Abstract
Kindled seizures are widely used as a model for epileptogenesis. Although the achievement of kindling criterion is known to require time to develop, the precise developmental period has not been identified. We now report that optimal achievement of the kindling criterion in the Sprague-Dawley rat is associated with a critical inter-stimulus interval of 24 to 26 days. We show that highly efficient kindling can be achieved with only two subconvulsive doses of pentylenetetrazole so long as they are given 25 days apart. Using Northern blot hybridization we show that the increased seizure susceptibility at 25 days coincides with an increased expression of the plasticity-associated proteins, growth-associated protein-43 (GAP-43), microtubule-associated protein 1B (MAP1B), and tissue plasminogen activator (tPA) mRNAs in the hippocampus. By in situ hybridization and immunocytochemistry on tissue sections, we also show an increased expression for GAP-43 in the polymorphic layer of the dentate gyrus, mossy fibers, and pyramidal cells in the CA3 region of the hippocampus. The demonstration of a long, defined developmental interval for inducing the kindling criterion should enable a dissection of the cellular and genetic events underlying this phenomenon in the rat.
Epilepsy is a chronic disorder characterized by recurrent seizures occurring over long periods of time. 1 The most studied model of chronic limbic epilepsy is kindled seizures, a condition achieved by the repeated administration of an initially subconvulsant stimulus that ultimately results in the generation of seizure activity. 2 Kindled seizures are achieved by electrical or chemical stimulation. 3,4,5 Examples of chemicals producing kindling in rats include pentylenetetrazole (PTZ), FG-7142, and picrotoxin.
The achievement of the kindling criterion takes a long time, usually between 15 and 38 days, depending on the kindling procedure and animal strain. 6,7 Although the genetic events underlying the development and achievement of the kindling criterion are not fully understood, it is conceivable that the repeated administration of the stimulus will lead to a succession of similar genetic events the summation of which ends with changes in the brain network such that the animals are very susceptible to developing seizure activity on exposure to a subconvulsive dose of that stimulus.
One major drawback of the kindling model is that periods of seizure susceptibility alternate with periods of seizure refractoriness 6,8 making it difficult to choose time points at which to study gene expression. We reasoned that disturbing the unfolding of gene expression before completion by a second application of the same stimulus should result in a new wave of gene expression leading to resistance to drug application. If this is so, it follows that there must be a critical time window of seizure susceptibility whereby an application of a subconvulsive dose of the stimulus would evoke seizure activity only if the previous cycle of gene expression is complete.
In this article we report results from a series of experiments aimed at finding this time window using PTZ as a convulsant and growth-associated protein-43 (GAP43), microtubule-associated protein 1B (MAP1B), and tissue plasminogen activator (tPA) as markers of neuronal plasticity. 9-12
Materials and Methods
For all procedures, ethical approval was granted by the university animal experimentation ethics board according to the requirements of the German National Act on the Use of Experimental Animals.
Administration of Pentylenetetrazole
Three-month-old male Sprague-Dawley rats (Charles River, Sulzfeld, Germany) were maintained on a 12-hour light/dark cycle and allowed free access to food and water. The body weights ranged between 320 to 400 grams. Seizures were induced by intraperitoneal (i.p.) injection of PTZ (50 mg/kg, between 9 and 11 a.m.). With a dose of PTZ at 50 mg/kg, rats had seizures within 3 to 5 minutes and had fully recovered by the time of sacrifice. For the identification of the time window of seizure susceptibility, rats were then treated with a subconvulsive dose of PTZ (30 mg/kg) at various times thereafter until day 30. After the time window had been established, kindling criterion was induced by two subconvulsive doses of PTZ (30 mg/kg) given 25 days apart. Seizure severity was scored according to Racine, 13 whereby a score of 5 means rearing and falling accompanied by generalized seizures. Control rats received physiological saline i.p.
Histology
After survival times of 3-, 7-, 14-, 25- and 30-days after the last kindling session, the rats were deeply anesthetized with 3% halothane in 75% nitrous oxide/25% oxygen, and perfused with buffered saline. The brains were then removed and bisected mid-sagitally. One half of each brain (left and right hemispheres were collected alternately) was fixed in 4% paraformaldehyde, 50 mmol/L phosphate buffer (pH 7.2) for 24 hours, cryoprotected in 20% sucrose, 10 mmol/L phosphate-buffered saline, (pH 7.2), and stored at −70°C. This hemisphere was used for immunocytochemistry and in situ hybridization experiments; the hippocampus from the other hemisphere was used for Northern- and immunoblotting.
Preparation of cRNA Probes
A full-length rat cDNA clone for GAP-43 was subcloned into a pBluesscript [pBSSK(I)+] vector. MAP1B, a rat cDNA (clone 36a in pUC vector), was kindly provided by Dr. C.C. Garner (Zentrum für Molekulare Neurobiologie, University of Hamburg, Hamburg) and subcloned into a pBluescript [pBSSKI+] vector. Tissue-plasminogen activator, a rat cDNA coding for tPA (in pGEM-1 vector), was a gift from Dr. Tor Ny (UME University, Stockholm, Sweden). These plasmids allowed the synthesis of both radioactive and non-radioactive antisense and sense RNA probes using [2P]-UTP or digoxygenin-11-UTP. For in situ hybridization experiments the GAP-43 riboprobe was hydrolyzed to an average size of 300 bp by incubating in 1 mol/L NaHCO3/Na2CO3 buffer at 60°C for 60 minutes.
Northern Blots
Total RNA was extracted from individual hippocampi by acid guanidinium thiocyanate-phenol-chloroform extraction, resolved on formaldehyde/agarose gels and linked to the membrane by ultraviolet (UV) irradiation using established procedures. After electrophoresis, the RNA (5 μg) was capillary-blotted to positively charged Nylon-Plus membranes (Schleicher & Schuell) and linked to the membrane by UV cross-linking. A [32P]-labeled cRNA probe coding for the 18S RNA (Ambion, Austin, TX, nucleotides 794–715) was used to correct for differences between the lanes. Blots were hybridized with 2 × 106 cpm/ml in 50% formamide, 1.5X sodium saline phosphate (SSPE), 1% sodium dodecyl sulfate (SDS), 0.5% dry milk, 100 μg/ml yeast total RNA, and 300 μg/ml salmon sperm DNA at 55°C for 15 hours. After hybridization, filters were washed in 2X standard saline citrate (SSC), 0.2% SDS at room temperature and then at high stringency in 0.1X SSC, 0.2% SDS at 75°C, and exposed to preflashed Amersham MP film with intensifying screens at −70°C. The optical density of a band was determined by computerized videodensitometry with background substraction (Kontron, Munich, Germany). Each value was then normalized with respect to the loaded 18S rRNA. After exposure to a film, the filters were stripped of the GAP-43 riboprobe and used for further hybridizations with the MAP1B and tPA riboprobes.
Non-Radioactive in Situ Hybridization
A 1.1 kb digoxigenin-11-UTP-labeled antisense RNA probe was synthesized using a kit supplied by Boehringer Mannheim (Mannheim, Germany) according to the manufacturer’s specifications. cRNA quality and quantity were evaluated by detection of the digoxigenin-labeled cRNA with anti-digoxigenin alkaline phophatase-F(ab′) fragment (Boehringer Mannheim) conjugate followed by color development using the nitro-blue tetrazolium (NBT) and 5-bromo-4-chloro-3-indolyl-phosphate (BCIP) system. A digoxigenin-labeled neurofilament-68 cRNA probe of known size and concentration was also used as a reference probe. Finally the riboprobe was hydrolyzed to an average size of 300 bp by incubating in 1 mol/L NaHCO3/Na2CO3 buffer at 60°C for 60 minutes. Sixty-μm sections were cut on a freezing microtome and processed for in situ hybridization as previously described. 9,14 For the detection of signal, slides were incubated at 37°C in darkness for 1 hour in a chromogen solution consisting of 330 μg/ml NBT, 150 μg/ml of BCIP, and 250 μg/ml levamisole in alkaline phosphatase buffer.
Controls
The specificity of the rat GAP-43 antisense RNA probe was assessed by Northern blot analysis. Selected tissue sections were hybridized with a hydrolized sense probe.
Immunoblotting
For immunoblotting, SDS-PAGE separated proteins were transferred to nitrocellulose membranes by semi-dry blotting. The membrane was then incubated with a GAP-43 monoclonal mouse antibody (clone GAP-7B10, 1:2000, Sigma, Munich, Germany) and the antigen-antibody complex was detected using the ABC system (Vectastain Elite kit, Vector) and 3′,3′ diaminobenzidine/NiCl2 as a substrate.
Immunocytochemistry
Twenty-five-μm sections were processed for immunohistochemistry as free-floating material as previously described 9,14 . The following antibodies were used: GAP-43 (1:4000, mouse clone GAP-7B10, Sigma); mouse monoclonals to neurofilament polypeptide 160 kd (NF-M, 1:2000, clone NN18, Roche); and mouse monoclonal to neuron-specific nuclear protein (NeuN; 1:2000, Chemicon). Color development ensued using either 3,3′-diaminobenzidine/hydrogen peroxide (ABC system, Vectastain Elite kit, Vector) or 3,3′-diaminobenzidine/glucose oxidase. 15 The diaminobenzidine/glucose oxidase method allows for longer staining by keeping the background at low levels, and is therefore more suitable for mossy fiber staining in this model.
Light Microscopy
For light microscopy, a Nikon microscope was used. Images (768 × 1024 pixels) were captured electronically using a CCD camera (Optronics). The digital images were arranged and labeled using Adobe Photoshop and printed using a Kodak XLS 8000 digital printer. For a group of micrographs the camera setting for exposure, gain, and contrast enhancement was similar.
Quantitative Analysis of Tissue Sections
For in situ hybridization, integrated optical densities of DIG-labeled neurons over the entire CA3 and polymorphic subregion of the hippocampus were collected at three different levels for each rat using the National Institutes of Health (NIH) software PC Image (NIH, Bethesda, MD), and a mean value was computed. After background substraction, the data were expressed as relative optical density ± SD.
Statistical Analysis
Differences between the groups were evaluated for statistical significance using analysis of variance followed by the Tukey test. For those experiments implicating both time and dose as variables, we used two-way analysis of variance followed by the Tukey post-hoc analysis using SigmaStat software (SPSS Inc., Chicago, IL). The level of significance was set at P < 0.05.
Results
Involvement of a Critical 25-Day Period in the Development of PTZ-Induced Kindling Criterion
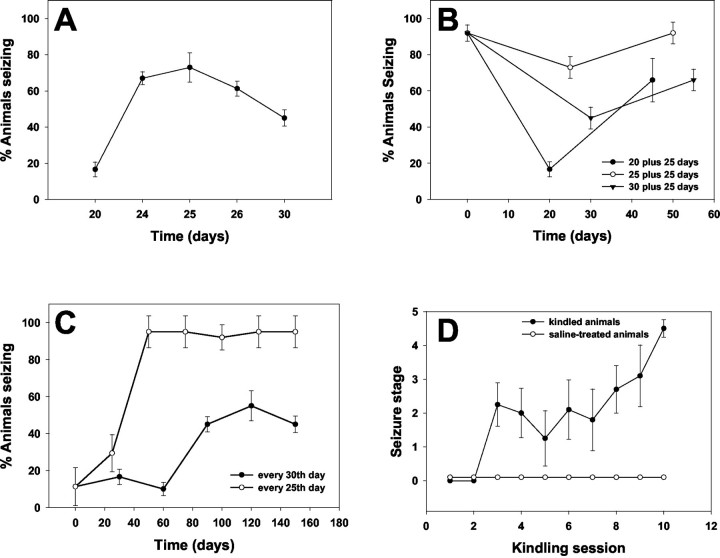
To find a critical time window of seizure susceptibility, we injected Sprague-Dawley rats with a convulsive dose of PTZ (50 mg/kg) and then searched for a time point thereafter when the injection of a subconvulsive dose (30 mg/kg) would result in a seizure. Based on preliminary data we searched between days 20 and 30 and found that 71% of the animals had acquired the full kindling criterion (seizure score of 5) at 24 to 26 days after the initial injection (Figure 1A) ▶ . Furthermore, 90% of animals given one further subconvulsive dose of 30 mg/kg PTZ on day 50 had acquired the full kindling criterion (Figure 1B) ▶ . If the groups with lower incidences of full kindling criterion (ie, days 20 and 30) were further treated with a 30 mg/kg PTZ dose 25 days thereafter, the percentage of animals with full kindling criterion was lower for both time points (66.3%, P < 0.02, Figure 1B ▶ ), indicating that 24 to 26 days are critical to obtaining full kindling with the highest efficiency.
Figure 1.
A: Delimitation of a critical time window of seizure susceptibility by treating the animals with a subconvulsive dose of PTZ (30 mg/kg) at various times after the initial, convulsive dose of 50 mg/kg. Data are means ± SD from three replicates for each PTZ concentration. N, 7 to 9 animals per group. Note that about 70% of animals have reached a full seizure syndrome at 24 to 26 days. B: Further administration of a subconvulsive dose at 25 days to animals previously treated with subconvulsive doses of 30 mg/kg at 20 days (filled circles) and 30 days (filled triangles) greatly increases the number of fully kindled animals. For comparison, the 25- plus 25-day treatment schedule is shown (open circles). N, 7 to 9 animals per group. C: Treatment cycles of 25 days at a dose of 30 mg/kg (open circles) are much more effective in inducing full kindling status than are 30-day cycles at the same dose (filled circles). Note that about 90% of the animals are fully kindled by day 50. N, 8 animals per group. Data are means ± SD from three replicates for each treatment. D: A subconvulsive dose of 20 mg/kg induces seizure behavior (score of 4.5) in 62% of rats treated every 25th day for 250 days (filled circles). Control animals show no seizure-like behavior over the same period (open circles). N, 10 animals per group. Data are means ± SD from two replicates.
We then asked if the initial convulsive dose is essential. For this purpose, we treated an additional group of rats with three subconvulsive (30 mg/kg) doses of PTZ at 25-day intervals. The last PTZ injection induced full tonic-clonic convulsions in 90% of the treated animals (Figure 1C) ▶ . However, treating animals with a subconvulsive dose of 30 mg/kg PTZ at 30-day intervals resulted in a lower incidence of animals with the full kindling criterion (about 50%, Figure 1C ▶ ), confirming that the 25-day interval is critical to achieving a high rate of fully kindled animals. We then tried an even lower dose of 20 mg/kg, and found that 10 PTZ injections, given every 25th day, were required to achieve an average score of 4.5 in 62% of treated animals (Figure 1D) ▶ .
Occasionally, rats experiencing recurrent seizures died, except in cases where we lowered the subconvulsive dose to 20 mg/kg. Otherwise, the mortality rate of rats in this model was less than 5%.
Up-Regulation of tPA, MAP1B, and GAP-43 mRNA Expression in the Hippocampus during the Critical (Susceptibility) Period
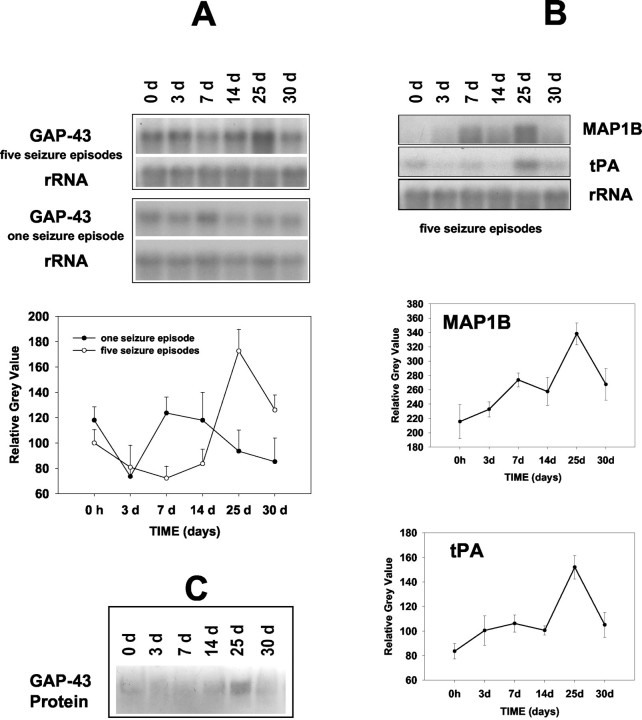
Rats were first treated with a subconvulsive dose of PTZ (30 mg/kg) followed by six similar subconvulsive doses given 25 days apart. Following the last kindling session, the levels of tPA, MAP1B, and GAP-43 mRNAs in the hippocampus reached a maximum at 25 days and began to decline by 30 days. Representative data from individual animals assayed in one experiment are shown in Figure 2 ▶ . A quantitative analysis of the time course of PTZ-mediated mRNA induction revealed that the increase in tPA transcripts was 1.8-fold (P < 0.015; Figure 2B ▶ ), the increase in MAP1B mRNA was 1.6-fold (P < 0.02; Figure 2B ▶ ), and the increase in GAP-43 mRNA was 1.7-fold over control levels (P < 0.001; Figure 2A ▶ ).
Figure 2.
A and B: Northern blot and quantitative analysis of the time course of PTZ-induced GAP-43, MAP1B, and tPA mRNA expression in the hippocampus of adult rats. Rats experienced either a single seizure episode or five seizure episodes. The 18S rRNA subunit was chosen both as a loading control and as a non-changing parameter. Note the increase in prevalence of GAP-43, MAP1B, and tPA transcripts at day 25 following five full seizure episodes after the rats had been primed with two initial subconvulsive doses. The animals at time point 0 hours represent non-seizure animals. C: Immunoblotting of GAP-43. Representative immunoblot shows GAP-43 immunoreactivity in hippocampal homogenates after the fifth seizure episode. Note the increase in the prevalence of GAP-43 protein at 25 days. Data are means ± SD for two replicate experiments. N, 7 animals per group.
We then asked if a single, convulsive dose of PTZ would elicit the same effect as in the repeated seizure episodes. We found, however, that there was a significant reduction (1.6-fold, P < 0.05) in the levels of GAP-43 mRNA at 3 days post-seizure with recovery by day 7 (Figure 2A) ▶ .
Hippocampal homogenates also were examined for GAP-43-like immunoreactivity using Western blot analysis. Immunoblotting with a monoclonal anti-GAP-43 antibody demonstrated an increase of immunoreactive protein in rat hippocampus at 25 days following the seventh PTZ administration (Figure 2C) ▶ . It should be noted that the reported increases represent merely global increases. A region- and time-specific distribution of GAP-43 prevalence was further investigated using in situ hybridization and immunohistochemistry.
The levels and regional distribution of GAP43 mRNA in the hippocampus are shown in Figure 3 ▶ . Hippocampal GAP-43 mRNA of control rats was highly expressed in the pyramidal cells of the CA3 and CA1 regions and, to a lesser extent, in neurons of the supragranular layer. The granule cells of the dentate gyrus were almost devoid of signal (Figure 3A) ▶ . At 25 days after the last episode of generalized seizures, there was a strong up-regulation of GAP-43 transcripts in the polymorphic layer of the dentate gyrus(7.5-fold increase, P < 0.001, analysis of variance, Figure 3, B and C ▶ ) and in pyramidal cells of the CA3 region (1.8-fold increase, P < 0.001, analysis of variance), as compared to the corresponding region of control animals (not shown). However, the granule cells remained devoid of expression. By 30 days, the levels and distribution of GAP-43 transcripts were reminiscent of those of control rats except in the polymorphic layer, where GAP-43 mRNA expression remained significantly above that of controls (not shown). Hybridization with a sense probe at the 30-day time point gave almost no signal (Figure 3D) ▶ .
Figure 3.
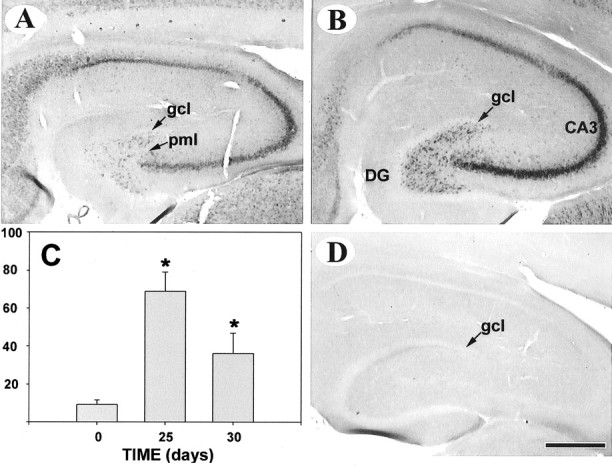
In situ hybridization of GAP-43 mRNA at 25 days after the last of five seizure episodes. A and B: Note the strong increase in the prevalence of GAP-43 transcripts in the polymorphic layer of the dentate gyrus and CA3 region of the hippocampus (B) versus controls (A). A sense probe yielded no signal (D). C: Integrated optical densities for GAP-43 mRNA in the polymorphic layer of the dentate gyrus at 25- and 30-days post-seizure. Data are presented as percent ± SD for two replicate experiments (N, 7 rats; *, P < 0.001 versus controls by analysis of variance). Abbreviations: gcl, granule cell layer; DG, dentate gyrus; pml, polymorphic layer. Bar, 500 μm.
We used immunohistochemistry for GAP-43, which is involved in neurite outgrowth and anatomical plasticity, to follow structural changes in the hippocampus during the critical period. The distribution of GAP-43 in the dentate gyrus is shown in Figure 4 ▶ . In control animals, moderate staining was observed at the periphery of the granule cell layer and the molecular layer of the dentate gyrus (Figure 4B) ▶ . At 25 days following the last PTZ administration, there was a robust increase in GAP-43 immunoreactivity in the mossy fibers (Figure 4, E and G) ▶ and in the inner molecular layer of the dentate gyrus (Figure 4E) ▶ . In contrast, staining of axonal processes with the neuronal cytoskeleton antigen NF-M revealed no differences between control and PTZ-treated animals, both in the polymorphic region (Figure 4C ▶ versus Figure 4F ▶ ) and in mossy fibers of the stratum lucidum (not shown), suggesting that the increases in GAP-43 immunostaining in these structures at 25 days post-seizure were specific.
Figure 4.
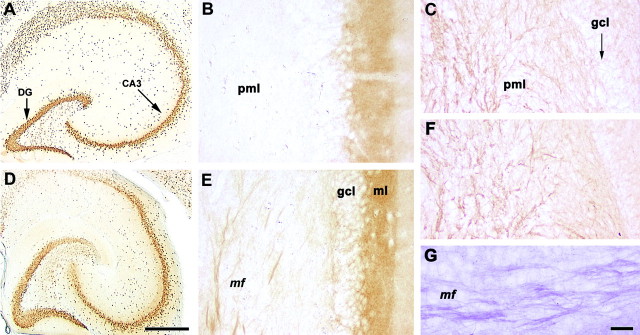
GAP-43, NeuN, and NF-M immunohistochemistry in the hippocampus of rats at 25 days after the last of five seizure episodes. NeuN immunohistochemistry revealed no evidence of microscopic lesions (A, controls; D, treated animals). Note the robust increase in GAP-43 immunoreactivity in the mossy fibers and, to a lesser extent, in the inner molecular layer of the dentate gyrus (E). An enlarged image of the GAP-43-immunopositive mossy fibers in stratum lucidum stained by the glucose oxidase method is also shown (G). In control animals, moderate staining was observed at the periphery of the granule cell layer and in the molecular layer of the dentate gyrus (B). There was no significant difference between NF-M-stained mossy fiber axons in the polymorphic region of controls (C) and the corresponding areas of PTZ-treated rats (F). Five episodes of full seizure syndrome had no obvious morphological effect on neurons in the dentate gyrus (A, controls versus D, kindled rats). Abbreviations: CA3, DG, denote hippocampal regions; mf, mossy fibers; pml, polymorphic layer; gcl, granule cell layer; ml, molecular layer. Bar, 500 μm (A and D); 50 μm (B and E); 33 μm (G).
Finally, five episodes of full seizure activity did not induce obvious microscopic lesions as judged by staining with NeuN, a sensitive marker of neuronal degeneration (Figure 4A ▶ , controls versus Figure 4D ▶ ).
Discussion
In this study, we have shown that the application of the chemoconvulsant PTZ initiates a chain of events with a 25-day-period, at the end of which the rat brain is highly susceptible to the development of generalized seizures induced by a subconvulsive dose of PTZ. We also have shown that the high susceptibility period is associated with increased expression of a protease (tPA), a structural protein (MAP1B), and an axonal growth-associated protein (GAP43) in the hippocampus.
Initially, we sought a critical time window of seizure susceptibility by using a convulsive dose of PTZ followed by a second, subconvulsive dose given at various intervals thereafter. However, we then found that a high proportion (over 90%) of animals reached full kindling criterion after only two PTZ subconvulsive doses (30 mg/kg) given 25 days apart, and therefore dubbed it the “critical time window kindling model.” Although it is possible to achieve the kindling criterion by treating rats with an even smaller dose of PTZ (20 mg/kg), this procedure takes a much longer time to achieve (about 250 days) and has a lower efficiency (62% versus 90%). In this instance, the results of using a lower PTZ dose may be confounded by the aging process, which is known to alter the time course of gene expression after seizure activity in rats. 14 Despite these technical drawbacks, our findings support the hypothesis that periodic exposure to even low levels of stimuli may lead to kindling criterion-like conditions. Our results suggest that the timing of these episodes may influence the likelihood that the epileptic condition will develop.
Previous studies have documented a complex pattern of long-term changes in plasticity-associated protein expression after seizure activity in the rat brain. For example, a single, convulsive dose of PTZ leads to transient increases in MAP1B immunoreactivity in granule cells and the dentate inner and mid-molecular layers, as well as in the stratum lucidum, including the mossy fiber pathway of the CA3 region. 9 Tissue plasminogen activator is a serine protease that is induced as an immediate-early gene during pharmacologically induced seizures. 10,16 It is conceivable that the proteolytic capacity of tPA could be implicated in brain remodeling by facilitating the establishment of new synaptic connections by removal of previous connections.
Our findings confirm previous reports that GAP-43 immunoreactivity increases in the dentate gyrus of adult rats following chemically induced seizures. 17,18 However, although it has been reported that seizure activity induces GAP-43 mRNA expression in granule cells of the dentate gyrus, 19 we could not detect GAP-43 mRNA in these cells, suggesting that the cells contributing GAP-43 mRNA are located in the polymorphic/supragranular layer of the dentate gyrus. Another possibility is that the GAP-43 mRNA is rapidly transported from the cell body to the mossy fibers. Targeting of mRNAs to dendrites is well documented. However, there is increasing evidence that mRNAs can be targeted to axons as well. 20,21 We did, however, detect GAP-43 mRNA in the pyramidal cells of the CA3 region, suggesting an interplay, mediated by mossy fibers, between neurons of the polymorphic/supragranular layer and those of the CA3 region in response to seizure. 15,22-26 Finally, a single convulsive injection of PTZ failed to induce significant increases in GAP-43 mRNA expression in the rat hippocampus. On the contrary, GAP-43 mRNA was decreased at 3 days post-seizure. Although the significance of this decrease is not yet evident, these results suggest that there is a difference between the genetic events required for achievement of the critical period of seizure susceptibility and the 25 days “cycle of gene expression” that occurs during “kindling maintenance” after seven PTZ doses.
Overall these results suggest that persistent expression of several different classes of brain plasticity-associated proteins may be required for the maintenance of the kindling criterion. Although we do not know what other specific genetic events are involved in this phenomenon, our findings support the concept of quantal duration of memories, ie, each new stimulus initiates its own molecular clock in the brain. 27 While we cannot rule out the possibility that the observed 25-day inter-stimulus intervals could be an effect that is specific to the PTZ induction method, 27 with the critical period kindling model, it should be possible to answer cardinal questions about the initiation and persistence of seizure susceptibility, such as: (1) what are the genetic events leading to the kindling criterion? (2) are there critical time points in the development of the kindling criterion? and (3) what brain regions are involved in the development of the kindling criterion? The results of these studies could have important implications for the development of new treatments for seizure disorders, and for our understanding of the neurogenetic substrate of information retention in the nervous system.
Footnotes
Address reprint requests to Aurel Popa-Wagner, Ph.D., Department of Neurology, Ernst-Moritz-Arndt-Universität Greifswald, Ellernholzstr. 1–2, 17487 Greifswald, Germany. E-mail: wagnerap@neurologie.uni-greifswald.de.
Supported by a grant from Deutsche Forschungsgemeinschaft (Po359/5–1) to A.P.-W.
References
- 1.Lowenstein DH: Status epilepticus. N Engl J Med 1998, 338:970-976 [DOI] [PubMed] [Google Scholar]
- 2.Lothman EW, Bertram EH, Stringer JL: Functional anatomy of hippocampal seizures. Prog Neurobiol 1991, 37:1-82 [DOI] [PubMed] [Google Scholar]
- 3.Goddard GV, McIntyre DC, Leech CK: A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol 1969, 25:295-330 [DOI] [PubMed] [Google Scholar]
- 4.Sloviter RS, Damiano BP: Sustained electrical stimulation of the perforant path duplicates kainate-induced electrophysiological effects and hippocampal damage in rats. Neurosci Lett 1981, 24:279-284 [DOI] [PubMed] [Google Scholar]
- 5.Bekenstein JW, Lothman EW: Dormancy of inhibitory interneurons in a model of temporal lobe epilepsy. Science 1993, 259:97-100 [DOI] [PubMed] [Google Scholar]
- 6.Atack JR, Cook SM, Hutson PH, File SE: Kindling induced by pentylenetetrazole in rats is not directly associated with changes in the expression of NMDA or benzodiazepine receptors. Pharmacol Biochem Behav 2000, 65:743-750 [DOI] [PubMed] [Google Scholar]
- 7.Becker A, Grecksch G, Thiemann W, Hollt V: Pentylenetetrazole-kindling modulates stimulated dopamine release in the nucleus accumbens of rats. Pharmacol Biochem Behav 2000, 66:425-428 [DOI] [PubMed] [Google Scholar]
- 8.Craig CR, Colasanti BK: A study of pentylenetetrazol kindling in rats and mice. Pharmacol Biochem Behav 1988, 31:867-870 [DOI] [PubMed] [Google Scholar]
- 9.Popa-Wagner A, Fischer B, Schmoll H, Platt D, Kessler C: Increased expression of microtubule-associated protein 1B in the hippocampus, subiculum, and perforant path of rats treated with a high dose of pentylenetetrazole. Exp Neurol 1997, 148:73-82 [DOI] [PubMed] [Google Scholar]
- 10.Qian Z, Gilbert ME, Colicos MA, Kandel E, Kuhl D: Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling, and long-term potentiation. Nature 1993, 361:453-457 [DOI] [PubMed] [Google Scholar]
- 11.Frey D, Laux T, Xu L, Schneider C, Caroni P: Shared and unique roles of CAP23 and GAP43 in actin regulation, neurite outgrowth, and anatomical plasticity. J Cell Biol 2000, 149:1443-1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bomze HM, Bulsara KR, Iskandar BJ, Caroni P, Pate Skene JH: Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nat Neurosci 2001, 4:38-43 [DOI] [PubMed] [Google Scholar]
- 13.Racine RJ: Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol 1972, 32:281-294 [DOI] [PubMed] [Google Scholar]
- 14.Popa-Wagner A, Fischer B, Schmoll H, Platt D, Kessler C: Anomalous expression of microtubule-associated protein 1B in the hippocampus and cortex of aged rats treated with pentylenetetrazole. Neuroscience 1999, 94:395-403 [DOI] [PubMed] [Google Scholar]
- 15.Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH: Dentate granule cell nurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci 1997, :3727-3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carroll P, Tsirka S, Richards W, Frohman M, Strickland S: The mouse plasminogen activator gene 5í flanking region directs appropriate expression during development and a seizure-enhanced response in the CNS. Development 1994, 120:3173-3183 [DOI] [PubMed] [Google Scholar]
- 17.Bendotti C, Baldessari S, Pende M, Southgate T, Guglielmetti F, Samanin R: Relationship between GAP-43 expression in the dentate gyrus and synaptic reorganization of hippocampal mossy fibres in rats treated with kainic acid. Eur J Neurosci 1997, 9:93-101 [DOI] [PubMed] [Google Scholar]
- 18.Naffah-Mazzacoratti MG, Funke MG, Sanabria ER, Cavalheiro EA: Growth-associated phosphoprotein expression is increased in the supragranular regions of the dentate gyrus following pilocarpine-induced seizures in rats. Neuroscience 1999, 91:485-492 [DOI] [PubMed] [Google Scholar]
- 19.McNamara RK, Routtenberg A: NMDA receptor blockade prevents kainate induction of protein F1/GAP-43 mRNA in hippocampal granule cells and subsequent mossy fiber sprouting in the rat. Brain Res Mol Brain Res 1995, 133:22-28 [DOI] [PubMed] [Google Scholar]
- 20.Mohr E, Richter D: Axonal mRNAs: functional significance in vertebrates and invertebrates. J Neurocytol 2000, 29:783-791 [DOI] [PubMed] [Google Scholar]
- 21.Aronov S, Aranda G, Behar L, Ginzburg I: Axonal τ mRNA localization coincides with τ protein in living neuronal cells and depend on axonal targeting signal. J Neurosci 2001, 21:6577-6587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parent JM, Lowenstein DH: Mossy fiber reorganization in the epileptic hippocampus. Curr Opin Neurol 1997, 10:103-109 [DOI] [PubMed] [Google Scholar]
- 23.Lynch M, Sutula T: Recurrent excitatory connectivity in the dentate gyrus of kindled and kainic acid-treated rats. J Neurophysiol 2000, 83:693-704 [DOI] [PubMed] [Google Scholar]
- 24.Scharfman HE, Goodman JH, Sollas AL: Granule-like neurons at the hillar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J Neurosci 2000, 20:6144-6158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassan H, Pohle W, Ruthrich H, Brodemann R, Krug M: Repeated long-term potentiation induces mossy fibre sprouting and changes the sensibility of hippocampal granule cells to subconvulsive doses of pentylenetetrazol. Eur J Neurosci 2000, 12:1509-1515 [DOI] [PubMed] [Google Scholar]
- 26.Buckmaster PD, Dudek FE: Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol 1997, 385:385-404 [PubMed] [Google Scholar]
- 27.Chew SJ, Vicario DS, Nottebohm F: Quantal duration of auditory memories. Science 1996, 274:1909-1914 [DOI] [PubMed] [Google Scholar]