Abstract
Dystroglycan (DG) is an adhesion molecule responsible for crucial interactions between extracellular matrix and cytoplasmic compartment. It is formed by two subunits, α-DG (extracellular) and β-DG (transmembrane), that bind to laminin in the matrix and dystrophin in the cytoskeleton, respectively. In this study we evaluated by Western blot analysis the expression of DG in a series of human cancer cell lines of various histogenetic origin and in a series of human primary colon and breast cancers. Decreased expression of DG was observed in most of the cell lines and in both types of tumors and correlated with higher tumor grade and stage. Analysis of the mRNA levels suggested that expression of DG protein is likely regulated at a posttranscriptional level. Evaluation of α-DG expression by immunostaining in a series of archival cases of primary breast carcinomas confirmed that α-DG expression is lost in a significant fraction of tumors (66%). Loss of DG staining correlated with higher tumor stage (P = 0.022), positivity for p53 (P = 0.033), and high proliferation index (P = 0.045). A significant correlation was also observed between loss of α-DG and overall survival (P = 0.013 by log-rank test) in an univariate analysis. These data indicate that DG expression is frequently lost in human malignancies and suggest that this glycoprotein might play an important role in human tumor development and progression.
Cellular interactions with the extracellular matrix are important factors in the development and progression of many types of cancer. Originally characterized as a member of the so-called dystrophin-glycoprotein complex in muscle sarcolemma, dystroglycan (DG) is a transmembrane glycoprotein expressed in a wide variety of tissues at the interface between the basement membrane and cell membrane linking the extracellular matrix to the intracellular cytoskeleton. 1,2 DG is encoded by a single gene and is formed on cleavage of a precursor protein into two mature proteins that form a tight noncovalent complex. 2,3 The cell surface-associated (extracellular) α-DG binds extracellular matrix molecules, such as laminins and agrin, whereas the transmembrane β-DG anchors α-DG to the cell membrane and is linked to the cytoskeleton actin via dystrophin or its paralogue utrophin. 2,4,5 Thus, it has been proposed that DG forms a continuous link from the extracellular matrix to the actin cytoskeleton, providing structural integrity and perhaps transducing signal, in a manner similar to integrins. 2,6,7 DG knockout mice undergo premature death early in embryogenesis and genetic defects of DG have not been reported as causes of hereditary diseases in humans. 8 Disruption of the DG-dystrophin interaction, however, has been described in several forms of hereditary neuromuscular disease such as Duchenne muscular dystrophy. 9,10
The highly and heterogeneously glycosylated cell surface-associated α-DG has a size that ranges from 120 to 180 kd, depending on different amounts of glycosylation. β-DG has been identified as a 43-kd band, sometimes accompanied by a 31-kd band that likely originates from proteolytic fragmentation. 11,12 We recently demonstrated that the 31-kd band is expressed at an increased level in a series of human breast cancer cell lines compared with normal mammary epithelial cells. 11 Increased expression of the 31-kd band was usually associated with a reduced expression of the 43-kd band. Interestingly, expression of α-DG was reduced or absent in most of the cell lines in which β-DG was found as a 31-kd band. Indeed, we demonstrated that both the 43-kd and 31-kd α-DG bands harbor their transmembrane segment. 11 Thus, the cleavage likely occurs within the extracellular domain of β-DG and disrupts the integrity of the DG complex that is strictly dependent on noncovalent interactions between the extracellular domain of β-DG and the C-terminal domain of α-DG. 13 The accumulation of the 31-kd band was interpreted as the consequence of posttranscriptional events as demonstrated by reverse transcriptase-polymerase chain reaction (RT-PCR) experiments that allowed to exclude other phenomena, such as mRNA alternative splicing. 11 We hypothesized that an aberrant processing of DG might play an important role in tumor development by altering the interactions between cells and the surrounding matrix. Thus, a reduction of DG function could influence the formation of strong contacts between basement membranes and the cytoskeleton of cells, thus eventually favoring tumor development and invasiveness. Indeed, Henry and colleagues 14 recently reported a reduction in the expression levels of DG in human primary prostate and breast cancers that was most pronounced in high-grade disease. Yamada and co-workers 12 also recently confirmed that the 31-kd form of β-DG is the product of a proteolytic processing of the extracellular domain of β-DG and reported evidence that this processing is because of the membrane-associated matrix metalloproteinase (MMP) activity and disintegrates the DG complex. Taken together these data suggest that reduced expression of DG may lead to abnormal cell-extracellular matrix interactions and thus contribute to progression to metastatic disease and justify the needs for further studies on the regulation of DG in epithelial cells and its alterations in tumor cells.
In this study we analyzed the expression of DG by Western blot analysis in a series of human cancer cell lines and primary breast and colon cancers. We also investigated the expression of α-DG in a series of breast carcinomas and evaluated its relation with other tumor characteristics and with the clinical outcome of the patients. The results obtained suggest that loss of DG is a frequent event in human tumors with potential prognostic significance and warrant further studies on the involvement of this protein in tumor development.
Materials and Methods
Cell Culture
The 184B5 human immortalized mammary epithelial cells were grown in complete serum-free mammary epithelial cell growth medium (MEGM) medium supplemented with growth factors (Clonetics-BioWhittaker, East Rutherford, NJ), as previously described. 15 All of the other cell lines used for this study were obtained from the American Type Culture Collection (Rockville, MD) and cultured according with the instructions of the supplier.
Patient Characteristics and Tissue Samples
Samples were from patients who underwent surgery in our institution. No patient received preoperative chemotherapy or radiotherapy. Surgical specimens were snap-frozen in liquid nitrogen immediately after surgery and stored at −80°C until use. For colon normal mucosa samples, biopsies were taken where the mucosa appeared free from cancer at a distance of at least 5 cm to resection site. For tumor samples, only fragments containing at least 80% tumor cells, as assessed by hematoxylin and eosin staining, were used for subsequent analyses. Histological tumor grading and staging were assessed according to standard criteria. Other clinicopathological parameters, such as age and gender, were also recorded. The samples were coded and the names of the patients were not revealed.
For immunohistochemical studies, samples were obtained from a series of consecutive, unselected patients who underwent routine surgery for breast cancer at the Division of Surgery, County Hospital, Modena, Italy, from June 1989 to April 1991 and for whom clinicopathological data were available. After excluding cases with previous personal and/or familiar tumor history and patients with evidence of distant metastatic disease at diagnosis or who received any treatment before surgery or lost to follow-up, a cohort of 102 patients was selected for this study with a mean age at diagnosis of 59 years (range, 28 to 83 years) and a mean follow-up of 72 months (range, 2 to 135 months). Treatment remained reasonably consistent during the study period. Briefly, all patients had either a mastectomy or a segmental resection followed by radiation therapy. Axillary dissection with histopathological examination was performed in all patients and if metastases were detected adjuvant therapy was given in the form of tamoxifen to postmenopausal and chemotherapy treatment to premenopausal patients. No further treatment was given to node-negative patients and no patients received any therapy before surgery. The selection did not require approval by an Institutional Review Board because the samples were coded and the names of the patients were not revealed. Our series included 96 ductal and 6 lobular carcinomas. Histological grading and staging were assessed according to standard criteria. 16,17 Tumor stage was pT1 in 38, pT2 in 44, pT3 in 4, and pT4 in 16. Five (5.2%) cases were classified as well (G1), 59 (61.5%) as moderately (G2), and 32 (33.3%) as poorly (G3) differentiated tumors.
Total Protein Extraction
Frozen tissues were washed twice with ice-cold phosphate-buffered saline (PBS) containing 1 mmol/L of MgCl2, finely diced on ice with a surgical blade, and spun down in a microcentrifuge at 4°C to recover pellets. After removing the supernatants, pellets were resuspended in 3 to 5 volumes of sonication buffer containing proteases and phosphatase inhibitors (20 mmol/L Tris-HCl, pH 7.4, 2 mmol/L EGTA, 6 mmol/L β-mercaptoethanol, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate, 50 mmol/L NaF, 15 μg/ml benzamidine, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mmol/L phenylmethyl sulfonyl fluoride) and sonicated at 4°C with a Sonifier Cell Disruptor (Ultrasonic Instruments International, Inc., Farmingdale, NY). Homogenates were incubated on ice for 30 minutes and then centrifuged at 14,000 rpm in a microcentrifuge for 15 minutes at 4°C. The supernatants were assayed for protein content by the BioRad protein assay method (BioRad Laboratories GmbH, München, Germany) and stored at −80°C. For cell lines, exponentially growing cultures of each cell lines were washed with cold PBS, collected by cell scraping and cell pellets were added to 3 to 5 volumes of sonication buffer and processed as for tumor pellets.
Western Blot Analysis
For Western blotting, 50 μg of protein from each sample were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to Immobilon-P membranes (Millipore, Bedford, MA) at 100 V for 1 hour at 4°C. Total cell lysate from SW620 cells was always included and was used as an internal positive control for standardization of the different blots. Immunodetection was performed using the enhanced chemiluminescence kit for Western blotting detection (Amersham Pharmacia Biotech, Freiburg, Germany) and multiple film exposure for different lengths of time were made to establish a linear range. Bands were analyzed on the image analysis system Gel Doc 200 System (BioRad) and quantitated using the Quantity One Quantitation Software (BioRad). The monoclonal antibody to α-DG (clone VIA4-1) was obtained from Upstate Biotechnology (Lake Placid, NY) and was used diluted at 1:2000. The monoclonal antibody to β-DG (clone 43DAG/8D5) was from Novocastra (Newcastle, UK) and was used diluted at 1:50. Experimental validation of anti-DG antibody reactivity was done using a cell line engineered to overexpress an exogenous DG cDNA (data not shown). Protein extraction was independently performed two times for each sample. Similar results were obtained when the two protein extracts from each sample were independently tested. The densitometric data shown are the mean values of the results obtained in four separate experiments (two runs for each protein extract) after correction for the internal control and are expressed as the ratios of DG to β-actin bands. Two values were arbitrarily considered identical when the difference between them was less than 10%.
Semiquantitative RT-PCR Analysis
Total RNA was extracted from exponentially growing cultures of each cell line using the RNeasy Mini kit (Qiagen, Hilden, Germany), in accordance with the manufacturer’s instructions. For reverse transcriptase reaction, the RNA samples were reverse transcribed using the One-Step RT-PCR kit (Qiagen).
PCR was performed using the Gene Amp PCR Systems 9600 (Perkin-Elmer Corp., Norwalk, CT). The following DG-specific primers for the human DG sequence were used: DAGMH (forward) 5′-GGAGAACCCAACCAGCGCCCAGAGC-3′ and DAGAH (reverse) 5′-CGGGTGATATTCTGCAGGGTGATGG-3′ that amplify a 485-bp region encompassing both α-DG and β-DG. A second primer pair: forward 5′-TCACCCACACTGTGCCCATCT-3′ and reverse 5′-ACGGAGTACTTGCGCTCAGG-3′ were used to amplify a 550-bp region of the β-actin transcript. After an initial denaturation at 95°C for 15 minutes, 20 cycles of PCR amplification were performed, each consisting of a denaturing step of 94°C for 45 seconds, annealing at 55°C for 30 seconds, and extension at 72°C for 1 minute, followed by a final step at 72°C for 10 minutes. The number of 20 cycles was selected because in preliminary experiments we verified that with this number of cycles the reaction was still in a linear range for both genes (data not shown). The amplified fragments were detected by 3% (w/v) agarose gel electrophoresis and staining with 0.3 mg/ml of ethidium bromide (Sigma, St. Louis, MO). Each band was quantitated and the specific gene expression level was determined semiquantitatively by calculating the ratio of densitometric value from the DG band in relation to the internal standard represented by β-actin. The densitometric data shown are the mean values of the results obtained in three independent experiments.
Immunohistochemistry
All immunohistochemical analyses were performed on routinely processed, formalin-fixed, paraffin-embedded tissues using an avidin-biotin complex immunoperoxidase technique (Vectastain ABC kit; Vector Laboratories, Burlingame, CA), as previously described. 18-20 Briefly, successive 5-μm tissue sections were cut from blocks selected for the presence of representative tumor tissue. Sections were dewaxed, rehydrated, and then microwave pretreated (10 minutes at 800 W in 1 mmol/L of ethylenediaminetetraacetic acid buffer, pH 8.0), followed by incubation with 0.3% hydrogen peroxide in methanol for 30 minutes to block endogenous peroxidase. After blocking with horse serum for 1 hour at room temperature, the primary antibodies were applied overnight at 4°C in a high-humidity chamber. Binding was visualized using the Vectastain diaminobenzidine kit (Vector Laboratories) and counterstaining was performed with 1% modified Harris hematoxylin, as described. 18-20 The following antibodies were used diluted in PBS: monoclonal anti-α-DG antibody (clone VIA4-1) (Upstate Biotechnology) diluted 1:100; monoclonal anti-Ki67 antibody (clone MIB-1; DAKO, Glostrup, Denmark) diluted 1:100; and monoclonal anti-p53 DO7 antibody (DAKO, Milan, Italy) diluted 1:750. Detection of estrogen and progesterone receptor status (ER, PR) was also performed by immunohistochemistry and has been previously reported. 21 Controls for specificity of staining were performed by immunostaining duplicate sections in the absence of the primary antibody. A breast carcinoma with known positive immunostaining served as a positive control for each antibody. Positive and negative control slides were included within each batch of slides. For the anti-α-DG antibody only a clear staining of the cell membrane was regarded as positive. For p53 and Ki67 immunostaining nuclei were considered positive when they showed a distinct brown color in the absence of cytoplasmic and background staining. The fraction of positive cells was scored by examining at least 10 random high-power fields (×400) for each sample and the percentage of cells with positive reaction was calculated semiautomatically by means of a computer-assisted cellular image analyzer (Image-Pro Plus; Media Cybernetics, Silver Spring, MD) on a total of at least 1000 tumor cells per case. All scoring and interpretations of the results were made by two of the authors independently (AS and MM) without knowledge of clinical outcome or other clinicopathological variables. To assess interobserver variation, the results of the two measurements were compared by paired t-test and no statistical differences were found (data not shown). The few cases with discrepant scoring were re-evaluated jointly on a second occasion, and agreement was reached. The cutoff values for ER and PR (20% positive cells) and for Ki67 (15% positive cells) are routinely used for therapeutic and prognostic evaluation of breast cancer patients and were selected following the indications of the Italian Multicentric Study of Breast Cancer. 21 The figure 20% was used as the cutoff for p53 positivity because it has been mostly used in previous publications regarding primary breast cancers. 22,23
Analysis of DNA Content
DNA analysis was performed on tumor samples as previously reported. 24,25 Briefly, 50-μm tissue sections were cut from paraffin blocks, dewaxed, rehydrated, and digested in a solution of 0.5% pepsin (Sigma). After washings cell pellets were resuspended in a hypotonic citrate-propidium iodide solution (0.05 mg/ml) containing Nonidet P-40 (50 μg/ml) and RNase (50 μg/ml) and were incubated at 4°C in the dark overnight. The cell suspension was then filtered and analyzed for DNA content on a FACScan flow cytometer (Becton Dickinson, San Jose, CA). Normal human peripheral white blood cells were stained with propidium iodide under the same conditions and were used as the internal reference. Tumor ploidy was determined by the ratio of the mean peak values for the sample and the internal reference, according to international conventions. 26 Four cases were not used for this study because of the scarcity of the material or because the coefficient of variation was not acceptable in repeated analyses.
Statistical Analysis
The association between DG expression and other molecular and clinicopathological parameters were calculated using contingency table methods and tested for significance using the Pearson’s chi-square test. Median values were compared using the Mann-Whitney test. Survival curves were calculated using the Kaplan-Meier method and the log-rank test was used for the analysis. Univariate and multivariate relative risks were calculated using Cox proportional hazards regression. Two patients who died accidentally for causes unrelated to tumor disease were not included in the survival analyses. For grade and stage the G1 grade and the pT1 stage were used as baseline, respectively. The proportional hazard assumption was tested following the generalization by Grambsch and Therneau. 27 All calculations were performed using the STATA 6.0 statistical software package (Stata Corporation, College Station, TX) and the results were considered statistically significant when the P value was ≤0.05.
Results
DG Is Differentially Expressed in a Series of Cancer Cell Lines
We previously reported the accumulation of an aberrant β-DG-related band of ∼31 kd in tumor cell lines and tissues compared with normal counterparts. 11 The presence of the 31-kd band was frequently associated with a reduction in the expression level of the expected 43-kd β-DG band and with a reduced or absent expression of the α-DG (see Introduction). A reduction or loss of α-DG expression was also observed in some cell lines expressing normal levels of the 43-kd β-DG band (data not shown). 11 We hypothesized that loss of DG, and mainly α-DG, might be related with cell transformation and might play an important role in the process of tumor development.
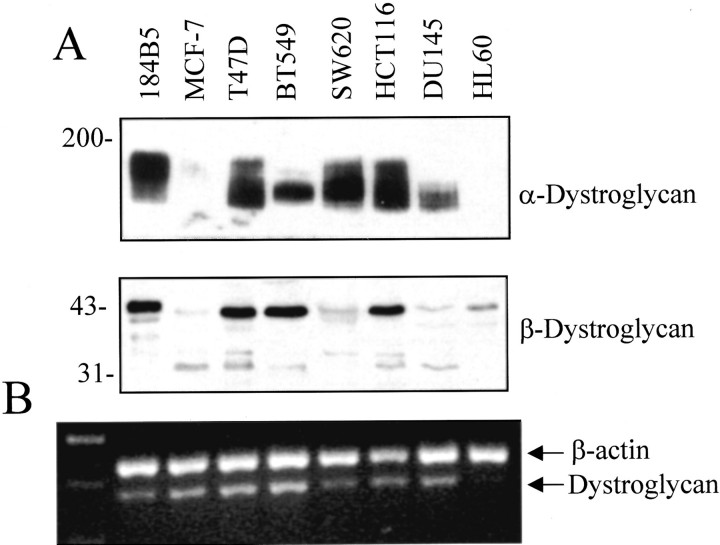
To test this hypothesis we analyzed the expression of both α-DG and β-DG in a series of human cancer cell lines of various histogenetic origin. As shown in Figure 1 ▶ , α-DG was expressed at a high level in the T47D but its expression was reduced in the MCF-7 and BT549 breast cancer cell lines compared with the 184B5 nontumorigenic immortalized human mammary cell line. A reduced expression of α-DG was also observed in the DU145 prostate cancer cells and in the HL60 promyelocytic leukemic cells (it is noteworthy that α-DG was also not detectable in cell extracts of human peripheral white blood cells, data not shown). α-DG was expressed in the SW620 and HCT116 colon cancer cell lines but its loss was previously observed in the HT29 colon cancer cell line. 11 A reduced expression of the 43-kd β-DG band was observed in the MCF-7, SW620, DU145, and HL60 cell lines whereas the β-DG related 31-kd band was clearly visible in the MCF-7, T47D, HCT116, and DU145 cell lines (Figure 1) ▶ . To evaluate whether variations in the expression of the DG protein were related to changes in the levels of DG mRNA, primers were designed that amplify a sequence of 485 bp corresponding to the C-terminal region of α-DG, next to the posttranslational proteolytic site, and the N-terminal region of β-DG. These primers were used for PCR amplification of cDNA prepared from total RNA isolated from the same cell lines. Assessment of DG mRNA expression by RT-PCR was determined semiquantitatively by calculating the ratio of densitometric values from the DG band in relation to the internal standard represented by β-actin (Figure 1) ▶ . As shown in Table 1 ▶ , the expression of DG mRNA was not related to the expression of the corresponding protein thus suggesting that variations in the expression of DG protein in different cell lines are likely because of posttranscriptional events.
Figure 1.
A: Levels of expression of α- and β-DG in a series of human normal and cancer cell lines as assessed by Western blot analysis. B: RT-PCR analysis of DG gene expression in the same cell lines amplifying β-actin (as internal control) with DG. In lane 1 the DNA molecular marker standard is shown. The sizes of the specific RT-PCR products were 550 bp for β-actin and 485 bp for DG.
Table 1.
Expression of Dystroglycan mRNA and Protein in Human Normal and Cancer Cell Lines*
| Cell line | mRNA | α-Dystroglycan | β-Dystroglycan |
|---|---|---|---|
| 184B5 | 0.21 ± 0.014 | 8.67 ± 0.776 | 2.60 ± 0.248 |
| MCF-7 | 0.33 ± 0.092 | 0.01 ± 0.002 | 0.50 ± 0.025 |
| T-47D | 0.38 ± 0.120 | 10.64 ± 1.224 | 3.40 ± 0.214 |
| BT-549 | 0.41 ± 0.134 | 3.03 ± 0.114 | 2.26 ± 0.255 |
| SW-620 | 0.22 ± 0.014 | 6.27 ± 0.856 | 0.46 ± 0.055 |
| HCT116 | 0.25 ± 0.042 | 12.46 ± 1.280 | 2.79 ± 0.187 |
| DU145 | 0.25 ± 0.028 | 2.93 ± 0.086 | 0.27 ± 0.023 |
| HL60 | 0.06 ± 0.013 | 0.05 ± 0.007 | 1.07 ± 0.059 |
*Values are ratios of dystroglycan to β-actin (mean ± SD). Cell lines include the 184B5 normal mammary epithelium; the MCF-7, T-47D, and BT-549 breast; the SW-620 and HCT116 colon; the DU145 prostate adenocarcinomas; and the HL60 promyelocytic leukemia cells.
DG Expression Is Frequently Reduced in Human Colon Cancers
The results obtained with the cell lines suggested that DG is differentially expressed in tumor cell lines. To evaluate whether this reduction also occurs in primary tumors and how it relates with tumor features, the expression levels of α-DG and β-DG were analyzed by Western blot analyses in a series of 43 human primary colon cancers and paired adjacent normal colonic mucosa samples and the results obtained were quantitated by densitometry (Figure 2) ▶ . According to Duke’s criteria tumors were classified as 3 stage A, 19 stage B, 13 stage C, and 8 stage D. We observed a variable expression of both subunits in normal and tumor samples. However, expression of α-DG was considered reduced, unchanged, or increased in 30 (70%), 6 (14%), and 7 (16%) of 43 tumors, respectively, compared with normal adjacent mucosa. This reduction was less frequent in well (G1) (5 of 9, 55%) than in moderately (14 of 20, 70%) and poorly (11 of 14, 79%) differentiated tumors but the difference was not significant. The reduced expression of α-DG was observed in all 3 Duke’s A (100%), in 9 of 19 (47%) Duke’s B, 11 of 13 (85%) Duke’s C, and in 7 of 8 (87%) Duke’s D tumors. These differences were not significant.
Figure 2.

Levels of expression of α- and β-DG in representative cases of primary colon carcinomas (T) and paired normal adjacent mucosa (M) as assessed by Western blot analysis.
The expression level of the 43-kd β-DG band was also reduced in 17 (39%) tumors whereas it was considered unchanged or increased in 8 (19%) and 18 (42%) tumors, respectively, compared with normal adjacent mucosa samples. Tumors with reduced expression of β-DG included three G1 (33%), eight G2 (40%), and six G3 (43%) tumors and, in terms of tumor stage, one Duke’s A (33%), eight Duke’s B (42%), six Duke’s C (46%), and two Duke’s D (25%) tumors. These differences were not significant. The truncated 31-kd β-DG band was clearly detectable in 22 of the 43 paired samples (51%) and it was increased in 13 (59%) of them which included 3 of 7 G1 (43%), 3 of 8 G2 (37%), and 7 of 7 G3 (100%) tumors. In terms of disease stage, increased expression of the 31-kd band of DG was observed in two of two (100%) Duke’s A, two of eight Duke’s B (25%), seven of eight (87%) Duke’s C, and all (100%) Duke’s D tumors.
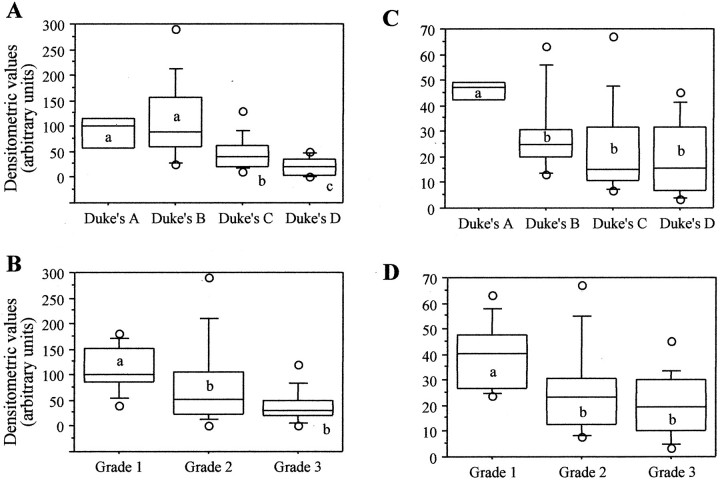
We then analyzed the absolute values of the expression of α-DG and β-DG in the same tumors. The mean densitometric values for α-DG and β-DG expression in the tumor samples were 73.1 (range, 1.0 to 290.0) and 26.6 (range, 3.5 to 67.0), respectively. According to Duke’s stages, the mean densitometric values for α-DG were 87.0 (range, 41.0 to 120.0), 110.5 (range, 25.0 to 290.0), 47.2 (range, 10.0 to 130.0), and 21.4 (range, 1.0 to 50.0) for stages A, B, C, and D, respectively. According to tumor grade, the mean densitometric values for α-DG were 112.4 (range, 41.0 to 180.0), 76.8 (range, 2.0 to 290.0), and 40.2 (range, 1.0 to 120.0) for G1, G2, and G3 tumors, respectively (Figure 3, A and B) ▶ .
Figure 3.
Box-plot of relative density values of α-DG (A and B) and β-DG (C and D) expression in primary colon carcinomas stratified according with tumor stage (A and C) and grade (B and D). The values shown represent the average of four experiments as shown in Figure 2 ▶ . The intensities of each of the bands on the Western blots were quantitated and adjusted according with the value of the internal positive control represented by total cell extract of the SW620 cells (not shown). For each group, the bottom and top edges of the box are the 25th and 75th percentiles, respectively. Median values are shown by the lines within the boxes. The circles indicate the minimum and maximum values, respectively. Whiskers are obtained by not including the data points in the extreme 20% of the observed values. Within each set (A–D) groups of values not sharing the same letter were significantly different (P < 0.05).
Similarly, the mean densitometric values for the 43-kd band β-DG were 45.7 (range, 40.5 to 49.6), 28.8 (range, 12.0 to 67.0), 23.5 (range, 7.0 to 67.0), and 19.6 (range, 3.5 to 45.0) for stages A, B, C, and D, respectively. According to tumor grade, the mean densitometric values for β-DG were 39.8 (range, 24.0 to 63.0), 25.4 (range, 7.0 to 67.0), and 20.0 (range, 3.5 to 45.0) for G1, G2, and G3 tumors, respectively (Figure 3, C and D) ▶ . Thus, we observed a significant progressive reduction of α-DG and β-DG expression with increased tumor grade and stage.
Assessment of DG mRNA expression in a series of selected colon cancer samples by RT-PCR demonstrated that, as previously reported for cell lines, the expression of DG mRNA is not related to the expression of the corresponding protein thus suggesting that variations in the expression of DG protein in colon carcinomas are also likely because of posttranscriptional events (Figure 4) ▶ .
Figure 4.
A: α-DG expression was assayed by Western blot analysis in a series (1 to 7) of selected primary colon cancer samples. Cell lysate from the SW620 cell line was also included for comparison (first lane). B: Expression of DG mRNA was evaluated in the same tumors by RT-PCR (see Figure 1 ▶ ). Values of ratios of DG to β-actin are shown in the associated graphs.
DG Expression in Human Primary Breast Cancers
The expression levels of DG were analyzed by Western blot analyses in a series of primary breast cancers. Total cell extracts were prepared from 10 breast ductal carcinomas including three G1, four G2, and three G3 tumors. As shown in Table 2 ▶ , these tumors expressed variable levels of both α-DG (range, 1.96 to 10.48) and β-DG (range, 0.11 to 1.21) protein. The mean densitometric values for α-DG were 8.47 (range, 5.76 to 10.48), 5.18 (range, 3.21 to 7.98), and 2.96 (range, 1.96 to 4.18) for grade G1, G2, and G3 cancers, respectively. The corresponding values for β-DG were 0.72 (range, 0.6 to 0.85), 0.71 (range, 0.25 to 1.21), and 0.40 (range, 0.11 to 0.89), respectively. Thus, as for colon cancers we observed a progressive reduction of α-DG and β-DG expression with increased tumor grade in this limited series of cases. We also evaluated the expression of DG in three samples of normal breast epithelium obtained during reduction mammoplasty. The median values of α-DG and β-DG were 9.9 ± 4.2 and 1.1 ± 0.36 (mean ± SD), respectively, thus confirming that expression of DG tends, indeed, to be reduced in breast cancers compared with normal mammary tissue.
Table 2.
Expression of Dystroglycan Protein in Human Primary Breast Cancers*
| No. | Grade | α-DG | 43-kd β-DG | 31-kd β-DG |
|---|---|---|---|---|
| 1 | G1 | 10.48 ± 2.36 | 0.718 ± 0.03 | 0.60 ± 0.06 |
| 2 | G1 | 5.76 ± 1.67 | 0.60 ± 0.03 | ND |
| 3 | G1 | 9.18 ± 3.12 | 0.85 ± 0.71 | 0.12 ± 0.03 |
| 4 | G2 | 7.98 ± 1.21 | 0.25 ± 0.01 | ND |
| 5 | G2 | 4.20 ± 0.95 | 1.21 ± 0.06 | 0.18 ± 0.01 |
| 6 | G2 | 5.35 ± 1.31 | 0.67 ± 0.21 | ND |
| 7 | G2 | 3.21 ± 1.01 | 0.70 ± 0.06 | ND |
| 8 | G3 | 4.18 ± 1.01 | 0.19 ± 0.01 | 0.13 ± 0.06 |
| 9 | G3 | 2.75 ± 0.61 | 0.89 ± 1.03 | 0.31 ± 0.08 |
| 10 | G3 | 1.96 ± 0.231 | 0.11 ± 0.01 | 0.10 ± 0.01 |
*Values are ratios of dystroglycan to β-actin (mean ± SD). ND, not detectable.
To further investigate the significance of DG in human breast cancer, the expression of α-DG was evaluated by immunostaining in a series of 102 primary human breast carcinomas. We decided to analyze the expression of α-DG because the available anti-β-DG antibody recognizes the cytoplasmic tail of β-DG that is common to both the 43-kd and the 31-kd β-DG-related bands. 11 Thus, the staining attainable using this antibody would have been confused by the simultaneous assessment of both bands being not possible to determine the contribution of each of them. Only cells with a clear membranous staining were regarded as positive. In normal glands a strong staining of mammary epithelial cells was always observed (Figure 5) ▶ . In tumor cells immunostaining was frequently heterogeneous within one specimen, both in terms of percentage of positive cells and staining intensity with few cases displaying well-differentiated areas with clear positive staining and poorly differentiated areas showing decrease or loss of α-DG expression (data not shown). The percentage of positive cells ranged from 0 to 85% with a median value of 0 (mean, 13.6; SD, 24.9).
Figure 5.

Immunohistochemical staining of α-DG in representative cases of breast carcinomas. A: Immunostaining shows a clear membranous staining in normal ductal epithelial cells. B: Representative case of ductal carcinoma with positive membranous staining. C: Loss of α-DG expression in high-grade ductal carcinoma (left). Normal lobules (right) display a pagetoide invasion with positive staining in the residual normal ducts but not in the infiltrating neoplastic cells. Original magnifications: ×100 (A and B); ×200 (C); ×800 (insets in A and B).
Expression of α-DG was not detectable in tumor cells in 67 (66%) specimens despite the fact that adjacent normal glands were stained positively (Figure 5C) ▶ . No correlation was observed with age (P = 0.19), tumor grade (P = 0.65), tumor invasion (P = 0.14), lymph node status (P = 0.08), progesterone (P = 0.8) and estrogen (P = 0.5) receptor status, and DNA ploidy (P = 0.09). On the other hand, a significant association was observed with tumor stage. In fact, negative staining for α-DG was observed in 19 of 38 (50%) pT1, 32 of 44 (73%) pT2, 3 of 4 (75%) pT3, and 14 of 16 (87%) pT4 tumors (P = 0.014) (Table 3) ▶ . A weak association was also observed with Ki67 staining and p53 expression. In fact, loss of expression was observed in 44 of 72 (61%) tumors with a low Ki67 index and in 23 of 28 (82%) tumors with a high Ki67 index (P = 0.045) (Table 3) ▶ . Negative staining for α-DG was found in 47 of 79 (59.5%) tumors negative for p53 protein expression and in 17 of 20 (85%) p53-overexpressing tumors (P = 0.033) (Table 3) ▶ .
Table 3.
α-Dystroglycan Expression and Clinicopathological Parameters in Human Primary Breast Cancers
| Total | α-Dystroglycan expression | P value | ||
|---|---|---|---|---|
| Positive (%) | Negative (%) | |||
| Age (yr) | ||||
| >60 | 50 | 14 (28%) | 36 (72%) | |
| ≤60 | 52 | 21 (40%) | 31 (60%) | 0.19 |
| Tumor grade† | ||||
| G1/G2 (5/59) | 64 | 24 (38%) | 40 (62%) | |
| G3 | 32 | 10 (31%) | 22 (69%) | 0.65 |
| Tumor stage | ||||
| pT1 | 38 | 19 (50%) | 19 (50%) | |
| pT2 | 44 | 12 (27%) | 32 (73%) | |
| pT3/T4 (4/16) | 20 | 3 (15%) | 17 (85%) | 0.014 |
| Nodal status | ||||
| Negative | 32 | 15 (47%) | 17 (53%) | 0.114 |
| Positive | 70 | 20 (29%) | 50 (71%) | |
| p53 expression* | ||||
| Positive (≥20%) | 20 | 3 (15%) | 17 (85%) | |
| Negative (<20%) | 79 | 32 (41%) | 47 (59%) | 0.033 |
| Ki67 expression* | ||||
| High (≥15%) | 28 | 5 (18%) | 23 (82%) | |
| Low (<15%) | 72 | 28 (39%) | 44 (61%) | 0.045 |
| DNA ploidy* | ||||
| Diploid | 67 | 27 (40%) | 40 (60%) | |
| Aneuploid | 31 | 7 (23%) | 24 (77%) | 0.09 |
| ER expression* | ||||
| High (>20) | 46 | 30 (65%) | 16 (35%) | |
| Low (≤20) | 52 | 37 (71%) | 15 (29%) | 0.53 |
| PR expression* | ||||
| High (>20) | 45 | 16 (36%) | 29 (64%) | |
| Low (≤20) | 54 | 18 (33%) | 36 (67%) | 0.82 |
Statistical analyses were performed by the Pearson chi-square test. P < 0.05 was considered significant.
*Not all parameters were available for all cases.
†Six lobular carcinomas were not graded.
Prognostic Significance of α-DG Expression in Primary Breast Cancers
Twelve of 34 tumors (35%) positive for α-DG and 28 of 66 (42%) negative cases recurred in our series of breast cancers during the period of follow-up. This difference was not significant (P = 0.49). On the other hand, 8 of 34 tumors (23%) positive for α-DG and 32 of 66 (48%) negative cases died of disease during the period of follow-up. This difference was significant (P = 0.016). Thus, death for disease occurred more frequently in patients of our series whose cancer did not express α-DG compared with positive tumors.
The Kaplan-Meier curves of overall survival within patients with negative versus positive cancers showed a significant separation (P = 0.013 by log-rank test) which, as expected, was not significant in terms of disease-free survival (P = 0.35 by log-rank test) (Figure 6) ▶ .
Figure 6.
Kaplan-Meier curves for disease-free (A) and overall (B) survival in 100 patients who underwent surgery for breast carcinoma stratified according to α-DG expression.
In an univariate analysis high tumor stage (P = 0.0002 by log-rank test), lymph node involvement (p = 0.0006), high tumor grade (P = 0.0008), high Ki67 index (P = 0.001), low progesterone receptor expression (P = 0.002), DNA ploidy (P = 0.03) but not age, estrogen receptor, and p53 expression were significantly associated with a shorter overall survival in our series of patients. 28-30
Multivariate analysis of the variables was performed using two different models. Lymph node involvement (P = 0.006; relative risk, 3.790), loss of α-DG expression (P = 0.044; relative risk, 2.286), and high tumor grade (P = 0.001; relative risk, 3.099) confirmed to be independent predictors of overall survival when a Cox proportional hazards model was constructed that only included these three parameters. However, when a second model was built by adding age of patients at the diagnosis and all of the other parameters that were significant at the univariate analyses, only lymph node involvement (P = 0.008; relative risk, 3.766), high tumor grade (P = 0.008; relative risk, 2.872), and low progesterone receptor expression (P = 0.026; relative risk, 2.464) appeared to be independent predictors of overall survival (Table 4) ▶ . Loss of α-DG expression was not an independent predictor of survival although it remained not far from significance with a relative risk of 1.842. Similar results were also obtained when DNA ploidy was included in the model or when age and/or Ki67 index were not included in the analysis (data not shown). Tumor stage was not included in the model because it was significantly correlated with lymph node status and models with both variables were heavily affected by collinearity.
Table 4.
Contribution of Various Potential Prognostic Factors to Overall Survival by Cox Regression Analysis in Breast Cancers
| Variable | Risk ratio | 95% Confidence Interval | P value |
|---|---|---|---|
| Age | 1.440 | 0.692–2.995 | 0.329 |
| Grade | 2.872 | 1.323–6.236 | 0.008 |
| Nodal status* | 3.766 | 1.417–10.008 | 0.008 |
| Dystroglycan† | 1.842 | 0.762–4.454 | 0.175 |
| Ki67‡ | 1.257 | 0.564–2.807 | 0.576 |
| Progesterone† | 2.464 | 1.115–5.445 | 0.026 |
*The risk ratio is given as node-positive versus node-negative patients.
†The risk ratio is given as negative versus positive tumors.
‡The risk ratio is given as high (>15% positive cells) versus low proliferation index.
In an univariate analysis lymph node involvement (P = 0.003), high tumor stage (P = 0.005 by log-rank test), high tumor grade (P = 0.02), high Ki67 index (P = 0.013), low progesterone receptor expression (P = 0.007) but not age, estrogen receptor, DNA ploidy and, finally, α-DG expression were significantly associated with a shorter disease-free survival in our series of patients.
When a Cox proportional hazards model was constructed that included age of patients at the diagnosis, lymph node involvement, tumor grade, Ki67 index, DG, and progesterone receptor expressions, only lymph node involvement (P = 0.006; relative risk, 3.497) and progesterone receptor expression (P = 0.015; relative risk, 2.435) confirmed to be independent predictors of disease-free survival (Table 5) ▶ . Similar results were also obtained when age and/or α-DG expression were not included in the analysis (data not shown). Tumor stage was not included in the model because of the high collinearity with lymph node involvement.
Table 5.
Contribution of Various Potential Prognostic Factors to Disease-Free Survival by Cox Regression Analysis in Breast Cancers
| Variable | Risk ratio | 95% Confidence Interval | P value |
|---|---|---|---|
| Age | 1.174 | 0.593–2.322 | 0.645 |
| Grade | 1.812 | 0.915–3.588 | 0.088 |
| Nodal status* | 3.497 | 1.443–8.475 | 0.006 |
| Dystroglycan† | 1.058 | 0.508–2.204 | 0.880 |
| Ki67‡ | 1.634 | 0.806–3.310 | 0.173 |
| Progesterone† | 2.435 | 1.188–4.994 | 0.015 |
*The risk ratio is given as node-positive versus node-negative patients.
†The risk ratio is given as negative versus positive tumors.
‡The risk ratio is given as high (>15% positive cells) versus low proliferation index.
Discussion
DG is a transmembrane glycoprotein expressed in a wide variety of tissues at the interface between the basement membrane and cell membrane linking the extracellular matrix to the actin cytoskeleton, providing structural integrity and perhaps transducing signal, in a manner similar to integrins. 1,2,7 This is the first study that demonstrates that DG expression is frequently lost in human cancer cells and might have prognostic significance in breast cancer patients.
We previously reported the accumulation of an aberrant form of the β-subunit of DG in a series of human cancer cell lines and in rat mammary tumors. We observed that the presence of this aberrant band was associated with the reduction or loss of expression of α-DG. However, α-DG was also lost in some cell lines despite the presence of a normal β-DG band. 11 We hypothesized that loss of DG might be associated with cell transformation and might play a role in cancer development. In this study the expression of α- and β-DG were analyzed by Western blot analyses in a series of human cancer cell lines of various histogenetic origin. We found that DG is differentially expressed in various cell lines and demonstrated that expression of DG protein is not related with the level of the corresponding mRNA thus suggesting that regulation of DG expression mainly occurs at a posttranscriptional level (Figure 1 ▶ and Table 1 ▶ ). The series also included one breast normal (184B5) and three breast cancer (MCF-7, BT549, and T47-D) cell lines and it is of interest that DG expression was reduced in two of the three breast cancer cell lines compared with the normal counterpart (Figure 1) ▶ .
We also determined by Western blot analyses the expression of DG in a series of human primary colon and breast cancers and found that its expression varies significantly among tumors. Expression of α- and β-DG were considered reduced in 70% and 39% of colon cancers, respectively, compared with normal adjacent mucosa. Reduced expression of DG was more frequent in less differentiated (G3) and in more advanced cases compared with well-differentiated and earlier lesions (Figure 3) ▶ . These findings are in agreement with a previous report demonstrating that a reduction in the expression levels of DG is a frequent event in human primary prostate and breast cancers and is most pronounced in high-grade disease. 14 We also demonstrated that the reduced expression of DG is not associated with a reduced level of the corresponding mRNA thus suggesting that, as previously demonstrated for cell lines, regulation of DG expression in colon cancers mainly occur at a posttranscriptional level (Figure 4) ▶ . The truncated 31-kd β-DG band was clearly detectable in 22 of 43 (51%) colon cancer and in 6 of 10 (60%) breast cancer samples. We could not perform a statistical analysis because of the small number of cases. These results suggest, however, that an altered processing of β-DG might play an important role in the observed reduced expression of both α-DG and β-DG because, as previously mentioned, the 31-kd band lacks the extracellular domain of β-DG essential for the interaction with α-DG. 13
Western blot analysis demonstrated that DG expression is also reduced in human breast cancers and that its loss is associated with higher tumor grade (Table 2) ▶ . To extend this analysis, we determined by immunostaining the expression of α-DG in a series of archival human primary breast cancers and found that expression of α-DG is lost in 66% of tumors. Loss of α-DG expression was significantly associated with high tumor stage (P = 0.01), high Ki67 index (P = 0.045), and p53 overexpression (P = 0.033) but not with other clinicopathological features (Table 3) ▶ . The association with p53 and Ki67 might depend on the association with stage because both high Ki67 index and positivity for p53 were associated with higher tumor stage (data not shown).
Survival analyses by Kaplan-Meier demonstrated that in an univariate analysis loss of α-DG was associated with an increased risk of death for disease (P = 0.013 by log-rank test) but not with a shorter disease-free survival (Figure 6) ▶ . Loss of α-DG expression confirmed to be an independent predictor of overall survival (P = 0.044; relative risk, 2.286) when a Cox proportional hazards model was constructed that only included α-DG expression, lymph node involvement, and tumor grade. When a second model was built by including all of the parameters that were significant at the univariate analyses, loss of α-DG expression was no longer an independent predictor of survival but it remained not far from significance with a relative risk of 1.842 (Table 4) ▶ .
Our series of patients included both node-negative and node-positive patients. It is well known, as also demonstrated by this study, that lymph node involvement is one of the strongest prognostic markers for breast cancer patients. Adjuvant therapy is the standard of care for women with axillary node involvement. Efficacy of adjuvant therapy in node-negative patients is still uncertain and the decision is currently based on risk factors such as tumor stage, estrogen receptor status, and proliferation index. However, in most cases there are no clear indications for treatment choice and there is a great need for useful prognostic markers able to identify node-negative patients with high risk of recurrence and death. Thus, it will be of interest to evaluate the prognostic significance of α-DG expression in the subgroup of node-negative patients. We could not perform this analysis because the small number of eligible cases in our series did not allow a reliable statistical analysis.
To our knowledge this is the first study investigating the expression of DG protein in a series of human cancer cell lines and primary tumors. We provide evidence that the expression of both DG subunits is frequently reduced in human cancer cell lines and in primary colon and breast cancers, being associated with higher grade, higher stage diseases. Henry and colleagues 14 previously evaluated the immunohistochemical expression of DG in breast and prostate cancer and found that reduced expression of DG is a consistent feature of both types of tumors. They, however, evaluated only the expression of β-DG and their series was too limited to perform any statistical analysis. In this study, the expression of α-DG was evaluated by immunostaining in a large series of primary breast carcinomas. We found that α-DG expression is frequently lost in breast cancer cells and observed a progressive loss of its expression with advancing tumor stage. It is noteworthy that although most of the tumors display a negative staining it is likely that, as suggested by the Western blot analysis (Table 2) ▶ , they still express α-DG although at a level that is undetectable by the immunohistochemical technique. Our data, however, suggest that loss of α-DG expression may be an early event in the multistep process of breast carcinogenesis because it was already detected in ∼50% of pT1 tumors and was also observed in some cases of in situ carcinoma (Table 3 ▶ and data not shown). It has been demonstrated that the 31-kd form of β-DG is the product of a proteolytic processing of the extracellular domain of β-DG. 11,12 This processing is because of the membrane-associated MMP activity and disintegrates the DG complex with loss of α-DG. 12 We could, indeed, demonstrate that α-DG is released in the medium of the MCF-7 breast cancer cell line (unpublished data), although the underlying molecular mechanisms are still unknown. Compelling evidence has demonstrated that MMP activity can enhance tumor growth, invasion, and metastasis. Indeed MMPs are invariably up-regulated in the stromal compartment of invasive epithelial cancers. 31 Further in vitro and in vivo studies are required to elucidate whether MMP activity is also responsible for the reduced expression of α-DG observed in breast carcinomas. It is of interest, however, that inhibitors of such activity have proved effective for the treatment of human cancers 32 and it cannot be excluded, on the basis of our results, that some of their beneficial effects might be in part because of the reduced processing of DG after their treatment that could contribute to halt tumor cell growth and spreading.
The exact significance of reduced expression of DG in human tumors remains unknown. We are convinced, however, that our results warrant further studies on the role of this glycoprotein in human tumor development and on its potential prognostic significance in breast and in other cancers. Our preliminary data suggest that its evaluation could have prognostic value, possibly adding information to that obtained from conventional prognostic parameters. In fact, an unexpected finding of the present study was that loss of expression of DG in tumor cells is associated with shorter overall survival. It is tempting to hypothesize that tumors with reduced expression of DG have an enhanced metastatic potential that would be responsible for the poor outcome of the patients. Thus, further investigation on the significance of DG expression level in a more homogeneous group of patients (ie, young node-negative cases) could provide greater insights intro the prognostic significance of DG expression.
Intracellular signal transduction pathways activated by the adhesion of cells to other cells or to the extracellular matrix play crucial roles in cellular differentiation, migration, and proliferation and their alterations might play an important role in the process of tumor development. Indeed, defects in extracellular matrix organization with perturbations of the basement membrane separating the epithelial and stromal compartments have long been considered a hallmark of malignant tumors, particularly adenocarcinomas. 33,34 Moreover, transformed cells have altered relationship with the extracellular matrix, showing decreased association of fibronectin and laminin with their surfaces compared with their normal counterparts. 35,36 Taken together our data demonstrate the presence of a large variations in the expression of DG among tumors and suggest that reduced expression of DG is a frequent event in human tumorigenesis and might contribute to tumor progression. Our hypothesis is that an aberrant expression of DG might play a role in tumor development by altering the interactions between cells and the surrounding matrix. A reduction of DG function, in fact, may lead to abnormal cell-extracellular matrix interactions and thus contribute to tumor progression and metastasis rather than merely being a consequence of neoplastic transformation. It has been also proposed that DG might be involved in signal transduction pathways, in a manner similar to integrins. Indeed, β-DG can bind Grb2, 7,37 the growth factor receptor bound adapter protein involved in the activation of several signaling pathways, including the recruitment to plasma membrane and subsequent activation of the Ras oncogene. 38
In conclusion, further studies on the role played by DG in the regulation of epithelial cell growth and transformation and on its possible role as a tumor suppressor gene are warranted and we believe that they might provide insights into the mechanisms of human tumor pathogenesis and contribute to our understanding of tumor cell growth and invasiveness.
Footnotes
Address reprint requests to Alessandro Sgambato, M.D., Istituto di Patologia Generale, Centro di Ricerche Oncologiche “Giovanni XXIII,” Università Cattolica del Sacro Cuore, Largo Francesco Vito 1, 00168 Rome, Italy. E-mail: asgambato@rm.unicatt.it.
Supported in part by grants from Cofinanziamento Ministero Istruzione, Università e Ricerca (MIUR) 2001 (to A.C. and G.P.T.), the Università Cattolica and MIUR (Programma Oncologia L 444/99) (to A.C.), and the Compagnia di San Paolo (to A.S.).
References
- 1.Durbeej M, Henry MD, Ferletta M, Campbell KP, Ekblom P: Distribution of dystroglycan in normal adult mouse tissues. J Histochem Cytochem 1998, 46:449-457 [DOI] [PubMed] [Google Scholar]
- 2.Winder SJ: The complexities of dystroglycan. Trends Biochem Sci 2001, 26:118-124 [DOI] [PubMed] [Google Scholar]
- 3.Holt KH, Crosbie RH, Venzke DP, Campbell KP: Biosynthesis of dystroglycan: processing of a precursor peptide. FEBS Lett 2000, 468:79-83 [DOI] [PubMed] [Google Scholar]
- 4.Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP: Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature 1992, 355:696-702 [DOI] [PubMed] [Google Scholar]
- 5.Ibraghimov-Beskrovnaya O, Mialtovich A, Ozcelik T, Yang B, Koepnick K, Francke U, Campbell KP: Human dystroglycan: skeletal muscle cDNA, genomic structure, origin of tissue specific isoforms and chromosomal localization. Hum Mol Genet 1993, 2:1651-1657 [DOI] [PubMed] [Google Scholar]
- 6.Ervasti JM, Campbell KP: Dystroglycan: an extracellular matrix receptor linked to the cytoskeleton. Curr Opin Cell Biol 1996, 8:625-631 [DOI] [PubMed] [Google Scholar]
- 7.Russo K, Di Stasio E, Macchia G, Rosa G, Brancaccio A, Petrucci TC: Characterization of the beta-dystroglycan-growth factor receptor 2 (Grb2) interaction. Biochem Biophys Res Commun 2000, 274:93-98 [DOI] [PubMed] [Google Scholar]
- 8.Williamson RA, Henry MD, Daniels KJ, Hrstka RF, Lee JC, Sunada Y, Ibraghimov-Beskrovnaya O, Campbell KP: Dystroglycan is essential for early embryonic development: disruption of Reichert’s membrane in Dag1-null mice. Hum Mol Genet 1997, 6:831-841 [DOI] [PubMed] [Google Scholar]
- 9.Ervasti JM, Ohlendieck K, Kahl SD, Gaver MG, Campbell KP: Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature 1990, 345:315-319 [DOI] [PubMed] [Google Scholar]
- 10.Ohlendieck K, Matsumura K, Ionasescu VV, Towbin JA, Bosch EP, Weinstein SL, Sernett SW, Campbell KP: Duchenne muscular dystrophy: deficiency of dystrophin-associated proteins in the sarcolemma. Neurology 1993, 43:795-800 [DOI] [PubMed] [Google Scholar]
- 11.Losasso C, Di Tommaso F, Sgambato A, Ardito R, Cittadini A, Giardina B, Petrucci TC, Brancaccio A: Anomalous dystroglycan in carcinoma cell lines. FEBS Lett 2000, 484:194-198 [DOI] [PubMed] [Google Scholar]
- 12.Yamada H, Saito F, Fukuta-Ohi H, Zhong D, Hase A, Arai K, Okuyama A, Maekawa R, Shimizu T, Matsumura K: Processing of β-dystroglycan by matrix metalloproteinase disrupts the link between the extracellular matrix and cell membrane via the dystroglycan complex. Hum Mol Genetics 2001, 10:1563-1569 [DOI] [PubMed] [Google Scholar]
- 13.Sciandra F, Schneider M, Giardina B, Baumgartner S, Petrucci TC, Brancaccio A: Identification of the β-dystroglycan binding epitope within the C-terminal region of α-dystroglycan. Eur J Biochem 2001, 268:4590-4597 [DOI] [PubMed] [Google Scholar]
- 14.Henry MD, Cohen MB, Campbell KP: Reduced expression of dystroglycan in breast and prostate cancer. Hum Pathol 2001, 32:791-795 [DOI] [PubMed] [Google Scholar]
- 15.Sgambato A, Doki Y, Schieren I, Weinstein IB: Effects of cyclin E overexpression on cell growth and response to TGF-β depend on cell context and p27Kip1 expression. Cell Growth Differ 1997, 8:393-405 [PubMed] [Google Scholar]
- 16.Bearhs OH, Henson DE, Hutter RVP, Kennedy BJ: Manual for Staging of Cancer. 1992. J. B. Lippincott, Philadelphia
- 17.Elston CW, Ellis IO: Pathological prognostic factors in breast cancer. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991, 19:403-410 [DOI] [PubMed] [Google Scholar]
- 18.Sgambato A, Ratto G, Faraglia B, Merico M, Ardito R, Schinzari G, Romano G, Cittadini A: Reduced expression and altered subcellular localization of the CDK inhibitor p27Kip1 in human colon cancer. Mol Carcinog 1999, 26:172-179 [DOI] [PubMed] [Google Scholar]
- 19.Sgambato A, Zhang Y-J, Arber N, Hibshoosh H, Doki Y, Ciaparrone M, Santella RM, Cittadini A, Weinstein IB: Deregulated expression of p27Kip1 in human breast cancers. Clin Cancer Res 1997, 3:1879-1887 [PubMed] [Google Scholar]
- 20.Sgambato A, Migaldi M, Faraglia B, Garagnani L, Romano G, De Gaetani C, Ferrari P, Capelli G, Trentini GP, Cittadini A: Loss of p27Kip1 expression correlates with tumor grade and with reduced disease-free survival in primary superficial bladder cancers. Cancer Res 1999, 13:3245-3250 [PubMed] [Google Scholar]
- 21.Zunarelli E, Nicoll JA, Migaldi M, Trentini GP: Apolipoprotein E polymorphism and breast carcinoma: correlation with cell proliferation indices and clinical outcome. Breast Cancer Res Treat 2000, 63:193-198 [DOI] [PubMed] [Google Scholar]
- 22.Railo M, Lundin J, Haglund C, von Smitten K, von Boguslawsky K, Nordling S: Ki67, p53, ER receptors, ploidy and S-phase as prognostic factors in T1 node negative breast cancer. Acta Oncol 1997, 36:369-374 [DOI] [PubMed] [Google Scholar]
- 23.Mirza AN, Mirza NQ, Vlastos G, Singletary SE: Prognostic factors in node-negative breast cancer. Ann Surg 2002, 235:10-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hedley DW, Friedlander ML, Taylor IW, Rugg CA, Musgrove EA: Method for analysis of cellular DNA content of paraffin-embedded pathological material using flow cytometry. J Histochem Cytochem 1983, 31:1933-1935 [DOI] [PubMed] [Google Scholar]
- 25.Sgambato A, Migaldi M, Faraglia B, De Aloysio G, Ferrari P, Ardito R, De Gaetani C, Capelli G, Cittadini A, Trentini GP: Cyclin D1 expression in papillary superficial bladder cancer: its association with other cell cycle-associated proteins, cell proliferation and clinical outcome. Int J Cancer 2002, 97:671-678 [DOI] [PubMed] [Google Scholar]
- 26.Hiddemann W, Schumann J, Andreeff M, Barlogie B, Herman CJ, Leif RC, Mayall BH, Murphy RF, Sandberg AA: Convention on nomenclature for DNA cytometry. Cytometry 1984, 5:445-446 [DOI] [PubMed] [Google Scholar]
- 27.Grambsch PM, Therneau TM: Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994, 81:515-526 [Google Scholar]
- 28.Clark GM: Interpreting and integrating risk factors for patients with primary breast cancer. J Natl Cancer Inst Monogr 2001, 30:17-21 [DOI] [PubMed] [Google Scholar]
- 29.Costa SD, Lange S, Klinga K, Merkle E, Kaufmann M: Factors influencing the prognostic role of oestrogen and progesterone receptor levels in breast cancer—results of the analysis of 670 patients with 11 years of follow-up. Eur J Cancer 2002, 38:1329-1334 [DOI] [PubMed] [Google Scholar]
- 30.Schnitt SJ: Traditional and newer pathologic factors. J Natl Cancer Inst Monogr 2001, 30:22-26 [DOI] [PubMed] [Google Scholar]
- 31.Hanahan D, Weinberg RA: The hallmark of cancer. Cell 2000, 100:57-70 [DOI] [PubMed] [Google Scholar]
- 32.Maekawa R, Maki H, Yoshida H, Hojo K, Tanaka H, Wada T, Uchida N, Takeda Y, Kasai H, Okamoto H, Tsuzuki H, Kambayashi Y, Watanabe F, Kawada K, Toda K, Ohtani M, Sugita K, Yoshioka T: Correlation of antiangiogenic and antitumor efficacy of N-biphenylo sulfonyl-phenylalanine hydroxiamic acid (BPHA), an orally-active, selective matrix metalloproteinase inhibitor. Cancer Res 1999, 59:1231-1235 [PubMed] [Google Scholar]
- 33.Bonkhoff H, Wernert M, Dhom G, Remberger K: Distribution of basement membranes in primary and metastatic carcinomas of the prostate. Hum Pathol 1992, 23:408-421 [DOI] [PubMed] [Google Scholar]
- 34.Ozzello L: The behavior of basement membranes in intraductal carcinoma of the breast. Am J Pathol 1959, 35:887-900 [PMC free article] [PubMed] [Google Scholar]
- 35.Hayman EG, Engvall E, Ruoslahti E: Concomitant loss of cell surface fibronectin and laminin from transformed rat kidney cells. J Cell Biol 1981, 88:352-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hynes RO, Wyke JA: Alterations in surface proteins in chicken cells transformed by temperature-sensitive mutants of Rous sarcoma virus. Virology 1975, 64:492-504 [DOI] [PubMed] [Google Scholar]
- 37.Yang B, Jung D, Motto D, Meyeer J, Koretzky G, Campbell KP: SH3 domain mediated interaction of dystroglycan and Grb2. J Biol Chem 1995, 270:11711-11714 [DOI] [PubMed] [Google Scholar]
- 38.Chardin P, Cussac D, Maignan S, Ducruix A: The Grb2 adaptor. FEBS Lett 1995, 369:47-51 [DOI] [PubMed] [Google Scholar]