Abstract
To investigate the potential involvement of the nitric oxide (NO) pathway in colorectal carcinogenesis, we correlated the expression and the activity of inducible nitric oxide synthase (iNOS) with the degree of tumor angiogenesis in human colorectal cancer. Tumor samples and adjacent normal mucosa were obtained from 46 surgical specimens. Immunohistochemical expression of iNOS, vascular endothelial growth factor (VEGF), and CD31 was analyzed on paraffin-embedded tissue sections. iNOS activity and cyclic GMP levels were assessed by specific biochemical assays. iNOS protein expression was determined by Western blot analysis. iNOS and VEGF mRNA levels were evaluated using Northern blot analysis. Both iNOS and VEGF expressions correlated significantly with intratumor microvessel density (rs = 0.31, P = 0.02 and rs = 0.67, P < 0.0001, respectively). A significant correlation was also found between iNOS and VEGF expression (P = 0.001). iNOS activity and cyclic GMP production were significantly higher in the cancer specimens than in the normal mucosa (P < 0.0001 and P < 0.0001, respectively), as well as in metastatic tumors than in nonmetastatic ones (P = 0.002 and P = 0.04, respectively). Western and Northern blot analyses confirmed the up-regulation of the iNOS protein and gene in the tumor specimens as compared with normal mucosa. NO seems to play a role in colorectal cancer growth by promoting tumor angiogenesis.
Nitric oxide (NO) is a bioactive agent that mediates a number of actions, such as vasodilatation, neurotransmission, and immune response. 1-3 NO is synthesized by a family of three NO synthase (NOS) isoenzymes that convert l-arginine into l-citrulline in the presence of molecular oxygen yielding free NO. 4,5 The endothelial NOS and the neuronal NOS are Ca2+ and calmodulin-dependent isoforms and are constitutively expressed. The third isoform, inducible NOS (iNOS), is Ca2+-independent and requires induction in response to cytokines and proinflammatory agents. Only iNOS is capable of producing sustained NO concentrations in the micromolar range. 6 NO released by iNOS is generated by the cells of the immune system among others and it has been shown to be both cytostatic and cytotoxic to tumor cells and a variety of microorganisms. 7
Recent studies have investigated the expression and the activity of iNOS in human cancer. An increased level of iNOS expression and/or activity has been found in the tumor cells of gynecological malignancies, 8 in the stroma of breast cancer, 9 and in the tumor cells of head and neck cancer. 10,11 Although iNOS expression and activity have been demonstrated even in colorectal cancer, results are still controversial. Several studies on this subject have shown an increase of iNOS expression in tumor tissue when compared to normal mucosa. 12-17 However, opposite results have been found by Moochhala and colleagues. 18 Contrasting findings have been found even regarding the cellular localization of iNOS within the tumor samples. Some studies 14-17 have shown the presence of iNOS expression in tumor epithelial cells, whereas Ambs and colleagues 12,13 demonstrated that iNOS is expressed mainly by the inflammatory mononuclear cells that infiltrate colorectal adenomas and adenocarcinomas.
A specific cause-effect relationship between the up-regulation of the iNOS gene and colon cancer has been recently demonstrated by Ahn and Oshima. 19 The knocking out of the iNOS gene in APC (Min/+) mice (an animal model for human adenomatous polyposis) resulted in a marked reduction in the number of intestinal polyps. This observation indicates that iNOS and NO may play a relevant role in colorectal carcinogenesis. It is likely that several molecular mechanisms are involved. It is known that NO reaction with oxygen or superoxide may result in toxic compounds capable of causing tissue damage and genotoxicity. 20 Direct damage includes DNA base deamination and single-strand breaks in DNA. All these alterations may have potential carcinogenic effects.
A number of in vitro and in vivo investigations indicate the possible involvement of NO in promoting tumor angiogenesis. 21 It has been demonstrated that NO induces endothelial cell growth and regulates the tumor blood flow. 22 Moreover, vascular endothelial growth factor (VEGF)-mediated angiogenesis is dependent on NO production and requires the activation of the NO/cGMP pathway within the endothelial compartment. 23,24
An indirect promotion of tumor angiogenesis through the activation of the cyclooxygenase (COX) enzyme pathway may also be hypothesized for NO. Peroxynitrite, the coupling product of NO and superoxide, activates COX activity and prostaglandin biosynthesis. 25 We have recently shown the relationship between COX-2 overexpression and tumor angiogenesis in colorectal cancer. 26
The aim of this study was to determine the expression of iNOS and its activity in human colorectal tumors. To investigate the possible role of the NO pathway in tumor angiogenesis, these two parameters were correlated with tumor stage, intratumor microvessel density (MVD), the production of cGMP and prostaglandin E2 (PGE2), and the expression of VEGF and nitrotyrosine (NT), which is a marker of peroxynitrite.
Materials and Methods
Patients and Tissue Collection
Tissue samples were obtained from 46 patients (20 males, 26 females; median age, 66 years; age range, 46 to 81 years) who had consecutively undergone surgical resections for primary sporadic colorectal adenocarcinomas at the Department of General Surgery, University of Florence, Florence, Italy. All patients were thoroughly informed about the aims of the study and gave written consent for the investigation in accordance with the ethical guidelines of the University of Florence. Nine tumors were located in the proximal colon (up to the splenic flexure), 20 in the distal colon, and 17 in the rectum. Forty tumors were classified as adenocarcinomas and six tumors were classified as mucinous carcinomas (when more than 50% of the tumor volume was composed of mucin). Adenocarcinomas were classified according to the World Health Organization classification 27 as well differentiated (n = 1), moderately differentiated (n = 34), and poorly differentiated (n = 5). Tumors were classified into four stages according to the American Joint Committee on Cancer staging system: 28 stage I (T1-T2, N0, M0) (n = 5); stage II (T3-T4, N0, M0) (n = 19); stage III (any T, N1–2, M0) (n = 18), and stage IV (any T, any N, M1) (n = 4). Cancer tissue (from the edge of the tumor) and adjacent normal mucosa (at least 10 cm from the tumor) were excised from each surgical specimen. The samples were washed in phosphate-buffered saline (PBS) and then flash-frozen in liquid nitrogen for Northern blot analysis; frozen at −80°C for Western blot analysis; and frozen at −20°C for iNOS activity, cGMP, and PGE2 production evaluation until processing. Other samples were fixed in 4% formaldehyde and embedded in paraffin for immunohistochemical analysis.
iNOS activity, cGMP levels, and PGE2 production were also evaluated in normal colon mucosa biopsies from 10 patients (controls) (seven males, three females; median age, 53 years; age range, 18 to 65 years) who had undergone colonoscopy for altered bowel habits or abdominal pain. Four biopsies were obtained from the proximal colon, three from the distal colon, and three from the rectum. These patients had also given written consent regarding the investigation.
Immunohistochemistry
Four-μm-thick sections were cut from the formalin-fixed and paraffin-embedded tissue blocks and processed as previously described. 26 Immunohistochemical staining was performed using the streptavidin-biotin-peroxidase method. Staining for CD31, an endothelial antigen, was used to highlight the endothelial cells and determine MVD. The following antibodies were used: a monoclonal mouse antibody (JC 70; DAKO, Milan, Italy) for CD31 at a 1:10 dilution at room temperature for 30 minutes; two different rabbit polyclonal antibodies for iNOS, one from Alexis (Läufelfingen, Switzerland) at a 1:400 dilution at 4°C overnight, the other from Biomol (Plymouth Meeting, PA) at a 1:600 dilution at 4°C overnight, and an affinity-purified rabbit polyclonal antibody (A-20; Santa Cruz Biotechnology, Santa Cruz, CA) for VEGF at a 1:100 dilution at room temperature for 1 hour. Ten sections underwent immunostaining with a rabbit polyclonal antibody against NT (Upstate Biotechnology, Buckingham, UK) at a 1:120 dilution at 4°C overnight. Positive controls for iNOS and VEGF immunostaining included fragments of inflammatory nasal polyps and breast carcinoma, respectively. Tissue sections treated with nonimmune rabbit serum in place of the primary antibodies were used as negative controls.
Evaluation of Immunostaining and Microvessel Counting
Two pathologists (LM and SN) independently evaluated the immunostained specimens. The extent of iNOS, VEGF, and NT immunostaining was recorded semiquantitatively using a three-grade system, based on the percentage of stained tumor epithelial cells: grade 0, 1 to 20%; grade 1, 21 to 70%; grade 2, more than 70%. Because iNOS is known to be expressed in inflammatory cells, 12,14 we also evaluated the percentage of tumor-infiltrating inflammatory cells that express iNOS.
MVD was evaluated according to the method first described by Weidner and colleagues. 29 The entire tumor section was first carefully scanned at low magnification (×100) to find the areas that showed the most intense neovascularization, ie, the highest density of CD31-positive cells (hot spots). The hot spots were included in the MVD counts but only if they had been in the stroma surrounded by malignant glands and if they had not been found within any areas of granulation tissue, such as those near the surface of ulcerated tumors. Individual microvessels in each hot spot were then counted in a single ×250 field. Any immunoreactive endothelial cell or endothelial cell cluster that was clearly separated from adjacent microvessels was considered as a single countable vessel. No vessel lumina or red blood cells were used to define a microvessel. The occasionally found CD31-positive lymphocytes, macrophages, and plasma cells were excluded on the basis of the staining pattern and cell morphology. MVD in each tumor was expressed as the microvessel count of the hot spot with the highest number of microvessels.
Assay for NOS Activity
Fragments of tissue were homogenized at 0 to 4°C in buffer containing 0.32 mol/L sucrose, 20 mmol/L HEPES (pH 7.2), 0.5 mol/L EDTA, and 1 mmol/L dithiothreitol. Total NOS activity was measured in tissue homogenates by the [3H]arginine conversion assay, as previously described. 30 The activity of the calcium-calmodulin-independent isoform (iNOS) was identified in the supernatant of tumor homogenates by measuring the enzymatic activity in buffer without calcium and containing 1 mmol/L of EGTA and the calmodulin inhibitor trifluoperazine (100 μmol/L) as previously reported. 30 Protein concentration in the tissue samples was determined according to the method described by Lowry and colleagues. 31 Bovine serum albumin was used as the standard. iNOS activity was expressed as pmol of [3H]citrulline formed per minute per mg protein.
Measurement of cGMP Content
cGMP levels were measured in the aqueous phase of the tissue homogenates extracted from 10% trichloroacetic acid with 0.5 mol/L tri-n-octylamine dissolved in 1,1,2-trichlorotrifluoroethane. cGMP was measured with commercially available radioimmunoassay kits (Amersham, Buckinghamshire, UK). The assay is based on the competition between unlabeled cGMP and [125I]cGMP for binding to a limited quantity of an antibody raised with a high specificity to cGMP. 32,33 Determinations were performed in duplicate. cGMP values were expressed as pmol per minute per μg protein.
Measurement of PGE2 Production
Tissue fragments were homogenized at 0 to 4°C in the presence of 10 μmol/L of indomethacin so as to prevent PG production during the procedure and then centrifuged at 600 × g. Five hundred μl of supernatant were used for PGE2 determination using a specific radioimmunoassay. 34 Protein concentration in the tissue samples was determined according to the method described by Lowry and colleagues. 31 Bovine serum albumin was used as the standard. PGE2 values were expressed as μg/mg protein in the tissue samples.
Western Blot Analysis
Tumor and normal mucosa samples were processed as previously described. 26 The samples were mixed 1:1 with sample buffer (20 mmol/L Tris-HCl , pH 6.8, 20% glycerol, 2% sodium dodecyl sulfate, 5% β-mercaptoethanol, and 0.025% bromphenol blue) and boiled. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed using 8% and 5% acrylamide for the separating gel and the stacking gel, respectively. Proteins were transferred onto nitrocellulose membranes (Pierce Chemical Co., Rockford, IL). Blots were blocked with Blocker bovine serum albumin 5% in PBS (Pierce Chemical Co.) and incubated overnight at 4°C with a human polyclonal antibody for inducible iNOS (Chemicon International, Inc., Temecula, CA) at 1:1000 dilution. Signal detection was performed as previously described. 26
Human colon cancer cell line SW620 constitutively expresses a high level of iNOS. 35 These cells served as a positive control. No iNOS expression was demonstrated in WiDr cells. 35 These cells served as a negative control. Cells were processed and analyzed as previously described. 36
Northern Blot Analysis
Total RNA from tumor and normal mucosa specimens was extracted using the guanidinium-isothiocyanate method and analysis was performed as previously described. 26 As iNOS and VEGF165 cDNA probes, we used polymerase chain reaction product fragments obtained by amplification of sequences obtained from the National Institutes of Health–GenBank. The primers used were: iNOS sense, 5′-GTCTTGGTCAAAGCTGTGCTC-3′ and iNOS anti-sense, 5′-CAAAGGCTGTGAGTCCTGCA-3′, which gave rise to a 633-bp product; VEGF sense, 5′ CCGGTCGGGCCTCCGAAACCATGAACTTTCT-3′ and VEGF anti-sense, 5′-TCACCGCCTCGGCTTGTCACATCTGCAAGT-3′, which gave rise to a 590-bp product. These products were then purified using the QIAquick Gel Extraction kit (Qiagen Inc., Valencia, CA). Each probe was labeled with α-[32P]dCTP, using random priming reaction (Boehringer Mannheim, Mannheim, Germany). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe was used as an internal control to adjust for differences in the amount of RNA loaded in each lane. It was obtained by a polymerase chain reaction using the following primer: GAPDH sense, 5′-CCATGGAGAAGGCTGGGG-3′ and GAPDH anti-sense, 5′-CAAAGTTGTCATGGATGACC-3′ that gave rise to a 196-bp product. The filters were exposed to Kodak Xar-5 film (Eastman Kodak, Rochester, NY) for autoradiography for 1 to 6 days at −80°C. The level of expression in the tissue samples was assessed using a GS-670 Imaging Densitometer (Bio-Rad, Hercules, CA). The densitometric percentage of the autoradiographic signal was evaluated according to Pertschuk and colleagues. 37
SW620 and WiDr cells served as iNOS-positive and -negative controls, respectively. 35 Constitutive expression of VEGF has been reported in human colon cancer cell line HCT116. 38 HCT116 cells served as a VEGF-positive control. Low expression of VEGF has been reported in LoVo cells. 39 LoVo cells served as a VEGF-negative control. Cells were processed and analyzed as previously described. 36
Statistical Analysis
MVD values were reported as mean values ± SE. The relationships among MVD, iNOS activity, cGMP levels, PGE2 production, iNOS, NT, and VEGF immunostaining were evaluated using the Spearman correlation coefficients (rs). iNOS activity, cGMP production, and PGE2 levels were expressed as median values and range. Differences in iNOS activity, cGMP production, and PGE2 levels in tumor and normal mucosa specimens and in tumors with and without metastases were analyzed using the Mann-Whitney test. Statistical analysis was performed using Stata Statistic Software (release 5.0; Stata Corporation, College Station, TX). All P values resulted from the use of two-sided statistical tests; P values that were less than 0.05 were considered statistically significant.
Results
Correlations among iNOS, VEGF, and CD31 Immunoreactivity
The source of iNOS was determined by immunohistochemistry with two different polyclonal antibodies. No significant differences were observed concerning immunostaining extent and distribution with the two antibodies tested. iNOS was observed mainly in the tumor epithelial cells where it was localized within the cytoplasm. Most of the tumors showed extensive staining for iNOS: 11 tumors (23.9%) were grade 0, 13 (28.7%) were grade 1, and 22 (47.8%) were grade 2 (Figure 1; A, C, and E) ▶ . iNOS staining was homogeneous within each tumor section. iNOS was also found in tumor-infiltrating inflammatory cells. The staining of inflammatory infiltrate was classified as grade 0 in 19 tumors (41.3%), grade 1 in 18 (39.2%), and grade 2 in 9 (19.5%). Normal mucosa specimens were negative for iNOS staining.
Figure 1.

iNOS and VEGF immunostaining. Successive sections of three representative cancer specimens classified as grade 0 (A, iNOS; B, VEGF), grade 1 (C, iNOS; D, VEGF) and grade 2 (E, iNOS; F, VEGF). iNOS immunoreactivity was detected mainly within the cytoplasm of the tumor epithelial cells. Hematoxylin counterstain; original magnifications, ×250.
VEGF staining was found in both the cytoplasm and the membranes of the tumor epithelial cells. Most of the tumors showed extensive staining for VEGF: 10 tumors (21.8%) were grade 0, 18 (39.1%) were grade 1, and 18 (39.1%) were grade 2 (Figure 1; B, D, and F) ▶ . VEGF staining was homogenous within the tumors and heterogeneity was observed in only a few cases. No immunoreactivity for VEGF was detected in the normal mucosa specimens.
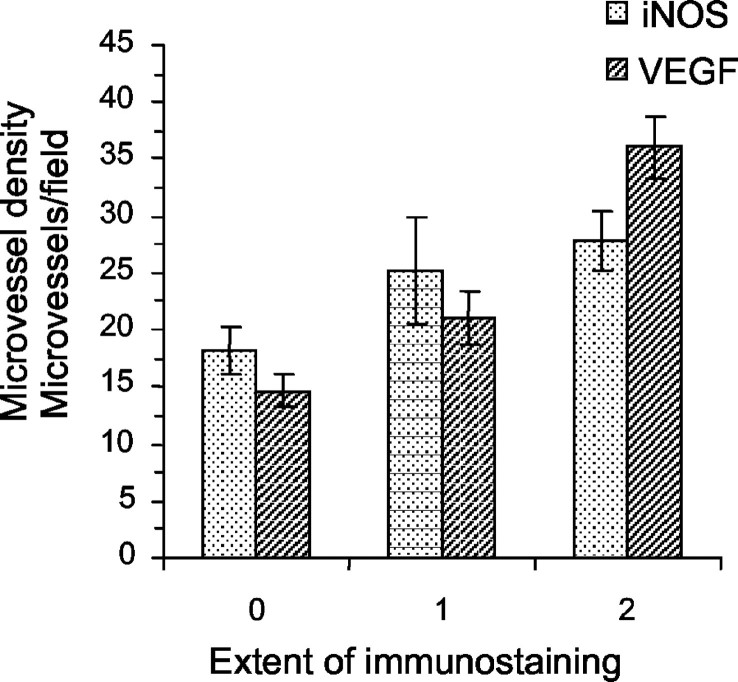
When all of the cases were considered, the mean value of MVD was 25.3 (±1.93). The expression of iNOS and VEGF significantly correlated with MVD (rs = 0.31, P = 0.02; rs = 0.67, P < 0.0001, respectively). The mean values (±SE) of MVD in each grade of iNOS and VEGF immunostaining are indicated in Figure 2 ▶ . A significant correlation was found between iNOS and VEGF expression (rs = 0.44, P = 0.001). iNOS and VEGF staining did not significantly differ in the metastatic (stage III and IV) and nonmetastatic (stage I and II) tumors. The percentage of iNOS-expressing inflammatory cells in the tumors correlated with neither MVD nor VEGF expression.
Figure 2.
MVD (mean values ± SE) in relation to iNOS- and VEGF-immunostaining grade. MVD increases as the extent of iNOS and VEGF staining increases (rs = 0.31, P = 0.02; rs = 0.67, P < 0.0001, respectively).
NT Immunoreactivity
The expression of NT was evaluated in 10 tumor specimens representative of the three grades of iNOS immunostaining (three tumors were grade 0, three were grade 1, and four were grade 2). Tumor epithelial cells expressing iNOS stained positive for NT (Figure 3) ▶ . The extent of NT immunostaining was classified as grade 0 in one tumor, grade 1 in four tumors, and grade 2 in five tumors. NT was also found in the tumor-infiltrating inflammatory cells. A significant correlation was found between iNOS and NT expression (rs = 0.76, P = 0.005).
Figure 3.

iNOS and NT immunostaining. Two successive sections of a representative tumor specimen: iNOS-expressing tumor cells (A) stained positive for NT (B). Hematoxylin counterstain; original magnifications, ×250.
iNOS activity, cGMP Content, and PGE2 Production
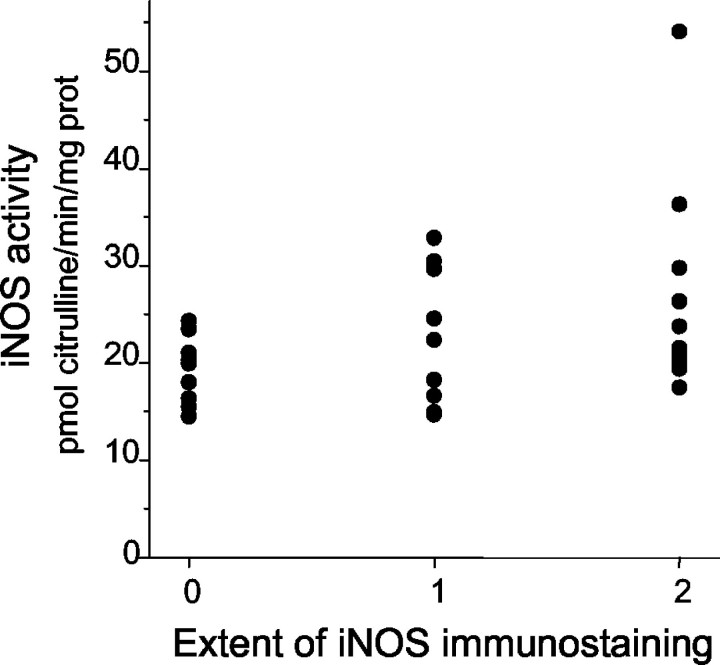
iNOS activity, cGMP levels, and PGE2 production in the normal mucosa of the controls did not significantly differ from those in the normal mucosa of the surgically resected specimens. iNOS activity and cGMP production were significantly higher in the cancer specimens than in the normal mucosa obtained at 10 cm from the tumor [21.17 (11.92 to 54.07) versus 9.40 (5.90 to 15.31), P < 0.0001; 14.79 (7.50 to 32.52) versus 6.64 (3.51 to 14.24), P < 0.0001, respectively] (Figure 4, A and B) ▶ . iNOS activity was significantly correlated with cGMP levels (rs = 0.34, P = 0.01). Both iNOS activity and cGMP levels were significantly related to the tumor stage: they were higher in tumors with lymph node or distant metastases (stage III to IV) than in those tumors without metastases (stage I to II) [24.32 (14.76 to 54.07) versus 19.45 (11.92 to 35.52), P = 0.002; 18.30 (8.17 to 32.50) versus 14.07 (7.50 to 29.55), P = 0.04, respectively] (Figure 4, A and B) ▶ . A significant correlation was found between iNOS activity and the iNOS immunostaining grade (rs = 0.30, P = 0.02) (Figure 5) ▶ . The percentage of iNOS expressing inflammatory cells did not correlate with iNOS activity.
Figure 4.

iNOS activity and cGMP levels in normal mucosa and in tumor samples. iNOS activity was expressed as pmol of [3H] citrulline formed per minute per mg protein. cGMP levels were expressed as pmol per minute per μg protein. iNOS activity (A) and cGMP levels (B) were significantly higher in tumor samples than in normal mucosa (P < 0.0001 and P < 0.0001, respectively). iNOS activity (A) and cGMP levels (B) were significantly higher in metastatic (stage III and IV) than in nonmetastatic (stage I and II) tumors (P = 0.002 and P = 0.04, respectively). Bars, Min-Max; □, 25th to 75th percentile; —, median value.
Figure 5.
iNOS activity versus iNOS immunostaining. iNOS activity correlates with iNOS immunostaining grade (rs = 0.30, P = 0.02).
PGE2 levels were significantly higher in the cancer specimens than in the normal mucosa obtained at 10 cm from the tumor [8.30 (3.47 to 26.3) versus 2.92 (0.31 to 6.65), P < 0.0001]. iNOS activity significantly correlated with PGE2 production (rs = 0.60, P < 0.0001).
iNOS Protein Expression
Western blotting was used to examine and confirm the up-regulation of iNOS protein expression that had been detected in 13 colorectal cancer specimens by immunohistochemistry. A semiquantitative analysis was used to allow for the measurement of the percentage increase in iNOS in the tumor tissue when compared to the corresponding normal tissue. Immunoblot analysis indicated a higher amount of protein in the neoplastic tissue of 11 specimens than in the normal mucosa (Figure 6) ▶ . No iNOS protein expression was detected in two tumor specimens. Very low levels of iNOS protein were detected in the normal mucosa of three specimens. However, the level of iNOS in normal tissue never exceeded that found in the carcinoma.
Figure 6.
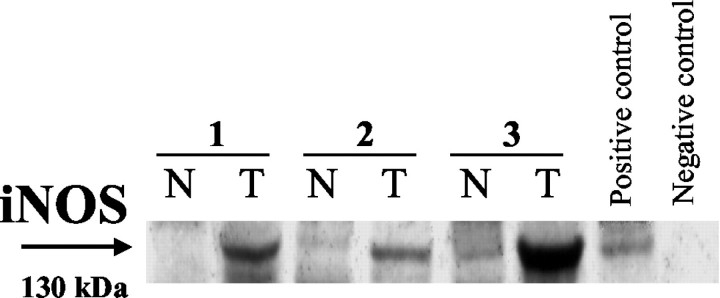
Western blot analysis. Expression of iNOS in representative paired tissues from tumor and adjacent normal mucosa. A higher amount of protein was found in the neoplastic tissue than in the normal mucosa. SW620 and WiDr human colon cancer cell lines served as positive and negative controls, respectively. T, tumor; N, normal; kd, kilodalton.
iNOS and VEGF mRNA Expression
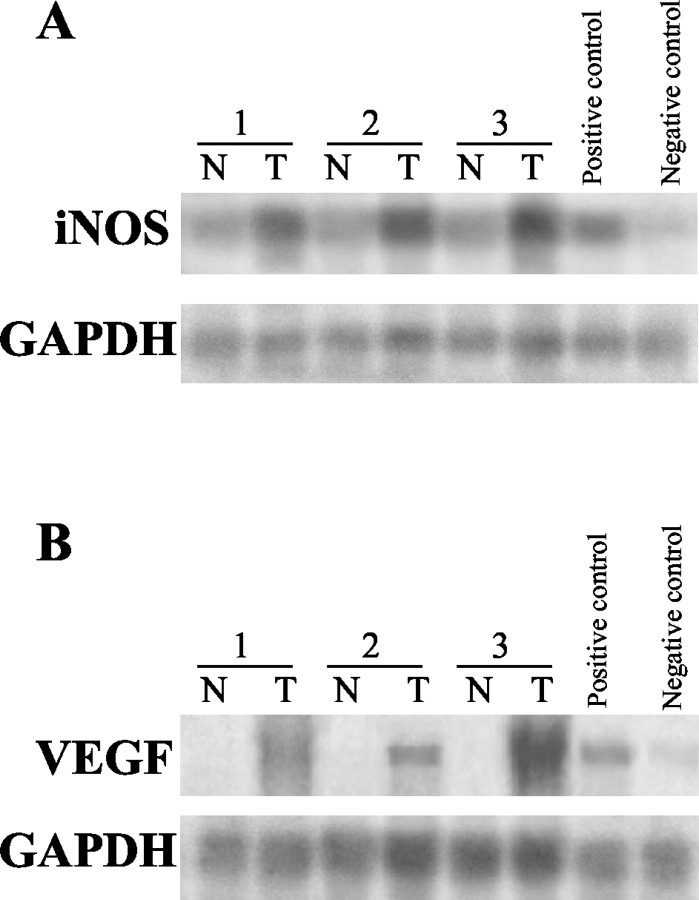
Using Northern hybridization analysis, iNOS and VEGF gene expressions were evaluated in tumor and corresponding normal mucosa samples obtained from seven surgically resected specimens. iNOS and VEGF mRNA levels were undetectable in the normal mucosa of all seven specimens. Up-regulation of both iNOS and VEGF mRNA levels was detected in all of the cancer specimens studied (Figure 7) ▶ .
Figure 7.
Northern blot analysis. Expression of iNOS and VEGF mRNA in representative paired tissue from tumor and adjacent normal mucosa. Up-regulation of both iNOS (A) and VEGF (B) mRNA levels was found in the cancer specimens when compared to normal mucosa. SW620 and WiDr human colon cancer cell lines served as iNOS-positive and -negative controls (A), respectively; HCT 116 and LoVo cell lines served as VEGF-positive and -negative controls (B), respectively. T, tumor; N, normal; GAPDH, glyceraldehyde phosphate dehydrogenase.
Discussion
The precise pathophysiological functions of NO in colorectal carcinogenesis are not well defined. This is mainly because of the dose-dependent effects of NO and to the conflicting data in literature regarding the possible source of NO in colorectal cancer. In one study, 18 iNOS expression and activity were detected in the normal colonic epithelium, whereas they were decreased or absent in tumor tissue. Ambs and colleagues 12 investigated iNOS expression and activity in human colon normal mucosa, adenomas, adenocarcinomas, and metastases. They found very low levels of iNOS activity in the normal tissue adjacent to the neoplastic lesions, whereas the highest level was detected in the adenomas. Immunohistochemical analysis identified tumor-infiltrating mononuclear cells as the major source of iNOS protein in their tumor specimens. In accordance with the majority of the previously published studies, 14-17 our results showed that colorectal tumor cells had strong iNOS positivity, as did tumor-infiltrating inflammatory cells. Western blot and Northern blot analyses confirmed the up-regulation of the iNOS protein and gene expression in tumor specimens as compared to normal mucosa. We also investigated iNOS activity and the production of cGMP, which mediates the response of the cells to NO. 40 iNOS activity and cGMP levels in tumor tissue increased twofold when compared to normal colon mucosa. Taken together, these observations suggest a relevant role of NO in the pathogenesis of colorectal cancer.
One of the potential mechanisms by which NO can promote tumor growth is the stimulation of tumor angiogenesis. Jenkins and colleagues 22 demonstrated that tumors derived from human colon adenocarcinoma cells engineered to generate NO continuously (iNOS-19) are significantly more vascularized and more invasive than the wild-type controls. The authors also suggested that NO might have different actions depending on the local concentration of the molecule. Low concentrations of NO (≈20 pmol per min per mg of protein) promote tumor growth and angiogenesis whereas at high concentrations (1 to 2 orders of magnitude higher) NO has anti-tumor activity by inducing cytotoxicity 7 and apoptosis. 41
Ziche and colleagues 23 demonstrated that NO controls angiogenesis by modulating the activity of some angiogenic factors, such as VEGF, released by tumor cells. These authors also showed that breast carcinoma cells that overexpress VEGF require the NO pathway to promote angiogenesis in vivo. To our knowledge, the present study is the first to show a strong correlation among iNOS expression, MVD, and VEGF expression in human colorectal cancer. The extent of both iNOS and VEGF immunostaining was significantly related to a progressive increase in tumor MVD. Moreover, iNOS expression was significantly associated to that of VEGF. On the basis of our immunohistochemical data, we cannot definitively establish the effect of NO on VEGF expression: it is likely that NO participates in the induction of VEGF or, alternatively, VEGF and iNOS may have a common induction pathway. However, our results suggest that VEGF should most likely be considered one of the most important mediators involved in the stimulation of tumor angiogenesis promoted by iNOS activity in colorectal cancer.
The possible role of iNOS in promoting VEGF-mediated angiogenesis in human tumors has already been suggested by some previously published studies. Marrogi and colleagues 42 showed that iNOS expression correlated positively with both MVD and VEGF expression in non-small-cell lung carcinoma. Ambs and colleagues 12 found increased VEGF protein levels in adenomas that expressed iNOS when compared to the VEGF level in the adjacent normal mucosa that was low in iNOS. These authors therefore hypothesized that the excessive production of NO by infiltrating adenoma inflammatory cells might stimulate VEGF production and thus, contribute to the necessary vascularization of colon adenoma at the point of transition into adenocarcinoma. However, our results do not seem to have confirmed this hypothesis: iNOS expression by tumor-infiltrating inflammatory cells did not correlate with either MVD or VEGF expression. This finding suggests that NO-related tumor angiogenesis is mainly mediated by the activity of neoplastic epithelial cells and only marginally by the inflammatory host response to the tumor.
We also investigated NT expression in a limited number of our samples. NT is the stable product of the action of peroxynitrite on tyrosine-containing proteins. Peroxynitrite is a powerful oxidant that seems to mediate some of the pathobiological effects of NO. 13,43 Landino and colleagues 25 demonstrated that NO stimulates COX activity and prostaglandin biosynthesis through the intermediacy of peroxynitrite. Recent studies have shown the relevant role of COX-2 and prostaglandins in promoting tumor angiogenesis of colorectal cancer. 26,44 We found a strong immunoreactivity of the tumor cells for NT and this expression was correlated with that of iNOS. A strong correlation was also found between iNOS activity and PGE2 production in the same tumor specimens. These data collectively suggest that NO may indirectly promote tumor angiogenesis through the COX pathway activation. This hypothesis seems to be supported by the in vivo demonstration that the chemopreventive effect of SC-51, a selective iNOS inhibitor, is increased by the combined administration of celecoxib, a selective inhibitor of COX-2. 45
Tumor vascularization is considered an absolute requirement for growth of solid tumors 46 and its extent correlates with tumor metastasis. 47,48 Both MVD and VEGF expression have been shown to be of prognostic significance in colorectal cancer. 49,50 Understanding the NO pathway may improve our knowledge of the biochemical mechanisms responsible for tumor angiogenesis and provide new potential anti-angiogenic drugs. The effects of a highly selective iNOS inhibitor, 1400W, has been tested against human colon adenocarcinoma cells (iNOS-19) genetically engineered to express iNOS. 51 The continuous infusion of 1400W was able to reduce the growth rate of the iNOS-19 cells to the level of the wild-type clone (w/tR) that has undetectable iNOS activity.
The hypothesis of a direct link between the NO pathway and a more aggressive phenotype of colorectal cancer cells is also supported by our findings on iNOS activity and cGMP production. We found a significant correlation between iNOS immunostaining and iNOS activity, as well as between iNOS activity and the production of cGMP. Interestingly, iNOS activity and cGMP levels were higher in tumor specimens with lymph node or distant metastases than in those without any metastases. The median value of iNOS activity in our tumor specimens (21.17 pmol per min per mg of protein) is similar to the value found by Jenkins and colleagues. 22 At these low concentrations, NO is likely to promote tumor growth and enhance tumor cell aggressiveness. Our data suggest that iNOS activity rather than immunoreactivity may be regarded as a novel biological marker for colorectal cancer prognosis. The iNOS activity found in tumor tissue extracts represents the combined activity of the enzyme expressed in a variety of cell types, ie, tumor cells and inflammatory cells. The total of iNOS activity can actually promote tumor progression and this might explain the association between iNOS activity and tumor staging.
In conclusion, our data demonstrated the up-regulation of iNOS gene expression in colorectal carcinoma. A strong correlation was found between iNOS expression and tumor angiogenesis. Even if we cannot provide a definite explanation, it is likely that VEGF is one of the most important promoters of the NO-mediated angiogenesis pathway. The association between increased iNOS activity and the presence of lymph node or distant metastases raises the possibility that NO offers a positive growth advantage for colorectal tumor cells and points to the inhibitors of iNOS as potential anti-neoplastic drugs.
Footnotes
Address reprint requests to Fabio Cianchi, M.D., Dipartimento di Area Critica Medica e Chirurgica, Viale Morgagni 85, 50134 Firenze, Italy. E-mail: cianchif@mail.unifi.it.
Supported by grants from the Italian Ministry of University, Scientific, and Technological Research; the University of Florence; and the Ente Cassa di Risparmio di Firenze.
References
- 1.Moncada S, Palmer RM, Higgs E: Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 1991, 43:109-142 [PubMed] [Google Scholar]
- 2.Nathan C, Xie QW: Nitric oxide synthase: roles, tolls, and controls. Cell 1994, 78:915-918 [DOI] [PubMed] [Google Scholar]
- 3.Bredt DS, Snyder SH: Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem 1994, 63:175-195 [DOI] [PubMed] [Google Scholar]
- 4.Marletta MA: Nitric oxide synthase structure and mechanism. J Biol Chem 1993, 268:12231-12234 [PubMed] [Google Scholar]
- 5.Forstermann U, Kleinert H: Nitric oxide synthase: expression and expressional control of the three isoforms. Naunyn-Schmiedebergs Arch Pharmakol 1995, 352:351-364 [DOI] [PubMed] [Google Scholar]
- 6.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA: Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 1990, 87:1620-1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hibbs JB, Taintor RR, Vavrin Z, Granger DL, Drapier J-C, Amber IJ, Lancaster JR: Synthesis of nitric oxide from a terminal guanidino nitrogen atom of L-arginine: a molecular mechanism regulating cellular proliferation that targets in intracellular iron. Moncada S Higgs EA eds. Nitric Oxide from L-Arginine: A Bioregulatory System. 1990:pp 189-223 Elsevier Amsterdam
- 8.Thomsen LL, Lawton FG, Knowles RG, Beesley JE, Riveros-Moreno V, Moncada S: Nitric oxide activity in human gynecological cancer. Cancer Res 1994, 54:1352-1354 [PubMed] [Google Scholar]
- 9.Thomsen LL, Miles DW, Happerfield L, Bobrow LG, Knowles RG, Moncada S: Nitric oxide synthase activity in human breast cancer. Br J Cancer 1995, 72:41-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallo O, Masini E, Morbidelli L, Franchi A, Fini-Storchi I, Vergari WA, Ziche M: Role of nitric oxide in angiogenesis and tumor progression in head and neck cancer. J Natl Cancer Inst 1998, 90:587-596 [DOI] [PubMed] [Google Scholar]
- 11.Franchi A, Gallo O, Paglierani M, Sardi I, Magnelli L, Masini E, Santucci M: Inducible nitric oxide synthase expression in laryngeal neoplasia: correlation with angiogenesis. Head Neck 2002, 24:16-23 [DOI] [PubMed] [Google Scholar]
- 12.Ambs S, Merriam WG, Bennett WP, Felley-Bosco E, Ogunfusika MO, Oser SM, Klein S, Shields PG, Billiar TR, Harris CC: Frequent nitric oxide synthase-2 expression in human colon adenomas: implication for tumor angiogenesis and colon cancer progression. Cancer Res 1998, 58:334-341 [PubMed] [Google Scholar]
- 13.Ambs S, Bennett WP, Merriam WG, Ogunfusika MO, Sean MO, Harrington AM, Shields PG, Felley-Bosco E, Hussain SP, Harris CC: Relationship between p53 mutations and inducible nitric oxide synthase expression in human colorectal cancer. J Natl Cancer Inst 1999, 91:86-88 [DOI] [PubMed] [Google Scholar]
- 14.Ropponen KM, Kellokoski JK, Lipponen PK, Eskelinen MJ, Alanne L, Alhava EM, Kosma VM: Expression of inducible nitric oxide synthase in colorectal cancer and its association with prognosis. Scand J Gastroenterol 2000, 11:1204-1211 [DOI] [PubMed] [Google Scholar]
- 15.Yagihashi N, Kasajima H, Sugai S, Matsumoto K, Ebina Y, Morita T, Murakami T, Yagihashi S: Increased in situ expression of nitric oxide synthase in human colorectal cancer. Virchows Arch 2000, 436:109-114 [DOI] [PubMed] [Google Scholar]
- 16.Kojima M, Morisaki T, Tsukahara Y, Uchiyama A, Matsunari Y, Mibu R, Tanaka M: Nitric oxide synthase expression and nitric oxide production in human colon carcinoma tissue. J Surg Oncol 1999, 70:222-229 [DOI] [PubMed] [Google Scholar]
- 17.Hao XP, Pretlow TG, Rao JS, Pretlow TP: Inducible nitric oxide synthase (iNOS) is expressed similarly in multiple aberrant crypt foci and colorectal tumors from the same patients. Cancer Res 2001, 61:419-422 [PubMed] [Google Scholar]
- 18.Moochhala S, Chhatwal VJ, Chan ST, Ngoi SS, Chia YW, Rauff A: Nitric oxide synthase activity and expression in human colorectal cancer. Carcinogenesis 1996, 17:1171-1174 [DOI] [PubMed] [Google Scholar]
- 19.Ahn B, Ohshima H: Suppression of intestinal polyposis in Apc(Min/+) mice by inhibiting nitric oxide production. Cancer Res 2001, 61:8357-8360 [PubMed] [Google Scholar]
- 20.Felley-Bosco E: Role of nitric oxide in genotoxicity: implication for carcinogenesis. Cancer Met Rev 1998, 17:25-37 [DOI] [PubMed] [Google Scholar]
- 21.Fukumura D, Jain RK: Role of nitric oxide in angiogenesis and microcirculation in tumor. Cancer Met Rev 1998, 17:77-89 [DOI] [PubMed] [Google Scholar]
- 22.Jenkins DC, Charles IG, Thomsen LL, Moss DW, Holmes LS, Baylis SA, Rhodes P, Westmore K, Emson PC, Moncada S: Roles of nitric oxide in tumor growth. Proc Natl Acad Sci USA 1995, 92:4392-4396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ, Bicknell R: Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest 1997, 99:2625-2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziche M, Morbidelli L, Masini E, Granger HJ, Geppetti P, Ledda F: Nitric oxide promotes DNA synthesis and cyclic GMP formation in endothelial cells from postcapillary venules. Biochem Biophys Res Commun 1993, 15:437-442 [DOI] [PubMed] [Google Scholar]
- 25.Landino LM, Crews BC, Timmons MD, Morrow JD, Marnett LJ: Peroxynitrite, the coupling product of nitric oxide and superoxide, activates prostaglandin biosynthesis. Proc Natl Acad Sci USA , 93:15069-15074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cianchi F, Cortesini C, Bechi P, Fantappiè O, Messerini L, Vannacci A, Sardi I, Baroni G, Boddi V, Mazzanti R, Masini E: Up-regulation of cyclooxygenase-2 gene expression correlates with tumor angiogenesis in human colorectal cancer. Gastroenterology 2001, 121:1339-1347 [DOI] [PubMed] [Google Scholar]
- 27.Jass JR, Sobin LH: Histological typing of intestinal tumors. WHO International Histological Classification of Tumors. 1989. Springer-Verlag Berlin
- 28.Fleming ID, Cooper JS, Henson DE, Hutter RJP, Kennedy BJ, Murray GP, Sobin LH, Yarbro JW: AJCC Cancer Staging Manual. 1997. Lippincott Philadelphia
- 29.Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparrini G: Tumour angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst 1992, 84:1875-1887 [DOI] [PubMed] [Google Scholar]
- 30.Bani D, Masini E, Bello MG, Bigazzi M, Bani Sacchi TB: Relaxin activates the L-arginine-nitric oxide pathway in human breast cancer cells. Cancer Res 1995, 55:5272-5275 [PubMed] [Google Scholar]
- 31.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurement with the folin phenol reagent. J Biol Chem 1951, 193:265-275 [PubMed] [Google Scholar]
- 32.Steiner AL, Parker CW, Kipnis DM: Radioimmunoassay for cyclic nucleotides. J Biol Chem 1972, 247:1106-1113 [PubMed] [Google Scholar]
- 33.Harper JF, Brooker G: Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2′Ο acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res 1975, 1:207-218 [PubMed] [Google Scholar]
- 34.Gentilini P, Laffi G, Meacci E, La Villa G, Cominelli F, Pinzani M, Buzzelli G: Effects of OKY 046, a thromboxane synthase inhibitor on renal function in non azotemic cirrhotic patients with ascites. Gastroenterology 1988, 94:1470-1477 [DOI] [PubMed] [Google Scholar]
- 35.Jenkins DC, Charles SA, Baylis SA, Lelchuk R, Radomski MW, Moncada S: Human colon cancer cell lines show a diverse pattern of nitric oxide synthase gene expression and nitric oxide generation. Br J Cancer 1994, 70:847-849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fantappiè O, Masini E, Sardi I, Raimondi L, Bani D, Solazzo M, Vannacci A, Mazzanti R: The MDR phenotype is associated with the expression of COX-2 and iNOS in a human hepatocellular carcinoma cell line. Hepatology 2002, 35:843-851 [DOI] [PubMed] [Google Scholar]
- 37.Pertschuk LP, Feldman JG, Kim DS, Nayeri K, Eisenberg KB, Carter AC, Thelmo WT, Rhong ZT, Benn P, Grossman A: Steroid hormone receptor immunohistochemistry and amplification of c-myc protooncogene. Relationship to disease-free survival in breast cancer. Cancer 1993, 71:162-171 [DOI] [PubMed] [Google Scholar]
- 38.Rak J, Mitsuhashi Y, Bayko L, Filmus J, Shirasawa S, Sasazuki T, Kerbel RS: Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res 1995, 55:4575-4580 [PubMed] [Google Scholar]
- 39.Kondo Y, Arii S, Mori A, Furutani M, Chiba T, Imamura M: Enhancement of angiogenesis, tumor growth, and metastasis by transfection of vascular endothelial growth factor into LoVo human colon cancer cell line. Clin Cancer Res 2000, 6:622-630 [PubMed] [Google Scholar]
- 40.Ignarro LJ: Signal transduction mechanisms involving nitric oxide. Biochem Pharmacol 1991, 41:485-490 [DOI] [PubMed] [Google Scholar]
- 41.Xie K, Huang S, Dong Z, Juang S-H, Gutman M, Xie Q-W, Nathan C, Fidler IJ: Transfection with the inducible nitric oxide synthase gene suppresses tumorigenicity and abrogates metastasis by K-1735 murine melanoma cells. J Exp Med 1995, 181:1333-1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marrogi AJ, Travis WD, Welsh JA, Khan MA, Rahim H, Tazelaar H, Pairolero P, Trastek V, Jett J, Caporaso NE, Liotta LA, Harris CC: Nitric oxide synthase, cyclooxygenase 2, and vascular endothelial growth factor in the angiogenesis of non-small cell lung carcinoma. Clin Cancer Res 2000, 6:4739-4744 [PubMed] [Google Scholar]
- 43.Lala PK: Significance of nitric oxide in carcinogenesis, tumor progression and cancer therapy. Cancer Met Rev 1998, 17:1-6 [DOI] [PubMed] [Google Scholar]
- 44.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN: Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 1998, 93:705-716 [DOI] [PubMed] [Google Scholar]
- 45.Rao CV, Indranie C, Simi B, Manning PT, Connor JR, Reddy BS: Chemopreventive properties of a selective inducible nitric oxide synthase inhibitor in colon carcinogenesis, administered alone or in combination with celecoxib, a selective cyclooxygenase-2 inhibitor. Cancer Res 2002, 62:165-170 [PubMed] [Google Scholar]
- 46.Folkman J, Watson K, Ingber D, Hanahan D: Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature 1989, 338:58-61 [DOI] [PubMed] [Google Scholar]
- 47.Blood CH, Zetter BR: Tumor interaction with the vasculature: angiogenesis and tumor metastases. Biochim Biophys Acta 1990, 1032:89-118 [DOI] [PubMed] [Google Scholar]
- 48.Fidler IJ, Ellis LM: The implications of angiogenesis for the biological and therapy of cancer metastasis. Cell 1994, 79:185-188 [DOI] [PubMed] [Google Scholar]
- 49.Tanigawa N, Amaya H, Matsumura M, Lu C, Kitaoka A, Matsuyama K, Muraoka R: Tumor angiogenesis and mode of metastasis in patients with colorectal cancer. Cancer Res 1997, 57:1043-1046 [PubMed] [Google Scholar]
- 50.Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM: Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res 1995, 55:3964-3968 [PubMed] [Google Scholar]
- 51.Thomsen LL, Scott JMJ, Topley P, Knowles RG, Keerie A-J, Frend AJ: Selective inhibition of inducible nitric oxide synthase inhibits tumor growth in vivo. Studies with 1400W, a novel inhibitor. Cancer Res 1997, 57:3300-3304 [PubMed] [Google Scholar]