Abstract
Inflammation underlines all major bladder pathologies and represents a defense reaction to injury involving a mandatory participation of mast cells and sensory nerves. Mast cells are particularly frequent in close proximity to epithelial surfaces where they are strategically located in the bladder and release their mediators in response to inflammation. Tryptase is specifically produced by mast cells and modulates inflammation by activating protease-activated receptors (PARs). We recently found that PAR-4 mRNA is up-regulated in experimental bladder inflammation regardless of the initiating stimulus. Because it has been reported that PAR-1, PAR-2, and PAR-3 may also be involved in the processes of inflammation, we used immunohistochemistry to characterize the expression of all known PARs in normal, acute, and chronic inflamed mouse bladder. We found that all four PARs are present in the control mouse bladder, and follow a unique distribution. All four PARs are co-expressed in the urothelium, whereas PAR-1 and PAR-2 are predominant in the detrusor muscle, and PAR-4 is expressed in peripheral nerves and plexus cell bodies. The strong expression of PARs in the detrusor muscle indicates the need for studies on the role of these receptors in motility whereas the presence of PAR-4 in nerves may indicate its participation in neurogenic inflammation. In addition, PARs are differentially modulated during inflammation. PAR-1 and PAR-2 are down-regulated in acute inflammation whereas PAR-3 and PAR-4 are up-regulated. Bladder fibroblasts were found to present a clear demarcation in PAR expression secondary to acute and chronic inflammation. Our findings provide evidence of participation of PARs in the urinary system, provide a working model for mast cell tryptase signaling in the mouse bladder, and evoke testable hypotheses regarding the roles of PARs in bladder inflammation. It is timely to understand the role of tryptase signaling and PARs in the context of bladder biology.
Inflammation underlines all major bladder pathologies and represents a defense reaction to injury caused by physical damage, chemical substances, microorganisms, or other agents. In consequence to inflammation, products of mast cell degranulation such as tryptase can be found in the urine of both cancer and cystitis patients. 1 Recently, we presented experimental evidence suggesting the existence of a recursive molecular pathway supporting bladder inflammatory responses. 2 One such pathway involves the participation of serine proteases thrombin, trypsin, and tryptase that bladder cells secrete into the extracellular space to mediate processes such as cellular invasion, extracellular matrix degradation, angiogenesis, and tissue remodeling. Thrombin, tryptase, and trypsin responses are modulated by protease-activated receptors (PARs), a unique class of G protein-coupled receptors that use a fascinating mechanism to convert an extracellular proteolytic cleavage event into a trans-membrane signal: these receptors carry their own ligands that remain cryptic until unmasked by receptor cleavage. 3-5 PARs may be involved in various biological responses, such as hemostasis, regulation of smooth muscle tone, and inflammation. To date, four PARs have been identified 3,6-8 with distinct N-terminal cleavage sites and tethered ligand pharmacology. 9 Each receptor has been shown to modulate a variety of physiological processes such as cytokine and mediator release, vasodilation, platelet aggregation, cellular proliferation, and smooth muscle contraction or relaxation. 3,9 PAR-1, PAR-3, and PAR-4 are activated by thrombin, whereas PAR-2 is activated by mast cell tryptase or trypsin. 10 In addition, PAR-1 mediates thrombin-dependent, cell-mediated renal inflammation in crescentic glomerulonephritis, 11 and gastrointestinal inflammation. 12 In the urinary bladder, PAR-1-activating peptide (PAR-AP) stimulated Evans blue extravasation that is abolished by neurokinin-1 receptor antagonist. 13 PAR-2 is expressed in the gastrointestinal tract, pancreas, kidney, liver, airways, prostate, ovary, and eye. 14 PAR-3 is the second thrombin receptor. 6 In humans it is expressed in the bone marrow, heart, brain, placenta, liver, pancreas, thymus, small intestine, stomach, lymph nodes, and trachea. The highest levels of PAR-4 mRNA have been detected in lung, pancreas, thyroid, testis, and small intestine. PAR-4 is relatively insensitive to thrombin and trypsin. 7 PAR-4 mRNAs were found up-regulated in response to cardiac inflammation 15 and a functionally active PAR-4 is present in smooth muscle and might contribute to thrombin-induced mitogenesis. 16 In the urinary bladder, we found that PAR-4 mRNA was up-regulated in response to lipopolysaccharide (LPS), substance P (SP), or antigen (Ag) challenge. 2
Another event tied to inflammation is the tissue repair process. Thrombin is known to play a role in tissue repair by enhancing neovascularization, collagen deposition, and wound healing. 17-19 The stimulatory effects of thrombin on growth factor production by fibroblasts can be reproduced by PAR-APs. 20,21 Another current study suggested that thrombin, through the activation of PAR, participates in tissue remodeling by regulating the release of matrix metalloproteinases, 22 a mechanism that involves activation of both MAP kinase and nuclear factor-κB. 22
As described above, PARs are widely distributed in a variety of tissues such as skin, intestine, blood vessels, myenteric plexus, 4 and colonic myocytes 23 and mast cells. 24 However, the expression and relative distribution of PAR-1, -2, -3, and -4 in bladder tissue has not been extensively described in their histological context. The aim of this study was to characterize the expression of all PARs in normal and inflamed tissue using immunohistochemistry (IHC) and to correlate the relative alteration in PAR expression and distribution with the degree of acute and chronic inflammation. Our findings provide evidence of the presence of all four PARs in the mouse urinary bladder and that experimental inflammation leads to alteration in PAR expression.
Materials and Methods
Animals
All animal experimentation described here was performed in conformity with the Guiding Principles for Research Involving Animals and Human Beings (OUHSC Animal Care & Use Committee protocol no. 00-109). Groups of 10- to 12-week-old female C57BL/6J mice were used in these experiments. Animals were maintained in housing facilities and allowed food and water ad libitum.
Ag Sensitization Protocol
One group of mice in this study was sensitized with intraperitoneal injections of 1 μg of dinitrophenol-human serum albumin (DNP4-HSA) in 1 mg of alum on days 0, 7, 14, and 21. This protocol induces sustained levels of IgE antibodies up to 56 days after sensitization 25 and its specificity resides in the fact that this response is abolished by antibodies to IgE. 25 One week after the last sensitization, cystitis was induced (see Induction of Cystitis) by intravesical challenge with Ag DNP4-ovalbumin (DNP-OVA) to induce bladder mast cell degranulation. 2,26,27
Induction of Cystitis
Acute cystitis was induced as we described previously. 2,26-29 Briefly, female mice were anesthetized (40 mg/kg i.p. ketamine and 2.5 mg/kg, i.p. xylazine), then transurethrally catheterized (24 Ga, 3/4 in, Angiocath; Becton Dickinson, Sandy, UT), and the urine was drained by applying slight digital pressure to the lower abdomen. The urinary bladders were instilled with 150 μl of one of the following substances: pyrogen-free saline, SP (10 μmol/L), Escherichia coli LPS strain 055:B5 (100 μg/ml; Sigma, St. Louis, MO), or Ag DNP4-OVA (1 μg/ml) in actively sensitized mice. Substances were infused at a slow rate to avoid trauma and vesicoureteral reflux. 2 To ensure consistent contact of substances with the bladder, infusion was repeated twice within a 30-minute interval and a 1-ml Tb syringe was maintained on the catheter end-retained intravesical solution for at least for 1 hour. After that the catheter was removed and mice were allowed to void normally. For acute inflammation, 24 hours after instillation, mice were sacrificed with pentobarbital (100 mg/kg, i.p.) and bladders were removed rapidly and placed in neutral buffered formalin.
Chronic cystitis was induced by LPS (100 μg/ml) instillations performed every 24 hours for 4 days. Mice were sacrificed 24 hours after the last instillation. Control mice for this group received the same volume of pyrogen-free saline at the same time points and will be denoted as saline chronic hereafter. Previous results indicated that this protocol induces chronic inflammation characterized by a predominate infiltrate of macrophages/monocytes and lymphocytes into the bladder. 2
Alterations at Histological Level
Mouse bladders were immediately placed in 10% neutral buffered formalin for a minimum of 48 hours and then placed in phosphate-buffered saline (PBS, pH 7.8) and dehydrated in graded alcohol and xylene. All bladders of the same group (n = 6) were then embedded together in paraffin as a multitissue block according to conventional methods. Five-μm sections were serially cut (8-μm apart), mounted onto SuperFrost Plus (Fisher Scientific, Pittsburgh, PA) microscopic slides, and dried overnight. Mast cells were staining according to Luna. 30 Briefly, paraffin tissue sections were routinely dewaxed and hydrated. After placing slides in dH2O, they were placed in 0.5% toluidine blue solution for 1 hour at room temperature. The slides were then briefly rinsed in 0.5% alcoholic hydrochloric acid until sections appeared colorless. The slides were dipped in 0.01% eosin ∼20 times and then were dehydrated and mounted for microscopic analysis. Mast cells appeared blue and were counted in all areas of the tissues. Histology slides were scanned using a Nikon digital camera (DXM1200; Nikon, Tokyo, Japan) mounted on a Nikon microscope (Eclipse E600, Nikon). Image analysis was performed using a MetaMorph Imaging System (Universal Imaging Corporation, West Chester, PA).
Quantification of Inflammation
The urinary bladder was evaluated for inflammatory cell infiltrates, mast cell numbers, and the presence of interstitial edema. A semiquantitative score using defined criteria of inflammation severity was used to evaluate edema formation as follows: 1+, mild (little or no interstitial edema); 2+, moderate interstitial edema; 3+, severe interstitial edema. 2
IHC
The protocols for routine single IHC have been described previously. 31 Tissue sections on microscopic slides were dewaxed and rehydrated. Slides were microwaved in Target buffer (DAKO, Carpinteria, CA), cooled, placed in PBS (pH 7.4) and treated with 3.0% H2O2 for 10 minutes at room temperature. All incubations (30 minutes) and washes were performed at room temperature. Normal blocking serum (Vector Laboratories, Burlingame, CA) was placed on all slides for 10 minutes. After a brief rinse in PBS, sections were treated with the polyclonal antibodies that have been previously characterized [PAR-1 (Robert Wood Johnson Pharmaceutical Research & Development (RWJPRD), Spring House, PA 31 ); PAR-2 (RWJPRD 32 ); PAR-3 (Santa Cruz Biotechnology, Santa Cruz, CA 33 ); PAR-4 (Santa Cruz Biotechnology 33 )]. We also used the following antibodies to further characterize the mouse bladders: smooth muscle actin (SMA, DAKO), proliferating cell nuclear protein (PCNA, DAKO), myeloperoxidase (DAKO), and macrophage marker MAC-3 (Pharmingen, San Diego, CA). In addition, we used the following antibodies to characterize nerve elements: neuronal-specific enolase (Chemicon, Temecula, CA), S100, a Schwann cell marker (Sigma, St. Louis, MO), and glial fibrillary acid protein (DAKO). Slides were then washed in PBS and treated with goat anti-rabbit biotinylated secondary antibodies (Vector Laboratories). After washing in PBS, the avidin-biotin-horseradish peroxidase complex reagent (Vector Laboratories) was added. Slides were washed and were treated with 3,3′-diaminobenzidine (Biomeda, Foster City, CA) two times for 5 minutes, rinsed in dH2O, and counterstained with hematoxylin. Negative controls included replacement of the primary antibody with the antibody diluent (Zymed Laboratories, South San Francisco, CA) or use of the nonimmune serum (Vector Laboratories). Preabsorption controls including preincubation of the primary antibody (overnight at 4°C) with its specific Ag (10-fold titer excess) have been previously described for the PAR antibodies. 31-33
PAR IHC Analysis
Urinary bladders isolated from control, acutely inflamed [LPS, SP, and Ag (in sensitized mice)], and chronic LPS-stimulated mice were scored for relative intensity of PAR-1, PAR-2, PAR-3, and PAR-4 immunolabeling in the urothelium and submucosa, detrusor smooth muscle, stromal fibroblasts, and inflammatory cells. For each tissue, the presence of PAR immunoreactivity was ranked under a ×20 objective according to the following criteria: 1) no immunoreactivity (score = 0); 2) weak, light brown immunoreactivity (score = 1); 3) moderate brown immunolabeling (score = 2); and 4) intense, dark brown immunoreactivity (score = 3).
The statistical analysis of histological data and IHC was performed using Wilcoxon’s rank sum test. Results are expressed as mean ± SEM. The n values reported refer to the number of animals used for each experiment. In all cases, a value of P < 0.05 was considered indicative of significant difference.
Results
Morphological Consequences of LPS- (Acute and Chronic), Ag-, and SP-Challenged Conditions
We examined whether the mouse bladder elicits an inflammatory response to all three stimuli by morphological analysis of tissue edema, detection of polymorphonuclear neutrophils (PMNs) (anti-myeloperoxidase), and macrophages (anti-MAC-3) using IHC, and staining of mast cells using the Luna’s toluidine blue method (Figure 1, A and B) ▶ . In addition, the effect of inflammation on urothelial and stromal cell proliferation was determined by PCNA immunolabeling (Figure 1C) ▶ . LPS (acute and chronic conditions)-, Ag-, and SP-challenged conditions induced slightly different degrees of acute inflammation characterized by vasodilation, edema, and intense PMN infiltration in the mucosa and submucosal layers (Figure 1A) ▶ . Confirming our results obtained with LPS, 2,28 Ag, 2,27 and SP, 2,29 both edema formation and PMN migration were observed as early as 4 hours (acute) and were stabilized 24 hours after stimulation (data not shown). In a separate study, we also examined additional time points at 48 and 72 hours after LPS stimulation to ensure maximal PMN infiltration and edema was obtained at 24 hours. 29 A differential response was observed on tissue mast cell numbers. Ag-challenged as well as the acute LPS and chronic LPS models induced an increase in tissue mast cell numbers whereas SP-challenged had no effect (Figure 1B) ▶ . All three acute stimuli decreased PCNA labeling of urothelial cells (Figure 1C) ▶ .
Figure 1.
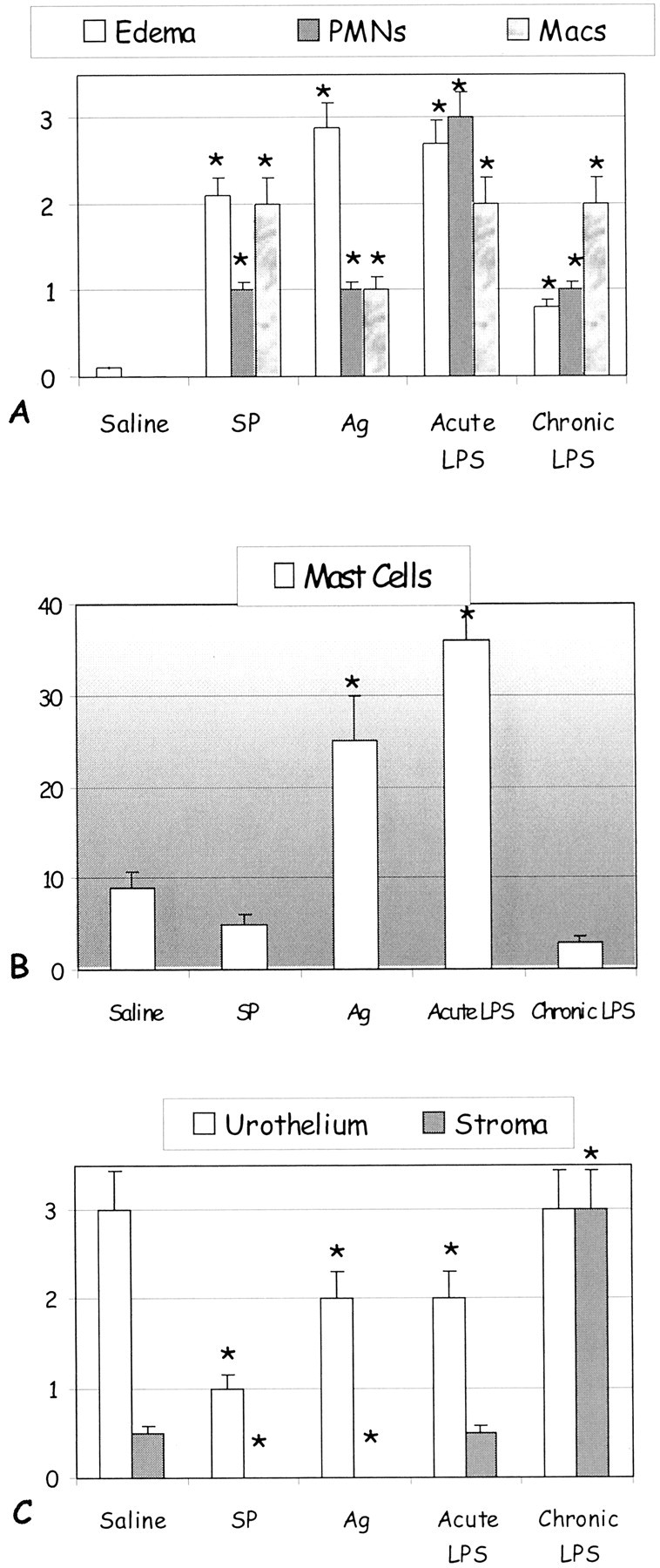
Quantification of inflammation induced by LPS (acute and chronic)-, Ag-, and SP-challenged conditions. Acute inflammation was induced by single stimulation with SP, Ag (in sensitized mice), or LPS. Chronic LPS inflammation was provoked by repeated bladder instillation with LPS every 24 hours throughout a 4-day period. Control mice were instilled with saline. Tissues were removed 24 hours after the last instillation. A semiquantitative score using defined criteria of inflammation severity was used to evaluate edema formation as follows: 1+, mild (little or no interstitial edema); 2+, moderate interstitial edema; 3+, severe interstitial edema (A). A: IHC was used for detection of PMNs (anti-myeloperoxidase) and macrophages (anti-MAC-3). B: Mast cells were counted per cross-section of Luna’s toluidine blue. C: The effect of inflammation on urothelial and stromal cell proliferation was determined by PCNA immunolabeling. Results are the average and SEM of six experiments. Asterisks indicate a statistical significant difference (P < 0.05) from bladders treated with saline.
Chronic LPS inflammation was provoked by repeated bladder instillation with LPS every 24 hours throughout a 4-day period. In a previous work we presented evidence that during chronic inflammation cross-sections of the mouse bladder presented a mixed inflammatory cell infiltrate containing predominantly macrophages, lymphocytes, and plasma cells, with some PMNs as minor components. 2 In the present work, a dramatic alteration of chronic bladder stimulation with LPS was the decrease of bladder mast cell numbers (Figure 1B) ▶ and PMNs concomitantly with an increased presence of macrophages/monocytes in the suburothelial and submucosal layers (Figure 1A) ▶ . In addition, chronic inflammation restored urothelial cell proliferation that was decreased by acute LPS stimulation (Figure 1C) ▶ . During chronic inflammation, PCNA labeling was increased in both urothelial and stromal cells. The PCNA immunolabeling was similarly increased in the urothelium in the Ag- and SP-challenged conditions like the responses observed in the LPS models (Figure 1C) ▶ . However, no PCNA immunolabeling was detected in the stromal fibroblasts in the Ag- and SP-challenged models (Figure 1C) ▶ .
Analysis of PAR Immunoreactivity
PAR immunolabeling was presented as brown staining in the tissues. No detectable immunolabeling was observed in all of the negative controls, and as a positive antigenicity control antibody, α-SMA immunolabeling was detected in all of the tissues. As mentioned in the Materials and Methods section, the PAR immunolabeling was scored according to the intensity of the labeling (strong dark brown = 3, moderate = 2, weak = 1, and absence of detectable immune label = 0).
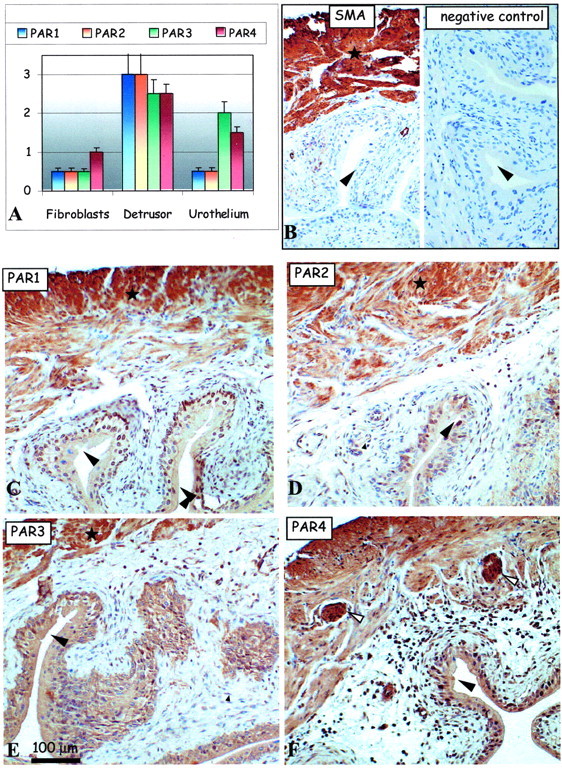
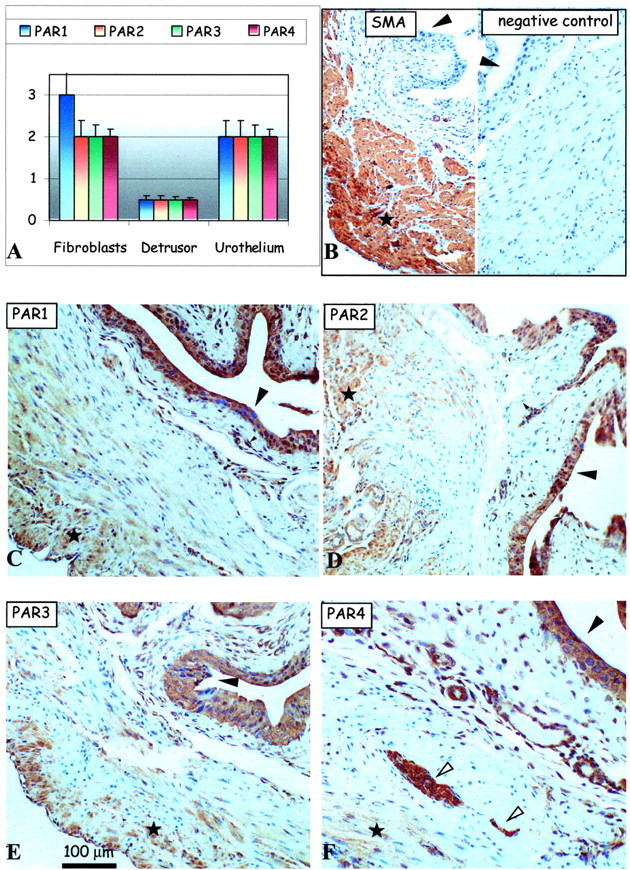
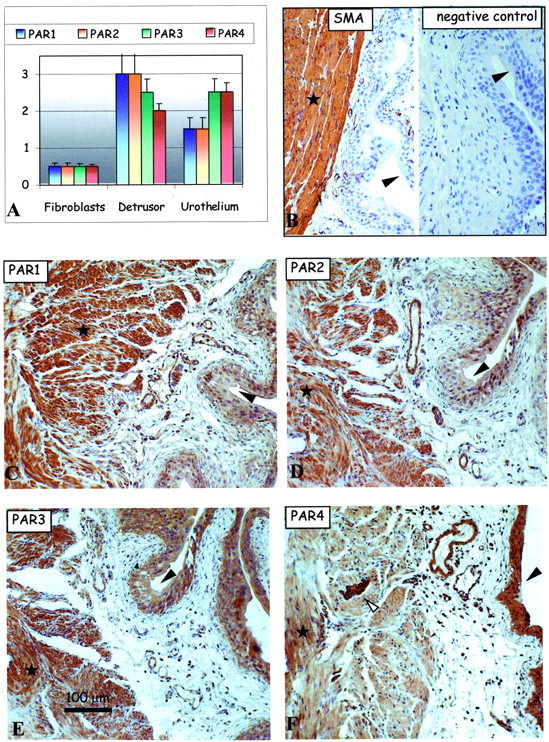
We characterized the presence of PAR-1, PAR-2, PAR-3, and PAR-4 in the mouse bladder tissues in various conditions including the saline-control condition, and other conditions of inflammation. The presence and the intensity of the immunolabeling (see above) of the PARs were analyzed in the various cells types of the bladder tissue: urothelium, detrusor smooth muscle, as well as in the stromal fibroblasts of the lamina propria in the subepithelial region (Table 1) ▶ . Generally, all four PARs were detected in most cell types of the mouse bladder in all of the conditions. However, the intensity of the PAR immunolabeling varied considerably and is presented in the form of bar charts for each condition (Figures 2A to 6) ▶ ▶ ▶ . Positive control antibody, SMA (Figure 2B ▶ , left), and negative control antibody (Figure 2B ▶ , right) accompany each figure to demonstrate, respectively, tissue antigenicity and to rule out potential false-positive immunoreactivity because of the detection system reagents. Representative images of PAR-1 (Figure 2C) ▶ , PAR-2 (Figure 2D) ▶ , PAR-3 (Figure 2E) ▶ , and PAR-4 (Figure 2F) ▶ immunolabeling in the serial sections (5 μm) of urinary bladder tissue isolated from saline-control (Figure 2) ▶ , acute LPS (Figure 3) ▶ and chronic LPS (Figure 4) ▶ inflamed bladders, as well as Ag-challenged (Figure 5) ▶ and SP-challenged (Figure 6) ▶ models are presented.
Table 1.
Distribution of PAR Immunoreactivity in Mouse Bladder Tissues
| PAR | Cell type | Saline-control | Acute LPS | Chronic LPS | Ag-challenged | Substance P-challenged |
|---|---|---|---|---|---|---|
| PAR1 | Fibroblasts | −/+ | −/+ | +++ | −/+ | −/+ |
| Detrusor muscle | +/++ | +++ | −/+ | +++ | −/+ | |
| Urothelium | ++ | −/+ | ++ | +/++ | −/+ | |
| PAR2 | Fibroblasts | − | −/+ | ++ | −/+ | −/+ |
| Detrusor muscle | +/++ | +++ | −/+ | +++ | −/+ | |
| Urothelium | ++/+++ | + | ++ | +/++ | + | |
| PAR3 | Fibroblasts | −/+ | −/+ | ++ | −/+ | −/+ |
| Detrusor muscle | −/+ | ++/+++ | −/+ | ++/+++ | −/+ | |
| Urothelium | ++/+++ | ++ | ++ | ++/+++ | + | |
| PAR4 | Fibroblasts | −/+ | + | ++ | −/+ | + |
| Detrusor muscle | −/+ | ++/+++ | −/+ | ++ | −/+ | |
| Urothelium | ++ | +/++ | ++ | ++/+++ | +/++ |
No detectable PAR immunoreactivity (−); weak, light brown immunoreactivity (1+); moderate brown immunoreactivity (2+); intense, dark brown immunoreactivity (3+); double scores (i.e. ++/+++) indicate mixed population of cells.
Figure 2.
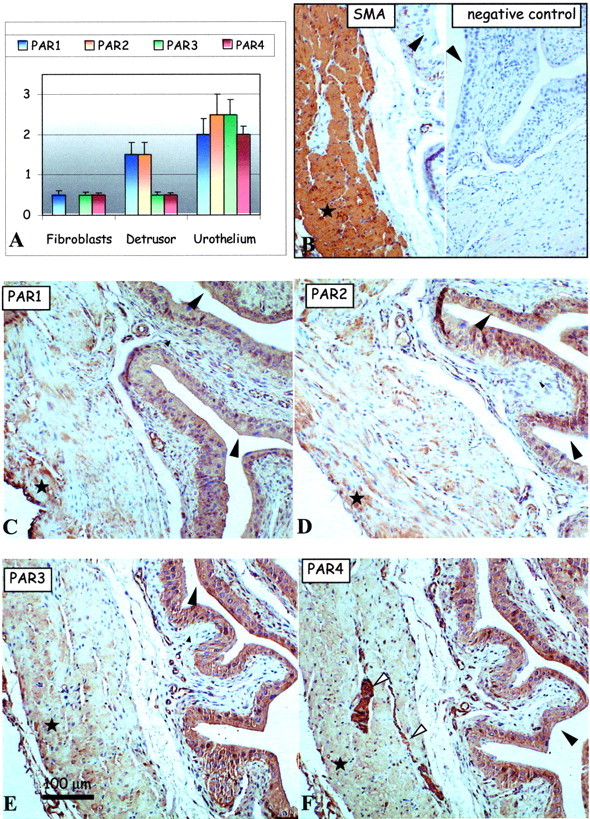
PAR expression in the urinary bladder of saline-treated mice. A: Bar charts show average intensities of immunolabeling for PAR-1 (blue), PAR-2, PAR-3, and PAR-4 in the mouse bladder tissues. B–F: Representative immunohistochemical micrographs show detection of positive control SMA (B, left) and the lack of any immunolabeling using the negative control antibody (B, right) in consecutive sections of mouse bladder tissue. Remaining representative images show immunolabeling patterns for the detection of PAR-1 (C), PAR-2 (D), PAR-3 (E), and PAR-4 (F) expression in serial mouse bladder sections. Large arrowheads identify the urothelium, small arrowheads identify the fibroblasts of the lamina propria in the subepithelial region of the bladder, white arrows identify nerve fibers, and asterisks show areas of the detrusor muscle. Original magnifications, ×150.
Figure 3.
Effect of acute LPS treatment on PAR expression in the mouse urinary bladder. A: Bar charts show average intensities of immunolabeling for PAR-1 (blue), PAR-2, PAR-3, and PAR-4 in the mouse bladder tissues. B–F: Representative immunohistochemical micrographs show detection of positive control SMA (B, left) and the lack of any immunolabeling using the negative control antibody (B, right) in consecutive sections of mouse bladder tissue. Remaining representative images show immunolabeling patterns for the detection of PAR-1 (C), PAR-2 (D), PAR-3 (E), and PAR-4 (F) expression in serial mouse bladder sections. Large arrowheads identify the urothelium, small arrowheads identify the fibroblasts of the lamina propria in the subepithelial region of the bladder, white arrows identify nerve fibers, and asterisks show areas of the detrusor muscle. Original magnifications, ×150.
Figure 4.
Effect of chronic LPS treatment on PAR expression in the mouse urinary bladder. A: Bar charts show average intensities of immunolabeling for PAR-1 (blue), PAR-2, PAR-3, and PAR-4 in the mouse bladder tissues. B–F: Representative immunohistochemical micrographs show detection of positive control SMA (B, left) and the lack of any immunolabeling using the negative control antibody (B, right) in consecutive sections of mouse bladder tissue. Remaining representative images show immunolabeling patterns for the detection of PAR-1 (C), PAR-2 (D), PAR-3 (E), and PAR-4 (F) expression in serial mouse bladder sections. Large arrowheads identify the urothelium, small arrowheads identify the fibroblasts of the lamina propria in the subepithelial region of the bladder, white arrows identify nerve fibers, and asterisks show areas of the detrusor muscle. Original magnifications, ×150.
Figure 5.
Effect of acute Ag treatment on PAR expression in the sensitized mouse urinary bladder. A: Bar charts show average intensities of immunolabeling for PAR-1 (blue), PAR-2, PAR-3, and PAR-4 in the mouse bladder tissues. B–F: Representative immunohistochemical micrographs show detection of positive control SMA (B, left) and the lack of any immunolabeling using the negative control antibody (B, right) in consecutive sections of mouse bladder tissue. Remaining representative images show immunolabeling patterns for the detection of PAR-1 (C), PAR-2 (D), PAR-3 (E), and PAR-4 (F) expression in serial mouse bladder sections. Large arrowheads identify the urothelium, small arrowheads identify the fibroblasts of the lamina propria in the subepithelial region of the bladder, white arrows identify nerve fibers, and asterisks show areas of the detrusor muscle. Original magnifications, ×150.
Figure 6.

Effect of acute SP treatment on PAR expression in the mouse urinary bladder. A: Bar charts show average intensities of immunolabeling for PAR-1 (blue), PAR-2, PAR-3, and PAR-4 in the mouse bladder tissues. B–F: Representative immunohistochemical micrographs show detection of positive control SMA (B, left) and the lack of any immunolabeling using the negative control antibody (B, right) in consecutive sections of mouse bladder tissue. Remaining representative images show immunolabeling patterns for the detection of PAR-1 (C), PAR-2 (D), PAR-3 (E), and PAR-4 (F) expression in serial mouse bladder sections. Large arrowheads identify the urothelium, small arrowheads identify the fibroblasts of the lamina propria in the subepithelial region of the bladder, white arrows identify nerve fibers, and asterisks show areas of the detrusor muscle. Original magnifications, ×150.
Distribution of the PARs in Control, Saline-Treated, Bladder
As presented in the bar chart (Figure 2A) ▶ , PAR-1, PAR-2, PAR-3, and PAR-4 were mostly co-expressed in the bladder urothelium (Figure 2A ▶ , large arrowheads) with much weaker PAR immunoreactivity in the detrusor muscle and fibroblast cells of the saline-control mice (Figure 2) ▶ . However, PAR-1 and PAR-2 were the predominant receptors in the detrusor muscle (asterisks) over the weakly detectable PAR-3 and PAR-4 (Figure 2) ▶ . Bladder fibroblasts, which did not express detectable PAR-2 immunoreactivity, expressed very low levels of PAR-1, PAR-3, and PAR-4 immunolabeling (Figure 2) ▶ . Interestingly, there was intense PAR-4 immunolabeling on or in the peripheral nerve fibers and plexus cell bodies within the detrusor muscle fibers (Figure 2F ▶ , arrows). In total, we believe these data represent basal levels of PAR expression in the mouse bladder.
Effect of Acute LPS Inflammation on PAR Expression
The summary of the PAR immunolabeling in the acute LPS model of inflammation in the mouse bladder is similarly summarized in a bar chart (Figure 3A) ▶ along with representative images (Figure 3; B to F) ▶ . Generally, the most dramatic changes of the intensity of the PAR immunolabeling occurred in the detrusor muscle (asterisks) and urothelial (large arrowheads) cells (Figure 3) ▶ as compared to the saline-control tissues (Figure 2) ▶ . The intensity of the PAR immunolabeling increased dramatically (from weakly positive to moderate/strong immunolabeling for all four PARs) in the detrusor muscle cells (asterisks). Conversely, the immunolabeling patterns for the PARs (Figure 3) ▶ , generally decreased in the urothelium (large arrowheads; PAR-1, PAR-2, and PAR-4), an observation similarly observed with PCNA immunolabeling (Figure 1C) ▶ . As compared to the saline-control tissues, we did not observe significant changes of PAR expression in the fibroblasts (Figure 3) ▶ .
Effect of Chronic LPS Inflammation on PAR Expression
Data presented in Figure 5 ▶ shows various PAR expression patterns. As compared to the saline-control and acute LPS mouse bladder tissues, the most dramatic change in the PAR immunolabeling was the down-regulation of PAR immunolabeling in the detrusor muscle (asterisks) cells (Figure 4) ▶ . Conversely, despite decreased PAR expression patterns in the detrusor cells there were pronounced increases of PAR immunoreactivity (saline-like levels) in the urothelium cells as compared to the acute LPS model (Figure 4) ▶ . Surprisingly, we observed dramatic increases of PAR expression in the reactive stromal fibroblasts of the lamina propria in the subepithelial region of the chronic LPS mouse bladder model. The up-regulation of the PARs in the urothelium and stromal fibroblasts correlated to the observed increases of PCNA immunolabeling (Figure 1C) ▶ .
PAR Immunoreactivity in the Ag-Challenged Condition
The PAR expression patterns in the Ag-challenged mouse bladder partially mimicked those responses in the acute and chronic LPS mouse model tissues (Figure 5) ▶ . The PAR-immunolabeling patterns of the fibroblasts and detrusor muscle cells resembled those PAR patterns in the acute LPS model of inflammation (Figure 5) ▶ . Conversely, the PAR immunolabeling patterns in the urothelium resembled that of the PAR-immunoreactive patterns in the chronic LPS inflammation model (Figure 5) ▶ . Up-regulation of the PARs in the urothelium resembled those patterns observed for PCNA (Figure 1C) ▶ .
PAR Immunoreactivity in the SP-Challenged Condition
Data presented in Figure 6 ▶ shows a somewhat down-regulation of PAR immunolabeling in most of the cell types in the SP-challenged mouse model. Although the PAR expression patterns resembled the basal, saline-control levels, the immunoreactive patterns in the detrusor muscle were less than that observed in the control tissues for PAR-1 and PAR-2, but similarly resembled the PAR levels in the chronic LPS model suggesting similar consequences in the muscle cells. The PAR expression patterns in the urothelium of the SP-challenged tissues were significantly much less than those observed in the control tissues, and somewhat resembled the PAR-1 and PAR-2 immunolabeling patterns in the acute LPS model tissues.
Collective Analysis of PAR Immunoreactivity
In an effort to compare the data collectively, we organized the PAR expression data in the fibroblasts (Figure 7A) ▶ , detrusor muscle cells (Figure 7B) ▶ , and urothelium (Figure 7C) ▶ as a function of each condition. The bar chart easily shows at a glance the changes of PAR expression patterns in all of the conditions. For example, it became obvious that the changes of PAR expression were dramatically up-regulated in the chronic LPS model for all PARs in the fibroblasts (Figure 7A) ▶ , which correlated to increased PCNA immunolabeling as well (Figure 1C) ▶ . Generally, no remarkable changes were observed in all of the other models of inflammation as compared to the basal levels detected in the saline-control mice stromal fibroblasts (Figure 7A) ▶ . Although the levels of PAR expression appeared at first to be without a trend in the detrusor muscle cells (Figure 7B) ▶ , generally, the PAR-labeling patterns were up-regulated as well but only in the acute LPS and Ag-challenged models of inflammation as compared to the saline-control, chronic LPS and SP conditions. Similarly, the PAR data of the urothelial cells appeared at first without trend, but what appears most dramatic is the down-regulation of the PAR-1 and PAR-2 immunolabeling in the acute LPS and SP-challenged models of inflammation (Figure 7C) ▶ , which was correlated to decrease PCNA immunolabeling (Figure 1C) ▶ . These data suggest multiple compensatory mechanisms of PAR expression in the models of inflammation, most specifically among the urothelial and detrusor muscle cells types. Also, the regulation patterns of the PARs appeared to correlate to the PCNA-immunolabeling patterns in many of the conditions and cell types.
Figure 7.
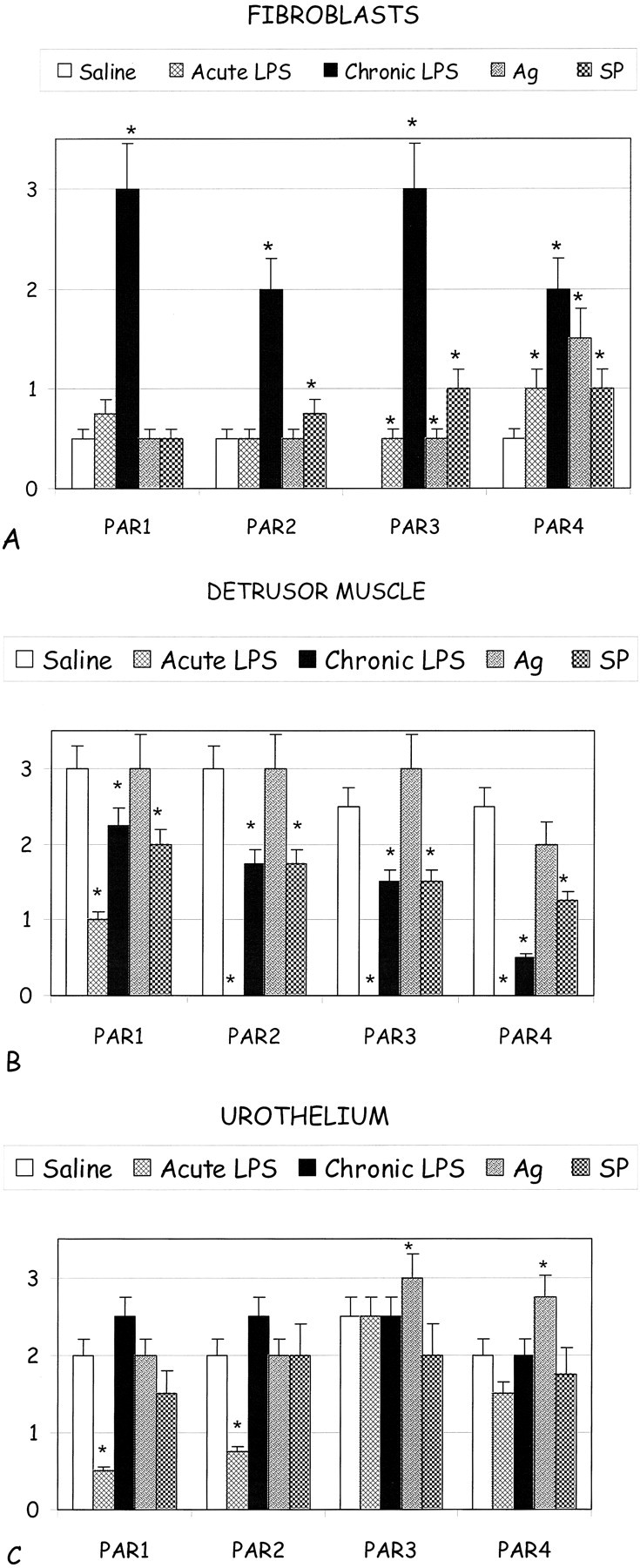
Bar graphs showing comparative PAR-immunolabeling patterns in the various conditions (saline control, acute LPS, chronic LPS, Ag-challenged, SP-challenged) in the fibroblasts of the lamina propria in the subepithelium (A) and in the detrusor muscle (B) and urothelial (C) cells. Asterisks indicate a statistical significant difference (P < 0.05) from bladders treated with saline.
Prominent PAR-4 Expression in Peripheral Nerve Fibers
In an effort to focus on the observation of PAR-4 in the nerve fibers of the detrusor muscle in the bladder, we present a panel of serially sectioned saline-control mouse bladder tissues, which show relatively unremarkable PAR-1 (Figure 8A) ▶ , PAR-2 (Figure 8B) ▶ , and PAR-3 (Figure 8C) ▶ immunolabeling in the same areas of prominent PAR-4 (Figure 8D) ▶ immunolabeling in the nerve fibers (arrowheads). For comparison, note the lack of SMA immunolabeling (Figure 8E) ▶ in the same area of the nerve fibers (arrowheads) as well as the absence of any detectable immunolabeling with the negative control antibody (Figure 8F) ▶ . Additional representative micrographs show similar patterns of PAR-4 immunolabeling in the acute LPS (Figure 8G ▶ , left), chronic LPS (Figure 8G ▶ , middle panel), and Ag-challenged (Figure 8G ▶ , right) tissues (similar data for SP-challenged, data not presented), suggesting that although PAR-4 may be present in the nerve fibers and cell bodies, the expression patterns of PAR-4 did not appear to be affected by conditions of inflammation, which is contrary to the myriad of altering PAR-immunolabeling intensities in the detrusor muscle. The nerve elements expressing PAR-4 (Figure 9A) ▶ were also positively stained with neuronal-specific enolase (Figure 9B) ▶ , but did not express similar labeling patterns of the S100 protein, a marker of myelin sheath (Figure 9C) ▶ , suggesting that PAR-4 immunoreactivity corresponds to peripheral nerve fibers and not the supportive Schwann cell fiber tracks. Furthermore, the same PAR-4-positive fibers did not express glial fibrillary acid protein (data not presented). Figure 9D ▶ shows the lack of any detectable immunolabeling in the absence of primary antibody. Together these results confirmed that, in the mouse urinary bladder, PAR-4 is present in peripheral neurons and not glial elements.
Figure 8.

Representative images (serial sections) of an enlarged region of the detrusor muscle show the lack of remarkable PAR-1 (A), PAR-2 (B), and PAR-3 (C) immunolabeling in the same area with prominent PAR-4 (D) immunolabeling of the nerve fibers (arrowheads). For control, lack of immunolabeling is observed using the α-SMA (E) and negative control (F) antibodies in the identical areas of the nerve fibers (arrowheads). Additional representative images of consistently intense PAR-4 immunolabeling in the nerve bundles and plexus cell bodies (arrowheads) are presented in the mouse bladder throughout the detrusor muscle layer (G), which did not vary in the acute LPS (left), chronic LPS (middle), and Ag-challenged (right) inflammation conditions. Original magnifications: ×600 (A–F); ×300 (G).
Figure 9.
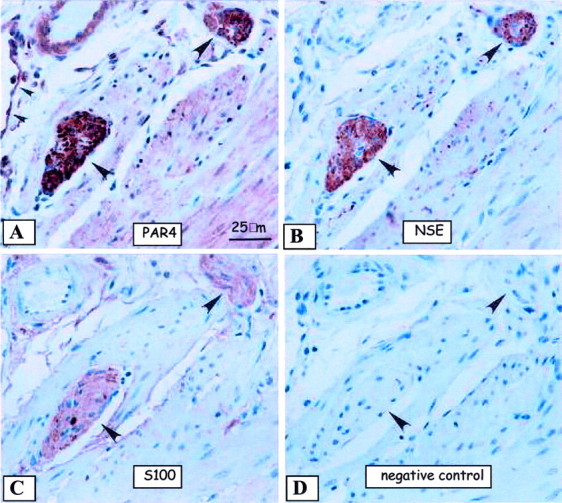
Representative immunohistochemical images to detect neuronal elements of serially sectioned mouse bladder are presented. A: Presence of PAR-4 appears to be in peripheral nerve bundles (large arrowheads). Note the nearby PAR-4-positive endothelium (small arrowheads). B: Presence of neuronal-specific enolase-positive peripheral nerve fibers (large arrowheads) of the same serially sectioned PAR-4-positive (A) fibers confirms the presence of PAR-4 in peripheral nerve. C: The same PAR-4-, neuronal-specific enolase-positive peripheral nerve fibers do not share the same labeling patterns as S100 (large arrowheads). D: The lack of primary antibody shows the lack of any detectable immunolabeling in the same serially sectioned nerve fibers. Original magnifications, ×600.
Discussion
Acute and Chronic Bladder Inflammation
During the acute phase of bladder inflammation, the predominantly migrated cells were neutrophils and mast cells. This migration of inflammatory cells into inflamed bladder is one of the critical events of inflammation. Indeed, we have published comprehensive evidence of an indication for a role for mast cell-sensory nerve communication during bladder inflammation. 2,26-29 Activation of bladder mast cells during inflammation leads to a release of tryptase, which constitutes the major protein released during human mast cell degranulation and accounts for increased tryptase levels in the urine of patients with interstitial cystitis and bladder carcinoma. 1 The observation that tryptase in vitro can cleave and activate PAR suggests a role for this receptor in inflammatory states in humans that are associated with mast cell degranulation. 34 Because bladder mast cells are closely associated with nerves, 35 mast cell proteases, including tryptase, are potential candidates for the activation of PAR on neurons. Taken together, these recent studies have revealed that the local release of tryptase and other proteases can, in principle, cause bladder inflammation by a neurogenic mechanism.
In the subsequent chronic phase, we observed a decrease of mast cell numbers and an increase in macrophages/monocytes. It has been proposed that the chemotactic activity of mast cell-derived chymase may have a role in switching acute inflammation to chronic inflammation. 36 It remains to be determined whether an increase in macrophages is the cause or consequence of a decrease in mast cell numbers observed during chronic inflammation. In addition, as PAR-1 and PAR-2 have been localized in the mast cell membranes, 32 a decrease in mast cell numbers could contribute to a reduced expression of PARs during chronic inflammation.
In addition to cellular infiltrate, we found that PCNA levels in the urothelium are reduced during acute inflammation and restored during chronic LPS stimulation. During chronic inflammation, an increase in PCNA levels was observed both in urothelial as well as stromal cells. PCNA has been used by other investigators to access the status of bladder urothelial cells. During development the mouse bladder presents a gradual increase in PCNA-positive cells in the superficial and basal cell layers of the urothelium. 37 In the adult, bladder urothelium responds to trauma, 38 cyclophosphamide-induced cystitis, 39 and experimental carcinogenesis 40 with alterations in PCNA-positive cells. In this study we did not investigate whether the alterations observed in PCNA were counter balanced by apoptosis. However, our studies indicate that such an approach is warranted. 39
PAR Distribution
Previous results from this laboratory demonstrated that during bladder inflammation, PAR-4 receptor mRNA is up-regulated. 2 In the present study, we showed that all four PARs are present in the mouse bladder, follow a unique distribution, and are differentially modulated by inflammation. The strong expression of PAR receptors in the detrusor muscle indicates the need for further studies on the role of those receptors on bladder function. In addition, PAR expression in bladder fibroblast seems to represent the most dramatic transition between acute and chronic inflammation. PAR receptors were found also in all inflammatory cells in the bladder as well as mast cells and may provide an answer to the question of how mast cell products, such as tryptase, produce signals and induce bladder inflammation. In regard to PAR-1, its widespread tissue distribution, and now urinary tract, predicts a multifactorial role for PAR-1, including neurogenic inflammatory responses 3,10,13,31 and adds another dimension to the potential roles that PAR-1 may play in the setting of bladder pathophysiology. 41 Like PAR-1, mRNA for PAR-2 can be detected in quite a number of tissues including the kidney, with a comparable distribution in murine and human tissues. 8,14 In addition, PAR-2 has been localized on the enteric nerves. 24 PAR-3 IHC was present primarily in the bladder urothelium, with a week presence in the detrusor muscle, and absent from control fibroblast. In the mouse, in addition to urinary bladder, PAR-3 is expressed by megakaryocytes. 6 To date, the expression of PAR-4 in different tissues has not been compared with the other PARs using an immunohistochemical approach. 3 In the mouse bladder PAR-4 was expressed in all layers. The tissue distribution of PAR-4 mRNA in the mouse appears to differ from that in the human, with larger amounts found in spleen and bone marrow but relatively low expression in liver or testis. 42 There may, therefore, be marked differences between species in the distribution of PAR-4, thus, apart from its function in platelets, the diverse physiological role that PAR-4 may play represents a most interesting topic for further investigation.
The present work indicates that primarily PAR-4 is present in high density on bladder nerves. Although, we have not determined the nature of those nerves, others have shown that PARs are present in dorsal root ganglia in cells co-expressing the inflammatory neuropeptides, calcitonin gene-related protein (CGRP) and SP. By measuring CGRP and SP release from superfused spinal dorsal horn and urinary bladder, it has been shown that PAR-2 agonists (eg, PAR-2-APs and trypsin) can stimulate the release of CGRP and SP from the central and peripheral projections of spinal afferent neurons in a Ca2+-dependent manner. 43 In addition, others have found that PAR activation involves the release of an endothelium-derived hyperpolarizing factor, possibly a lipoxygenase-derived eicosanoid, and activation of vanilloid receptors on sensory C fibers. 44
PAR Expression during Acute Inflammation
Our results indicate PARs are differentially modulated during inflammation. After acute inflammation caused by SP, LPS, or Ag, PAR-1 and PAR-2 are down-regulated in acute inflammation whereas PAR-3 and PAR-4 are up-regulated. In the present work, we used SP at high concentration (10 μmol/L). The reason for such a high concentration is that our preliminary results indicated an up-regulation of neutral endopeptidase (NEP) in the bladder of inflamed mice (MR Saban and MR D’Andrea, unpublished observations). A concern is that at high concentrations, SP may elicit effects independent of neurokinin receptor activation. The latter may be the case for even smaller concentrations of SP because its effects on mast cells seems to be independent of NK1 receptor activation. 26
Transition between Acute and Chronic Inflammation
Inflammation is a powerful innate defense against infection and injury but, uncontrolled, it can cause considerable damage. Chronic inflammatory diseases illustrate the importance of the coordinated resolution of inflammation. 45 In some cases, acute bladder inflammation is followed by repair, fibrosis, 46 or loss of function as indicated by a reduced bladder capacity in patients with chronic inflammation. 47 An interesting finding was observed in the present work indicating that bladder fibroblasts presented the most dramatic change in PAR expression during the transition between acute to chronic inflammations. Others have proposed that fibroblasts play a critical role in the switch from acute inflammation to chronic inflammation because of a failure to switch off their inflammatory program. 48
Although much attention has focused on proinflammatory pathways, relatively little is known about the mechanisms that switch off inflammation. We published evidence suggesting the existence of a pathway that is always activated when the bladder mounts an inflammatory response. 2 This pathway should protect the bladder from a noxious stimulus and, once the stimulus ceases, gene expression should down-regulate to avoid tissue damage and chronic inflammation. A possible mechanism for self-limiting inflammation is the down-regulation of receptors such as observed for PAR-1 and PAR-2 in the bladder urothelium. The first hypothesis is that PAR down-regulation in the urothelium is a consequence of the intense vacuolization and shedding of the bladder urothelial cells during LPS-induced acute inflammation. 29 A second hypothesis is that PAR activation leads to receptor down-regulation. Indeed, repeated application of an agonist of PAR-2, such as trypsin, caused marked desensitization of this response, which is due in part to irreversible cleavage of the receptor by trypsin and protein kinase C-mediated termination of signaling. 49 The mechanisms involved in PAR desensitization and internalization were recently reviewed and seems to involve the dynamin- and clathrin-dependent pathway that is independent of arrestins. 50,51 Thus, cleavage of surface receptors by thrombin, tryptase, or trypsin renders cells unresponsive to proteases for considerable periods, until the plasma membrane is replenished with intact receptors. 9 PAR is rapidly resensitized by mechanisms that depend on de novo synthesis of new protein as well as on trafficking of preformed receptors from intracellular pools such as Golgi stores. 52,53 Down-regulation of PAR-2 may alter the course of bladder inflammation because PAR-2 ligands have been proposed to have an anti-inflammatory effect in the treatment of inflammatory bowel diseases. 54,55 Whether specific stimulation of PAR-2 would also decrease bladder inflammation remains to be determined. Nevertheless, our results strongly suggest that both protein and RNA should be investigated in separate layers of urothelium, fibroblasts, and detrusor muscle.
Implications of Our Findings to Bladder Inflammatory Responses
A strong body of evidence has described a role for PARs in the inflammatory process in several tissues and organs. The present work is the first to demonstrate that all four PARs are present in the urinary bladder and that their expressions are altered during inflammation and injury. In addition, the inflammatory cells themselves, whenever present in the bladder tissue, expressed PAR immunoreactivity. The latter is in agreement with findings indicating that PAR-2 is highly expressed on inflammatory cells such as neutrophils and mast cells, where it might play a role in cell activation and degranulation. 5,56 Furthermore, the fact that LPS, an Escherichia coli endotoxin, induced alteration in PAR expression is in agreement with an emerging body of evidence implicating PARs as part of the body’s defense to invading pathogens. 5,57 In this context, the findings that the PAR-1 cascade co-modulates the inflammatory responses to activated protein C further implicate a role for PARs in sepsis and infections. 58
Conclusion
The present results confirm our previous findings that indicate that during bladder inflammation PAR-4 mRNA is up-regulated. 2 In the present study, we provide further evidence that all four PARs are present in the mouse bladder urothelium whereas PAR-1 and PAR-2 are found in the detrusor smooth muscle. In addition, PAR-4 is expressed in peripheral nerves and plexus cell bodies. The strong expression of PARs in the detrusor muscle indicates the need for studies on the role of these receptors in motility whereas the presence of PAR-4 in nerves may indicate its participation in neurogenic inflammation, as previously suggested. 13 In addition, PARs are differentially modulated during inflammation. As mast cells and tryptase are common features of intense bladder remodeling observed in cancer 1 and interstitial cystitis, 59 it is tempting to suggest that tryptase generated during the mast cell degranulation along with an up-regulation of PA receptors may contribute to abnormal neurotransmission and motility in the inflamed bladder. Future studies using mice deficient in genes encoding PARs should provide new insights into the role of proteases and PARs in inflammation and pain.
Acknowledgments
We thank Patti A. Reiser, BS, MT, HT (ASCP); Norah A. Gumula, HT (ASCP); Brenda M. Hertzog, BS, MT (ASCP); Debbie Polkovitch, BS, HT (ASCP); and Barbara Branchide of the Johnson & Johnson Pharmaceutical Research and Development’s MorphoMetrics department for excellent histological and immunohistochemical technical expertise.
Footnotes
Address reprint requests to Ricardo Saban, D.V.M., Ph.D., Associate Professor, Department of Physiology, College of Medicine, Oklahoma University Health Sciences Center, 940 SL Young Blvd., Room 605, Oklahoma City, OK 43104. E-mail: ricardo-saban@ouhsc.edu.
Supported by the National Institutes of Health (grant DK 55828-01 to R. S.).
References
- 1.Okragly A, Niles A, Saban R, Schmidt D, Hoffman R, Warner T, Moon T, Uehling D, Haak-Frendscho M: Elevated tryptase, NGF, NT-3, and GDNF levels in the urine of interstitial cystitis and bladder cancer patients. J Urol 1999, 161:434-441 [PubMed] [Google Scholar]
- 2.Saban M, Nguyen N-B, Hammond T, Saban R: Gene expression profiling of mouse bladder inflammatory responses to LPS, substance P, and antigen-stimulation. Am J Pathol 2002, 160:2095-2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollenberg D, Compton S: International Union of Pharmacology. XXVIII. Proteinase-Activated Receptors. Pharmacol Rev 2002, 54:203-217 [DOI] [PubMed] [Google Scholar]
- 4.Vergnolle N, Wallace JL, Bunnett NW, Hollenberg MD: Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol Sci 2001, 22:146-152 [DOI] [PubMed] [Google Scholar]
- 5.Vergnolle N: Proteinase-activated receptors—novel signals for gastrointestinal pathophysiology. Aliment Pharmacol Ther 2000, 14:257-266 [DOI] [PubMed] [Google Scholar]
- 6.Ishihara H, Connolly AJ, Zeng D, Kahn ML, Zheng YW, Timmons C, Tram T, Coughlin SR: Protease-activated receptor 3 is a second thrombin receptor in humans. Nature 1997, 386:502-506 [DOI] [PubMed] [Google Scholar]
- 7.Xu WF, Andersen H, Whitmore TE, Presnell SR, Yee DP, Ching A, Gilbert T, Davie EW, Foster DC: Cloning and characterization of human protease-activated receptor 4. Proc Natl Acad Sci USA 1998, 95:6642-6646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asokananthan N, Graham PT, Fink J, Knight DA, Bakker AJ, McWilliam AS, Thompson PJ, Stewart GA: Activation of protease-activated receptor (PAR)-1, PAR-2, and PAR-4 stimulates IL-6, IL-8, and prostaglandin E2 release from human respiratory epithelial cells. J Immunol 2002, 168:3577-3585 [DOI] [PubMed] [Google Scholar]
- 9.Macfarlane S, Seatter M, Kanke T, Hunter G, Plevin R: Proteinase-activated receptors. Pharmacol Rev 2001, 53:245-282 [PubMed] [Google Scholar]
- 10.Carr MJ, Schechter NM, Undem BJ: Trypsin-induced, neurokinin-mediated contraction of guinea pig bronchus. Am J Respir Crit Care Med 2000, 162:1662-1667 [DOI] [PubMed] [Google Scholar]
- 11.Cunningham MA, Rondeau E, Chen X, Coughlin SR, Holdsworth SR, Tipping PG: Protease-activated receptor 1 mediates thrombin-dependent, cell-mediated renal inflammation in crescentic glomerulonephritis. J Exp Med 2000, 191:455-462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawabata A, Kuroda R, Nagata N, Kawao N, Masuko T, Nishikawa H, Kawai K: In vivo evidence that protease-activated receptors 1 and 2 modulate gastrointestinal transit in the mouse. Br J Pharmacol 2001, 133:1213-1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Garavilla L, Vergnolle N, Young SH, Ennes H, Steinhoff M, Ossovskaya VS, D’Andrea MR, Mayer EA, Wallace JL, Hollenberg MD, Andrade-Gordon P, Bunnett NW: Agonists of proteinase-activated receptor 1 induce plasma extravasation by a neurogenic mechanism. Br J Pharmacol 2001, 133:975-987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nystedt S, Emilsson K, Wahlestedt C, Sundelin J: Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci USA 1994, 91:9208-9212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton JR, Frauman AG, Cocks TM: Increased expression of protease-activated receptor-2 (PAR2) and PAR4 in human coronary artery by inflammatory stimuli unveils endothelium-dependent relaxations to PAR2 and PAR4 agonists. Circ Res 2001, 89:92-98 [DOI] [PubMed] [Google Scholar]
- 16.Bretschneider E, Kaufmann R, Braun M, Nowak G, Glusa E, Schror K: Evidence for functionally active protease-activated receptor-4 (PAR-4) in human vascular smooth muscle cells. Br J Pharmacol 2001, 132:1441-1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carney DH, Mann R, Redin WR, Pernia SD, Berry D, Heggers JP, Hayward PG, Robson MC, Christie J, Annable C, Fenton JW, II, Glenn KC: Enhancement of incisional wound healing and neovascularization in normal rats by thrombin and synthetic thrombin receptor-activating peptides. J Clin Invest 1992, 89:1469-1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dabbagh K, Laurent GJ, McAnulty RJ, Chambers RC: Thrombin stimulates smooth muscle cell procollagen synthesis and mRNA levels via a PAR-1 mediated mechanism. Thromb Haemost 1998, 79:405-409 [PubMed] [Google Scholar]
- 19.Stiernberg J, Redin WR, Warner WS, Carney DH: The role of thrombin and thrombin receptor activating peptide (TRAP-508) in initiation of tissue repair. Thromb Haemost 1993, 70:158-162 [PubMed] [Google Scholar]
- 20.Chambers RC, Dabbagh K, McAnulty RJ, Gray AJ, Blanc-Brude OP, Laurent GJ: Thrombin stimulates fibroblast procollagen production via proteolytic activation of protease-activated receptor 1. Biochem J 1998, 333:121-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chambers RC, Leoni P, Blanc-Brude OP, Wembridge DE, Laurent GJ: Thrombin is a potent inducer of connective tissue growth factor production via proteolytic activation of protease-activated receptor-1. J Biol Chem 2000, 275:35584-35591 [DOI] [PubMed] [Google Scholar]
- 22.Vliagoftis H, Schwingshackl A, Milne CD, Duszyk M, Hollenberg MD, Wallace JL, Befus AD, Moqbel R: Proteinase-activated receptor-2-mediated matrix metalloproteinase-9 release from airway epithelial cells. J Allergy Clin Immunol 2000, 106:537-545 [DOI] [PubMed] [Google Scholar]
- 23.Corvera CU, Dery O, McConalogue K, Bohm SK, Khitin LM, Caughey GH, Payan DG, Bunnett NW: Mast cell tryptase regulates rat colonic myocytes through proteinase-activated receptor 2. J Clin Invest 1997, 100:1383-1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Andrea MR, Rogahn CJ, Andrade-Gordon P: Localization of protease-activated receptors-1 and -2 in human mast cells: indications for an amplified mast cell degranulation cascade. Biotech Histochem 2000, 75:85-90 [DOI] [PubMed] [Google Scholar]
- 25.Haak-Frendscho M, Saban R, Shields RL, Jardieu PM: Anti-immunoglobulin E antibody treatment blocks histamine release and tissue contraction in sensitized mice. Immunology 1998, 94:115-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saban R, Gerard N, Saban M, Nguyen N-B, DeBoer D, Wershil B: Mast cells mediate substance P-induced bladder inflammation through a neurokinin-1 receptor-independent mechanism. Am J Physiol 2002, 160:F2095-F2110 [DOI] [PubMed] [Google Scholar]
- 27.Saban R, Saban M, Nguyen N-B, Lu B, Gerard C, Gerard N, Hammond T: Neurokinin-1 (NK-1) receptor is required in antigen-induced cystitis. Am J Pathol 2000, 156:775-780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saban M, Hellmich H, Nguyen N-B, Winston J, Hammond T, Saban R: Time course of LPS-induced gene-expression in a mouse model of genitourinary inflammation. Physiol Genomics 2001, 5:147-116 [DOI] [PubMed] [Google Scholar]
- 29.Saban M, Saban R, Hammond T, Haak-Frendscho M, Steinberg H, Tengowski M, Bjorling D: LPS-sensory peptide communication in experimental cystitis. Am J Physiol 2002, 282:F202-F210 [DOI] [PubMed] [Google Scholar]
- 30.Luna L: Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. 1992:p 311 American Histolabs, Inc., Publications Division, Gaithersburg
- 31.D’Andrea MR, Derian CK, Santulli RJ, Andrade-Gordon P: Differential expression of protease-activated receptors-1 and -2 in stromal fibroblasts of normal, benign, and malignant human tissues. Am J Pathol 2001, 158:2031-2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Andrea MR, Derian CK, Leturcq D, Baker SM, Brunmark A, Ling P, Darrow AL, Santulli RJ, Brass LF, Andrade-Gordon P: Characterization of protease-activated receptor-2 immunoreactivity in normal human tissues. J Histochem Cytochem 1998, 46:157-164 [DOI] [PubMed] [Google Scholar]
- 33.Lourbakos A, Potempa J, Travis J, D’Andrea MR, Andrade-Gordon P, Santulli R, Mackie EJ, Pike RN: Arginine-specific protease from Porphyromonas gingivalis activates protease-activated receptors on human oral epithelial cells and induces interleukin-6 secretion. Infect Immun 2001, 69:5121-5130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molino M, Barnathan ES, Numerof R, Clark J, Dreyer M, Cumashi A, Hoxie JA, Schechter N, Woolkalis M, Brass LF: Interactions of mast cell tryptase with thrombin receptors and PAR-2. J Biol Chem 1997, 272:4043-4049 [DOI] [PubMed] [Google Scholar]
- 35.Keith IM, Jin J, Saban R: Nerve-mast cell interaction in normal guinea pig urinary bladder. J Comp Neurol 1995, 363:28-36 [DOI] [PubMed] [Google Scholar]
- 36.Tani K, Ogushi F, Shimizu T, Sone S: Protease-induced leukocyte chemotaxis and activation: roles in host defense and inflammation. J Med Invest 2001, 48:133-141 [PubMed] [Google Scholar]
- 37.Erman A, Jeyernik K: Mouse urothelial cells in early postnatal development—proliferation and apical plasma membrane specialization. Pfluegers Arch 2000, 440:R183-R184 [PubMed] [Google Scholar]
- 38.Li B, Kanamaru H, Saikawa S, Fukuda M, Okada K: Increased cell proliferation of urothelium in rat bladder following unilateral nephrectomy. J Urol 1997, 158:265-268 [DOI] [PubMed] [Google Scholar]
- 39.Romih R, Koprivec D, Martincic DS, Jezernik K: Restoration of the rat urothelium after cyclophosphamide treatment. Cell Biol Int 2001, 25:531-537 [DOI] [PubMed] [Google Scholar]
- 40.Nakanishi K, Kawai T, Aida S, Kasamatsu H, Aurues T, Ikeda T: Expression of p27(Kip1) protein in transitional cell carcinoma of the upper urinary tract. Mod Pathol 2001, 14:371-376 [DOI] [PubMed] [Google Scholar]
- 41.Coughlin S: Thrombin receptor function and cardiovascular disease. Trends Cardiovasc Med 1994, 4:77-83 [DOI] [PubMed] [Google Scholar]
- 42.Kahn ML, Zheng YW, Huang W, Bigornia V, Zeng D, Moff S, Farese RV, Jr, Tam C, Coughlin SR: A dual thrombin receptor system for platelet activation. Nature 1998, 394:690-694 [DOI] [PubMed] [Google Scholar]
- 43.Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, Trevisani M, Hollenberg MD, Wallace JL, Caughey GH, Mitchell SE, Williams LM, Geppetti P, Mayer EA, Bunnett NW: Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med 2000, 6:151-158 [DOI] [PubMed] [Google Scholar]
- 44.McLean PG, Aston D, Sarkar D, Ahluwalia A: Protease-activated receptor-2 activation causes EDHF-like coronary vasodilation: selective preservation in ischemia/reperfusion injury: involvement of lipoxygenase products, VR1 receptors, and C-fibers. Circ Res 2002, 90:465-472 [DOI] [PubMed] [Google Scholar]
- 45.Bell J: Inflammation: dousing the flames. Nat Rev Immunol 2001, 1:173 [Google Scholar]
- 46.Lemack GE, Zimmern PE: Interstitial cystitis: reevaluation of patients who do no respond to standard treatments. Prog Urol 2001, 11:239-244 [PubMed] [Google Scholar]
- 47.Messing E: Interstitial cystitis and related syndromes. Walsh P Retik A Stamey T Vaughan JE eds. Campbell’s Urology. 1992:p 982 W. B. Saunders Co., Philadelphia
- 48.Buckley CD, Pilling D, Lord JM, Akbar AN, Scheel-Toellner D, Salmon M: Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol 2001, 22:199-204 [DOI] [PubMed] [Google Scholar]
- 49.Bohm SK, Kong W, Bromme D, Smeekens SP, Anderson DC, Connolly A, Kahn M, Nelken NA, Coughlin SR, Payan DG, Bunnett NW: Molecular cloning, expression and potential functions of the human proteinase-activated receptor-2. Biochem J 1996, 314:1009-1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paing MM, Stutts AB, Kohout TA, Lefkowitz RJ, Trejo J: Beta-arrestins regulate protease-activated receptor-1 desensitization but not internalization or down-regulation. J Biol Chem 2002, 277:1292-1300 [DOI] [PubMed] [Google Scholar]
- 51.Trejo J, Altschuler Y, Fu HW, Mostov KE, Coughlin SR: Protease-activated receptor-1 down-regulation: a mutant HeLa cell line suggests novel requirements for PAR1 phosphorylation and recruitment to clathrin-coated pits. J Biol Chem 2000, 275:31255-31265 [DOI] [PubMed] [Google Scholar]
- 52.Dery O, Corvera CU, Steinhoff M, Bunnett NW: Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am J Physiol 1998, 274:C1429-C1452 [DOI] [PubMed] [Google Scholar]
- 53.Bohm SK, Khitin LM, Grady EF, Aponte G, Payan DG, Bunnett NW: Mechanisms of desensitization and resensitization of proteinase-activated receptor-2. J Biol Chem 1996, 271:22003-22016 [DOI] [PubMed] [Google Scholar]
- 54.Fiorucci S, Distrutti E: Role of PAR2 in pain and inflammation. Trends Pharmacol Sci 2002, 23:153-155 [DOI] [PubMed] [Google Scholar]
- 55.Fiorucci S, Mencarelli A, Palazzetti B, Distrutti E, Vergnolle N, Hollenberg MD, Wallace JL, Morelli A, Cirino G: Proteinase-activated receptor 2 is an anti-inflammatory signal for colonic lamina propria lymphocytes in a mouse model of colitis. Proc Natl Acad Sci USA 2001, 98:13936-13941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saban R, Undem BJ, Keith IM, Saban MR, Tengowski MW, Graziano FM, Bjorling DE: Differential release of prostaglandins and leukotrienes by sensitized guinea pig urinary bladder layers upon antigen challenge. J Urol 1994, 152:544-549 [DOI] [PubMed] [Google Scholar]
- 57.Hollenberg M: Protease-mediated signaling: new paradigms for cell regulation and drug development. Trends Pharmacol Sci 1996, 17:3-6 [DOI] [PubMed] [Google Scholar]
- 58.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W: Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science 2002, 296:1880-1882 [DOI] [PubMed] [Google Scholar]
- 59.Tomaszewski J, Landis J, Russack V, Williams T, Wang L, Hardy C, Brensinger C, Matthews Y, Abele S, Kusek J, Nyberg L: The Interstitial Cystitis Database Study Group. Biopsy features are associated with primary symptoms in interstitial cystitis: results from the interstitial cystitis database study. Urology 2001, 57:67-81 [DOI] [PubMed] [Google Scholar]


