Abstract
Objective:
To identify sociodemographic and clinical predictors of patient selection in bariatric surgery.
Summary Background Data:
Population-based studies suggest that bariatric surgery patients are disproportionately privately insured, middle-aged white women. It is uncertain whether such disparities are due to surgeon decisions to operate, differences among morbidly obese individuals in access to surgery, or patients’ personal preferences regarding surgical treatment.
Methods:
We conducted a national survey of 1343 U.S. bariatric surgeons. The questionnaire contained clinical vignettes generated using a balanced fractional factorial design. For each of 3 hypothetical patients unique in age, race, gender, body mass index (BMI), comorbidities, social support, functional status, and insurance, respondents were asked if they would operate. Logistic regression was used to determine the odds of selection for each characteristic while controlling for the other 7 characteristics. Subset analyses were also performed using combinations of BMI and comorbidities.
Results:
A total of 62.5% of eligible surgeons responded (n = 820). Patient race did not influence surgeon decisions to operate. Hypothetical patient age, BMI, and social support were most influential. In the subgroup of patients who did not meet current NIH BMI and comorbidity criteria for bariatric surgery, male sex (odds ratio [OR], 0.33; 95% confidence interval [CI], 0.14–0.76) was associated with decreased odds of selection. Overall, younger age (OR, 0.09; 95% CI, 0.07–0.11), older age (OR, 0.70; 95% CI, 0.56–0.90), limited functional status (OR, 0.66; 95% CI, 0.52–0.82), poor social support (OR, 0.37; 95% CI, 0.30–0.47), self-pay (OR, 0.72; 95% CI, 0.57–0.91), and public insurance (OR, 0.54; 95% CI, 0.43–0.67) were associated with decreased odds of selection. BMI and comorbidity criteria influenced the magnitude of these effects.
Conclusions:
Patient race did not play a role in surgeon decisions to operate. Further research should examine the roles of unequal access to bariatric surgery and differing socio-cultural perceptions of morbid obesity on racial disparities. The influence of patient age, gender, insurance status, social support, and functional status on decisions to operate was mitigated by BMI and comorbidities. Policy-makers currently debating BMI and comorbidity criteria for bariatric surgery should also consider guidelines pertaining to these sociodemographic issues that influence patient selection in bariatric surgery.
Population-based studies have shown racial, gender-based, and socioeconomic disparities among bariatric surgery patients. A national survey of U.S. bariatric surgeons using clinical vignettes reveals that age, body mass index, and social support are the chief determinants of patient selection for bariatric surgery. Comorbidities, functional status, and payment source influence surgeon decisions to a lesser extent with little or no influence from patient sex or race.
Morbid obesity is a public health crisis in the United States. The National Institutes of Health (NIH) defines morbid obesity as a body mass index (BMI) between 35 kg/m2 and 40 kg/m2 with obesity-related comorbidities such as diabetes, hypertension, and obstructive sleep apnea, or as a BMI greater than 40 kg/m2 with or without comorbidities.1 Population-based estimates of the prevalence of morbid obesity have ranged from 2.8% to 5.1%.2,3 Based on these estimates, well over 5 million Americans meet NIH clinical criteria for bariatric surgery.
The sociodemographic characteristics of morbidly obese Americans, however, do not match those of the bariatric surgery patient population in the United States. Livingston and Ko demonstrated that blacks, lower income groups, less educated groups, and publicly insured patients were underrepresented among the bariatric surgery patient population.2 Women disproportionately utilize bariatric surgery relative to sex-specific prevalence of morbid obesity.4–7 Nationally, the bariatric surgery patient population from 1998 to 2002 was increasingly likely to be female, privately insured, and from the highest income bracket.8
Although the sources of sociodemographic disparities in the prevalence of obesity have been extensively studied and debated,9–13 the sources of sociodemographic disparities in bariatric surgery utilization have not been examined. Differential access to health care, variation in patient valuation of health and appearance, or divergent practice styles when surgeons select patients for surgery may contribute to these observations. To examine the latter possibility, we conducted a national survey of bariatric surgeons using clinical vignettes to determine the influence of patient sociodemographic and clinical factors on surgeon decisions to perform bariatric surgery.
METHODS
The study was approved by the University of Chicago Institutional Review Board.
Survey Development and Content
Survey content was based on extended open-ended interviews, until theme saturation, with community and university bariatric surgeons (n = 12) and an expert focus group (n = 6). Community and university bariatric surgeons (n = 8) and health services researchers (n = 12) pilot tested the survey. The final survey contained questions about respondent characteristics, preoperative selection criteria, and methods of patient evaluation. It also included 3 clinical vignettes that are the subject of this paper.
The vignettes were structurally identical and asked if the respondent would operate given a hypothetical patient’s age, race, sex, BMI, comorbidities, functional status, social support, and payment source. Table 1 shows the values that for each trait. Referent values were chosen to be the most common category based on reports of bariatric surgery patient demographics,2,4–8,14 clinical criteria within current NIH guidelines that imply minimal clinical risk,1 and idealized social parameters.15,16 Qualitative interviews and pilot testing identified comparison values that were unlikely to be considered absolute contraindications to surgery and would elicit data on physician decisions to operate in the face of clinical uncertainty.
TABLE 1. Characteristics for Variables Used in Vignettes
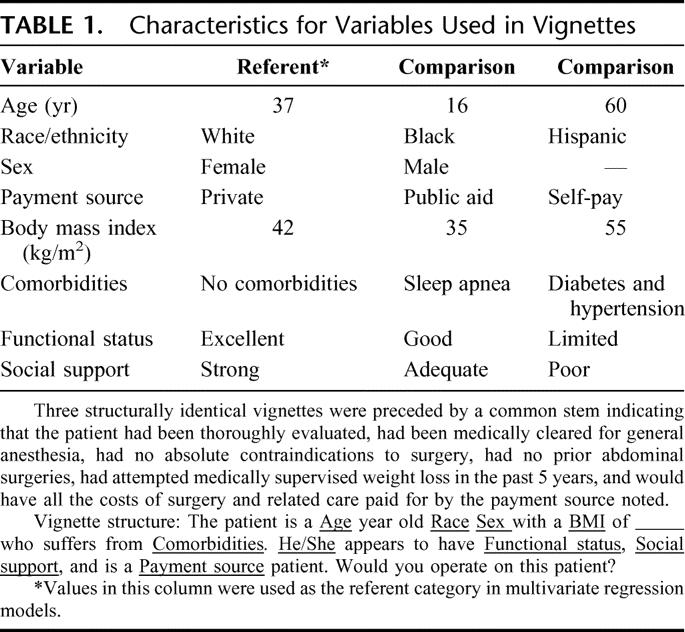
To minimize social desirability bias, hypothetical patient characteristics were randomly varied across all 3 vignettes using a fractional factorial design that allows for pairwise balance between covariates such that no patient characteristic value was over-represented in our sample.17,18 According to this design, each respondent had a totally unique set of vignettes, and no characteristic value (except sex) was duplicated in the set. There were over 3 million unique hypothetical patients for respondents to consider for bariatric surgery. To ensure that respondents based their decisions on the types of operations they were prepared to offer, they were asked which procedure they would perform if the decision to operate was “yes.” To indicate that the hypothetical patient was otherwise eligible for surgery and force respondents to seriously consider the patient’s characteristics, vignettes were also preceded by a set of common assumptions about the patient’s medical appropriateness for surgery and ability to cover all costs of surgery and related care with the payment source noted.
Respondent Identification and Recruitment
All active surgeon members of the American Society of Bariatric Surgery (ASBS) as of August 2004 (n = 1161) were selected as our first pool of potential respondents. Surgical residents and fellows as well as practicing surgeons who were not yet full members of the ASBS were excluded. Then, to identify a representative sample of surgeons performing bariatric procedures who were not in the ASBS, a postcard asking whether or not the respondent performed bariatric surgery was sent in 2 waves to a 50% random sample of all U.S. general surgeons (n = 10,500) listed on the March 2004 American Medical Association Physician Masterfile (AMA Masterfile). Of the 2855 responders (response rate = 27.2%), 12.5% reported to be practicing bariatric surgeons (n = 358). Only half belonged to the ASBS.19 A follow-up telephone survey of 100 postcard nonresponders revealed that only 4% were practicing bariatric surgeons. Since bariatric surgeons were over-represented in our postcard responders, we elected not to conduct a third wave of mailing since it would have yielded few additional bariatric surgeons to survey. After combining ASBS members with the bariatric surgeons identified through the postcard mailing, there were a total of 1343 bariatric surgeons to survey; 82.4% were ASBS members.
From September 2004 to December 2004, the questionnaire was sent in 3 waves to these 1343 bariatric surgeons. The first wave included a small incentive, a laser pointer pen.
Statistical Analysis
For analytic purposes, each hypothetical patient was treated as an individual observation. The outcome of interest was whether or not the respondent chose to operate. Since a single respondent was responsible for the outcome of 3 observations, we used population averaged logistic regression (estimated with Generalized Estimation Equations with robust standard errors [GEE]) to analyze responses.20 The model generated estimates and standard errors for operating on a hypothetical patient across all observations and all surgeons while appropriately accounting for correlation across respondents. It allowed us to determine the independent effect of each characteristic on the odds of patient selection across the population of bariatric surgeons represented by our respondents. We calculated the odds ratios for selection of hypothetical patients for bariatric surgery given any single characteristic value while controlling for the other 7 characteristics in the model. We also determined the overall influence of each characteristic on surgeon decision to operate. We tested all possible 2-way interactions between covariates but none were significant.
Next, we analyzed subsets of hypothetical patients based on BMI and comorbidity combinations to better understand how patient characteristics influenced surgeon decisions to operate when BMI and comorbidity levels did and did not meet current NIH recommendations. Since each patient characteristic was only observed once by each respondent due to vignette design, these subset analyses did not have to account for correlation across respondents and were performed using ordinary logistic regression. However, these subset estimates could be compared with estimates from the full data analysis using GEE because the interpretation of point estimates is equivalent between GEE and ordinary logistic regression.
Regression analyses were weighted to account for the sampling frame. Respondents identified through the postcard mailing (a 50% random sample of U.S. general surgeons) were given a probability weight of 2, while those identified solely from the ASBS membership list were given a probability weight of 1. Analyses were performed using Stata/SE v.9 statistical package (Stata Corp., College Station, TX).
RESULTS
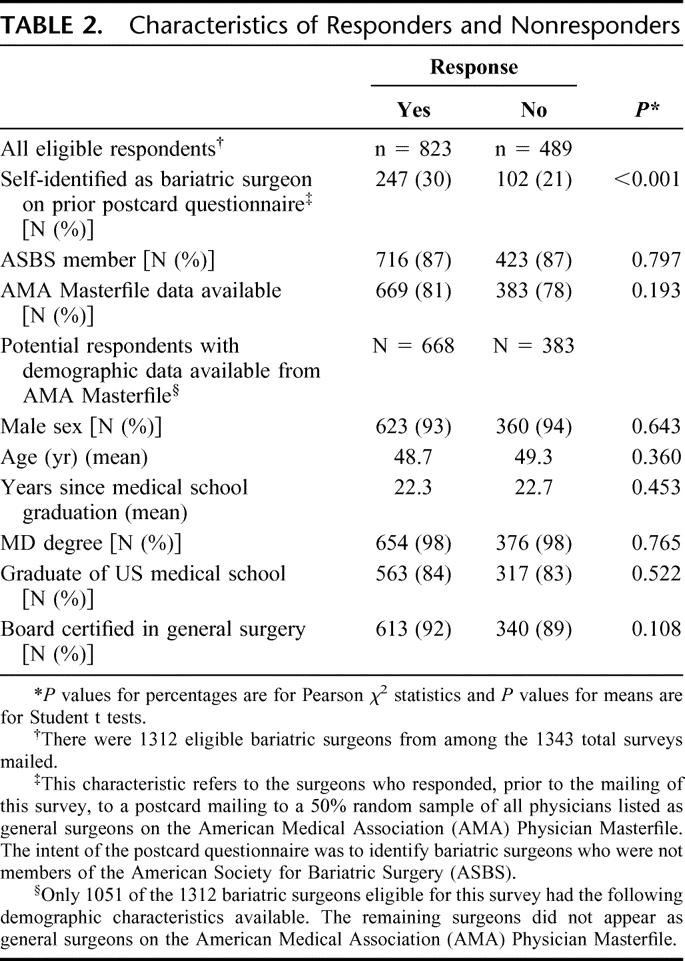
Of the 1343 surveys mailed, 31 (2.3%) were ineligible. Six (0.5%) were returned due to invalid or duplicate addresses, 19 (1.4%) were returned by retired surgeons, and 6 (0.5%) were returned by surgeons not yet practicing bariatric surgery. Among the 1312 eligible respondents, 80.1% had demographic data available from the AMA Masterfile. The response rate was 62.7% (823 of 1312); 26.6% of responders had also responded to the postcard mailing; 86.8% of responders were ASBS members. Table 2 compares responders with nonresponders. They only differed with respect to whether or not they had also responded to the postcard mailing (Pearson χ2, P value <0.001). There was no difference in ASBS membership, classification as a general surgeon on the AMA Masterfile, or demographic characteristics when such data were available for comparison.
TABLE 2. Characteristics of Responders and Nonresponders
Of the total respondents, 5 did not respond to any of the vignettes while 3 responded with marginal comments of “maybe.” These 18 vignettes were not analyzed, leaving a total of 2451 hypothetical patients from 818 respondents. Surgeons recommended bariatric surgery for 1417 hypothetical patients (57.8%). Table 3 shows the types of procedures they recommended. The majority (53%) recommended laparoscopic Roux-en-Y gastric bypass.
TABLE 3. Types of Bariatric Procedures Recommended for Hypothetical Patients (n = 1417) Selected for Surgery
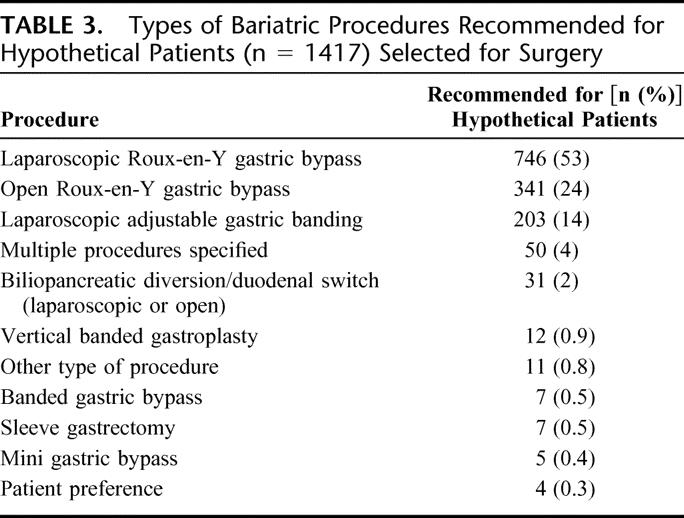
Figure 1 shows the probabilities of selection for surgery based on age, BMI, and comorbidities controlling for race, sex, functional status, social support, and payment source. Presence of comorbidities and increasing BMI increased the likelihood of selection. Notably, the rate of selecting patients with a BMI of 35 kg/m2 without comorbidities was 6% to 44% depending on age group. Similarly, for 16-year-olds without comorbidities, the rate of selection was 6% to 24% depending on BMI category.
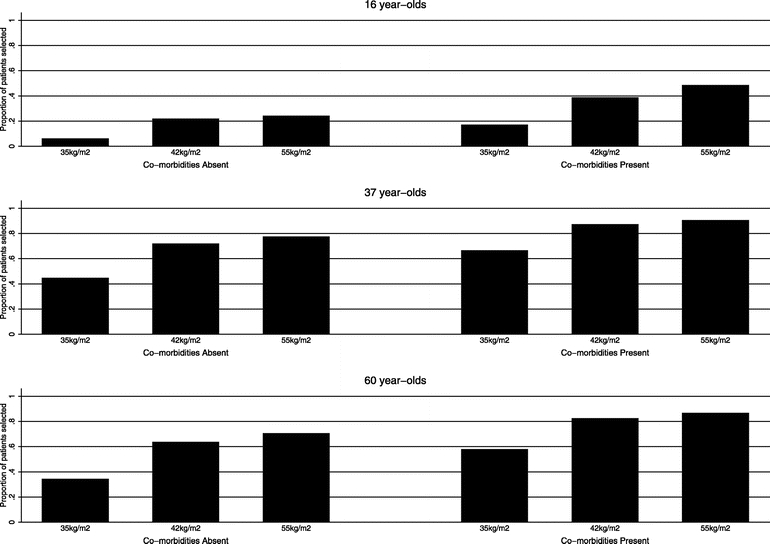
FIGURE 1. Rates of patient selection by age, BMI, and comorbidity groups. Rates are adjusted for patient race, sex, functional status, social support, and payment source.
Table 4 shows the adjusted odds ratios and probabilities for recommending bariatric surgery based on values of patient characteristics and the overall significance of each characteristic in surgeon decision-making. Younger age decreased the odds of selection more than older age. Public insurance decreased the odds of selection more than self-pay status. The combination of diabetes and hypertension increased the odds of selection more than sleep apnea alone. For the ordinal measures of functional status and social support, there was a threshold effect; only the lowest level significantly decreased the odds of selection. Age, BMI, and social support were the most influential factors. Sex and race did not influence surgeon decisions to operate.
TABLE 4. Patient Characteristics and Selection for Bariatric Surgery (n = 2448)
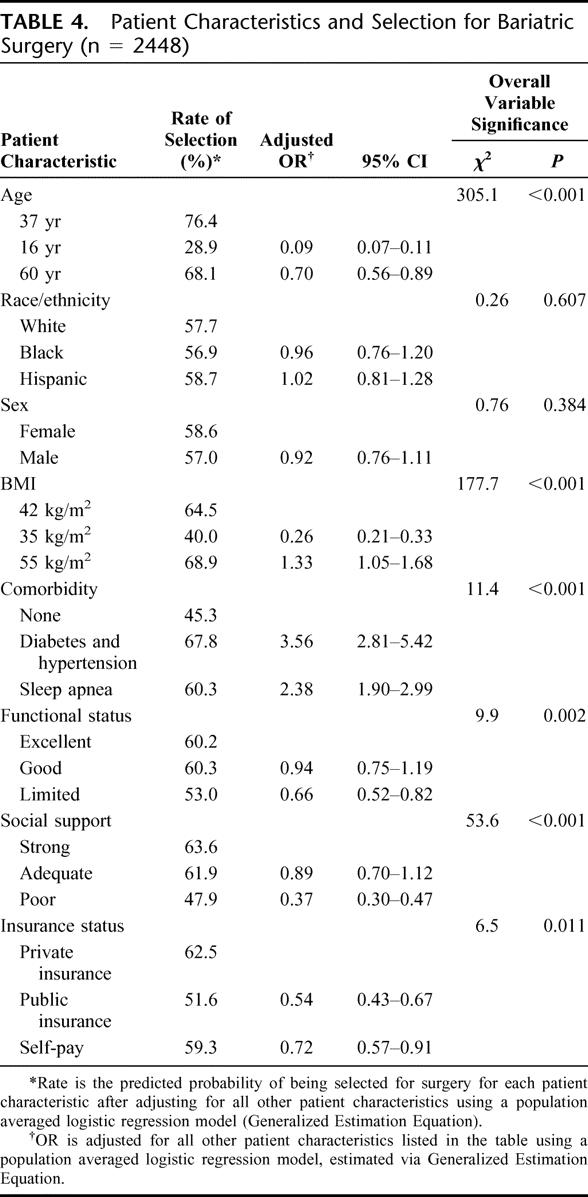
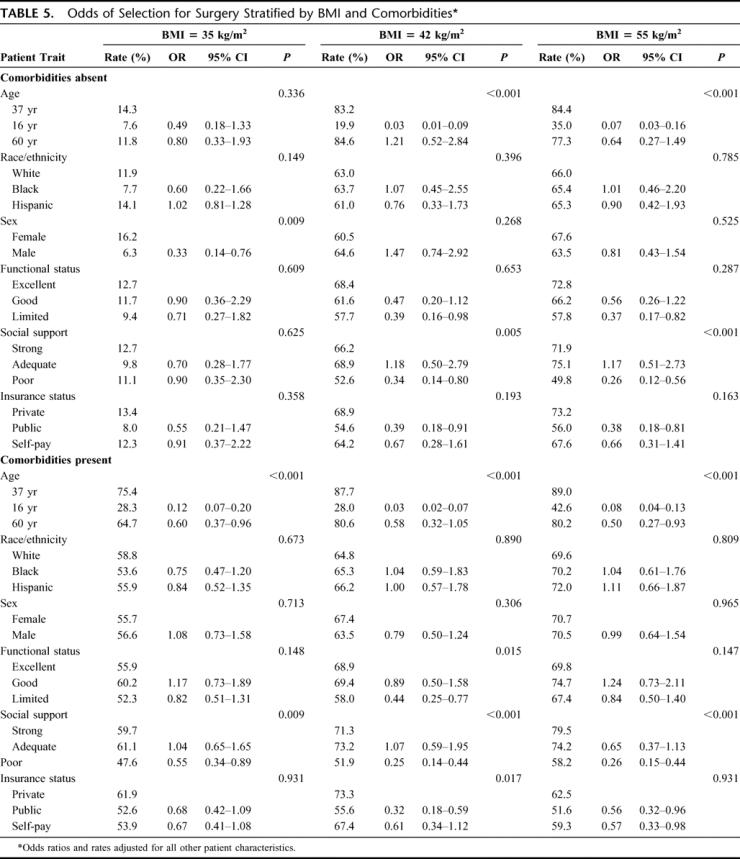
Table 5 shows the adjusted odds ratios and probabilities for recommending bariatric surgery when patients were stratified according to BMI and comorbidities. Race still did not influence patient selection. Sex influenced patient selection in the subgroup of patients with a BMI of 35 kg/m2 without comorbidities, with men having 67% decreased odds of selection. The odds of selection for this subgroup was unaffected by poor social support, a factor that was significant in all other subgroups. The odds of selection were substantially decreased among 16-year-olds compared with 37-year-olds in all BMI/comorbidity subgroups, but for 60-year-olds only in the presence of comorbidities. Public insurance did not impact the odds of selection among those with the lowest BMI level but did decrease the odds of selection for all other subgroups. Functional status and self-pay did not predictably affect the odds of selection in any subgroup.
TABLE 5. Odds of Selection for Surgery Stratified by BMI and Comorbidities
DISCUSSION
Through a series of vignettes that required respondents to consider patients’ demographic, clinical, functional, and socioeconomic characteristics, we found that age, BMI, and social support are the chief determinants of patient selection for bariatric surgery. Comorbidities, functional status, and payment source influence surgeon decisions to a lesser extent, with little or no influence from patient sex or race. However, BMI and comorbidities do mitigate the effect of patient functional and sociodemographic characteristics on surgeon decisions to operate.
Surgeon discretion on the basis of age was found to parallel current uncertainties within the field of bariatric surgery about operating at the extremes of age. Our finding that 16-year-olds were rarely selected shows that surgeons generally follow guidelines that adolescents should undergo bariatric surgery only in the presence of comorbidities and at pediatric specialty centers.21–23 However, it is concerning that 6% to 24% of 16-year-olds without comorbidities were selected for surgery by our sample that included only 2 pediatric surgeons.
Studies examining the relationship between older age and perioperative risks are mixed, with some showing that patients over 50 have higher rates of morbidity and mortality24,25 and others reporting no such differences.26–28 Our finding that 60-year-olds were less likely to be selected than 37-year-olds suggests that our respondents also perceive older age to be a marker of increased clinical risk. However, 68% of the patients in the older group were selected, indicating that the majority of our respondents still felt that the weight loss and health benefits of bariatric surgery warrant operative intervention in older patients despite the potential increased risk of morbidity and mortality. In contrast, surgeons do not seem to perceive comorbidities as markers of increased risk. Previous studies have shown higher rates of perioperative complications and mortality in patients with diabetes, hypertension, and sleep apnea.29–31 However, we found that these comorbidities increased the odds of patient selection. This is likely because most bariatric surgery guidelines include obesity-related comorbidities as criteria for patient selection.1,32–37
Functional status worsens with increasing BMI,38 and bariatric surgery results in substantial improvements in functional status.39,40 Thus, it was surprising that poor functional status decreased the odds of selection in the presence of comorbidities. Our respondents may believe that inability to ambulate postoperatively increases the risk of pulmonary embolism, the most frequent cause of perioperative mortality in bariatric surgery patients.37 They may also believe that decreased ability to exercise after surgery contributes to unsatisfactory long-term weight loss. Given that limitations in functional status are an important adverse consequence of morbid obesity, it is concerning that diminished functional status caused risk aversion among our respondents when the presence of medically defined comorbidities did not. Bariatric surgery programs that incorporate intensive preoperative and postoperative rehabilitation may better prevent complications related to decreased mobility and increase the numbers of patients with limited functional status who are selected for surgery.
According to current NIH recommendations, patients with BMI of 35 kg/m2 without comorbidities are not candidates for bariatric surgery.1 Thus, it was not surprising that patients in this group were least likely to be selected for surgery. However, they were still selected over 10% of the time across all age groups and as frequently as 44% of the time in the 37-year-old age group. Respondents who chose to operate on these patients might be in agreement with recent arguments to reduce the lower limit BMI threshold for bariatric surgery or eliminate the comorbidity requirement at lower BMI levels.32–34,41
Male sex has been shown to be a predictor of adverse outcomes after bariatric surgery.24,30 However, in all BMI and comorbidity subgroups that meet current NIH clinical guidelines, sex did not influence patient selection in our study. Therefore, surgeon discretion is unlikely to be the cause of the overrepresentation of women among the bariatric surgery patient population as described in epidemiologic reports.6,8,14 These studies’ findings that upwards of 80% of patients are female are more likely attributable to sociocultural norms about body size that encourage women to seek bariatric surgery out of proportion to men who have the same BMI and comorbidities. Unfortunately, these population-based studies lack BMI and comorbidity data with which to judge the clinical indications for bariatric surgery. We found that sex affected patient selection only in the subgroup of adult patients who did not meet the minimum NIH BMI and comorbidity criteria for bariatric surgery. This suggests that, in the absence of strong clinical indications, surgeons too may be consciously or subconsciously influenced by sociocultural pressure on women to be thin.
We found that poor social support strongly decreased the odds of selection for all BMI and comorbidity subgroups within current NIH clinical guidelines. It appears that bariatric surgeons’ experience regarding the importance of social support is consistent with research indicating that social support predicts successful weight loss after surgery.15,16 Social isolation increases with higher BMI,42–44 yet social isolation itself appears to be a contraindication for surgery among bariatric surgeons. Prospective patients meeting other criteria for bariatric surgery but lacking adequate social support may benefit from patient advocates, a “buddy system,” and variations on the widely used support group that might enable otherwise isolated individuals to achieve durable weight loss.
NIH BMI and comorbidity criteria are usually strictly enforced by most private insurers who deny coverage to those who do meet these criteria.45–47 We had hypothesized that prospective patients’ ability to pay out of pocket might, therefore, motivate patient selection. For patients who would be covered by insurance, self-pay decreases paperwork and likely results in higher total compensation for the surgeon. However, our respondents still chose privately insured patients more often than self-pay patients. Importantly, self-pay status did not increase odds of selection for patients who did not meet current NIH clinical criteria, indicating that financial opportunity did not motivate our respondents to operate in the absence of clinical indications.
We also found that private insurance was consistently favored over public insurance. Given the disproportionate reliance on public insurance by the morbidly obese,2 it is worrisome that this payment source is a negative predictor of selection for bariatric surgery. Our respondents may have been less likely to select publicly insured patients based on previous reports linking low socioeconomic status and public insurance with worse outcomes after bariatric surgery.15,48 However, our respondents may instead agree with the findings of Durkin et al that insurance status does not predict weight loss after surgery.49 It is possible that some respondents still did not select public insured patients because they doubted the adequacy of public insurance to cover the costs of surgery and related care even though it was included in the set of assumptions that preceded the vignettes. Whether due to concern about adverse outcomes or inadequate compensation, these results suggest that surgeon discretion plays a role in socioeconomic disparities in bariatric surgery.
Our study has some important limitations. First, we did not directly observe surgeons choices when selecting patients for bariatric surgery, and we required respondents to accept a set of assumptions that may not hold in actual practice. Direct observation would not have achieved significant sample sizes for the characteristics found less frequently in the current bariatric surgery patient population. Vignettes, however, have been shown to be a valid proxy for actual physician behavior through comparison with standardized patients and chart abstraction.50 Self-report would have been prone to socially desirability bias given the influence of patient sex, race, and socioeconomic status on physician decision-making.51 Appropriately constructed vignettes, however, can minimize social desirability bias and measure physician reactions to patient case-mix.52 A strength of the balanced fractional factorial design, in particular, is that it allows for the introduction of a larger number of covariates than in other types of vignettes.53 As confirmed by pilot testing of the survey instrument, combining social support, functional status, clinical criteria, and sociodemographic characteristics into a single vignette masked the variables that might trigger socially desirable responses. A number of other researchers have successfully used vignettes to study physician response to patient sociodemographic characteristics.54–59
Another limitation was that we tested a single socioeconomic variable, payment source. Insurance status correlates highly with physician treatment recommendations in primary care settings and has been used as a proxy for socioeconomic status in the bariatric surgery literature.48,49,54 We asked open-ended questions regarding patient socioeconomic factors during survey development and pilot-tested vignettes with a variety of proxies, including education level, income, and occupation. Based on this preliminary work, we concluded that payment source was the strongest proxy for socioeconomic status among practicing bariatric surgeons. Interestingly, we found that, among bariatric surgeons, “self-pay” does not suggest an indigent, uninsured patient but rather a person with the financial resources to pay out of pocket.
Our response rate of 62% was higher than most physician surveys.60 This high response rate and lack of demographic differences among responders and nonresponders suggest that our results are generalizable to the population of U.S. bariatric surgeons. However, our respondent pool included a majority of ASBS members, and specialty society memberships have been shown to correlate with variation in surgeon beliefs.61 Since we were concerned about bias related to ASBS membership, we previously attempted to identify bariatric surgeons who did not choose to belong to the ASBS through a postcard mailing to a 50% random sample of all U.S. general surgeons. While we appropriately weighted our analyses for this random sample, ASBS nonmembers accounted for less than 20% of our respondent pool. Our results therefore may reflect the influence of ASBS membership on bariatric surgery patient selection. Unfortunately, since bariatric surgery is not a board-certified field and no national registry exists for surgeons who perform bariatric procedures in the United States, we believe that our 2-pronged approach to respondent identification was the most comprehensive possible.
Despite these possible limitations, our study design allowed for the independent measurement of predictors of patient selection for bariatric surgery nationwide through systematic variation in vignette content across a representative sample of U.S. bariatric surgeons. Livingston and Ko demonstrated sociodemographic disparities in bariatric surgery utilization.2 Our results suggest that surgeon decisions are not contributing to racial disparities in bariatric surgery utilization. While decisions to operate outside of clinically recommended criteria may be driven by gender ideals, surgeon decisions did not contribute to sex-based disparities in other circumstances. This suggests that forces outside of surgeon discretion are primarily responsible for race- and sex-based disparities in bariatric surgery. Structural possibilities include race- and sex-based differences in access to health care, referral patterns for bariatric surgery, and proximity of facilities performing bariatric surgery. Population-based possibilities include differences in socio-cultural norms about body weight, appearance, and health.
These same structural and socio-cultural forces likely play a role in socioeconomic disparities in bariatric surgery. However, it is clear that surgeon decisions also play a role as evidenced by their consideration of payment source. Public insurance plans may not reimburse at the same level as private insurers. However, if financial motivations were the driving force behind surgeons’ decreased likelihood of operating on public aid patients, we should have found an increased likelihood of selecting self-pay patients. Therefore, we conclude that surgeon decisions related to public insurance are based partly on perceptions of socioeconomic status.
While open gastric bypass and vertical banded gastroplasty were the only procedures recommended in the 1991 NIH Consensus Statement, we found that surgeons are offering a variety of options, including laparoscopic techniques, biliopancreatic bypass, silastic banding, and other variations. However, gastric bypass, long regarded as the “gold standard” for surgical weight loss, remains the most widely offered procedure whether open or laparoscopic. Few surgeons currently offer vertical banded gastroplasty.
In recent years, this procedural innovation in bariatric surgery and exponential growth in procedure volume has outpaced bariatric surgical outcomes research and efforts to set forth new guidelines based on patient- and procedure-specific criteria.62,63 The NIH BMI and comorbidity criteria have come into question and the field of bariatric surgery is at an important crossroad. Policy-makers are currently reissuing best practice guidelines and identifying “Centers of Excellence.”64 Based on our findings, we suggest that future recommendations should incorporate specific guidelines for age and clinical criteria as well as issues of social support, functional status, and financial resources. Outside of bariatric surgery, efforts should be made to increase awareness among the public that morbid obesity is a health concern rather than a cosmetic concern. Healthcare providers should be educated about the indications, risks, and benefits of bariatric surgery so they can make appropriate referrals and fully participate in the care of bariatric surgery patients. Both perception and delivery of bariatric surgery must change if the only known durable option for weight loss in the morbidly obese is to be offered equitably to all Americans who might benefit from it.
ACKNOWLEDGMENTS
The authors would also like to acknowledge the following individuals for their assistance in this research: Colm A. O’Muircheartaigh, PhD, Michael Quinn, PhD, and Vivek N. Prachand, MD for their advice on survey development; Sumerah Bakhsh, David Munson, Olubukunola Tawose, and Terri Rossi for data entry and administrative support; and the many physicians and researchers who participated in the telephone interviews, focus group, and pilot testing in advance of this survey.
Footnotes
Dr. Santry undertook this research while she was a Robert Wood Johnson Clinical Scholar supported by the Robert Wood Johnson Foundation. Additional funding was provided by a pilot project grant from the Center on Aging at the University of Chicago (P30 AG-12857-08) and a research grant from the Dr. Paul Jordan Research Fund in Surgery at the University of Chicago.
Dr. Santry presented preliminary data from this paper at the Charles Huggins Research Conference at the University of Chicago in Chicago, IL on May 16, 2005 and at the Surgical Forum of the American College of Surgeons Clinical Congress in San Francisco, CA on October 19, 2005.
Reprints: Heena P. Santry, MD, Department of Surgery, University of Chicago, MC 6040, 5841 S. Maryland Avenue, Chicago, IL 60637. E-mail: hpatel@surgery.bsd.uchicago.edu.
REFERENCES
- 1.Anonymous. Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am J Clin Nutr. 1992;55(suppl):615–619. [DOI] [PubMed]
- 2.Livingston EH, Ko CY. Socioeconomic characteristics of the population eligible for obesity surgery. Surgery. 2004;135:288–296. [DOI] [PubMed] [Google Scholar]
- 3.Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. [DOI] [PubMed] [Google Scholar]
- 4.Erickson JL, Remington PL, Peppard PE. Trends in bariatric surgery for morbid obesity in Wisconsin. Wis Med J. 2004;103:32–37. [PubMed] [Google Scholar]
- 5.Zizza CA, Herring AH, Stevens J, et al. Bariatric surgeries in North Carolina, 1990 to 2001: a gender comparison. Obes Res. 2003;11:1519–1525. [DOI] [PubMed] [Google Scholar]
- 6.Pope GD, Birkmeyer JD, Finlayson SR. National trends in utilization and in-hospital outcomes of bariatric surgery. J Gastrointest Surg. 2002;6:855–860. [DOI] [PubMed] [Google Scholar]
- 7.Rand CS, Kuldau JM. Morbid obesity: a comparison between a general population and obesity surgery patients. Int J Obes Relat Metab Disord. 1993;17:657–661. [PubMed] [Google Scholar]
- 8.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005;294:1909–1917. [DOI] [PubMed] [Google Scholar]
- 9.Goldblatt PB, Moore ME, Stunkard AJ. Social factors in obesity. JAMA. 1965;192:1039–1044. [DOI] [PubMed] [Google Scholar]
- 10.Sobal J, Stunkard AJ. Socioeconomic status and obesity: a review of the literature. Psychol Bull. 1989;105:260–275. [DOI] [PubMed] [Google Scholar]
- 11.Winkleby MA, Gardner CD, Taylor CB. The influence of gender and socioeconomic factors on Hispanic/white differences in body mass index. Prev Med. 1996;25:203–211. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Q, Wang Y. Socioeconomic inequality of obesity in the United States: do gender, age, and ethnicity matter? Soc Sci Med. 2004;58:1171–1180. [DOI] [PubMed] [Google Scholar]
- 13.Gortmaker SL, Must A, Perrin JM, et al. Social and economic consequences of overweight in adolescence and young adulthood. N Engl J Med. 1993;329:1008–1012. [DOI] [PubMed] [Google Scholar]
- 14.Trus TL, Pope GD, Finlayson SR. National trends in utilization and outcomes of bariatric surgery. Surg Endosc. 2005;19:616–620. [DOI] [PubMed] [Google Scholar]
- 15.van Hout GC, Verschure SK, van Heck GL. Psychosocial predictors of success following bariatric surgery. Obes Surg. 2005;15:552–560. [DOI] [PubMed] [Google Scholar]
- 16.Ray EC, Nickels MW, Sayeed S, et al. Predicting success after gastric bypass: the role of psychosocial and behavioral factors. Surgery. 2003;134:555–563. [DOI] [PubMed] [Google Scholar]
- 17.Alexander CS, Becker HJ. The use of vignettes in survey research. Pub Opin Q. 1978;42:93–104. [Google Scholar]
- 18.Green P, Carroll J, Carmone F. Some new types of fractional factorial designs for marketing experiments. Res Marketing. 1978;1:99–122. [Google Scholar]
- 19.Santry HP, Alverdy JC, Lauderdale DS. Who are today’s bariatric surgeons and which ones join the ASBS? SOARD In press. [DOI] [PubMed]
- 20.Diggle PJ, Liang K-Y, Zeger SL. Analysis of Longitudinal Data. New York: Oxford University Press, 1994. [Google Scholar]
- 21.Inge TH, Krebs NF, Garcia VF, et al. Bariatric surgery for severely overweight adolescents: concerns and recommendations. Pediatrics. 2004;114:217–223. [DOI] [PubMed] [Google Scholar]
- 22.Apovian CM, Baker C, Ludwig DS, et al. Best practice guidelines in pediatric/adolescent weight loss surgery. Obes Res. 2005;13:274–282. [DOI] [PubMed] [Google Scholar]
- 23.Inge TH, Lawson L. Treatment considerations for severe adolescent obesity. Surg Obes Relat Dis. 2005;1:133–139. [DOI] [PubMed] [Google Scholar]
- 24.Livingston EH, Huerta S, Arthur D, et al. Male gender is a predictor of morbidity and age a predictor of mortality for patients undergoing gastric bypass surgery. Ann Surg. 2002;236:576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sosa JL, Pombo H, Pallavicini H, et al. Laparoscopic gastric bypass beyond age 60. Obes Surg. 2004;14:1398–1401. [DOI] [PubMed] [Google Scholar]
- 26.Sugerman HJ, DeMaria EJ, Kellum JM, et al. Effects of bariatric surgery in older patients. Ann Surg. 2004;240:243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papasavas PK, Gagne DJ, Kelly J, et al. Laparoscopic Roux-En-Y gastric bypass is a safe and effective operation for the treatment of morbid obesity in patients older than 55 years. Obes Surg. 2004;14:1056–1061. [DOI] [PubMed] [Google Scholar]
- 28.Silecchia G, Greco F, Bacci V, et al. Results after laparoscopic adjustable gastric banding in patients over 55 years of age. Obes Surg. 2005;15:351–356. [DOI] [PubMed] [Google Scholar]
- 29.Perugini RA, Mason R, Czerniach DR, et al. Predictors of complication and suboptimal weight loss after laparoscopic Roux-en-Y gastric bypass: a series of 188 patients. Arch Surg. 2003;138:541–545. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Mason EE, Renquist KE, et al. Factors influencing survival following surgical treatment of obesity. Obes Surg. 2005;15:43–50. [DOI] [PubMed] [Google Scholar]
- 31.Ballantyne GH, Svahn J, Capella RF, et al. Predictors of prolonged hospital stay following open and laparoscopic gastric bypass for morbid obesity: body mass index, length of surgery, sleep apnea, asthma, and the metabolic syndrome. Obes Surg. 2004;14:1042–1050. [DOI] [PubMed] [Google Scholar]
- 32.Balsiger BM, Luque de Leon E, Sarr MG. Surgical treatment of obesity: who is an appropriate candidate? Mayo Clinic Proc. 1997;72:551–557. [DOI] [PubMed] [Google Scholar]
- 33.Kral JG. Selection of patients for anti-obesity surgery. Int J Obes Relat Metab Disord. 2001;25(suppl):107–112. [DOI] [PubMed] [Google Scholar]
- 34.Kral JG. Patient selection for treatment of obesity. Surg Obes Relat Dis. 2005;1:126–132. [DOI] [PubMed] [Google Scholar]
- 35.Cowan GS Jr, Hiler ML, Buffington C. Criteria for selection of patients for bariatric surgery. Eur J Gastroenterol Hepatol. 1999;11:69–75. [DOI] [PubMed] [Google Scholar]
- 36.Sauerland S, Angrisani L, Belachew M, et al. Obesity surgery: evidence-based guidelines of the European Association for Endoscopic Surgery (EAES). Surg Endosc. 2005;19:200–221. [DOI] [PubMed] [Google Scholar]
- 37.Saltzman E, Anderson W, Apovian CM, et al. Criteria for patient selection and multidisciplinary evaluation and treatment of the weight loss surgery patient. Obes Res. 2005;13:234–243. [DOI] [PubMed] [Google Scholar]
- 38.Fontaine KR, Barofsky I. Obesity and health-related quality of life. Obes Rev. 2001;2:173–182. [DOI] [PubMed] [Google Scholar]
- 39.Barreto Villela N, Braghrolli Neto O, Lima Curvello K, et al. Quality of life of obese patients submitted to bariatric surgery. Nutr Hosp. 2004;19:367–371. [PubMed] [Google Scholar]
- 40.Melissas J, Kontakis G, Volakakis E, et al. The effect of surgical weight reduction on functional status in morbidly obese patients with low back pain. Obes Surg. 2005;15:378–381. [DOI] [PubMed] [Google Scholar]
- 41.Fobi M, Lee H, Igwe D, et al. Gastric bypass in patients with BMI < 40 but >32 without life-threatening co-morbidities: preliminary report. Obes Surg. 2002;12:52–56. [DOI] [PubMed] [Google Scholar]
- 42.Blame B, Williams Z. Belief in the controllability of weight and attributions to prejudice among heavyweight women. Sex Roles. 2004;51:79–86. [Google Scholar]
- 43.Puhl R, Brownell KD. Bias, discrimination, and obesity. Obes Res. 2001;9:788–805. [DOI] [PubMed] [Google Scholar]
- 44.Allon N. The stigma of overweight in everyday life. In: Wolman B, ed. Psychological Aspects of Obesity: A Handbook. New York: Van Nostrand Reinhold, 1982:130–174. [Google Scholar]
- 45.Lindstrom W Jr. Maximizing your chances of getting an insurance approval the first time. Obes Surg. 1997;7:449–453. [DOI] [PubMed] [Google Scholar]
- 46.Choban P, Lu B, Flancbaum L. Insurance decisions about obesity surgery: a new type of randomization? Obes Surg. 2000;10:553–556. [DOI] [PubMed] [Google Scholar]
- 47.Buchwald H. Mainstreaming bariatric surgery. Obes Surg. 1999;9:462–470. [DOI] [PubMed] [Google Scholar]
- 48.Renquist KE, Mason EE, Tang S, et al. Pay status as a predictor of outcome in surgical treatment of obesity. Obes Surg. 1996;6:224–232. [DOI] [PubMed] [Google Scholar]
- 49.Durkin AJ, Bloomston M, Murr MM, et al. Financial status does not predict weight loss after bariatric surgery. Obes Surg. 1999;9:524–526. [DOI] [PubMed] [Google Scholar]
- 50.Peabody JW, Luck J, Glassman P, et al. Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality. JAMA. 2000;283:1715–1722. [DOI] [PubMed] [Google Scholar]
- 51.van Ryn M, Burke J. The effect of patient race and socioeconomic status on physicians’ perceptions of patients. Soc Sci Med. 2000;50:813–828. [DOI] [PubMed] [Google Scholar]
- 52.Veloski J, Tai S, Evans AS, et al. Clinical vignette-based surveys: a tool for assessing physician practice variation. Am J Med Q. 2005;20:151–157. [DOI] [PubMed] [Google Scholar]
- 53.Plackett R, Burman J. The design of optimum multifactorial experiments. Biometrika. 1946;33:305–325. [Google Scholar]
- 54.Mort EA, Edwards JN, Emmons DW, et al. Physician response to patient insurance status in ambulatory care clinical decision-making: implications for quality of care. Med Care. 1996;34:783–797. [DOI] [PubMed] [Google Scholar]
- 55.Furth SL, Hwang W, Neu AM, et al. Effects of patient compliance, parental education and race on nephrologists’ recommendations for kidney transplantation in children. Am J Transplant. 2003;3:28–34. [DOI] [PubMed] [Google Scholar]
- 56.Thamer M, Hwang W, Fink NE, et al. U.S. nephrologists’ attitudes towards renal transplantation: results from a national survey. Transplantation. 2001;71:281–288. [DOI] [PubMed] [Google Scholar]
- 57.Thamer M, Hwang W, Fink NE, et al. US nephrologists’ recommendation of dialysis modality: results of a national survey. Am J Kidney Dis. 2000;36:1155–1165. [DOI] [PubMed] [Google Scholar]
- 58.Schulman KA, Berlin JA, Harless W, et al. The effect of race and sex on physicians’ recommendations for cardiac catheterization. N Engl J Med. 1999;340:618–626. [DOI] [PubMed] [Google Scholar]
- 59.Tamayo-Sarver JH, Dawson NV, Hinze SW, et al. The effect of race/ethnicity and desirable social characteristics on physicians’ decisions to prescribe opioid analgesics. Acad Emerg Med. 2003;10:1239–1248. [DOI] [PubMed] [Google Scholar]
- 60.Asch DA, Jedrziewski MK, Christakis NA. Response rates to mail surveys published in medical journals. J Clin Epidemiol. 1997;50:1129–1136. [DOI] [PubMed] [Google Scholar]
- 61.Virgo KS, Naunheim KS, Coplin MA, et al. Lung cancer patient follow-up: motivation of thoracic surgeons. Chest. 1998;114:1519–1534. [DOI] [PubMed] [Google Scholar]
- 62.Mitka M. Surgery for obesity: demand soars amid scientific, ethical questions. JAMA. 2003;289:1761–1762. [DOI] [PubMed] [Google Scholar]
- 63.Steinbrook R. Surgery for severe obesity. N Engl J Med. 2004;350:1075–1079. [DOI] [PubMed] [Google Scholar]
- 64.Champion JK, Pories WJ. Centers of excellence for bariatric surgery. Surg Obes Relat Dis. 2005;1:148–151. [DOI] [PubMed] [Google Scholar]


