Abstract
Serrated adenomas are characterized by a saw-toothed growth pattern with epithelial dysplasia (intraepithelial neoplasia). The CpG island methylator phenotype (CIMP) is a recently described mechanism for tumorigenesis in colorectal carcinomas and adenomas characterized by methylation of multiple CpG islands. The role of these epigenetic alterations in the pathogenesis of serrated adenomas is not clear. We therefore evaluated CIMP in 22 sporadic serrated adenomas and 6 serrated adenomas with multiple (6 to 10) hyperplastic polyps, including 5 with admixed hyperplastic glands and adenomatous glands, and compared the results with 34 conventional adenomas. Bisulfite methylation-specific polymerase chain reaction was used for the p16 and hMLH1 genes, and three MINT (methylated in tumor) loci (MINT1, MINT2, and MINT31). Patients with sporadic serrated adenomas had a higher frequency of hyperplastic polyps (1.3 ± 1.6) as compared to patients with tubular adenomas (0.4 ± 0.9, P = 0.02). Mean number of methylated sites was significantly higher in sporadic serrated adenomas (2.0 ± 1.7) than in tubular adenomas (0.8 ± 0.9, P = 0.00001). Sporadic serrated adenomas had significantly more frequent methylation of MINT1 (48%, 10 of 22) and MINT2 (71%, 15 of 21) than tubular adenomas (9%, 3 of 34, P = 0.001; and 18%, 6 of 34, P = 0.0001), respectively. Concordant methylation of two or more sites (CIMP-high) was also more frequent in sporadic serrated adenomas (68%, 15 of 22) than in tubular adenomas (18%, 6 of 34, P = 0.0005). All five serrated adenomas with admixed hyperplastic glands and adenomatous glands were CIMP-high. Our results indicate that CpG island methylation is common in sporadic serrated adenomas and may play an important role in their pathogenesis.
Colorectal cancer is the fourth most common cancer and the second most common cause of cancer death in the United States. 1 The molecular pathogenesis of colorectal adenocarcinoma has been one of the most extensively studied and well characterized. 2,3 The traditional pathogenic pathway, the adenoma-adenocarcinoma sequence, plays an important role in the majority of colorectal adenocarcinoma. 4,5 Adenocarcinoma arising in the setting of colorectal hyperplastic polyps or serrated adenomas, especially in patients with hyperplastic polyposis, or serrated adenomatous polyposis has also been described. 6-18 In addition, an alternative pathway of colorectal carcinogenesis with a hyperplastic polyp-serrated adenoma-adenocarcinoma sequence has been recently proposed. 6,16,19-24
Serrated adenoma/mixed hyperplastic adenomatous polyp is an uncommon colorectal neoplasm, 11,25 characterized by the saw-toothed architectural features of hyperplastic polyp, but with the presence of unequivocal epithelial dysplasia (intraepithelial neoplasia). Hyperplastic polyps have been considered to be nonneoplastic lesions without malignant potential, but recent reports have challenged these assumptions. Genetic alterations that are frequent in colorectal carcinoma, such as K-ras mutations, chromosome 1p loss, and microsatellite instability (MSI), have been reported in hyperplastic polyps. 19,26-28 These genetic alterations are also present in serrated adenomas. 19,22,27,29 It has been reported that serrated adenomas have frequent p53 gene mutations compared with hyperplastic lesions or tubular adenomas, 30 but have lower frequency of APC gene mutation than in sporadic tubular adenomas. 31
CpG islands are 0.5- to 2-kb regions rich in cytosine-guanine dinucleotides and are present in the 5′ region of approximately half of all human genes. 32 CpG island methylation (CIM) is a mechanism for suppression of transcription of genes in physiological and pathological settings including neoplasia. 33 It has been shown that methylation of CpG islands is a molecular defect common in colorectal adenocarcinoma. 34 The recently described CpG island methylator phenotype (CIMP) is a novel pathway characterized by methylation of multiple CpG islands in colorectal carcinomas and adenomas. CIMP includes methylation of genes known to be important in tumorigenesis, such as the p16 tumor suppressor gene and hMLH1 mismatch repair gene. 35,36 In addition, CIMP-high adenomas and carcinomas have a distinct genetic profile with frequent mutation of the K-ras gene, but uncommon p53 mutation. 35 In a previous study, we reported concordant CIM of multiple hyperplastic polyps in patients with serrated adenoma and/or right-sided hyperplastic polyposis. 37 However, the occurrence of CIM in sporadic serrated adenomas has not been studied in detail.
In this study, we examined the methylation status of serrated adenomas and compared the findings to tubular adenomas to determine whether CIMP plays a role in the pathogenesis of sporadic serrated adenomas.
Materials and Methods
Patients and Specimens
This study included endoscopically obtained biopsy specimens from 28 colorectal serrated adenomas, including 5 serrated adenomas with admixed hyperplastic glands and adenomatous glands (Figure 1) ▶ , 25 from 27 patients identified from the files of the Department of Pathology at the M. D. Anderson Cancer Center between the years 1993 to 2001. Six serrated adenomas associated with 6 to 10 hyperplastic polyps were classified as serrated adenomas with multiple hyperplastic polyps, and 22 serrated adenomas associated with or less than 5 hyperplastic polyps were classified as sporadic serrated adenomas. 6 Thirty-four colorectal tubular adenomas from 34 patients, and 17 specimens of colonic mucosa (six from serrated adenoma patients, three from tubular adenoma patients, and eight from patients without colonic polyps) randomly selected in 2000 or 2001 were also included in this study as controls. The demographics of the patient population and the polyp characteristics analyzed in the present study are summarized in Table 1 ▶ .
Figure 1.
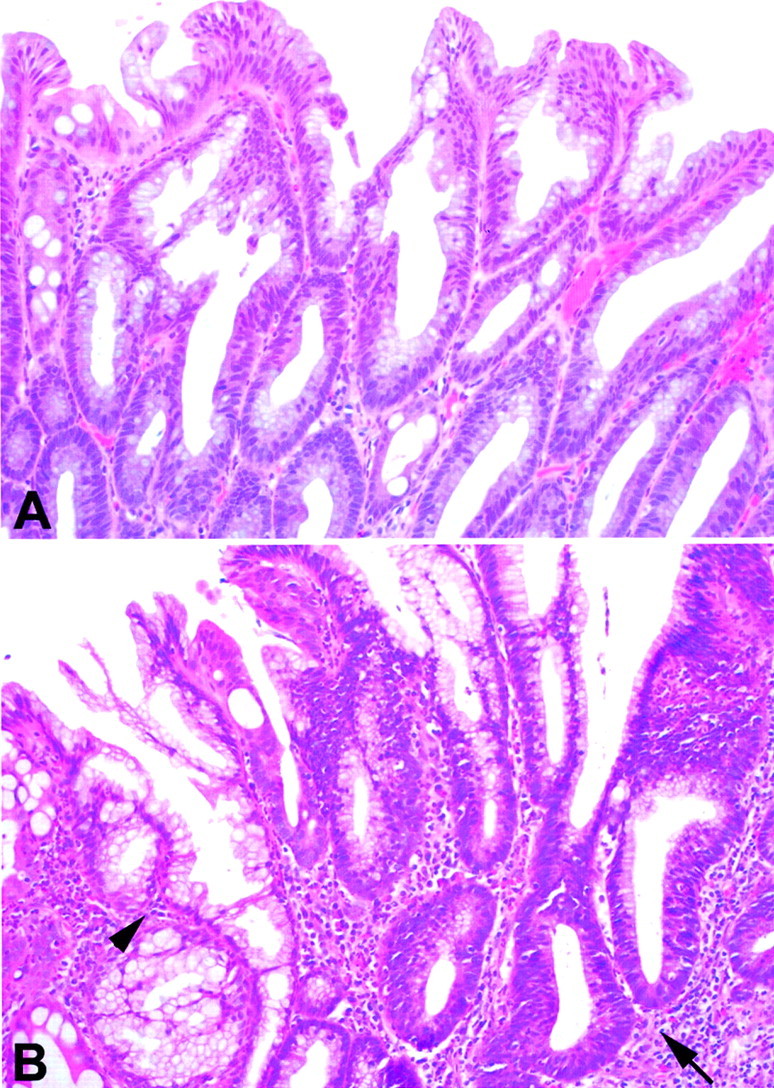
Histopathological appearance of serrated adenoma and serrated adenoma with admixed hyperplastic glands and adenomatous glands. A: Serrated adenoma with saw-toothed architecture and epithelial dysplasia. B: Serrated adenoma with admixed hyperplastic glands (arrowhead) and adenomatous glands (arrow) from case 2. Both hyperplastic and adenomatous glands showed methylations of all five loci (p16, MINT1, MINT2, MINT31, and hMLH1) and allelic shifts of BAT25, BAT26, and TGF-βRII.
Table 1.
Patient Demographics and Clinicopathological Characteristics
| Serrated adenomas | Tubular adenomas (n = 34) % (no.) | ||
|---|---|---|---|
| With multiple HPs* (n = 5)† % (no.) | Sporadic (n = 22) % (no.) | ||
| Age ± SD (years) | 57 ± 11 | 64 ± 12 | 61 ± 14 |
| Gender | |||
| Male | 80% (4) | 59% (13) | 59% (20) |
| Female | 20% (1) | 41% (9) | 41% (14) |
| Site | |||
| Right colon | 60% (3) | 45% (10)‡ | 50% (17) |
| Left colon or rectum | 40% (2) | 50% (11)‡ | 50% (17) |
| Previous hyperplastic polyps | |||
| 0 | 0% (0) | 45% (10)§ | 76% (26)§ |
| 1–3 | 0% (0) | 45% (9)§ | 21% (7)§ |
| 4–5 | 0% (0) | 14% (3)§ | 3% (1)§ |
| >5 | 100% (5) | ||
| Previous adenomas | |||
| 0 | 20% (1) | 50% (11) | 41% (14) |
| 1–3 | 40% (2) | 41% (9) | 50% (17) |
| >3 | 40% (2) | 9% (2) | 9% (3) |
| Previous serrated adenomas | |||
| 0 | 80% (4) | 95% (21) | 0% (0) |
| 1 | 20% (1) | 5% (1) | 0% (0) |
| History of colorectal cancer | 40% (2) | 27% (6) | 21% (7) |
| Right colon | 50% (1) | 50% (3) | 14% (1) |
| Left colon or rectum | 50% (1) | 50% (3) | 86% (6) |
| No. of colonoscopies | |||
| (Mean ± SD) | 3.0 ± 0.8‡ | 1.9 ± 1.1¶ | 1.7 ± 1.0 |
*HPs, hyperplastic polyps.
†One patient with two serrated adenomas.
‡Site not designated for one serrated adenoma.
§P = 0.048 between sporadic serrated adenoma and tubular adenoma;
¶P = 0.02.
DNA Extraction
Microdissection from paraffin-embedded tissue was performed on hematoxylin and eosin-stained slides. Genomic DNA was extracted from microdissected tissue as described previously. 38 For 23 serrated adenomas only the glands with unequivocal epithelial dysplasia and saw-toothed architecture were microdissected. For five serrated adenomas with admixed hyperplastic glands and adenomatous glands, the hyperplastic glands and adenomatous glands were microdissected and analyzed separately. Each specimen was treated with 50 μl of lysis buffer containing 0.5% Tween 20 (Boehringer Mannheim, Mannheim, Germany), 20 μg proteinase K (Boehringer Mannheim), 50 mmol/L trizma base at pH 8.9, and 2 mmol/L ethylenediaminetetraacetic acid. The samples were incubated at 56°C overnight. Proteinase K was inactivated by incubating the samples at 100°C for 10 minutes. The extracted DNA was stored at −80°C.
Bisulfite Treatment of DNA and Methylation-Specific Polymerase Chain Reaction (MSP)
The methylation status of p16, MINT1, MINT2, MINT31, and hMLH1 was determined by bisulfite treatment of DNA followed by methylation-specific polymerase chain reaction amplification (MSP-PCR), as described, with modification. 39 The selection of these loci was based on a previous study that showed these loci had high sensitivity and specificity for the detection of hypermethylation in cancer and offered excellent discrimination for CIMP status. 34 MINT1 is an island associated with a cDNA transcript of unknown function; MINT2 corresponds to a CpG island that is in the 5′ region of a cDNA with an open reading frame that has no protein homology, and MINT31 is 2 kb upstream of the CACNA1G, a T-type calcium channel gene. 36,40 In brief, 1 μg of microdissected genomic DNA was denatured with 2 mol/L NaOH at 37°C for 10 minutes, followed by incubation with 3 mol/L of sodium bisulfite (pH 5.0) at 50°C for 16 hours in the dark. After treatment, DNA was purified using the DNA cleanup kit (Promega, Madison, WI) as recommended by the manufacturer, incubated with 3 mol/L of NaOH at room temperature for 5 minutes, precipitated with 10 mol/L of ammonium acetate and 100% ethanol, washed with 70% ethanol, and finally resuspended in 20 μl of distilled water.
Methylation status of p16, MINT1, MINT2, MINT31, and hMLH1 was determined using 2 μl of bisulfite-treated DNA for bisulfite-PCR. PCR products from methylated and unmethylated reactions were electrophoresed on 6% acrylamide gels, and visualized by ethidium bromide staining. Primer sequences and conditions for PCR are summarized in Table 2 ▶ . DNA from the RKO colon cancer cell line (American Type Culture Collection, Manassas, VA) was used as a positive control for methylation. For quantitation of methylated alleles, gel images were digitized using a BioRad imager and quantitated using the manufacturer’s software (BioRad, Hercules, CA). MSP-PCR provided semiquantitative results. The loci (p16, MINT1, MINT2, MINT31, and hMLH1) were classified as unmethylated if the intensity of methylated band was <10% of the unmethylated band, or methylated if the intensity of methylated band was ≥10% of the unmethylated band.
Table 2.
Primer Sequences for Evaluation of Methylation at p16, MINT1, MINT2, and MINT31
| Site | Primers |
|---|---|
| p16 | |
| Methylated | |
| Sense | 5′-TTATTAGAGGGTGGGGCGGATCGC-3′ |
| Antisense | 5′-GACCCCGAACCGCGACCGTAA-3′ |
| Unmethylated | |
| Sense | 5′-TTATTAGAGGGTGGGGTGGATTGT-3′ |
| Antisense | 5′-CAACCCCAAACCACAACCATAA-3′ |
| MINT1 | |
| Methylated | |
| Sense | 5′-AATTTTTTTATATATATTTTCGAAGC-3′ |
| Antisense | 5′-AAAAACCTCAACCCCGCG-3′ |
| Unmethylated | |
| Sense | 5′-AATTTTTTTATATATATTTTTGAAGTGT-3′ |
| Antisense | 5′-AACAAAAAACCTCAACCCCACA-3′ |
| MINT2 | |
| Methylated | |
| Sense | 5′-TTGTTAAAGTGTTGAGTTCGTC-3′ |
| Antisense | 5′-AATAACGACGATTCCGTACG-3′ |
| Unmethylated | |
| Sense | 5′-GATTTTGTTAAAGTGTTGAGTTTGTT-3′ |
| Antisense | 5′-CAAAATAATAACAACAATTCCATACA-3′ |
| MINT31 | |
| Methylated | |
| Sense | 5′-TGTTGGGGAAGTGTTTTTCGGC-3′ |
| Antisense | 5′-CGAAAACGAAACGCCGCG-3′ |
| Unmethylated | |
| Sense | 5′-TAGATGTTGGGGAAGTGTTTTTTGGT-3′ |
| Antisense | 5′-TAAATACCCAAAAACAAAACACCACA-3′ |
| hMLH1 | |
| Methylated | |
| Sense | 5′-GATAGCGATTTTTAACGC-3′ |
| Antisense | 5′-TCTATAAATTACTAAATCTCTTCG-3′ |
| Unmethylated | |
| Sense | 5′-AGAGTGGATAGTGATTTTTAATGT-3′ |
| Antisense | 5′-ACTCTATAAATTACTAAATCTCTTCA-3′ |
Criteria for CIMP of Serrated Adenomas and Tubular Adenomas
CIMP status was determined for serrated and tubular adenomas with three or more evaluated loci. Serrated adenomas and tubular adenomas were classified as CIMP-negative if none of the evaluated loci were methylated, CIMP-low if one locus was methylated, and CIMP-high if two or more loci were methylated. 36 The sensitivity of any MINT locus to predict CIMP was 75% and specificity was 95%. The sensitivity of p16 to predict CIMP phenotype was 65% and specificity was 100%.
MSI Analysis
MSI was determined by PCR amplification using fluorescent dye-labeled (forward primer) and unlabeled (reverse primer) methodology for three mononucleotide repeats markers [BAT-25, BAT-26, and transforming growth factor-β type II receptor (TGF-βRII)]. The forward oligonucleotide was end-labeled with 6-FAM (Applied Biosystems, Foster City, CA). PCR was performed in 15-μl reaction volumes containing 40 ng of DNA, 9 μl ABI Prism True Allele PCR Premix (Applied Biosystems), and 5 pmol of 6-FAM-labeled forward primer and 10 pmol of unlabeled reverse primer. PCR was performed using the following cycling conditions: denaturation at 95°C for 6 minutes; 45 cycles (94°C for 45 seconds, 55°C for 45 seconds, 72°C for 1 minute), and extension at 72°C for 30 minutes. The PCR product was diluted further with 30 μl of H2O, and a 1.0-μl aliquot of each diluted fluorescent-labeled PCR product was combined with 12 μl of formamide and 0.5 μl of GeneScan 400HD (ROX) size standard (Applied Biosystems). The samples were then capillary electrophoresed on an ABI 3700 DNA Analyzer using GeneScan Analysis software (Applied Biosystems). Allelic shift (MSI) of a microsatellite marker was defined by the presence of at least one additional band in the DNA.
Statistical Analysis
Difference in frequency were evaluated with chi-square tests. Univariate analyses of the interaction between methylation and clinical parameters were performed with this test and with Fisher’s exact test when testing small samples. All P values presented are two-sided, and a P value of less than 0.05 was considered statistically significant.
All serrated and tubular adenomas in the study with a successful determination of the methylation status for at least three loci were included in the analysis. For serrated adenomas with admixed hyperplastic glands and adenomatous glands, the methylation status was scored as methylated if either the hyperplastic gland component or the adenomatous glands component had methylation. Patient characteristics included age, sex, and previous history of hyperplastic polyps, tubular adenomas, serrated adenomas, or colorectal cancers. Characteristics of serrated adenomas and tubular adenomas included location, size, and histology.
Results
Clinical Findings
There were no significant differences in the age or gender of the patients or the location of polyps between sporadic serrated adenomas and tubular adenomas (Table 1) ▶ . Patients with sporadic serrated adenomas had a higher frequency of hyperplastic polyps (1.3 ± 1.6) as compared to patients with tubular adenomas (0.4 ± 0.9, P = 0.02). There was no difference in the frequency of previous tubular adenomas, serrated adenomas, or colorectal cancer between the two groups. Patients with serrated adenomas with multiple hyperplastic polyps had a higher number of endoscopic examinations (3.0 ± 0.8) as compared to patients with sporadic serrated adenomas (1.9 ± 1.1, P = 0.02). There was no difference in the frequency of hyperplastic polyps between sporadic serrated adenoma patients with history of colorectal cancer (0.7 ± 0.8) or without (1.5 ± 1.8, P = 0.15).
Methylation at p16, MINT1, MINT2, and MINT31
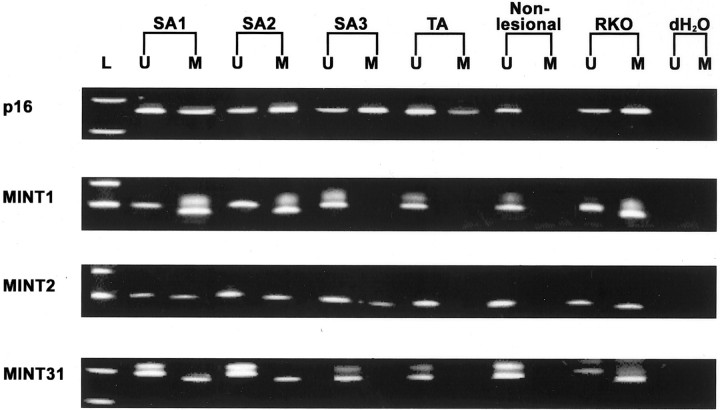
Figure 2 ▶ shows examples of methylation at p16, MINT1, MINT2, and MINT31. The methylation status of serrated adenomas and tubular adenomas status are summarized in Figure 3 ▶ . No methylation of p16 and MINT1 was present in nonlesional colonic mucosa, but methylation of MINT2 (5.9%, 1 of 17) and MINT31 (6.3%, 1 of 16) was each identified in one sample of nonlesional colonic mucosa. The frequency of methylation at p16, MINT1, MINT2, MINT31, and hMLH1 was 48% (10 of 21), 48% (10 of 21), 71% (15 of 21), 32% (7 of 22), and 14% (3 of 21), respectively, in sporadic serrated adenomas compared to 17% (1 of 6), 17% (1 of 6), 33% (2 of 6), 17% (1 of 6), and 20% (1 of 5) in serrated adenomas with multiple hyperplastic polyps, and 29% (10 of 34), 9% (3 of 34), 18% (6 of 34), 24% (8 of 34), and 0% (0 of 30) in tubular adenomas (Table 3) ▶ . The frequency of methylation of MINT1 and MINT2 was higher in sporadic serrated adenomas than in tubular adenomas [48% (10 of 21) versus 9% (3 of 34), P = 0.001; and 71% (15 of 21) versus 18% (6 of 34), P = 0.0001, respectively]. There was no difference between methylation of p16 and MINT1 between sporadic serrated adenomas and tubular adenomas.
Figure 2.
Examples of methylation-specific PCR (MSP) at p16, MINT1, MINT2, and MINT31 in serrated adenomas, tubular adenomas, and nonlesional mucosa. Methylation of p16, MINT1, MINT2, and MINT31 was evaluated by MSP using primers for methylated (M) and unmethylated (U) alleles of bisulfite-treated DNA. Gene or loci examined are indicated on the left of each gel and samples on top of the first gel. L, designates size marker; SA1, SA2, and SA3, three serrated adenomas; TA, tubular adenoma; nonlesional, nonlesional colonic mucosa; RKO, a colon cancer cell line used as a positive control; and dH2O, samples without DNA used as negative control. Methylation of p16 is present in all of the adenomas. Serrated adenomas (SA1 and SA2) have methylation of MINT1 and MINT31 and all three serrated adenomas have methylation of MINT2. The upper band of the double bands shown in MINT1 and MINT31 is a spurious band appearing only on 6% acrylamide gels but not on 2% agarose gels.
Figure 3.
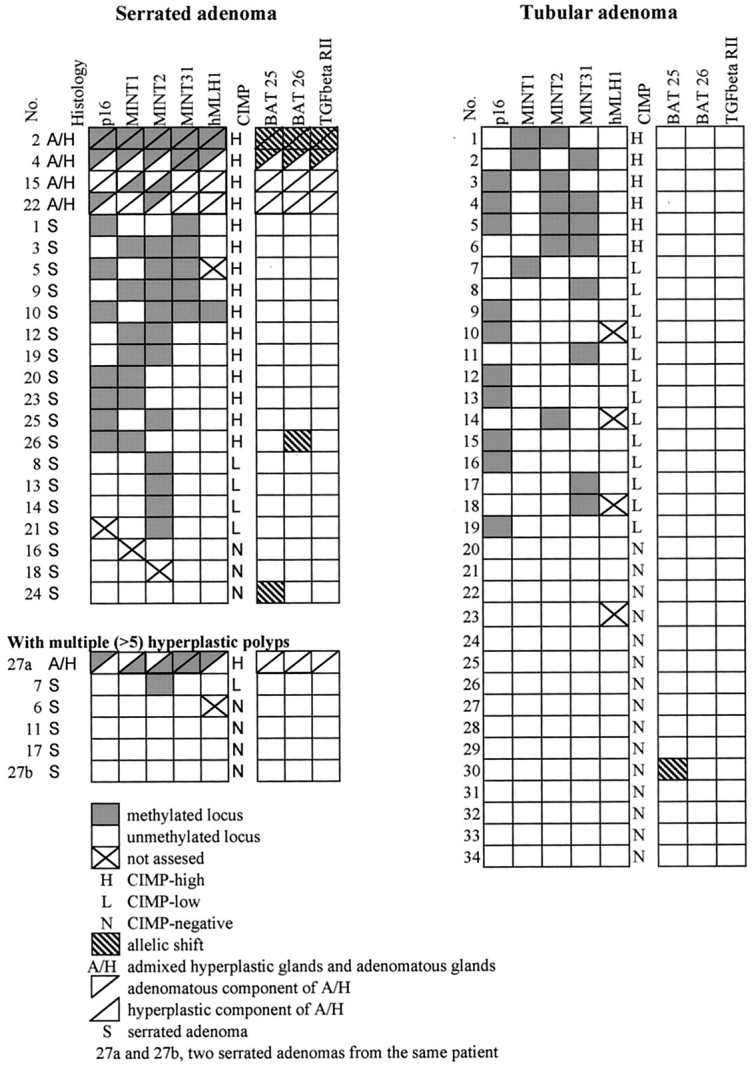
Summary of histology; methylation status at p16, MINT1, MINT2, MINT31, and hMLH1; CIMP status; and MSI in sporadic serrated adenomas, serrated adenomas with multiple (6 to 10) hyperplastic polyps, and tubular adenomas.
Table 3.
Frequency of Methylation and Microsatellite Instability Serrated Adenomas and Tubular Adenomas
| Alterations | Serrated adenomas | Tubular adenomas (n = 34) | Nonlesional mucosa (n = 17) | |
|---|---|---|---|---|
| With multiple HPs* (n = 6) | Sporadic (n = 22) | |||
| Methylation | ||||
| p16 | 17% (1/6) | 48% (10/21) | 29% (10/34) | 0% (0/16) |
| MINT1 | 17% (1/6) | 48% (10/21)† | 9% (3/34)† | 0% (0/16) |
| MINT2 | 33% (2/6) | 71% (15/21)‡ | 18% (6/34)‡ | 6% (1/17) |
| MINT31 | 17% (1/6) | 32% (7/22) | 24% (8/34) | 6% (1/16) |
| hMLH1 | 20% (1/5) | 14% (3/21) | 0% (0/30) | — |
| Allelic shift | ||||
| BAT25 | 0% (0/6) | 14% (3/22) | 3% (1/34) | — |
| BAT26 | 0% (0/6) | 14% (3/22) | 0% (0/34) | — |
| TGF-βRII | 0% (0/6) | 9% (2/22) | 0% (0/34) | — |
*HPs, hyperplastic polyps.
†P = 0.001.
‡P = 0.0001.
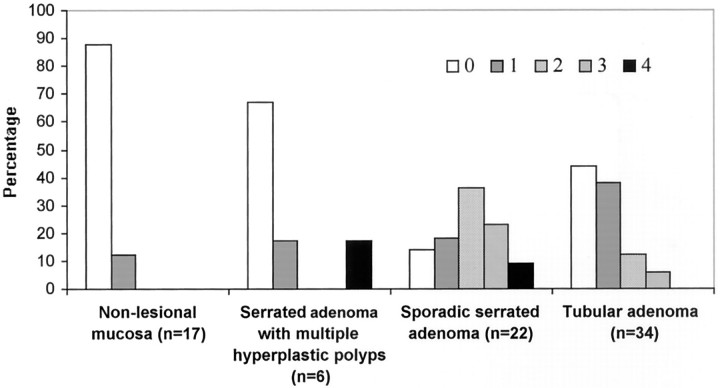
Sporadic serrated adenomas were more frequently methylated at multiple loci than tubular adenomas and serrated adenomas with multiple hyperplastic polyps, P = 0.002 and P = 0.03, respectively (Figure 4) ▶ . The mean number of methylated site was 2.0 ± 1.7 in sporadic serrated adenomas versus 0.8 ± 0.9 in tubular adenomas (P = 0.00001).
Figure 4.
The numbers of gene or loci methylated in nonlesional colonic mucosas, sporadic serrated adenomas, serrated adenomas with multiple (6 to 10) hyperplastic polyps, and tubular adenomas. Sporadic serrated adenomas showed more concordant methylation sites as compared to tubular adenomas (P = 0.002) and serrated adenomas with multiple hyperplastic polyps (P = 0.03).
There was discordance in methylation status of at least two loci between microdissected hyperplastic glands and adenomatous glands in four of five serrated adenomas with admixed hyperplastic glands and adenomatous glands (Figure 2) ▶ .
CIMP in Serrated Adenoma and Tubular Adenomas and Clinicopathological Associations
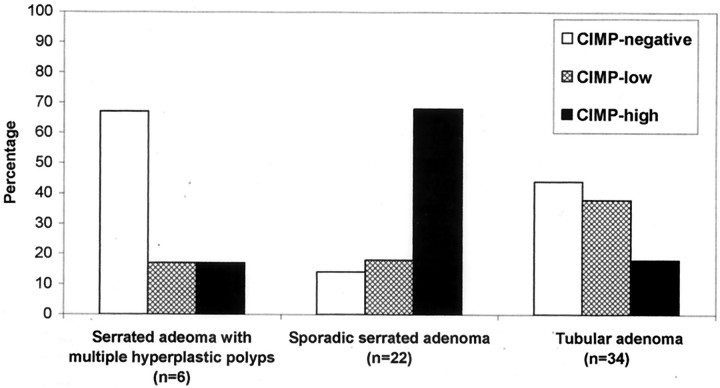
There was a significant difference in CIMP status between sporadic serrated adenomas and tubular adenomas (Figure 5) ▶ . The prevalence of CIMP-high, CIMP-low, and CIMP-negative was 68% (15 of 22), 18% (4 of 22), and 14% (3 of 22), respectively, in sporadic serrated adenomas as compared to 18% (6 of 34), 38% (13 of 34), and 44% (15 of 34), respectively, in tubular adenomas (P = 0.0005), and to 17% (1 of 6), 17% (1 of 6), and 66% (4 of 6), respectively, in serrated adenomas with multiple hyperplastic polyps (P = 0.03). All five serrated adenomas with admixed hyperplastic glands and adenomatous glands were CIMP-high as contrasted to 11 of 23 (48%) of serrated adenomas (P = 0.052).
Figure 5.
CIMP status of serrated adenomas and tubular adenomas. CIMP-high was more frequent in sporadic serrated adenomas than in tubular adenomas (P = 0.0005) and serrated adenomas with multiple hyperplastic polyps (P = 0.03).
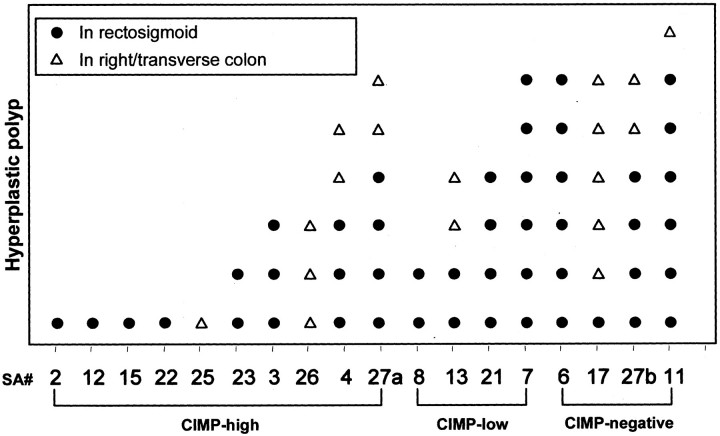
Patients with the CIMP-low sporadic serrated adenomas (100%, 4 of 4) or CIMP-negative sporadic serrated adenomas (33%, 1 of 7) more frequently had previous adenomas as compared to patients with the CIMP-high sporadic serrated adenomas (40%, 6 of 15, P = 0.04; Table 3 ▶ ). There was no correlation between CIMP status and other clinicopathological factors of patients with sporadic serrated adenomas (Table 4) ▶ or tubular adenomas (data not shown). There was no association between the CIMP status and the frequency of previous hyperplastic polyps in patients with serrated adenoma. In patients with serrated adenomas, the hyperplastic polyps were predominantly located in rectosigmoid colon in 4 of 5 patients with multiple (5 to 10) hyperplastic polyps and in 9 of 12 patients with less than five hyperplastic polyps (Figure 6) ▶ .
Table 4.
Correlation of CIMP Status and Clinicopathological Features of Sporadic Serrated Adenomas
| Characteristics | Total (no.) | CIMP-negative (n = 3) % (no.) | CIMP-low (n = 4) % (no.) | CIMP-high (n = 15) % (no.) | P value |
|---|---|---|---|---|---|
| Age ± SD (years) | 64 ± 12 | 59 ± 9 | 61 ± 7 | 66 ± 13 | NS |
| Gender | |||||
| Male | 13 | 15% (2) | 23% (3) | 62% (8) | NS |
| Female | 9 | 11% (1) | 11% (1) | 78% (7) | |
| Site | |||||
| Right colon | 10 | 0% (0) | 30% (3) | 70% (7) | NS |
| Left colon | 11 | 27% (3) | 9% (1) | 64% (7) | |
| Size | |||||
| ≤1.0 cm | 18 | 17% (3) | 22% (4) | 61% (11) | NS |
| >1.0 cm | 4 | 0% (0) | 0% (0) | 100% (4) | |
| History of hyperplastic polyps | |||||
| Present | 12 | 0% (0) | 22% (3) | 56% (9) | NS |
| Absent | 10 | 30% (3) | 10% (1) | 60% (6) | |
| History of conventional adenomas | |||||
| Present | 11 | 9% (1) | 36% (4) | 55% (6) | 0.04 |
| Absent | 11 | 18% (2) | 0% (0) | 82% (9) | |
| History of colorectal cancers | |||||
| Present | 6 | 0% (0) | 33% (2) | 67% (4) | NS |
| Absent | 16 | 19% (3) | 13% (2) | 69% (11) |
Figure 6.
CIMP status and distribution of hyperplastic polyps in patients with sporadic serrated adenomas and serrated adenomas with multiple (6 to 10) hyperplastic polyps (SA no. 6, 7, 11, 17, 27a, and 27b). Serrated adenomas (SA) no. 27a and 27b were from the same patient. The majority of the hyperplastic polyps were located in the distal colorectum (rectum and sigmoid colon).
MSI
There was no difference in prevalence of high levels of MSI between sporadic serrated adenomas (18%, 4 of 22) and tubular adenomas (3%, 1 of 34, P = 0.07; Figure 2 ▶ ). MSI was present in 2 of 5 (40%) serrated adenomas with admixed hyperplastic glands and adenomatous glands as compared to 2 of 23 (7%) of classic serrated adenomas (P = 0.13). Methylation of hMLH1 gene was present in two of four sporadic serrated adenomas with MSI (Figure 3) ▶ .
Discussion
The large majority of colorectal malignancies develop from adenomatous polyps following the classic adenoma-adenocarcinoma sequence. 3 In addition to classic adenomas, variants of adenomas such as serrated adenoma 11,25,41-44 and flat adenoma 45-47 have been described. Serrated adenomas are uncommon in the colorectum compared to conventional tubular adenomas. The role of serrated adenomas in colorectal carcinogenesis is under-recognized, but it has been reported that intramucosal carcinoma occurs in 11% of serrated adenomas and that colorectal cancer is common in patients with serrated adenomatosis. 7,25 In addition, adenocarcinomas can arise in association with hyperplastic polyps, especially in patients with hyperplastic polyposis. 4-13,18 The presence of dysplasia in serrated adenomas and the association of serrated adenomas and hyperplastic polyps with colorectal carcinomas have suggested the presence of a hyperplastic polyp-serrated adenoma-adenocarcinoma pathway in colorectal carcinogenesis. 6,16,19-24
We studied CIM in sporadic serrated adenomas and tubular adenomas. In our present study, methylation of two or more CpG islands (CIMP-high) was present in 68% of serrated adenomas but only in 18% of tubular adenomas, suggesting that CIM plays a more important role in the pathogenesis of sporadic serrated adenomas than in tubular adenomas. Aberrant methylation of CpG islands in the promoter region of tumor suppressor genes is associated with transcriptional inactivation of the genes and is thought to play an important role in carcinogenesis. 32,48 The recently discovered CIMP is a novel pathway characterized by methylation of multiple CpG islands in colorectal carcinomas and adenomas. 34 The methylation status of sporadic serrated adenomas had not been reported previously. Methylation at multiple loci (CIMP-high) is present in 40 to 50% of sporadic colorectal carcinomas and adenomas, 34-36 and in 43% of hyperplastic polyps and 75% of serrated adenomas from patients with hyperplastic polyposis. 37 Hyperplastic polyposis is morphologically and genetically heterogeneous, patients with hyperplastic polyposis can have chromosome 1p allelic loss, and patients with large right-sided hyperplastic polyps or small number of hyperplastic polyps tend to lack of 1p loss. 6 In the present study, CIMP-high was detected in only 17% (one of six) of serrated adenomas with multiple hyperplastic polyps. This discrepancy may be because of the different distribution pattern of hyperplastic polyps in these two studies. There is a predominance of right-sided hyperplastic polyps in the previous study of patients with hyperplastic polyposis and serrated adenomas, 37 this is in contrast of predominance of left-sided hyperplastic polyps even in patients with multiple (6 to 10) hyperplastic polyps in this study (Figure 6) ▶ .
Previous studies have shown heterogeneity of genetic alterations in serrated adenomas, including K-ras mutations, p53 gene mutations, and MSI. 6,18,19,29,30 The frequency of K-ras gene mutations has been reported to be as high as 58%, ie, higher than in tubular adenomas. 30 Overexpression of p53 protein of the type associated with p53 gene mutation (100%, 11 of 11) and mutation of p53 (47%, 9 of 19) have been demonstrated in serrated adenomas in one study. 30 However, other studies have shown a low rate of K-ras mutation and p53 overexpression. 49,50
In our study, MSI was present in 14% of serrated adenomas, and 75% of serrated adenomas with MSI had CIMP-high. The development of MSI in serrated adenoma 6,18,19,37 may have a role in the pathogenesis of colorectal carcinomas. Previous reports have shown more frequent MSI in colorectal carcinomas associated with a serrated adenoma compared to carcinomas without residual serrated adenoma. 17 An association between colorectal carcinomas with MSI and serrated adenomas and hyperplastic polyps has also been reported. 22 In addition, MSI develops because of methylation of hMLH1 in sporadic CIMP-high colorectal carcinomas, and in CIMP-high serrated adenomas, tubular adenomas, and carcinomas in patients with hyperplastic polyposis. 34,37 In the present study, methylation of hMLH1 gene was present in two of four sporadic adenomas with MSI.
Serrated adenomas with admixed hyperplastic glands and adenomatous glands have been described but not well characterized. Most studies have not separated these from the serrated adenomas. However, serrated adenomas with admixed hyperplastic glands and adenomatous glands are more frequently associated with K-ras mutation, p53 overexpression, and MSI in some studies. 6,19 In our present study, MSI was present in 40% of serrated adenomas with admixed hyperplastic glands and adenomatous glands but only in 8% of pure serrated adenomas, and all five serrated adenomas with hyperplastic glands and adenomatous glands had CIMP-high. The serrated adenomas with admixed hyperplastic glands and adenomatous glands showed heterogeneity between the adenomatous and hyperplastic components in genetic alterations such as K-ras mutations and p53 overexpression, 6 and in the methylation pattern (the present study). These observations are based on small numbers of cases and suggest that the molecular pathogenesis of serrated adenomas with admixed hyperplastic glands and adenomatous glands may be different from pure serrated adenomas, however, a larger series is needed to confirm these observations.
Previous studies have shown an association between serrated adenomas and hyperplastic polyps in patients with sporadic colorectal carcinomas, 22 and in patients with hyperplastic polyposis. 37 Similarly, in our current study hyperplastic polyps were more frequent in patients with serrated adenomas compared to patients with tubular adenomas. In our previous study, hyperplastic polyposis patients with serrated adenomas had concordant methylation of multiple hyperplastic polyps, 37 but in the present study of sporadic serrated adenomas we found no association between the presence and absence of previous history hyperplastic polyps and the CIMP status of the serrated adenoma. This suggests that the mechanism of methylation is lesion-specific in sporadic serrated adenomas and patient-specific in patients with hyperplastic polyposis.
In conclusion, our results demonstrated that concordant methylation occurs more frequently in sporadic serrated adenomas than in tubular adenomas and that epigenetic alterations because of methylation may play an important role in the pathogenesis of sporadic serrated adenomas.
Footnotes
Address reprint requests to Tsung-Teh Wu, M.D., Ph.D., Department of Pathology, G1.3595 C, Box 85, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX, 77030-4095. E-mail: twu@mdanderson.org.
References
- 1.: American Cancer Society: Cancer Facts and Figures—1997. 1997. American Cancer Society, Atlanta
- 2.Fearon ER, Vogelstein B: A genetic model for colorectal tumorigenesis. Cell 1990, 61:759-767 [DOI] [PubMed] [Google Scholar]
- 3.Potter JD: Colorectal cancer: molecules and populations. J Natl Cancer Inst 1999, 91:916-932 [DOI] [PubMed] [Google Scholar]
- 4.Morson BC: The polyp-cancer sequence in the large bowel. Proc R Soc Med 1974, 67:451-457 [PMC free article] [PubMed] [Google Scholar]
- 5.Hill MJ, Morson BC, Bussey HJ: Aetiology of adenoma-carcinoma sequence in large bowel. Lancet 1978, 1:245-247 [DOI] [PubMed] [Google Scholar]
- 6.Rashid A, Houlihan PS, Booker S, Peterson GM, Giardiello FM, Hamilton SR: Phenotypic and molecular characteristics of hyperplastic polyposis. Gastroenterology 2000, 119:323-332 [DOI] [PubMed] [Google Scholar]
- 7.Torlakovic E, Snover DC: Serrated adenomatous polyposis in humans. Gastroenterology 1996, 110:748-755 [DOI] [PubMed] [Google Scholar]
- 8.Goldman H, Ming S-C, Hickok DF: Nature and significance of hyperplastic polyps of the human colon. Arch Pathol 1970, 89:349-354 [PubMed] [Google Scholar]
- 9.Estrada RG, Spjut HJ: Hyperplastic polyps of the large bowel. Am J Surg Pathol 1980, 4:127-133 [DOI] [PubMed] [Google Scholar]
- 10.Williams GT: Metaplastic (hyperplastic) polyps of the large bowel: benign neoplasms after all? Gut 1997, 40:691-692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urbanski SJ, Kossakowska AE, Marcon N, Bruce WR: Mixed hyperplastic adenomatous polyps—an underdiagnosed entity. Am J Surg Pathol 1984, 8:551-556 [PubMed] [Google Scholar]
- 12.McCann BG: A case of metaplastic polyposis of the colon associated with focal adenomatous change and metachronous adenocarcinomas. Histopathology 1988, 13:700-702 [DOI] [PubMed] [Google Scholar]
- 13.Bengoechea O, Martinez-Penuela JM, Larrinaga B, Valerdi J, Borda F: Hyperplastic polyposis of the colorectum and adenocarcinoma in a 24-year-old man. Am J Surg Pathol 1987, 11:323-327 [DOI] [PubMed] [Google Scholar]
- 14.Teoh HH, Delahunt B, Isbister WH: Dysplastic and malignant areas in hyperplastic polyps of the large intestine. Pathology 1989, 21:138-142 [DOI] [PubMed] [Google Scholar]
- 15.Lieverse RJ, Kibbelaar RE, Griffioen G, Lamers CBHW: Colonic adenocarcinoma in a patient with multiple hyperplastic polyps. Neth J Med 1995, 46:185-188 [DOI] [PubMed] [Google Scholar]
- 16.Jass JR, Cottier DS, Pokos V, Parry S, Winship IM: Mixed epithelial polyps in association with hereditary non-polyposis colorectal cancer providing an alternative pathway of cancer histogenesis. Pathology 1997, 29:28-33 [DOI] [PubMed] [Google Scholar]
- 17.Makinen MJ, George SM, Jernvall P, Makela J, Vihko P, Karttunen TJ: Colorectal carcinoma associated with serrated adenoma—prevalence, histological features, and prognosis. J Pathol 2001, 193:286-294 [DOI] [PubMed] [Google Scholar]
- 18.Leggett BA, Devereaux B, Biden K, Searle J, Young J, Jass J: Hyperplastic polyposis: association with colorectal cancer. Am J Surg Pathol 2001, 25:177-184 [DOI] [PubMed] [Google Scholar]
- 19.Iino H, Jass JR, Simms LA, Young J, Leggett B, Ajioka Y, Watanabe H: DNA microsatellite instability in hyperplastic polyps, serrated adenomas, and mixed polyps: a mild mutator pathway for colorectal cancer? J Clin Pathol 1999, 52:5-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jass JR, Iino H, Ruszkiewicz A, Painter D, Solomon MJ, Koorey DJ, Cohn D, Furlong KL, Walsh MD, Palazzo J, Edmonston TB, Fishel R, Young J, Leggett BA: Neoplastic progression occurs through mutator pathways in hyperplastic polyposis of the colorectum. Gut 2000, 47:43-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jass JR: Serrated route to colorectal cancer: back street or super highway? J Pathol 2001, 193:283-285 [DOI] [PubMed] [Google Scholar]
- 22.Hawkins NJ, Ward RL: Sporadic colorectal cancers with microsatellite instability and their possible origin in hyperplastic polyps and serrated adenomas. J Natl Cancer Inst 2001, 93:1307-1313 [DOI] [PubMed] [Google Scholar]
- 23.Jass JR, Young J, Leggett BA: Hyperplastic polyps and DNA microsatellite unstable cancers of the colorectum. Histopathology 2000, 37:295-301 [DOI] [PubMed] [Google Scholar]
- 24.Jass JR, Biden KG, Cummings MC, Simms LA, Walsh M, Schoch E, Meltzer SJ, Wright C, Searle J, Young J, Leggett BA: Characterization of a subtype of colorectal cancer combining features of suppressor and mild mutator pathways. J Clin Pathol 1999, 52:455-460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longacre TA, Fenoglio-Preiser CM: Mixed hyperplastic adenomatous polyps/serrated adenomas. A distinct form of colorectal neoplasia. Am J Surg Pathol 1990, 14:524-537 [DOI] [PubMed] [Google Scholar]
- 26.Otori K, Oda Y, Sugiyama K, Hasebe T, Mukai K, Fujii T, Tajiri H, Yoshida S, Fukushima S, Esumi H: High frequency of K-ras mutations in human colorectal hyperplastic polyps. Gut 1997, 40:660-663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lothe RA, Andersen SN, Hofstad B, Meling GI, Peltomaki P, Heim S, Brogger A, Vatn M, Rognum TO, Borresen AL: Deletion of 1p loci and microsatellite instability in colorectal polyps. Genes Chromosom Cancer 1995, 14:182-188 [DOI] [PubMed] [Google Scholar]
- 28.Jen J, Powell SM, Papadopoulos N, Smith KJ, Hamilton SR, Vogelstein B, Kinzler KW: Molecular determinants of dysplasia in colorectal lesions. Cancer Res 1994, 54:5523-5526 [PubMed] [Google Scholar]
- 29.Fogt F, Brien T, Brown CA, Hartmann CJ, Zimmerman RL, Odze RD: Genetic alterations in serrated adenomas: comparison to conventional adenomas and hyperplastic polyps. Hum Pathol 2002, 33:87-91 [DOI] [PubMed] [Google Scholar]
- 30.Hiyama T, Yokozaki H, Shimamoto F, Haruma K, Yasui W, Kajiyama G, Tahara E: Frequent p53 gene mutations in serrated adenomas of the colorectum. J Pathol 1998, 186:131-139 [DOI] [PubMed] [Google Scholar]
- 31.Dehari R: Infrequent APC mutations in serrated adenoma. Tohoku J Exp Med 2001, 193:181-186 [DOI] [PubMed] [Google Scholar]
- 32.Bird AP: CpG-rich islands and the function of DNA methylation. Nature 1986, 321:209-213 [DOI] [PubMed] [Google Scholar]
- 33.Baylin SB, Herman JG, Graff JR, Vertino PM, Issa J-PJ: Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res 1998, 72:141-196 [PubMed] [Google Scholar]
- 34.Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa J-PJ: CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA 1999, 96:8681-8686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toyota M, Ohe-Toyota M, Ahuja N, Issa J-PJ: Distinct genetic profiles in colorectal tumors with or without the CpG island methylator phenotype. Proc Natl Acad Sci USA 2000, 97:710-715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rashid A, Shen L, Morris JS, Issa JPJ, Hamilton SR: CpG island methylation in colorectal adenomas. Am J Pathol 2001, 159:1129-1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan AO, Issa J-PJ, Morris JS, Hamilton SR, Rashid A: Concordant CpG island methylation in hyperplastic polyposis. Am J Pathol 2002, 160:529-536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moskaluk CA, Kern SE: Microdissection and polymerase chain reaction amplification of genomic DNA from histological tissue sections. Am J Pathol 1997, 150:1547-1552 [PMC free article] [PubMed] [Google Scholar]
- 39.Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB: Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA 1996, 93:9821-9826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toyota M, Ho C, Ohe-Toyota M, Baylin SB, Issa J-PI: Inactivation of CAGNA1G, a T-type calcium channel gene, by aberrant methylation of its 5′ CpG island in human tumors. Cancer Res 1999, 59:4535-4541 [PubMed] [Google Scholar]
- 41.Shimamoto F, Tanaka S, Tahara E: Pathogenesis of serrated adenoma of the colorectum: implication for malignant progression. Tahara E eds. Molecular Pathology of Gastroenterological Cancer. 1997:pp 93-106 Springer, Tokyo
- 42.Yao T, Nishiyama K, Oya M, Kouzuki T, Kajiwara M, Tsuneyoshi M: Multiple “serrated adenocarcinomas” of the colon with a cell lineage common to metaplastic polyp and serrated adenoma. Case report of a new subtype of colonic adenocarcinoma with gastric differentiation. J Pathol 2000, 190:444-449 [DOI] [PubMed] [Google Scholar]
- 43.Fujishima N: Proliferative activity of mixed hyperplastic adenomatous polyp/serrated adenoma in the large intestine, measured by PCNA (proliferating cell nuclear antigen). J Gastroenterol 1996, 31:207-213 [DOI] [PubMed] [Google Scholar]
- 44.Rubio CA, Jaramillo E: Flat serrated adenomas of the colorectal mucosa. Jpn J Cancer Res 1996, 87:305-309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kasumi A, Kratzer GL, Takeda M: Observations of aggressive, small, flat, and depressed colon cancer. Report of three cases. Surg Endosc 1995, 9:690-694 [DOI] [PubMed] [Google Scholar]
- 46.Yamagata S, Muto T, Uchida Y, Masaki T, Sawada T, Tsuno N, Hirooka T: Lower incidence of K-ras codon 12 mutation in flat colorectal adenomas than in polypoid adenomas. Jpn J Cancer Res 1994, 85:147-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minamoto T, Sawaguchi K, Mai M, Yamashita N, Sugimura T, Esumi H: Infrequent K-ras activation in superficial-type (flat) colorectal adenomas and adenocarcinomas. Cancer Res 1994, 54:2841-2844 [PubMed] [Google Scholar]
- 48.Jones PA: DNA methylation errors and cancer. Cancer Res 1996, 56:2463-2467 [PubMed] [Google Scholar]
- 49.Ajioka Y, Watanabe H, Jass JR, Yokota Y, Kobayashi M, Nishikura K: Infrequent K-ras codon 12 mutation in serrated adenomas of human colorectum. Gut 1998, 42:680-684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang M, Mitomi H, Sada M, Tokumitsu Y, Takahashi Y, Igarashi M, Katsumata T, Okayasu I: Ki-67, p53, and Bcl-2 expression of serrated adenomas of the colon. Am J Surg Pathol 1999, 23:1158-1160 [DOI] [PubMed] [Google Scholar]