Abstract
Objective:
To review present knowledge of the influence of hepatic steatosis in liver surgery as derived from experimental and clinical studies.
Summary Background Data:
Hepatic steatosis is the most common chronic liver disease in the Western world, and it is associated with obesity, diabetes, and metabolic syndrome. Fatty accumulation affects hepatocyte homeostasis and potentially impairs recovery of steatotic livers after resection. This is reflected clinically in increased mortality and morbidity after liver resection in patients with any grade of steatosis. Because of the epidemic increase of obesity, hepatic steatosis will play an even more significant role in liver surgery.
Methods:
A literature review was performed using MEDLINE and key words related to experimental and clinical studies concerning steatosis.
Results:
Experimental studies show the increased vulnerability of steatotic livers to various insults, attributed to underlying metabolic and pathologic derangements induced by fatty accumulation. In clinical studies, the severity of steatosis has an important impact on patient outcome and mortality. Even the mildest form of steatosis increases the risk of postoperative complications.
Conclusions:
Hepatic steatosis is a major factor determining patient outcome after surgery. Further research is needed to clarify the clinical relevance of all forms and severity grades of steatosis for patient outcome. Standardized grading and diagnostic methods need to be used in future clinical trials to be able to compare outcomes of different studies.
Hepatic steatosis significantly affects postoperative patient outcome after liver surgery. The increased susceptibility to injury of steatotic livers is attributed to underlying metabolic and pathologic derangements. This review addresses the morphology and mechanisms involved in damage occurring in steatotic livers.
Liver resection remains the only curative treatment of most patients with primary or secondary malignant liver tumors. Developments in surgical techniques and postoperative care have increased the number of resectable candidates and have enabled more extended anatomic and nonanatomic resections.1 Extended resections, however, stand a risk of postoperative liver failure. Liver dysfunction may be only transient if the liver has the ability to regenerate, but is prolonged when regeneration is impaired exposing the patient to potentially life-threatening complications. Mortality of posthepatectomy liver failure, despite intensive care treatment, remains as high as 60% to 90%.1 In particular, patients with parenchymal liver disease have an increased risk of postoperative mortality and morbidity because of the underlying pathogenic features affecting liver regeneration and recovery.2
Fatty liver or hepatic steatosis is a common histologic finding in human liver biopsy specimens, and it is estimated that more than 20% of the patients planned for liver resection have some degree of steatosis.2 The adverse effects of steatosis in liver surgery was at first acknowledged in transplantation studies reporting impaired outcome of steatotic grafts due to increased risk of primary nonfunction or dysfunction.3–6 Most recent data show that even the mildest form of steatosis increases the incidence of primary nonfunction and decreases patient survival after liver transplantation.7 Steatosis has also been gradually associated with an amplified postoperative morbidity and mortality after liver resection.8,9 The evolving knowledge about hepatic steatosis combined with the increasing prevalence in the future emphasizes understanding of the implications of steatosis for hepatic surgery.
This review focuses mainly on the aspects of steatosis associated with liver resection since the influence of steatosis in liver transplantation has been extensively reviewed in a number of publications.10–13 However, living-donor liver transplantation (LDLT) as a more recent modality of liver transplantation encompasses major liver resection on the part of the donor and will therefore be discussed here. Prevalence, pathogenesis, and diagnosis of steatosis are discussed to evaluate the impact of steatosis in liver surgery with particular emphasis on patient morbidity and survival. The data gained from experimental and clinical studies are discussed with the intention of clarifying the mechanisms behind the increased vulnerability of steatotic livers in liver surgery. Finally, different approaches including pharmacologic and surgical strategies to improve outcome of patients with liver steatosis after resection are presented and discussed.
Definition
Hepatic steatosis is characterized by an accumulation of lipids in the liver and is related to a spectrum of etiologic features such as obesity, diabetes, excessive use of alcohol, and a variety of drugs and toxins.14 Fatty accumulation is considered pathologic when the hepatic fat content, consisting mainly of triglycerides, exceeds 5% of the actual wet weight of liver.15 Steatosis can progress to a more severe inflammatory form as a consequence of excessive alcohol abuse or as in nonalcoholic fatty liver disease in nondrinkers. Nonalcoholic fatty liver disease is a clinical and histopathologic entity resembling alcohol-induced liver injury occurring in patients with little or no history of alcohol consumption.16 Recently, development of steatohepatitis has also been reported after neoadjuvant chemotherapy used to downstage patients with unresectable liver metastases.17 Steatohepatitis and nonalcoholic steatohepatitis are characterized by fat infiltration, hepatocyte ballooning, necroinflammatory changes together with progressive fibrosis and can eventually lead to cirrhosis in some patients.18
Incidence and Epidemiology of Hepatic Steatosis
Steatosis is the most common chronic liver disease in the world, affecting all racial, ethnic, and age groups without sex predilection. Even though the global prevalence has yet to be evaluated, studies report prevalence of 10% to 20% in lean population (body weight <110% of the ideal weight), 60% to 74% among the obese and over 90% in the morbidly obese (body weight >200% of ideal weight).19–22 Approximately 3% of lean children are affected and the prevalence increases up to 53% among obese children.23,24 Incidence of steatohepatitis ranges from 3% in lean population, to 18% among obese to almost 50% in morbidly obese individuals.25,26 The added risk to develop cirrhosis is 10% to 30% and is, to date, only seen in patients with steatohepatitis being associated with a decreased 5- and 10-year survival of 67% and 59%, respectively.27–29 The prevalence of steatosis and steatohepatitis is expected to dramatically increase in the near future due to increasing obesity among the Western population.
Clinical Manifestations
Clinical and Laboratory Abnormalities
Most patients have no clinical manifestations at the time of diagnosis and hepatomegaly is often the only finding on physical examination.19,25 Steatosis is usually an incidental finding as it is the most common cause of mild to moderate and asymptomatic elevation of plasma aminotransferases after other chronic liver diseases have been excluded.30 The ratio of aspartate aminotransferase to alanine aminotransferase is usually less than 1 in the presence of steatosis, but the predictive value of this ratio is poor in patients with severe steatosis and advanced parenchymal fibrosis. Serum alkaline phosphatase and gamma-glutamyltransferase are often above normal ranges and also elevated serum lipids and glucose concentrations are a common finding in up to 75% of all patients.31 Other possible laboratory abnormalities include hypoalbuminemia, prolonged prothrombin time, and hyperbilirubinemia.32 These parameters are, however, infrequently present in the patients with an advanced stage of disease.33 Anthropometric measurements such as a body mass index (BMI) = weight (kg)/height (m2) and hip-waist ratio have been shown to have some correlation with prevalence and severity of steatosis and might be useful in the assessment of patients for liver surgery.34,35
Methods of Quantifying Fatty Changes
Histopathology Findings
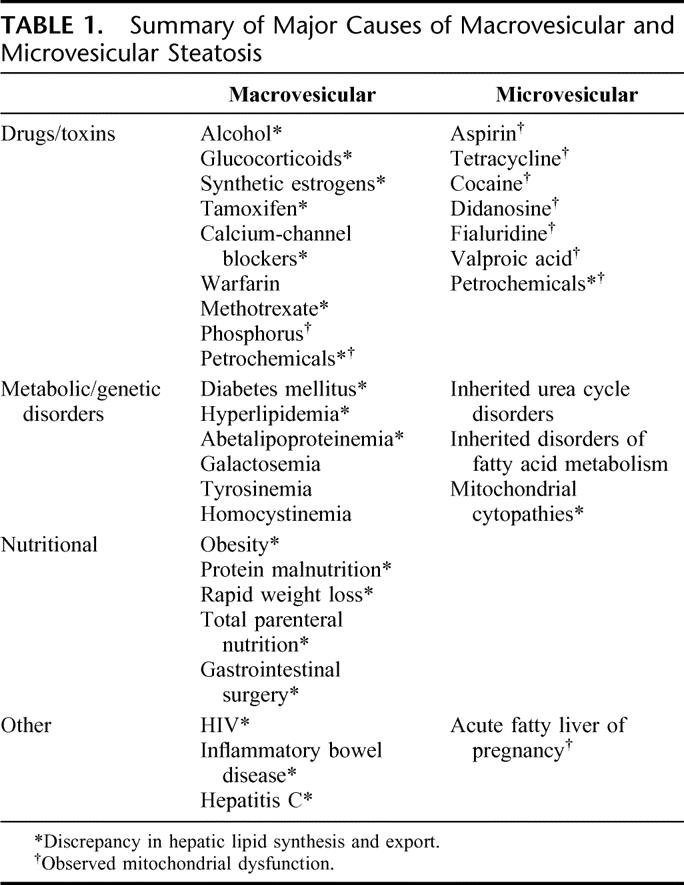
The gold standard of diagnosis is histopathologic evaluation of several liver biopsies, as a single biopsy can result in substantial misdiagnosis and staging inaccuracies.11,19–22,26,36 However, the risk of fatal bleeding after biopsy is estimated to be 0.4% and for nonfatal bleeding, 0.57%, and is therefore not routinely performed in patients without apparent complicated liver disease.37 Recently, a uniform quantitative grading for steatosis and steatohepatitis has been suggested, combining the identified key pathologic features. The severity is expressed as percentage of fatty hepatocytes of all hepatocytes. Further additional staging for steatohepatitis consists of the degree of portal and lobular inflammation, ballooning degeneration, Mallory bodies, and severity of fibrosis.38 Besides quantitative grading, steatosis can be classified qualitatively into microvesicular and macrovesicular forms. The most common clinical conditions causing these two forms of steatosis are summarized in Table 1. Most frequent is the macrovesicular one, in which the hepatocytes contain one single large fat vacuole, squeezing the nucleus into the cell periphery. This form of steatosis is frequently associated with obesity, non–insulin-dependent (type 2) diabetes, some dyslipidemias, and alcohol abuse. In microvesicular steatosis, the fat vacuoles are smaller than the cell nucleus and therefore remain central. This form is usually related to more acute conditions such as acute viral infections, metabolic disorders, and various toxins but also to acute fatty liver of pregnancy.39
TABLE 1. Summary of Major Causes of Macrovesicular and Microvesicular Steatosis
The histopathologic features of steatosis are evaluated in preoperative needle biopsies or operative wedge specimens that are frozen and/or deparaffinized.40 The staining methods currently used are hematoxylin and eosin with which the fatty changes are assessed by considering the nonstained regions. In addition, specific fat stains such as Oil Red O and Sudan IV are used. However, there are several problems in clinical application of these staining methods. The conventional techniques applying hematoxylin and eosin potentially underestimate the extent of fatty infiltration as they fail to identify microvesicular forms of steatosis.41 Also the fat specific stains have pitfalls, for example, in Oil Red O-stained liver tissue, the quality and quantity of the staining are highly operator-dependent and false-positive results or overestimation of the severity are possible because of unspecific sinusoidal staining.32,40
Imaging Studies
Despite widespread clinical use of imaging methods, ultrasound (US), computed tomography (CT), or magnetic resonance imagining (MRI) can only to some extent detect the degree of steatosis. On US, steatosis generates an area of diffusely increased echogenicity whereas on CT, a parenchyma with low-density is seen. Even though US is the least expensive and most easily available imaging technique, CT can be used for semiquantitative assessment of fat accumulation.42 Although liver density assessed by CT reflects the presence of steatosis and correlated with a positive biopsy, a false-negative rate of 24% has been reported.43 The advantage of MRI is the possibility to distinguish focal space-occupying lesions from focal fatty infiltrations.44 However, Saadeh et al reported in a study applying state-of-the-art equipment for US, CT, and MRI that only a hepatic fat accumulation above 25% to 30% can be reliably detected radiologically.45 Also, none of these modalities was able to either distinguish steatosis from steatohepatitis or to detect individual pathologic features important to establish steatohepatitis such as necroinflammatory changes, hepatocyte ballooning, and fibrosis.34 This study demonstrated the limited role of radiologic modalities in the management of patients with steatosis.
Clinical Impact of Steatosis on Hepatic Surgery
Background
The mortality rate associated with liver resections in the absence of parenchymal disease has declined to far below 5% during the last decade.46–48 Even zero mortality can be achieved with systematic preoperative patient selection as a recent study reported a cohort of 915 patients who were routinely screened for preoperative liver function to calculate the extent of safe resection.49 Also, the indications for liver resection have much changed and an increasing proportion of patients with extensive hepatobiliary malignancies, including patients with additional cirrhosis, may be curatively resected, sometimes using complex reconstructions of vascular structures.50 However, the clinical importance of other parenchymal liver diseases, such as steatosis, is unclear and a potentially increased risk of impaired postoperative recovery has been suggested. Especially, the clinical importance of the severity of steatosis and related underlying pathologic derangements remain undefined.
Effect of Steatosis on Posthepatectomy Morbidity and Mortality
There are few studies reporting the impact of steatosis on postoperative morbidity and mortality after liver resection (Table 2). Mortality is assessed as in-hospital mortality or 60 days mortality after operation, and there are no studies evaluating 5- or 10-year survival of steatotic patients with hepatic malignancy. As the first in 1998, Behrns et al evaluated in a retrospective study of 135 patients, the safety of major resection in patients with hepatic steatosis.8 They reported an increased postoperative mortality, morbidity, and blood transfusion together with longer operative time in the presence of steatosis. Furthermore, steatotic patients had increased plasma aminotransferases and bilirubin levels, reflecting postoperative liver dysfunction, and 14% had acute liver failure versus 4% in patients with normal liver parenchyma.5
TABLE 2. Clinical Studies Reporting the Impact of Steatosis on Patient Outcome
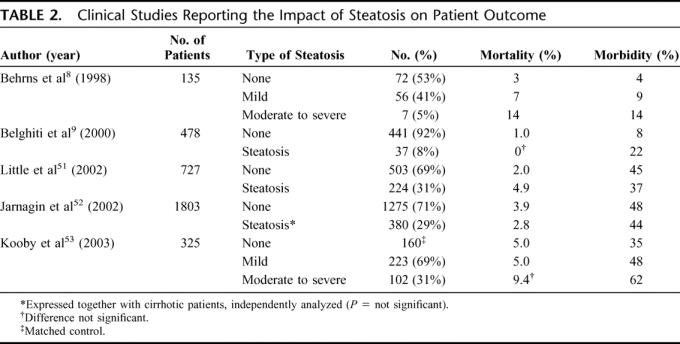
Belghiti et al, in a cohort of 478 elective liver resection patients including 37 patients with steatosis, showed that steatosis was an independent risk factor for postoperative complications.9 Complications occurred in 8% of patients with steatosis versus 2% in patients with normal parenchyma. However, in this cohort study, no effect on in-hospital mortality was seen. In contrast, Little et al51 reported a negative effect of steatosis on in-hospital mortality. The main objective of the latter study was to investigate the role of diabetes mellitus in postoperative mortality and morbidity rates within 30 days of operation. Interestingly, in a cohort of 727 patients, 224 patients (31%) had some degree of steatosis with mortality that was significantly increased (4.9% vs. 2.0%, in normal and steatotic patients, respectively). However, no differences were seen in postoperative complications (45% and 37%, in steatotic and nonsteatotic livers, respectively).51
Jarnegin et al published so far the largest cohort of 1803 liver resections performed in one institution.52 In 997 patients (55%) the non–tumor-bearing liver was histologically normal whereas steatosis was diagnosed in 325 patients (18%) without further staging of steatosis severity. In contrast to the previous studies of Belghiti et al9 and Behrns et al,8 Jarnegin et al52 did not report any effect of steatosis on perioperative outcome. However, the authors speculated that this was probably because of the small number of steatotic patients and the much larger proportion of patients with normal parenchyma in their study.52 Therefore, Kooby et al reviewed the above-mentioned cohort of 1803 patients in a later study.53 In this study, 160 patients with normal liver parenchyma were randomly selected to match the 325 patients with steatotic livers by age, comorbidity, and the extent of resection. Furthermore, the severity of steatosis was assessed; 223 patients had mild (<30%) hepatic steatosis, 64 had moderate (30%–60%) steatosis, 38 severe (>60%) steatosis, while patients with fibrosis were excluded. Patients with moderate and severe steatosis were combined in one group described as marked steatosis (>30%, n = 102). Total complications (62%, 48%, and 35%; in marked and mild steatosis and normal parenchyma, respectively) and infective complications (43%, 24%, and 14%) correlated with the degree of steatosis. However, no differences were observed in complications requiring major medical intervention, hospitalization time, or admission to the intensive care unit. In multivariate analysis, steatosis was an independent predictor of complications, and there was a nonsignificant trend toward higher 60-day mortality in patients with resection of one lobe or more and in patients with marked steatosis (9.4% mortality associated with marked steatosis vs. 5.0% in mild steatosis and 5.0% in control patients, respectively).
Impact of Steatosis in Living-Donor Liver Transplantation (LDLT)
LDLT is an option to increase organ availability in a time when the waiting lists for cadaveric liver transplantation are growing. LDLT was initially applied in the setting of pediatric liver transplantation in which donation of left lateral segments took place.54 In recent years however, LDLT has been successfully applied in adults as right lobe LDLT.55,56 Even though LDLT has several advantages, ie, optimal donor screening and planning of transplantation procedure, ethical concerns remain. For LDLT donors, several screening criteria exist, mainly consisting of clinical, biochemical, radiologic, histologic, and repeated psychologic evaluations.57 Currently, LDTD donors, as well cadaveric donors presenting with steatosis above 20% to 30%, are generally excluded obscuring the complete impact of steatosis in LDLT.55
Soejima et al reported in a series of 52 LDLT patients consisting of patients with no steatosis (n = 23), mild steatosis (n = 23), or moderate steatosis (n = 6), a comparable 1-year donor and graft survival in all groups; no primary nonfunction was observed in any patient group.58 Hayashi et al reported, in a cohort of 338 LDLT patients, that 41 patients that had received donor livers with varying degrees of steatosis (25 mild, 13 moderate, and 3 severe). Regarding the donor operation, there was no long-term morbidity or mortality, and the hospital stay was similar to those with normal liver. Furthermore, there was no difference in outcome between patients receiving steatotic or normal grafts.59 Also, Ito et al reported no impact of steatosis on cumulative donor survival at any of the United Network for Organ Sharing categories; complications were not analyzed in regard with degree of steatosis.60 Yoong et al showed in their study of 116 LDLT patients requiring retransplantation that severe steatosis had a serious negative effect on graft survival.61 Ten patients with a donor liver presenting with severe microvesicular steatosis (<66% of hepatocytes were affected) had a dramatically poorer 1-year graft survival of 20% compared with 57% in the nonsevere steatosis group. The graft failure rate was 100% in the severe group after a median survival of 1.5 months (vs. 59% in the nonsevere steatosis group). From these studies, even though limited in number, it can be concluded that mild steatosis does not seem to affect the prognosis of living donors and recipients, but livers with severe steatosis should not be transplanted.
Conditions Associated With Hepatic Steatosis
Insulin Resistance
Patients with nonalcoholic fatty liver disease have an increased prevalence of non–insulin-dependent diabetes, but the actual role of diabetes in postoperative recovery is unclear as studies report contradictory results. Non–insulin-dependent diabetes was identified as independent and significant variable predicting major postoperative complications in a cohort of 209 patients.62 However, contrary to this study, a study including 525 diabetic and nondiabetic HCC patients, reported no difference in perioperative morbidity or mortality after resection and no effect was observed in long-term prognosis.63 Although the impact of diabetes on postoperative complications remains unclear, an increased rate of wound infections in patients with disturbed glucose homeostasis is reported in an impressive cohort of over 20,000 patients.64
Obesity
Obesity is crucially linked with steatosis as the prevalence among obese is up to 75% and among morbidly obese up to 100%.16 In the past, obesity has been linked to increased perioperative technical complications leading to prolonged postoperative recovery. However, Dindo et al prospectively investigated a cohort of 6336 patients undergoing elective surgery and found no increase in postoperative morbidity and mortality between obese and nonobese, not even in morbidly obese patients with BMI above 40.65 In contrast to general obesity, body fat accumulation (subcutaneous or intraabdominal) has been reported to be independently associated with postoperative morbidity after gastric or colorectal surgery in a prospective study of 139 patients who underwent gastric or colorectal cancer surgery.66
Mechanisms of Fat Accumulation/Steatosis
Nonesterified fatty acids accumulate when the hepatic uptake exceeds the output, usually due to altered lipid ingestion and/or lipoprotein metabolism. This can be a consequence of an excessive supply of free fatty acids (FFAs) in the liver, diminished hepatic export of FFA, and/or impaired mitochondrial beta-oxidation of FFA.67,68 Nonesterified fatty acids inhibit beta-oxidation, subsequently decreasing the production of acetyl-coenzyme A, an important precursor of Krebs cycle and gluconeogenesis leading to depletion of 2 important energy sources, ie, beta-oxidation and gluconeogenesis in steatotic livers.69
Two-Hit Theory
The exact pathogenesis of steatohepatitis is unclear, but a two-“hit” theory proposed by Day and James is most widely supported.70 Fat accumulation is the essence and constitutes the first “hit.” Additionally, there are an increasing number of contributors recognized as the second “hits” that initiate and sustain the progression to steatohepatitis and subsequently cirrhosis in some patients. Increased oxidative stress, lipid peroxidation, mitochondrial p-450 cytochrome induction and distorted energy homeostasis, bacterial endotoxins, Kupffer cell dysfunction, and induction of Fas ligand promoting fibrinogenesis all play an important role.71,72
Oxidative Stress
Increased oxidative stress and lipid peroxidation are identified in the literature as the most prominent pathogenic features of injury in steatosis.73 Intracellular fatty acids induce oxidative stress by direct toxicity, or by activation of several microsomal cytochrome p-450 lipoxygenases or increase of peroxisomal beta-oxidation.74 Induction of hepatic cytochrome p-450 2E1 (CYP2E1) in a murine nonalcoholic steatohepatitis model is reported to be linked with a dramatic increase of total lipid peroxidation.75,76 As a consequence, lipid peroxidation and reactive oxygen species (ROS) deplete antioxidant enzymes, such as glutathione, rendering the liver susceptible to oxidative injury.77
Energy Homeostasis
Altered mitochondrial homeostasis contributes also to the pathogenesis of steatohepatitis by increasing oxidative stress. Mitochondria in steatotic hepatocytes produce excessive amounts of ROS leading to up-regulation of uncoupling protein-2, a mitochondrial inner membrane protein that decreases the mitochondrial adenosine triphosphate (ATP) production in fatty hepatocytes.78 Therefore, the efficacy of ATP synthesis in steatotic livers is compromised and ATP homeostasis insufficiently recovers after insults such as liver resection or ischemia-reperfusion injury (I/R) injury additionally consuming hepatic ATP reserves.79 Peroxisomes take over beta-oxidation of fatty acids, especially in fatty livers were the mitochondrial oxidative capacity is outdone. The peroxisomal enzymes are regulated by the nuclear hormone receptor proliferation activation receptor (PAPR)-alpha and transcriptional up-regulation is observed in obese and diabetic rodents.72,74 The up-regulation of PARP-α induces oxidative stress by increasing hydrogen peroxidase, a byproduct of increased beta-oxidation.80
Insulin Resistance
Other identified factors contribute to the development of steatohepatitis either by affecting hepatic lipid metabolism and/or inducing inflammatory response. There is increasing evidence of the crucial role of insulin resistance in the pathogenesis of hepatic steatosis as it is frequently observed in obese and type 2 patients with diabetes but also in lean patients with steatosis.81 Insulin resistance alters lipid metabolism by enhancing peripheral lipolysis and increasing triglyceride synthesis.82 Furthermore, increased insulin concentrations block mitochondrial fatty acid oxidation and together with all these features contribute to net retention of lipids within the liver.
Mechanisms of Injury During Hepatic Surgery in Steatotic Livers
Liver Resection
Liver resection is associated with a risk of mortality and morbidity closely related to volume and function of the remnant liver. Tolerance to warm I/R in case of hepatic pedicle clamping and sufficient postoperative regenerative capacity are crucial for an uncomplicated postoperative recovery of hepatic volume and function.83 I/R injury is considered as the main contributor to hepatocellular damage during liver resection as posthepatectomy liver failure is often caused by aggravated I/R injury.84 There are several mechanisms involved in I/R injury co-contributing to the increased susceptibility of the liver to I/R injury, thus delaying functional and morphologic recovery of steatotic livers after liver resection.
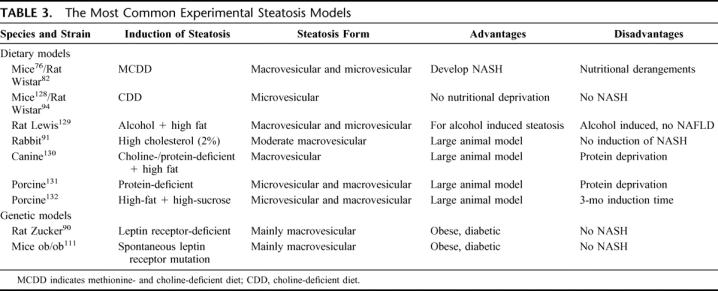
A variety of animal models of liver steatosis are applied in the studies investigating the impact of steatosis in liver surgery. The most widely used models are summarized in Table 3. Hepatic steatosis can be induced by genetic leptin mutation (Zucker rats, ob/ob mice) or by modulation of nutritional factors. The genetically modified rodents overeat due to lack of the controlling effect of leptin and consequently develop combined microvesicular and macrovesicular steatosis without inflammatory changes.72 The nutritional models are based either on diets of high fat percentage or of amino acid deficiency, ie, choline and methionine, essential for hepatic lipid excretion.75
TABLE 3. The Most Common Experimental Steatosis Models
Mechanism of Ischemia: Reperfusion Injury
Inflow occlusion by clamping of the hepatic pedicle (Pringle's maneuver) in combination with maintaining low central venous pressure is often applied in extensive hepatic surgery to reduce blood loss during parenchymal transection. The process of occlusion and reperfusion induces hepatic I/R injury as manifested during the reperfusion period when the blood flow recirculates the previously ischemic liver remnant.85,86 I/R injury is related to increased hepatic ROS production and cellular pH changes, increased inflammatory responses and reduced hepatic microcirculation by sinusoidal vasoconstriction.87–89 The increased vulnerability of the steatotic liver to I/R injury is suggested to be due to a combination of both microcirculatory blood flow and cellular changes.90
Microcirculation
Experimental studies indicate that the degree of steatosis shows an inverse correlation with hepatic blood flow and microcirculation. Even mild steatosis (<30% hepatocytes affected) reduces both total hepatic blood flow and microcirculatory flow.91 The degree of steatosis has more impact on the microcirculation than on total blood flow as demonstrated by decreased sinusoidal flow. This is attributable to narrowed sinusoidal lumens caused by swollen fatty hepatocytes.92 In addition, less visible vascular beds and distorted sinusoidal beds contribute to increased intrahepatic vascular resistance decreasing flow in fatty livers.90,93
As a result of decreased total perfusion, a continuous state of chronic cellular hypoxia persists in fatty hepatocytes predisposing the steatotic liver to I/R injury.94 In addition, sinusoidal lumens are narrowed by fibrin microthrombi and cellular debris during reperfusion and further decrease sinusoidal perfusion.95 Although Selzner et al did not find any differences in portal pressure in steatotic liver,96 it seems that microcirculatory failure plays an important role in the mainly necrotic cell death after I/R in fatty livers.97
Energy Homeostasis
The impaired energy homeostasis is considered to sensitize steatotic livers to further surgical stress. The total ATP synthesis is decreased in fatty hepatocytes because of decreased mitochondrial ATP synthase.98 Additionally, the depletion of beta-oxidation and gluconeogenesis compromises cellular integrity since glycogen is essential for the integrity maintenance by supplying glucose to ATP synthesis. In the absence of glycogen, for example, due to increased consumption by surgical stress, ATP depletion triggers irreversible hepatocellular necrosis.99,100 Furthermore, the ability to recover the depleted hepatic ATP storage is severely impaired in patients with obesity-related steatohepatitis.98,101 The mitochondrial dysfunction is speculated to be due to structural changes seen in mitochondrial matrix in electron microscopy studies.102,103
Cellular Consequences
Sinusoidal endothelial cells in steatotic livers show more leukocyte adherence contributing to sinusoidal congestion during reperfusion.104 Although sinusoidal endothelial cells in steatotic livers are more vulnerable to structural damage, it is unclear if this is due to changes in the sinusoidal endothelial cells or in the surrounding hepatocytes. Nonfatty hepatocytes are considered relatively resistant to oxidative stress during reperfusion with apoptosis as the main form of cell death after I/R. Apoptosis induced only minimal local response as no local proinflammatory response is activated. Steatotic hepatocytes, however, are not able to induce energy-consuming apoptosis but go through necrotic cell death probably as a result of impaired ATP homeostasis.94,102
The resident macrophages of the liver, ie, the Kupffer cells are activated in the early and late phases of reperfusion and further generate mediators such as cytokines as tumor necrosis factor alpha, interleukin (IL)-1β and chemokines initiating local and systemic inflammatory responses ultimately resulting in hepatocellular damage.105 Kupffer cells are activated by ROS leaking from damaged hepatocytes and endotoxin produced by bacterial translocation.106 Kupffer cells from obese steatotic mice demonstrated decreased phagocytosis capability and increased release of ROS and interleukins IL-6 and IL-1β.72 This Kupffer cell dysfunction amplifies the inflammatory response and further escalates the hepatocellular injury induced by I/R injury. In addition, increased chemokine-induced neutrophil chemoattractant contributes to hepatic injury by attracting neutrophils producing additional ROS and multiple proteases.72 I/R injury by itself causes cell swelling and leukocyte adhesion and the neutrophil accumulation further contributes to impaired regional and sinusoidal blood flow.107–109
Mechanisms of Impaired Liver Regeneration
Impaired liver regeneration is an important clinical complication of steatosis, manifesting as increased morbidity and mortality after partial hepatic resection.8,9
The mechanism(s) of impaired liver regeneration remain unclear, but investigations in different experimental models of steatosis have implicated abnormalities in the cell cycle progression.
The ob/ob mice show increased basal rates of hepatocyte proliferation and up-regulated antiapoptotic pathways; however, despite these features, ob/ob mice displayed impaired liver regeneration after endotoxin-mediated hepatocellular injury.110–112 For normal liver regeneration, several cytokine-dependent and cytokine-independent pathways are essential. In steatotic rats, impaired liver regeneration was found to be associated with interruption in the normal IL-6 signaling pathway, a critical pathway that primes the hepatocytes to respond to mitogenic signals.113,114 Furthermore, failure of signaling at the level of G1/S phase transition in the cell cycle was observed in fatty hepatocytes during hepatocyte proliferation. This arrest has been proposed to be due to a combination of factors such as the inhibition of induction of the cyclin D1 gene and MAP kinases in the G1 phase of cell cycle.110,115 The IL-6 signal is further transduced through the activation of Janus family of kinases, which in turn triggers the signal transducer and activator of transcription (STAT)-3.116,117 Levels of activated STAT-3 have been reported to be higher in ob/ob mice than in lean controls at baseline; however, decreased proliferation shown by BrdU labeling has also been observed. Therefore, even though STAT-3 activation is necessary for proliferation, it is not sufficient to induce cell cycle progression in fatty hepatocytes.110,118
Deranged ATP Homeostasis
During liver regeneration, ATP is crucial in several events required for cell cycle transition from G1 to S phase. These events include activation of certain ion channels, thymidine kinase, and chromatin remodeling enzymes.119 Therefore, ATP dysfunction most likely plays a crucial role in impairment of regeneration in fatty livers. Depletion of hepatic ATP synthesis in fatty hepatocytes has been reported to be the result of mitochondrial dysfunction.120 Mitochondria are among the critical cellular organelles damaged by intracellular fatty acids through oxidants derived from increased lipid peroxidation dissolving the lipid membranes.101 Interestingly, ATP depletion might, conversely, also protect steatotic livers from apoptotic cell death by directly inhibiting both caspase-3 activation and Jak kinase activation, preventing further caspase-3 activation.110,121 This might explain why the main form of death of fatty hepatocytes is necrosis instead of apoptosis as is the case in normal hepatocytes, when progression in cell cycle during proliferation is blocked.
DISCUSSION
Steatosis in hepatic surgery is step by step recognized as a clinically important feature that influences patient morbidity and mortality after hepatic resection. In the coming years, steatosis will become a major concern as the prevalence is closely linked to obesity, an epidemic phenomenon in Western countries. Surgeons are increasingly taking steatosis into account when planning the extent and type of hepatic surgery and are likely to consider possible preoperative and perioperative interventions to minimize the additional damage. However, there are certain pitfalls complicating preoperative assessment of steatotic patients. Currently, the single most reliable method to diagnose steatosis is a liver biopsy.45 However, core biopsies contain a risk of complications and even of mortality37 and, therefore, are not routinely performed in patients with normal or slightly elevated liver enzymes. This places a patient with steatosis at considerable risk as the current noninvasive imaging tests do not reliably exclude the presence of even severe steatosis. When a biopsy is available, after explorative laparotomy or as part of preoperative tumor staging, further assessment is further hampered by the unreliability of staining methods that might overestimate or underestimate the degree of steatosis.32,40 This is especially relevant in patients with a microvesicular component,31 as this form of steatosis is related to more deranged energy homeostasis39 and in a worst case scenario exposes the patient to severe postoperative complications and even mortality.8,51 The histologic evaluation and grading of steatosis should be standardized to avoid this pitfall. Furthermore, it is obvious that there is an urgent need for reliable noninvasive methods to detect steatosis and related pathologic features preoperatively.
Preoperative assessment targeted to identify steatosis is complicated because of the lack of specific diagnostic tools. Currently available laboratory workup and imaging modalities are too unspecific for accurate diagnosis.30,45 Presently, the most promising marker is the BMI as there is a correlation between hepatic steatosis and a BMI above 28 kg/m2.34 This index, together with clinical presentation, might help to select candidates for liver biopsy as histopathology remains the gold standard for the diagnosis of steatosis.
Even though the mechanisms behind the injurious effects of steatosis in hepatic surgery are becoming unraveled, the actual risk remains unclear. Animal models applied in experimental studies have all biases precluding the extrapolation of results to the clinical situation. In the genetically modified rodents with leptin deficiency, it seems that the reported injury mechanisms and impaired regeneration are due to disturbed leptin signaling per se instead of steatosis.122 In the nutrition-based rodent models, an imbalance of metabolic features is created that is not representative of the clinical situation. On the other hand, these rodents display the crucial pathogenic features for the development of steatohepatitis; therefore, these nutritional models better represent the clinical situation than the genetic ones that lack inflammatory changes.123 The development of clinically relevant experimental models is also hindered by the spectrum of patients with different etiologic factors.14 Different etiologic backgrounds lead to different forms of steatosis combined with a range of pathologic features unique to some etiologic factors. Furthermore, the clinical significance of the type and extent of steatosis is not clear as larger cohort studies applying uniform diagnostic criteria are missing. There are a few large cohort studies assessing the role of steatosis in postoperative recovery.8,9,51,52 However, there are some general problems in the reporting of these studies. The histopathology methods for diagnosis of steatosis are not frequently, if ever, mentioned. So, the reliability of diagnosis of steatosis remains uncertain, rendering the comparison of the results difficult. Uniform grading together with a more detailed description of the staining methods used and the number and sort of biopsies taken are important to compare different studies.
Various approaches have been proposed to improve the poorer postoperative outcome of patients with steatosis after liver surgery. The currently applied protective strategies are based on the increased susceptibility of steatotic liver to I/R injury96 and can be classified into pharmacologic and surgical strategies and gene therapy. A positive effect by preconditioning with mild hypothermia or hyperthermia has been described in experimental studies but is yet to be applied in clinical studies.124,125 Although none of these approaches is currently routinely applied, it seems that surgical strategies such as ischemic preconditioning are the most promising. Ischemic preconditioning (IPC) consists of introducing a brief ischemic period before the actual surgical procedure improving microcirculation and subsequently increasing the cellular oxygen supply after I/R.126,127 This beneficial effect has also been shown in patients with mild to moderate steatosis by Clavien et al.84 However, the beneficial effect of IPC in older patients and patients undergoing extensive resections is controversial as there are recent studies reporting specific negative side effects of IPC in these cohorts of patients.133 It is clear that more research is needed in this field of surgery as the prevalence of steatosis is dramatically increasing together with the more graying population.
CONCLUSION
Steatosis plays an important role in hepatic surgery as it is a major risk factor in patient outcome after liver resection. This is due to lipid accumulation deranging hepatic energy homeostasis and inducing hepatocellular damage subsequently affecting hepatocellular recovery. Further research is needed to clarify the clinical relevance of the broad spectrum of all forms and severity grades of steatosis on patient outcome. Standardized grading and diagnostic modalities need to be applied in future clinical trials to be able to compare outcomes of different studies.
Footnotes
Reprints: Thomas M. van Gulik, MD, Department of Surgery, Academic Medical Center, IWO-1, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands. E-mail: t.m.vangulik@amc.uva.nl.
REFERENCES
- 1.Redaelli CA, Wagner M, Krahenbuhl L, et al. Liver surgery in the era of tissue-preserving resections: early and late outcome in patients with primary and secondary hepatic tumors. World J Surg. 2002;26:1126–1132. [DOI] [PubMed] [Google Scholar]
- 2.Bernuau J, Rueff B, Benhamou JP. Fulminant and subfulminant liver failure: definitions and causes. Semin Liver Dis. 1986;6:97–106. [DOI] [PubMed] [Google Scholar]
- 2a.Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105–113. [DOI] [PubMed] [Google Scholar]
- 3.Wu CC, Ho WL, Yeh DC, et al. Hepatic resection of hepatocellular carcinoma in cirrhotic livers: is it unjustified in impaired liver function? Surgery. 1998;123:270–277. [DOI] [PubMed] [Google Scholar]
- 3a.Strasberg SM, Howard TK, Molmenti EP, et al. Selecting the donor liver: risk factors for poor function after orthotopic liver transplantation. Hepatology. 1994;20:829–838. [DOI] [PubMed] [Google Scholar]
- 4.Trevisani F, Colantoni A, Caraceni P, et al. The use of donor fatty liver for liver transplantation: a challenge or a quagmire? J Hepatol. 1996;22:114–121. [DOI] [PubMed] [Google Scholar]
- 5.Markin RS, Wisecarver JL, Radio SJ, et al. Frozen section evaluation of donor livers before transplantation. Transplantation. 1993;56:1403–1409. [DOI] [PubMed] [Google Scholar]
- 6.Adam R, Reynes M, Johann M, et al. The outcome of steatotic grafts in liver transplantation. Transplant Proc. 1991;23:1538–1540. [PubMed] [Google Scholar]
- 7.Ploeg RJ, D'Alessandro AM, Knechtle SJ, et al. Risk factors for primary dysfunction after liver transplantation: a multivariate analysis. Transplantation. 1993;55:8087–8013. [DOI] [PubMed] [Google Scholar]
- 8.Behrns KE, Tsiotos GG, DeSouza NF, et al. Hepatic steatosis as a potential risk factor for major hepatic resection. J Gastrointest Surg. 1998;2:292–298. [DOI] [PubMed] [Google Scholar]
- 9.Belghiti J, Hiramatsu K, Benoist S, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. [DOI] [PubMed] [Google Scholar]
- 10.Crowley H, Lewis WD, Gordon F, et al. Steatosis in donor and transplant liver biopsies. Hum Pathol. 2000;31:1209–1213. [DOI] [PubMed] [Google Scholar]
- 11.Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105–113. [DOI] [PubMed] [Google Scholar]
- 12.Imber CJ, St. Peter SD, Handa A, et al. Hepatic steatosis and its relationship to transplantation. Liver Transpl. 2002;8:415–423. [DOI] [PubMed] [Google Scholar]
- 13.Sauer P, Schemmer P, Uhl W, et al. Living-donor liver transplantation: evaluation of donor and recipient. Nephrol Dial Transplant. 2004;19:11–15. [DOI] [PubMed] [Google Scholar]
- 14.Burt AD, MacSween RNM, Peters TJ, et al. Nonalcoholic Fatty Liver: Causes and Complications. Oxford: Oxford University Press, 863–872. [Google Scholar]
- 15.Sherlock S. Acute fatty liver of pregnancy and the microvesicular fat diseases. Gut. 1983;24:260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angulo P. Non-alcoholic fatty liver disease. N Engl J Med. 2002;8:1221–1231. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez FG, Ritter J, Goodwin JW, et al. Effect of steatohepatitis associated with irinotecan or oxaplatin pretreatment on respectability of hepatic colorectal metastases. J Am Coll Surg. 2005;200:845–853. [DOI] [PubMed] [Google Scholar]
- 18.Saksena S. Natural history and determinants of disease progression in non-alcoholic fatty liver disease: good and bad news. Hepatology 2003;38:233A. [Google Scholar]
- 19.Bellentani S, Saccoccio G, Masutti F, et al. Prevalence of and risk factors for hepatic steatosis in northern Italy. Ann Intern Med. 2000;132:112–117. [DOI] [PubMed] [Google Scholar]
- 20.Nomura H, Kashiwagi S, Hayashi J, et al. Prevalence of fatty liver in a general population of Okinawa, Japan. Jpn J Med. 1988;27:142–149. [DOI] [PubMed] [Google Scholar]
- 21.Luyckx FH, Desaive C, Thiry A, et al. Liver abnormalities in severely obese subjects: effect of drastic weight lost after gastroplasty. Int J Obes. 1998;22:222–226. [DOI] [PubMed] [Google Scholar]
- 22.Silverman JF, O'Brien KF, Long S, et al. Liver pathology in morbidly obese patients with and without diabetes. Am J Gastroenterol. 1990;85:1349–1355. [PubMed] [Google Scholar]
- 23.Franzese A, Vajro P, Argenziano A, et al. Liver involvement in obese children: ultrasonography and liver enzyme levels at diagnosis and during follow-up in an Italian population. Dig Dis Sci. 1997;42:1428–1432. [DOI] [PubMed] [Google Scholar]
- 24.Tominaga K, Kurata JH, Chen YK, et al. Prevalence of fatty liver in Japanese children and relationship to obesity: an epidemiological ultrasonographic survey. Dig Dis Sci. 2002;40:2002–2009. [DOI] [PubMed] [Google Scholar]
- 25.Powell EE, Cooksley WG, Hanson R, et al. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74–80. [DOI] [PubMed] [Google Scholar]
- 26.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. [DOI] [PubMed] [Google Scholar]
- 27.Teli MR, James OF, Burt AD, et al. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995;22:1714–1719. [PubMed] [Google Scholar]
- 28.Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. [DOI] [PubMed] [Google Scholar]
- 29.Propst A, Propst T, Zangerl G, et al. Prognosis and life expectancy in chronic liver disease. Dig Dis Sci. 1995;40:1805–1815. [DOI] [PubMed] [Google Scholar]
- 30.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease: the most common cause of abnormal liver enzymes in the U.S. population. Gastroenterology 2001;120(suppl A). [Google Scholar]
- 31.Sheth SG, Gordon FD, Chopra S. Nonalcoholic steatohepatitis. Ann Intern Med. 1980;126:137–145. [DOI] [PubMed] [Google Scholar]
- 32.Diehl AM, Goodman Z, Ishak KG. Alcohol-like liver disease in nonalcoholics: a clinical and histologic comparison with alcohol-induced liver disease. Gastroenterology. 1988;95:1056–1062. [PubMed] [Google Scholar]
- 33.Angulo P, Keach JC, Batts KP, et al. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. [DOI] [PubMed] [Google Scholar]
- 34.Rinella ME, Alonso E, Rao S, et al. Body mass index as a predictor of hepatic steatosis in living liver donors. Liver Transpl. 2001;7:409–414. [DOI] [PubMed] [Google Scholar]
- 35.Kral J, Schaffner F, Pierson R, et al. Body fat topography as an independent predictor of fatty liver. Metabolism. 1993;42:548–551. [DOI] [PubMed] [Google Scholar]
- 36.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. [DOI] [PubMed] [Google Scholar]
- 37.McGill DB, Rakela J, Zinsmeister AR, et al. A 21-year experience with major hemorrhage after percutaneous liver biopsy. Gastroenterology. 1990;99:1396–1400. [DOI] [PubMed] [Google Scholar]
- 38.Brunt EM, Janney CG, Di Biseglie AM, et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. [DOI] [PubMed] [Google Scholar]
- 39.Sass DA, Chang P, Chopra KB. Nonalcoholic fatty liver disease: a clinical review. Dig Dis Sci. 2005;50:171–180. [DOI] [PubMed] [Google Scholar]
- 40.Markin RS, Wisecarver JL, Radio SJ, et al. Frozen section evaluation of donor livers before transplantation. Transplantation. 1993;56:1403–1409. [DOI] [PubMed] [Google Scholar]
- 41.Urena MA, Ruiz-Delgado FC, Gonzalez EM, et al. Hepatic steatosis in liver transplant donors: common feature of donor population? World J Surg. 1998;22:837–844. [DOI] [PubMed] [Google Scholar]
- 42.Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–1725. [DOI] [PubMed] [Google Scholar]
- 43.Nomura F, Ohnishi K, Ochiai T, et al. Obesity-related nonalcoholic fatty liver: CT features and follow-up studies after low calorie diet. Radiology. 1987;27:108–113. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell DG. Focal manifestations of diffuse liver disease at MR imaging. Radiology. 1992;185:10–11. [DOI] [PubMed] [Google Scholar]
- 45.Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. [DOI] [PubMed] [Google Scholar]
- 46.Taylor M, Forster J, Langer B, et al. A study of prognostic factors for hepatic resection for colorectal metastases. Am J Surg. 1997;173:467–471. [DOI] [PubMed] [Google Scholar]
- 47.Finch MD, Crosbie JL, Currie E, et al. An 8-year experience of hepatic resection: indications and outcome. Br J Surg. 1998;85:315–319. [DOI] [PubMed] [Google Scholar]
- 48.Iwatsuki S, Starzl TE. Personal experience with 411 hepatic resections. Ann Surg. 1988;208:421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Imamura H, Seyama Y, Kokudo N, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–1206. [DOI] [PubMed] [Google Scholar]
- 50.Kubota K, Makuuchi M, Sugawara Y, et al. Reconstruction of the hepatic and portal veins using a patch graft from the right ovarian vein. Am J Surg. 1998;176:295–297. [DOI] [PubMed] [Google Scholar]
- 51.Little SA, Jarnagin WR, DeMatteo RP, et al. Diabetes is associated with increased perioperative mortality but equivalent long-term outcome after hepatic resection for colorectal cancer. J Gastrointest Surg. 2002;6:88–94. [DOI] [PubMed] [Google Scholar]
- 52.Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kooby DA, Fong Y, Suriawinata A, et al. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034–1044. [DOI] [PubMed] [Google Scholar]
- 54.Broelsch CE, Burdeleski M, Rogiers X, et al. Living donor for liver transplantation. Hepatology. 1994;20:49. [DOI] [PubMed] [Google Scholar]
- 55.Miller CM, Gondolesi GE, Florman S, et al. One hundred nine living donor liver transplants in adults and children: a single center experience. Ann Surg. 2001;3:301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bak T, Wachs M, Trotter J, et al. Adult-to-adult living donor liver transplantation using right-lobe-grafts: results and lessons learned from a single center experience. Liver Transplant. 2001;7:680–686. [DOI] [PubMed] [Google Scholar]
- 57.American Society of Transplant Surgeons Ethics Committee. Position paper on living-donor transplant. Liver Transplant. 2000;6:815. [DOI] [PubMed] [Google Scholar]
- 58.Soejima Y, Shimada M, Suehiro T. Use of steatotic graft in living-donor liver transplantation. Transplantation. 2003;76:344–348. [DOI] [PubMed] [Google Scholar]
- 59.Hayashi M, Fujii T, Kiuchi K, et al. Effects of fatty infiltration of the graft on the outcome of living-related liver transplantation. Transplant Proc. 1999;31:403. [DOI] [PubMed] [Google Scholar]
- 60.Ito T, Kiuchi T, Egawa H, et al. Surgery-related morbidity in living donors of right-lobe liver graft: lessons from the first 200 cases. Transplantation. 2003;76:158–163. [DOI] [PubMed] [Google Scholar]
- 61.Yoong KF, Gunson BK, Neil DAH, et al. Impact of donor liver microvesicular steatosis on the outcome of liver retransplantation. Transplant Proc. 1999;31:550–551. [DOI] [PubMed] [Google Scholar]
- 62.Shimada M, Matsumata T, Akazawa K, et al. Estimation of risk of major complications after hepatic resection. Am J Surg. 1994;164:399–403. [DOI] [PubMed] [Google Scholar]
- 63.Poon R, Sheung-Tat Fan MS, Wong J. Does diabetes mellitus influence the perioperative outcome or long term prognosis after resection of hepatocellular carcinoma? Am J Gastroenterol. 2002;97:1480–1488. [DOI] [PubMed] [Google Scholar]
- 64.Cruse RJ, Foord R. A five-year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107:206–210. [DOI] [PubMed] [Google Scholar]
- 65.Dindo D, Muller MK, Weber M, et al. Obesity in general elective surgery. Lancet. 2003;361:2032–2035. [DOI] [PubMed] [Google Scholar]
- 66.Tsukada K, Miyazaki T, Kato H, et al. Body fat accumulation and postoperative complications after abdominal surgery. Am Surg. 2004;70:347–351. [PubMed] [Google Scholar]
- 67.Fong D, Nehra V, Lindor K, et al. Metabolic and nutritional considerations in nonalcoholic fatty liver. Hepatology. 2000;32:3–10. [DOI] [PubMed] [Google Scholar]
- 68.Fisher R. Hepatobiliary abnormalities associated with total parenteral nutrition. Gastroenterol Clin North Am. 1989;18:645–666. [PubMed] [Google Scholar]
- 69.McGarry J, Foster D. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. [DOI] [PubMed] [Google Scholar]
- 70.Day C, James O. Hepatic steatosis: innocent bystander or guilty party? Hepatology. 1998;27:1463–1466. [DOI] [PubMed] [Google Scholar]
- 71.Pessayre D, Berson A, Fromety B, et al. Mitochondria in steatohepatitis. Semin Liver Dis. 2001;21:57–69. [DOI] [PubMed] [Google Scholar]
- 72.Yang S, Zhu H, Lane M, et al. Obesity increases sensitivity to endotoxin liver injury; implication for the pathogenesis of steatohepatitis. Proc Natl Acad Sci USA. 1997;94:2557–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neushwander-Tetri BA, Caldwell AH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology. 2003;37:1202–1219. [DOI] [PubMed] [Google Scholar]
- 74.Fan C-Y, Pan J, Usuda N, et al. Steatohepatitis, spontaneous peroxisome proliferation and liver tumors in mice lacking peroxisomal fatty acyl-CoA oxidase: implications for peroxisomal proliferator activated receptor alpha natural ligand metabolism. J Biol Chem 1998;273:15639–15645. [DOI] [PubMed] [Google Scholar]
- 75.Weltman M, Farrell G, Liddle C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology. 1996;111:1645–1653. [DOI] [PubMed] [Google Scholar]
- 76.Leclerq I, Farrell G, Field J, et al. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxidation in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105:1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Strauss RS, Barlow SE, Dietz WH. Prevalence of abnormal serum aminotransferase values in overweight and obese adolescents. J Pediatr. 2000;136:727–733. [PubMed] [Google Scholar]
- 78.Yang S, Zhu H, Li H, et al. Mitochondrial adaptations to obesity-related oxidative stress. Arch Biochem Biophys. 2000;378:259–268. [DOI] [PubMed] [Google Scholar]
- 79.Rashid A, Wu TC, Huang C, et al. Mitochondrial proteins that regulate apoptosis and necrosis are induced in mouse fatty liver. Hepatology. 1999;29:1131–1138. [DOI] [PubMed] [Google Scholar]
- 80.Reddy JK, Goel SK, Nemali MR, et al. Transcriptional regulation of peroxisomal fatty acyl-CoA oxidase and enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase in rat liver by peroxisomal proliferators. Proc Natl Acad Sci USA. 1986;83:1747–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marchesini G, Brizi M, Morselli-Labate AM, et al. Metformin in nonalcoholic steatohepatitis. Lancet. 2001;358:893–894. [DOI] [PubMed] [Google Scholar]
- 82.Rinella ME, Green RM. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J Hepatol. 2004;40:47–51. [DOI] [PubMed] [Google Scholar]
- 83.Marcos A, Fisher R, Ham JM, et al. Liver regeneration and function in donor and recipient after right lobe adult to adult living donor liver transplantation. Transplantation. 2000;69:1375–1379. [DOI] [PubMed] [Google Scholar]
- 84.Clavien PA, Harvey PR, Strasberg SM. Preservation and reperfusion injuries in liver allografts: an overview and synthesis of current studies. Transplantation. 1992;53:957–978. [DOI] [PubMed] [Google Scholar]
- 85.Huguet C, Gavelli A, Chieco PA, et al. Liver ischemia for hepatic resection: where is the limit? Surgery. 1992;111:251–259. [PubMed] [Google Scholar]
- 86.Caldwell-Kinkel JC, Currin RT, Tanaka Y, et al. Reperfusion injury to endothelial cells following cold storage of rat liver. Hepatology. 1989;10:292–299. [DOI] [PubMed] [Google Scholar]
- 87.Jaeschke H. Mechanism of reperfusion injury after warm ischemia of the liver. J Hepatobiliary Pancreat Surg. 1998;21:402–408. [DOI] [PubMed] [Google Scholar]
- 88.Lemasters JJ. The mitochondrial permeability transition and the calcium, oxygen and pH paradoxes: one paradox after another. Cardiovasc Res. 1999;44:470–473. [DOI] [PubMed] [Google Scholar]
- 89.Pannen BH, Al-Adili F, Bauer M, et al. Role of endothelins and nitric oxide in hepatic reperfusion injury in the rat. Hepatology. 1998;27:755–764. [DOI] [PubMed] [Google Scholar]
- 90.Sun CK, Zhang XY, Zimmermann A, et al. Effect of ischemia-reperfusion injury on the microcirculation of the steatotic liver of Zucker rat. Transplantation. 2001;72:1625–1631. [DOI] [PubMed] [Google Scholar]
- 91.Seifalian AM, Piasecki C, Agarwal A, et al. The effect of graded steatosis on the flow in the hepatic parenchymal microcirculation. Transplantation. 1997;68:780–784. [DOI] [PubMed] [Google Scholar]
- 92.Sato N, Eguchi H, Inoue A, et al. Hepatic microcirculation in Zucker fatty rats. Adv Exp Med Biol. 1986;200:477–483. [DOI] [PubMed] [Google Scholar]
- 93.Teramoto K, Bowers JL, Kruskal JB, et al. Hepatic microcirculatory changes after reperfusion in fatty and normal liver transplantation in the rat. Transplantation. 1993;56:1076–1082. [DOI] [PubMed] [Google Scholar]
- 94.Hayashi M, Tokunaga Y, Fujita T, et al. The effects of cold preservation on steatotic graft viability in rat liver transplantation. Transplantation. 1993;56:282–296. [DOI] [PubMed] [Google Scholar]
- 95.McCuskey RS, Ito Y, Robertson GR, et al. Hepatic microvascular dysfunction during evolution of dietary steatohepatitis in mice. Hepatology. 2004;40:386–393. [DOI] [PubMed] [Google Scholar]
- 96.Selzner M, Rudiger HA, Sindram D, et al. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology. 2000;32:1280–1288. [DOI] [PubMed] [Google Scholar]
- 97.Hakamada K, Sasaki M, Takahashi K, et al. Sinusoidal flow block after warm ischemia in rat fed diet-induced fatty liver. J Surg Res. 1997;70:12–20. [DOI] [PubMed] [Google Scholar]
- 98.Rosser BG, Gores GJ. Liver cell necrosis: cellular mechanisms and clinical implications. Gastroenterology. 1995;108:252–275. [DOI] [PubMed] [Google Scholar]
- 99.Domenicali M, Vendemiale G, Serviddio G, et al. Oxidative injury in rat fatty liver exposed to ischemia-reperfusion is modulated by nutritional status. Dig Liver Dis. 2005;37:689–697. [DOI] [PubMed] [Google Scholar]
- 100.Cortez-Pinto H, Chatham J, Chacko VP, et al. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: a pilot study. JAMA. 1999;282:1659–1664. [DOI] [PubMed] [Google Scholar]
- 101.Chavin K, Yang S, Lin H, et al. Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion. J Biol Chem. 1999;274:5692–5700. [DOI] [PubMed] [Google Scholar]
- 102.Fukumori T, Ohkochi N, Tsukamoto S, et al. Why fatty liver is unsuitable for transplantation? Deterioration of mitochondrial ATP synthesis and sinusoidal structure during cold preservation of a liver with steatosis. Transplant Proc. 1997;29:412–415. [DOI] [PubMed] [Google Scholar]
- 103.Sanyal AJ, Campbell-Sargent C, Mirshahi F, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1196. [DOI] [PubMed] [Google Scholar]
- 104.McCuskey RS, Ito Y, Robertson GR, et al. Hepatic microvascular dysfunction during evolution of dietary steatohepatitis in mice. Hepatology. 2004;40:386–393. [DOI] [PubMed] [Google Scholar]
- 105.Wanner GA, Ertel W, Muller P, et al. Liver ischemia and reperfusion induces a systemic inflammatory response through Kupffer cell activation. Shock. 1996;5:34–40. [DOI] [PubMed] [Google Scholar]
- 106.Tsoulfas G, Takahashi Y, Ganster RW, et al. Activation of the lipopolysaccharide signaling pathway in hepatic transplantation preservation injury. Transplantation. 2002;74:7–13. [DOI] [PubMed] [Google Scholar]
- 107.Yadav SS, Howell DN, Steeber DA, et al. P-selectin mediates reperfusion injury through neutrophil and platelet sequestration in the warm ischemic mouse liver. Hepatology. 1999;29:1494–1502. [DOI] [PubMed] [Google Scholar]
- 108.Nakano H, Nagasaki H, Barama A, et al. The effects of n-acetylcysteine and anti-intercellular adhesion molecule-1 monoclonal antibody against ischemia-reperfusion injury of the steatotic liver produced by a choline-methionine-deficient diet. Hepatology. 1997;26:670–678. [DOI] [PubMed] [Google Scholar]
- 109.Hakamada K, Sasaki M, Takahashi K, et al. Sinusoidal flow block after warm ischemia in rats with diet-induced fatty liver. J Surg Res. 1997;70:12–20. [DOI] [PubMed] [Google Scholar]
- 110.Yang S, Lin HZ, Mandal AK, et al. Disrupted signaling and inhibited regeneration in obese mice with fatty livers: implications for nonalcoholic fatty liver disease pathophysiology. Hepatology. 2003;34:694–706. [DOI] [PubMed] [Google Scholar]
- 111.Torbenson M, Yang SQ, Liu HZ, et al. STAT-3 overexpression and p21 up-regulation accompany impaired regeneration of fatty livers. Am J Pathol. 2002;161:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang S, Lin H, Diehl AM. Fatty liver vulnerability to endotoxin-induced damage despite NF-kappaB induction and inhibited caspase 3 activation. Am J Physiol. 2001;281:G382–G392. [DOI] [PubMed] [Google Scholar]
- 113.Taub R, Greenbaum LE, Peng Y. Transcriptional regulatory signals define cytokine-dependent and -independent pathways in liver regeneration. Semin Liver Dis. 1999;19:117–127. [DOI] [PubMed] [Google Scholar]
- 114.Cressman DE, Greenbaum LE, DeAngelis RA, et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–1383. [DOI] [PubMed] [Google Scholar]
- 115.Selzner M, Clavien PA. Failure of regeneration of the steatotic rat liver: disruption at two different levels in the regeneration pathway. Hepatology. 2000;31:35–42. [DOI] [PubMed] [Google Scholar]
- 116.Darnell JE Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. [DOI] [PubMed] [Google Scholar]
- 117.Ihle JN. Cytokine receptor signaling. Nature. 1995;377:591–594. [DOI] [PubMed] [Google Scholar]
- 118.Cressman DE, Diamond RH, Taub R. Rapid activation of the Stat3 transcription complex in liver regeneration. Hepatology. 1995;21:1443–1449. [PubMed] [Google Scholar]
- 119.Lee DG, Bell SP. ATPase switches controlling DNA replication initiation. Curr Opin Cell Biol. 2000;12:280–285. [DOI] [PubMed] [Google Scholar]
- 120.Vendemiale G, Grattagliano I, Caraceni P, et al. Mitochondrial oxidative injury and energy metabolism alteration in fatty liver: effect of nutritional status. Hepatology. 2001;33:808–815. [DOI] [PubMed] [Google Scholar]
- 121.Eguchi Y, Shimazu S, Tsujimoto Y. Intracellular ATP levels determine cell death by apoptosis and necrosis. Cancer Res. 1997;57:1835–1840. [PubMed] [Google Scholar]
- 122.Leclercq IA, Field J, Farrell GC. Leptin-specific mechanisms for impaired liver regeneration in ob/ob mice after toxic injury. Gastroenterology. 2003;124:1451–1464. [DOI] [PubMed] [Google Scholar]
- 123.Green RM. NASH: hepatic metabolism and not simply the metabolic syndrome. Hepatology. 2003;38:14–17. [DOI] [PubMed] [Google Scholar]
- 124.Yamagami K, Enders G, Schauer RJ, et al. Heat-shock preconditioning protects fatty livers in genetically obese Zucker rats from microvascular perfusion failure after ischemia reperfusion. Transpl Int. 2003;16:456–463. [DOI] [PubMed] [Google Scholar]
- 125.Choi S, Noh J, Hirose R, et al. Mild hypothermia provides significant protection against ischemia/reperfusion injury in livers of obese and lean rats. Ann Surg. 2005;241:470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Peralta C, Hotter G, Closa D, et al. Protective effect of ischemic preconditioning on the injury associated to hepatic ischemia-reperfusion in the rat: role of nitric oxide and adenosine. Hepatology. 1997;25:934–937. [DOI] [PubMed] [Google Scholar]
- 127.Serafin A, Rosello-Catafau J, Prats N, et al. Ischemic preconditioning increases the tolerance of fatty liver to hepatic ischemia-reperfusion injury in the rat. Am J Pathol. 2002;161:587–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kulinski A, Vance DE, Vance JE. A choline-deficient diet in mice inhibits neither the CDP-choline pathway for phosphatidylcholine synthesis in hepatocytes nor apolipoprotein B secretion. J Biol Chem. 2004;4:23916–23924. [DOI] [PubMed] [Google Scholar]
- 129.Schemmer P, Schoonhoven R, Swenberg JA, et al. Gentle organ manipulation during harvest as a key determinant of survival of fatty livers after transplantation in the rat. Transplant Int. 1999;12:351–359. [DOI] [PubMed] [Google Scholar]
- 130.Takahashi K, Hakamada K, Totsuka E, et al. Warm ischemia and reperfusion injury in diet-induced canine fatty livers. Transplantation. 2000;27:2028–2034. [DOI] [PubMed] [Google Scholar]
- 131.Spannbauer MM, Oleszczuk A, Tannapfel A, et al. Micro- and macrovesicular steatotic liver model for transplantation induced by ethanol and protein-deficient diet. Transplant Proc. 2005;1:210–211. [DOI] [PubMed] [Google Scholar]
- 132.Yin W, Liao D, Kusunoki M, et al. NO-1886 decreases ectopic lipid deposition and protects pancreatic beta cells in diet-induced diabetic swine. J Endocrinol. 2004;180:399–408. [DOI] [PubMed] [Google Scholar]
- 133.Clavien PA, Selzner M, Rudiger HA, et al. A prospective, randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238:845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]


