearly the result of a technical error, if foreign body retrieval were coded on the same date as an abdominal infection procedure, we counted retained foreign body. Further, if a patient had codes for “management of postoperative hemorrhage” as well as “reopening of a recent laparotomy” on the same day, only the former would be counted since both may have occurred as part of the same case and “management of postoperative hemorrhage” is more descriptive of the case.
We determined the association between procedural intervention and the 2 established outcome measures for surgery, postoperative mortality, and length of stay. Mortality was measured during the first 30 days after operation, to capture mortality most likely to have been associated with operative technical errors. Length of stay was used as a proxy for resource use; prolonged hospitalization was defined as length of stay greater than 14 days.
Statistical Analysis
We determined the overall frequency of the 30-day postoperative procedures for treatment of complications. We examined patient demographic and environmental variables (age, gender, race/ethnicity, census-tract based median household income, rural/urban residence, SEER registry), clinical variables (emergency admission, obstruction, perforation, comorbidity), and tumor characteristics (AJCC stage) among those patients with and without postoperative procedures. We used logistic regression modeling to determine characteristics that predict postoperative procedural interventions. Adjusting for these characteristics, we used logistic regression modeling to examine the association between complications requiring postoperative procedures and postoperative mortality and prolonged length of stay. We also examined the association between multiple complications and mortality and length of stay. We corrected for clustering of patients by physician and hospital using general estimating equation techniques19 and found no substantial difference in results. Because some outcomes (eg, prolonged hospital stay) were common in the study population, the adjusted odds ratio derived from the logistic regression does not approximate the relative risk. We approximated relative risk from the adjusted odds ratios using published methods.20 All analyses were performed use SAS 8.02 software (SAS Institute, Inc., Cary, NC).
RESULTS
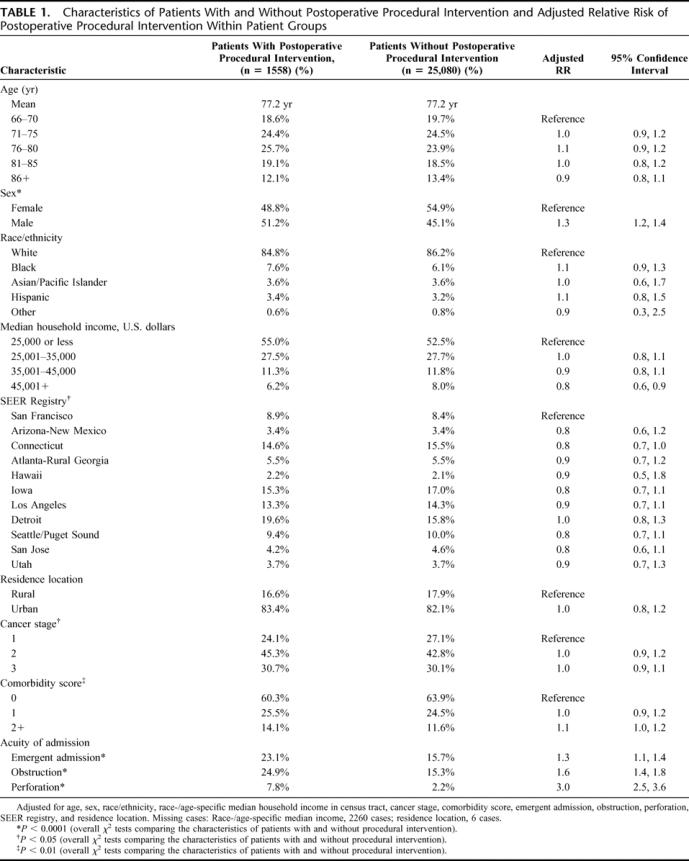
The study group was comprised of 26,638 individuals: 22,672 with colon cancer and 3966 with rectal cancer. A total of 1300 (5.7%) colon cancer patients and 258 (6.5%) rectal cancer patients had one or more postoperative procedures within 30 days of their colorectal resection, for a combined total of 1558 (5.8%). Demographic (age, race, income), environmental, tumor, and chronic clinical conditions had little impact on the frequency of postoperative procedures (Table 1). Male gender posed a slightly higher risk (adjusted relative risk [RR] = 1.3, 95% confidence interval [CI] = 1.2–1.4); median income in the census tract greater than $45,000/year conferred a protective effect (RR = 0.8; 95% CI = 0.6–0.9). Acute medical conditions were more predictive of the need for a postoperative intervention, most dramatically among patients who presented with bowel perforation (RR = 3.0; 95% CI = 2.5–3.6).
TABLE 1. Characteristics of Patients With and Without Postoperative Procedural Intervention and Adjusted Relative Risk of Postoperative Procedural Intervention Within Patient Groups
Overall, 5.8% of colorectal cancer patients experienced at least one postoperative complication that required a procedural intervention (Table 2). Among those, 1438 (93.1%) had a single complication. A total of 107 patients had 2 or more complications. After adjusting for other characteristics, a single complication requiring intervention was associated with a significant increase in the likelihood of postoperative mortality (RR = 2.1; 95% CI = 1.7–2.5) and risk of prolonged postoperative hospitalization (RR = 2.2; 95% CI = 2.1–2.3). More complications were associated with substantially increased risk of mortality (RR = 7.2; 95% CI = 5.1–9.7) and prolonged hospitalization (RR = 2.8; 95% CI = 2.3–3.2).
TABLE 2. Postoperative Procedural Intervention Frequency and Association With Mortality and Prolonged Hospital Stay
The most common postoperative procedures were related to treatment of intra-abdominal infection (31.7%), wound complications (21.1%), and organ injury, such as repair of viscera laceration (18.7%) (Table 3). Thirty-day mortality after these procedures varied considerably by the indication for intervention, from a low of 4.6% for wound infection to a high of 20.9% for an unspecified laparotomy. The postoperative procedure category associated with the greatest adjusted risk of postoperative death was unspecified reoperative laparotomy (RR = 4.2; 95% CI = 3.2–5.4), followed by abdominal infection (RR = 2.9; 95% CI = 2.3–3.7) and postoperative shock or hemorrhage (RR = 2.3; 95% CI = 1.5–3.4).
TABLE 3. Indication for Intervention Frequency and Association With Postoperative Mortality and Prolonged Hospital Stay
Prolonged hospitalization for more than 14 days was a relatively common event, occurring in 50.2% of patients who required postoperative interventions, and in 22.5% of those who did not. There was less variation in rates of prolonged hospitalization by indication for intervention, from a low of 38.9% for retained foreign body to a high of 63.0% for abdominal infection/abscess. The postoperative procedure category associated with the greatest adjusted risk of prolonged hospitalization was abdominal infection (RR = 2.7; 95% CI = 2.5–2.9), followed by the combined group of bowel obstruction, stoma complication, and fistula formation (RR = 2.6; 95% CI = 2.0–3.1), and unspecified reoperative laparotomy (RR = 2.2; 95% CI = 1.9–2.5).
DISCUSSION
In this study, we examined reoperation as an indicator of surgical quality in the context of colorectal cancer. Although colorectal cancer is common and the surgical treatment is well defined, a range of postoperative complications may occur and postoperative death is an infrequent but real risk.21–24 Moreover, previous studies demonstrate substantial variation in operative mortality following colorectal cancer surgery.23–25 Such outcome variation suggests a need for better standardization of care. Although hospital and surgeon volume have been established as predictors of perioperative mortality,2,24 both function as proxies for important resources or processes of care that have not yet been identified. Because perioperative mortality is rare, its use for identification of influential targets for quality improvement is limited. Establishing more precise and more common outcome measures could supplement the studies associating different provider characteristics (such as volume and accreditation) with outcomes, and permit identification of more effective processes of care that might be employed by all providers.
We found that postoperative procedural interventions were measurable using SEER-Medicare data and that procedures were usually associated with codes suggesting a corresponding complication diagnosis. Patient and tumor characteristics were less predictive of surgical complications than acuity of illness, especially bowel perforation. This finding supports the notion that postoperative complications requiring procedural intervention tend to be associated with acute intraoperative events, rather than baseline patient characteristics.
An earlier study of coronary bypass patients26 found no correlation between postoperative complications and mortality, concluding that postoperative complications are not an adequate measure of surgical quality. Our study applied a rigorous method to avoid overcounting and clearly demonstrated that complications requiring a procedural intervention were associated with increased mortality and increased resource use. Our results are supported by the further finding that more complications were associated with substantially greater mortality and prolonged length of stay.
Silber et al recently examined the extremely negative impact of postoperative complications on prognosis but focused primarily on medical complications.27 Return to the operating room was lumped into a single category. To our knowledge, this is the first study using population-based data to examine all postoperative procedural interventions and to distinguish the associated complications. We found that certain types of complications requiring procedural intervention confer greater risk of mortality and prolonged length of stay than others. Intra-abdominal infection contributed to about a third of the postoperative procedural interventions, increasing mortality risk by nearly 3-fold and risk of prolonged hospitalization 2.7-fold. In contrast, wound infection or dehiscence, the second most common reason for a postoperative procedure, conferred no significant additional mortality, although it did increase the likelihood of prolonged hospitalization.
Previously, administrative data, such as the Medicare files, have not provided adequate clinical information to serve as a resource for the analysis of surgical complications.26,28–30 Romano et al compared ICD-9-CM coded complications to those identified by record review among patients who underwent discectomy in 1990 to 1991.28 They found overall weighted sensitivity of claims was only 35%, although sensitivity of claims for reoperation was greater than 60%. We have minimized this limitation by using both ICD-9-CM and CPT codes to focus only on the subset of major surgical complications that require reoperation or other procedural intervention and are therefore more likely to be found in administrative claims. These procedures are generally performed to treat complications at the original operative site involving technical problems with the anastomosis, surgical wound, infections, and bleeding.5
In their study of patients at Dartmouth-Hitchcock Medical Center, Birkmeyer et al found that colectomy patients had an overall return to the operating room rate of 9%.5 Our report expands upon this study by using population-based data, and by including a broader range of postoperative procedures, such as percutaneous procedures. The postoperative procedural intervention rate for our population-based study group was 5.8%, or about 64% of the reoperation rate among the single center study patients. The lower postoperative procedure rate in the current study likely results from the fact that the Dartmouth series included patients undergoing colectomy for all indications, including emergent operations for bleeding, perforation, and inflammatory conditions. In such a group of patients, postoperative complications and reoperative procedures will be more common than in our study group, who underwent resection for colorectal cancer exclusively and therefore were more likely to undergo elective surgery.
Several other single-institution and localized studies found that major complication rates after surgery for colorectal cancer were similar to the current population-based study.25,31,32 Much like our study, these investigators found that major technical complications occur in a small subset of patients but are clinically significant events that often require reoperative surgery.
This study is subject to a number of limitations. First, there are obvious limitations to the identification of surgical complications based on administrative data sources. However, we think that one of the strengths of this study is the extensive use of Medicare part B data (Carrier file), which documents specific surgical procedures using CPT codes submitted by surgeons, rather than relying on codes entered by nonclinicians in hospital claims.18 Second, although surgical complications are traditionally accepted as the sequelae of errors in surgical technique,33 underlying patient factors likely play a contributing role and tools for case-mix adjustment are inherently limited. To mitigate this limitation, we chose the most widely used risk adjustment method among surgical outcome studies and studies of Medicare patients,34 a modified Charlson method, and further adjusted for acuity of illness. Third, medical or nonoperative complications are not captured by postoperative procedures. These include potentially life-threatening events such as pulmonary embolus, pneumonia, and myocardial infarction that can also be affected by the quality of care provided. However, our aim was to provide a measure of surgical technical care, which would be less likely to influence medical complications than complications requiring procedural intervention.
Despite these limitations, these data demonstrate a strong linkage between postoperative procedures and postoperative mortality, and suggest that complications that require such intervention are associated with a markedly increased risk of postoperative death. We supported validity of this measure by demonstrating an association with mortality that gained strength as the number of different unplanned reoperations increased. Although some complications, such as wound infection or dehiscence, did not result in increased mortality, these were still associated with increased length of stay and could have a major impact on recovery time, resource use, and quality of life. While the limitations of these measures lead us to caution against using them indiscriminately as components of surgical quality report cards for hospitals or surgeons, they may serve as important methods for safety audit and review within hospitals or departments of surgery. Prospective tracking and periodic review of postoperative procedural intervention rates are likely to identify processes of care that affect patient safety and outcome. Our results also suggest that this type of prospective evaluation and review process may be performed using data that are widely accessible and relatively inexpensive to use.
The greatest utility of identifying these postprocedural interventions may be in surgical outcomes research. This approach to identifying complications allows researchers to assess the frequency and pattern of a subset of some of the most clinically significant surgical complications across large population groups. Studies of this nature may focus on improving the delivery of surgical care to vulnerable patient groups or determination of the effect of evolving structures or processes of surgical care on complications.
Although we do not expect measuring unplanned reoperations to contribute directly to quality of care, we think that it provides a readily measurable outcome for more accurately identifying influential processes of care. For example, tracking procedures to treat postoperative infection may help to support or refute the efficacy of a protocol of tight glucose management applied across a large hospital system. These investigations will assist in clarifying the complex relationship between surgeon factors, health system factors, and outcomes in cancer surgery. By identifying the key processes of care that reduce the number of major surgical complications, further research will aim to increase the quality of surgical care in all healthcare settings.
Footnotes
Supported by Grant No. R01CA089544 from the National Cancer Institute, National Institutes of Health, Bethesda, MD.
The views expressed in this article are those of the authors and do not necessarily represent the views of the National Cancer Institute or the Department of Veterans Affairs.
Reprints: Arden M. Morris, MD, MPH, 1500 E Medical Center Dr., TC-2920, Ann Arbor, MI 48109-0331. E-mail: ammsurg@umich.edu.
REFERENCES
- 1.Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280:1747–1751. [DOI] [PubMed] [Google Scholar]
- 2.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. [DOI] [PubMed] [Google Scholar]
- 3.Zingmond D, Maggard M, O'Connell J, et al. What predicts serious complications in colorectal cancer resection? Am Surg. 2003;69:969–974. [PubMed] [Google Scholar]
- 4.Gawande AA, Thomas EJ, Zinner MJ, et al. The incidence and nature of surgical adverse events in Colorado and Utah in 1992. Surgery. 1999;126:66–75. [DOI] [PubMed] [Google Scholar]
- 5.Birkmeyer JD, Hamby LS, Birkmeyer CM, et al. Is unplanned return to the operating room a useful quality indicator in general surgery? Arch Surg. 2001;136:405–411. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Murray T, Samuels A, et al. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. [DOI] [PubMed] [Google Scholar]
- 7.Beart RW, Steele GD Jr, Menck HR, et al. Management and survival of patients with adenocarcinoma of the colon and rectum: a national survey of the Commission on Cancer. J Am Coll Surg. 1995;181:225–236. [PubMed] [Google Scholar]
- 8.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 9.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(suppl 8):3–18. [DOI] [PubMed] [Google Scholar]
- 10.Nattinger AB, McAuliffe TL, Schapira MM. Generalizability of the surveillance, epidemiology, and end results registry population: factors relevant to epidemiologic and health care research. J Clin Epidemiol. 1997;50:939–945. [DOI] [PubMed] [Google Scholar]
- 11.Zippin C, Lum D. Study of completeness of the surveillance, epidemiology and end results (SEER) program case ascertainment by hospital size and casefinding source. Health Rep. 1993;5:87–90. [PubMed] [Google Scholar]
- 12.Beahrs O, Henson D, Hutter R, et al, eds. AJCC Manual for Staging of Cancer, 4th ed. Philadelphia: Lippincott, 1992. [Google Scholar]
- 13.Rural-urban commuting area codes (RUCAs). WWAMI Rural Health Research Center. Available at: http://www.fammed.washington.edu/wwamirhrc/. Accessed April 20, 2004.
- 14.Morrill R, Cromartie J, Hart L. Metropolitan, urban, and rural commuting areas: toward a better depiction of the US settlement system. Urban Geography. 1999;20:727–748. [Google Scholar]
- 15.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075–1079; discussion 1081–1090. [DOI] [PubMed]
- 16.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 17.Lawthers AG, McCarthy EP, Davis RB, et al. Identification of in-hospital complications from claims data: is it valid? Med Care. 2000;38:785–795. [DOI] [PubMed] [Google Scholar]
- 18.Potosky AL, Warren JL, Riedel ER, et al. Measuring complications of cancer treatment using the SEER-Medicare data. Med Care. 2002;40(suppl 8):62–68. [DOI] [PubMed] [Google Scholar]
- 19.Liang KY ZS. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 20.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. [DOI] [PubMed] [Google Scholar]
- 21.Longo WE, Virgo KS, Johnson FE, et al. Outcome after proctectomy for rectal cancer in Department of Veterans Affairs Hospitals: a report from the National Surgical Quality Improvement Program. Ann Surg. 1998;228:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longo WE, Virgo KS, Johnson FE, et al. Risk factors for morbidity and mortality after colectomy for colon cancer. Dis Colon Rectum. 2000;43:83–91. [DOI] [PubMed] [Google Scholar]
- 23.Schrag D, Cramer LD, Bach PB, et al. Influence of hospital procedure volume on outcomes following surgery for colon cancer. JAMA. 2000;284:3028–3035. [DOI] [PubMed] [Google Scholar]
- 24.Schrag D, Panageas KS, Riedel E, et al. Hospital and surgeon procedure volume as predictors of outcome following rectal cancer resection. Ann Surg. 2002;236:583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McArdle CS, Hole D. Impact of variability among surgeons on postoperative morbidity and mortality and ultimate survival. BMJ. 1991;302:1501–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silber JH, Rosenbaum PR, Schwartz JS, et al. Evaluation of the complication rate as a measure of quality of care in coronary artery bypass graft surgery. JAMA. 1995;274:317–323. [PubMed] [Google Scholar]
- 27.Silber JH, Rosenbaum PR, Trudeau ME, et al. Changes in prognosis after the first postoperative complication. Med Care. 2005;43:122–131. [DOI] [PubMed] [Google Scholar]
- 28.Romano PS, Chan BK, Schembri ME, et al. Can administrative data be used to compare postoperative complication rates across hospitals? Med Care. 2002;40:856–867. [DOI] [PubMed] [Google Scholar]
- 29.Brailer DJ, Kroch E, Pauly MV, et al. Comorbidity-adjusted complication risk: a new outcome quality measure. Med Care. 1996;34:490–505. [DOI] [PubMed] [Google Scholar]
- 30.Iezzoni LI, Daley J, Heeren T, et al. Identifying complications of care using administrative data. Med Care. 1994;32:700–715. [DOI] [PubMed] [Google Scholar]
- 31.Lothian and Borders large bowel cancer project: immediate outcome after surgery. The consultant surgeons and pathologists of the Lothian and Borders Health Boards. Br J Surg. 1995;82:888–890. [DOI] [PubMed] [Google Scholar]
- 32.Read TE, Mutch MG, Chang BW, et al. Locoregional recurrence and survival after curative resection of adenocarcinoma of the colon. J Am Coll Surg. 2002;195:33–40. [DOI] [PubMed] [Google Scholar]
- 33.Donabedian A. The end results of health care: Ernest Codman's contribution to quality assessment and beyond. Milbank Q. 1989;67:233–256; discussion 257–267. [PubMed]
- 34.Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: an overview. Med Care. 2002;40(suppl 8):26–35. [DOI] [PubMed] [Google Scholar]