Abstract
It has been much disputed whether or not stress can cause hair loss (telogen effluvium) in a clinically relevant manner. Despite the paramount psychosocial importance of hair in human society, this central, yet enigmatic and controversial problem of clinically applied stress research has not been systematically studied in appropriate animal models. We now show that psychoemotional stress indeed alters actual hair follicle (HF) cycling in vivo, ie, prematurely terminates the normal duration of active hair growth (anagen) in mice. Further, inflammatory events deleterious to the HF are present in the HF environment of stressed mice (perifollicular macrophage cluster, excessive mast cell activation). This provides the first solid pathophysiological mechanism for how stress may actually cause telogen effluvium, ie, by hair cycle manipulation and neuroimmunological events that combine to terminate anagen. Furthermore, we show that most of these hair growth-inhibitory effects of stress can be reproduced by the proteotypic stress-related neuropeptide substance P in nonstressed mice, and can be counteracted effectively by co-administration of a specific substance P receptor antagonist in stressed mice. This offers the first convincing rationale how stress-induced hair loss in men may be pharmacologically managed effectively.
“An intense psychic shock may also exert pronounced effects on the skin, eg, graying and generalized loss of hair.” 1 This quote from Hans Selye, 1 a pioneer in research into stress more than half a century ago reflects in laymen and physicians the deeply rooted assumption that stress can be associated with hair loss and changes in hair color. Many anecdotal observations of sudden hair loss concomitant with emotional stress have been reported supporting the above quote. 2-6 Such observations prompted many retrospective studies, the majority of which date back to the 1950s or 1960s. 3,4,7-9 Although a medically benign condition, hair loss (alopecia areata, telogen effluvium) is nevertheless a significant psychosocial issue for most of the affected patients that greatly reduces quality of life and as such effectuates both additional stress perception and secondary morbidity. 3,10
Despite the fact that an association between stress and hair loss is well accepted among patients and clinicians, it remains to be convincingly demonstrated in appropriate stress models that a defined psychoemotional stressor can actually inhibit or damage hair growth in vivo. Moreover, in the absence of a clear-cut pathophysiological concept, no specific pharmacological intervention is currently available to manage stress-induced hair loss. To be effective, any such therapeutic intervention that attempts to counteract stress-induced telogen effluvium or alopecia areata would have to prevent premature termination of the growth phase of the hair cycle (anagen) and would have to inhibit the onset of catagen. 11
Recently, we have introduced an animal model for telogen effluvium that allows for the testing of whether stress-induced hair growth inhibition is fact or fiction, which offers the opportunity to gain insights into the pathomechanism involved in stress-triggered hair growth inhibition and that permits the exploration of various strategies for therapeutic intervention. 12 This animal model uses mice, because they display a highly synchronized hair cycle (which facilitates experimentation and allows to detect even subtle hair growth alterations by stress). Further the hair follicle (HF) cycle has nowhere been better characterized than in mice. 11,13-15 Exposure of mice to a well-defined experimental psychoemotional stressor (sound stress) 16 resulted in significantly increased keratinocyte apoptosis and decreased keratinocyte proliferation in resting (telogen) HFs in situ. 12 These data are further supported by observations from Aoki and colleagues, 17 who used a different stressor to investigate the effect of stress on the HF, the so-called intermittent foot shock, which prolonged the telogen stage in the murine hair cycle and may delay exogen. 17
These intriguing findings had pointed to the existence of a brain-hair follicle axis, 12 however, this pilot study had examined only resting (telogen) HFs and did not yet prove that stress can inhibit the growth of actually growing hair shaft producing (= anagen) HF by premature induction of HF regression (catagen), as necessary prerequisite for being capable of inducing telogen effluvium and massive catagen development, as it is seen in alopecia areata (AA) in man. 11,16 In the context of the ancient debate on whether or not, and how, stress can negatively impact hair growth, investigating the effect of a defined psychoemotional stressor on the cycling activity of anagen HF in vivo and therefore remaining the immediate research priority, based on the hypothesis that stress-induced immunoactivation causes apoptosis, which, in a rather mild expression, would lead to telogen effluvium and in a more profound manifestation associated with dysregulation of calcitonin gene-related peptide (CGRP), 18 triggers alopecia areata.
Our previous pilot study had suggested that the neuropeptide substance P (SP) is one candidate mediator along the brain-HF axis because the anti-proliferation and proapoptotic effects could be reproduced in fact by SP. 12 Also we had previously demonstrated that SP potently manipulates murine HF cycling in vivo and in skin organ cultures, 19,20 making SP a particularly attractive candidate mediator in these stress studies. After von Euler and Gaddum 21 had first described SP more than 70 years ago, much evidence has accumulated to suggest that this undecapeptide can indeed be considered as the prototypic stress-related neuropeptide. 19-25 This concept is further supported by two recent studies reporting that when the function of SP or its receptor is genetically disrupted, such mice show a significantly reduced response to moderate to intense pain. 26,27 In line with the distinct pharmacological properties of various neurokinins (NKs), which constitute a family of neuropeptides that comprises SP, three distinct receptors for SP have been cloned: the NK1 receptor, where SP is the preferred high affinity ligand, and the NK2 and NK3 receptors. 28-31 Because the NK1 receptor has become a model for how neuropeptides and corresponding receptor-blocking drugs interact, 32 more than 30 nonpeptide NK1 antagonists are available to date. 33-37 Availability of these selective and sensitive NK1 antagonists now allows to dissect the functional role of endogenous SP in stress-associated responses, including the effects stress may exert on HF cycling.
For this reason the present study aimed at dissecting the impact of both stress and SP on the anagen/catagen transition of the murine hair cycle. We used the most studied mouse strain in hair research, C57BL/6 mice, and CBA/J mice, which have previously been demonstrated to be highly susceptible to stress. 16,23 Specifically, we investigated whether sound stress 1) promotes premature catagen development in anagen HFs in C57BL/6 and/or CBA/J mice with an originally synchronized hair cycle, as it can be identified by the hair cycle score (HCS); 14,38 2) changes the physiological patterns of apoptosis in situ during the anagen/catagen transformation of HFs in both mouse strains, using terminal dUTP nick-end labeling (TUNEL) as markers; 37 3) alters the number, perifollicular location, and/or activation status of perifollicular macrophages and/or mast cells in murine back skin,assessed by quantitative (immuno-)histochemistry; 39,40 4) induces changes in the skin of both mouse strains that can be abrogated by using the highly selective NK1-receptor antagonist (NK1-RA) RP 67580; 35 5) in addition, we tested whether SP is up-regulated in murine skin after stress exposure and if the systemic administration of SP alone sufficed to reproduce the observed effects of stress on selected parameters of skin immunology and hair biology in C57BL/6 and CBA/J mice.
Materials and Methods
Animals
Six- to 8-week-old female CBA/J and C57BL/6 mice (Charles River, Sulzfeld, Germany) were chosen because mice at this age show the most reliable and profound stress response 16,23 and are in the telogen stage of the hair cycle. 38 The animals were housed in community cages at the animal facilities of the Charité, Virchow Hospital (Berlin, Germany) with 12-hour light periods, and were fed water and mouse chow ad libitum. Animal care and experimental procedures were approved by the state authority for animal research conduct (LaGetSi, Berlin, Germany) and performed in compliance with guidelines of the LaGetSi for the use of laboratory animals.
Anagen Induction
Anagen was experimentally induced by depilation, as previously published. 13,41 Briefly, a wax/rosin mixture was applied to the dorsal skin (from neck to tail) of mice with all HFs in telogen, as evidenced by the homogeneously pink skin color on the back. Peeling-off the wax/rosin mixture removes all hair shafts and immediately induces homogeneous anagen development over the entire depilated back skin area of the mouse, thus inducing a highly synchronized anagen development. After full anagen development, the consecutive stages (catagen and telogen) then develop spontaneously in a relatively homogeneous wave-like pattern starting in the neck region.
Application of Stress
The CBA/J and C57BL/6 mice were exposed to sound stress for the duration of 24 hours starting on day 14 after depilation, ie, when all back skin HFs were in late anagen. 38 The ultrasound stress was emitted by a rodent repellant device (Conrad Electronics, Berlin, Germany) at a frequency of 300 Hz in intervals of 15 seconds. The stress device was placed into the mouse cage so that the mice could not escape the sound perception. 12,16,23
Application of SP
Recombinant SP was dissolved in 2% dimethyl sulfoxide, diluted 1:10−5 in phosphate-buffered saline (PBS) and injected intraperitoneally at a concentration of 10 nmol/0.2 ml, as shown to be effective, 12 into nonstressed mice as a single dose on day 14 after depilation to mimic stress-triggered, SP-mediated effects.
Application of SP NK1 Receptor Antagonist (NK1-RA)
In one set of experiments, the highly selective NK1-RA named RP 67580 (Rhône-Poulenc, France) was injected intraperitoneally at a dose of 200 μg/200 μl PBS/mouse every other day from day 2 until day 16 after depilation in control and stressed mice.
Tissue Preparation
On day 16 after depilation, ie, at the time when control mice are just about to spontaneously enter the catagen transformation of their depilation-induced anagen HFs, 15,38 all mice were sacrificed. The mice were anesthetized by an intraperitoneal injection of a lethal overdose of ketanest and perfusion-fixed with a mixture of 4% paraformaldehyde and 14% saturated picric acid as previously described 42-44 or killed by cervical dislocation. Skin specimens from the neck region of murine back skin were harvested parallel to the vertebral line to obtain longitudinal sections through the hair units, which is an essential requirement for the quantitative histomorphology of the hair cycle, 45 folded, deep frozen in liquid nitrogen, and then covered with embedding medium, as described in detail. 40 Cryosections were then processed for immunohistochemistry and TUNEL staining.
Quantitative Histomorphometry of HF Cycling
A previously published effective technique for follicle classification based on the alkaline phosphatase histochemistry of dermal papilla and sebaceous gland has been performed on the tissue sections. 38,41 This allowed to accurately classify all HFs with respect to their exact stage in the transition from anagen VI via catagen I to VII to telogen. 15 For quantification, a previously described HCS was calculated: 14 every stage of anagen or catagen was assigned to an arbitrary unit in ascending numerical order (anagen VI, 0; catagen I, 1; catagen II, 2, and so forth). The total number of HFs in each specific hair cycle stage was then multiplied by its corresponding unit number. The resulting sum was divided by the total number of HFs counted, thus defining the average hair cycle stage of all HFs within the entire examined group.
Immunohistochemistry
Immunohistochemistry was performed following published protocols. 12,40 Anti-mouse MHC class II antibody (ER-TR; BMA, Germany) was used in a 1:100 dilution for immunodetection of MHC-II+ cell cluster within murine skin). In brief, cryostat sections (10 μm) were fixed in acetone (10 minutes at −20°C) and preincubated with ready-to-use blocking solution (Immunotech, Germany), followed by an incubation with the primary antibody. Development was performed using diaminobenzidine. The rat anti-mouse MHC II+ antibody was incubated for 45 minutes at room temperature, followed by rabbit anti-rat secondary antibody (1:70, 30 minutes; DAKO, Germany), and the alkaline phosphatase anti-alkaline phosphatase technique was used for development.
Fluoresence Immunohistochemistry for SP-Positive Nerve Fibers in the Skin
For visualization of nerve fibers, perfusion-fixed cryostat sections (14 μm) were incubated overnight at room temperature in a humidity chamber with the primary antiserum to SP (1:100, rat, monoclonal; Chemicon, Temecula, CA). This was followed by an incubation of 1 hour at 37°C with tetramethylrhodamine-isothiocyanate-conjugated F(ab)2 fragments of goat anti-rat IgG (Jackson ImmunoResearch, West Grove, PA) at a dilution of 1:200. 46 After incubation, sections were rinsed in 0.1 mol/L Tris-buffered saline, pH 7.4, three times for 15 minutes each. After washing in Tris-buffered saline, all sections were mounted and stored at −20°C until analyzed. Three types of negative controls were done: 1) slides were incubated with the secondary antibody alone; 2) slides were incubated with the primary antibody after preincubation with control peptides for the specific antisera (10 to 20 μg/ml, 37°C, 1 hour; Sigma, St. Louis, MO); 3) slides were incubated with rabbit/rat IgG as primary antibody. As positive controls, cryostat sections of whole-mount mouse fetuses were used containing brain, spinal cord, heart, and gut. Negative controls did not reveal specific immunoreactivity. Sections were examined at ×400 magnification under a Zeiss Axioscope fluorescence microscope. The appearance of nerve fibers in defined areas such as the epidermis, the dermis including the arrector pili muscle, and the subcutis was assessed and the number of single nerve fiber profiles in dermis and subcutis was quantified by histomorphometry, as previously described in detail. 46 For each stage of HF cycling, at least 10 microscopic fields each of four to five different mice containing a minimum of 30 different HFs per mouse, were studied (ie, more than 200 microscopic fields were studied for each immunocytochemicalreaction).
Giemsa Staining
The presence of mast cells in murine back skin was detected using Giemsa staining (Merck, Germany). In brief, Giemsa was applied at a 1:10 dilution with 2% sodium borate solution for 45 minutes at room temperature. Differentiation was achieved using 0.02% acetic acid under microscopic control, slides were dehydrated and mounted. Mast cells were classified as degranulated when eight or more granules could be found outside the cell membrane. 39,47
TUNEL Staining
To evaluate apoptotic cells in murine HFs, we used the TUNEL-staining method as described before. 48 Ten-μm cryostat sections of murine back skin were freshly prepared and fixed in formalin, postfixed in ethanol/acetic acid, and incubated with digoxigenin-dUTP in the presence of terminal desoxynucleotidyl transferase (TdT), using a commercially available kit (Intergen, Germany). TUNEL-positive cells were visualized by anti-digoxigenin fluorescein isothiocyanate-conjugated F(ab)2 fragments, counterstaining was performed using 4,6-diamidino-2-phenylindole (DAPI) dye (1 μg/ml methanol; Roche, Germany) in a subsequent incubation step. Finally, sections were mounted using VectaShield (Vector Laboratories, Germany). Negative controls for the TUNEL staining were made by omitting TdT, according to the manufacturer’s protocol.
Quantitative Histomorphometry and Statistical Analysis
All sections were analyzed at ×200 or ×400 magnification under a Zeiss Axioscope light microscope (Zeiss, Germany), photo documentation was performed using a KS400 camera (Zeiss, Germany). A mean of 79.3 single HFs per mouse (range, 40 to 110 HFs) was assessed from the neck region because catagen development occurs here first. 14,15,38 The total number of mice per group is given in the figures. Immunoreactive cells were counted in distinct HF compartments and around the hair units using a scaled eye piece. A fluorescence microscope (Zeiss, Germany) with appropriate excitation emission filter systems for fluorescence induced by DAPI and fluorescein isothiocyanate were used for the detection of apoptotic cells. The photo documentation was performed by a digital image analysis system (ISIS Metasystems, Altusheim, Germany). Means of all counts ±SEM were calculated. Differences were judged as significant if the P value was ≤0.05 (*), P ≤ 0.01 (**), and P ≤ 0.001 (***), as determined by the Mann-Whitney U/Wilcoxon rank tests.
Results
Stress Promotes Premature Catagen Development
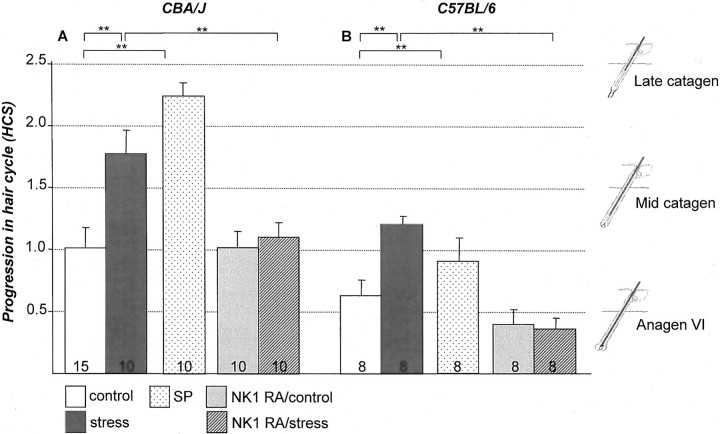
To test whether stress affects the timing of spontaneous catagen induction in depilation-induced anagen HFs in vivo, the onset of catagen development after initiation of an experimental hair cycle was examined in CBA/J and C57BL/6 mice exposed to sound stress compared to nonstressed control mice. As depicted in Figure 1 ▶ , a significant increase in the progression of the hair cycle was observed with most HFs of nonstressed mice still in anagen VI, and the majority of HFs in stressed mice in early catagen. Interestingly, this premature progression was more pronounced in the stress-prone CBA/J mice (Figure 1A) ▶ , compared to C57BL/6 mice (Figure 1B) ▶ . This indicates a premature development of catagen follicles in both mice strains exposed to stress. Representative skin section that were used to assess the HCS of control C57BL/6 mice are shown in Figure 2A ▶ ; areas with a significantly advanced progression of HCS (showing primarily catagen IV and V HFs) are presented in Figure 2B ▶ .
Figure 1.
In both mouse strains increased vulnerability of HFs toward an advanced catagen progression was detectable in stressed mice and could be mimicked by injection of SP in nonstressed mice and abrogated by injection of the highly specific NK1 receptor antagonist. The results for the CBA/J mice are shown on the left (A) and the data for C57BL/6 on the right (B). HCS was assessed as suggested by Maurer and colleagues. 14 In brief the y axis depicts the mean ± 1 SEM of histometric score assessed on day 16 after anagen induction. For every mouse a minimum of 100 individual HFs was assigned to defined hair cycle stages. On the right of the graph, representative hair cycle stages for each HCS are depicted, ie, anagen VI is the dominant hair cycle stage with a score of 0.5. **, P < 0.01. The number of mice per group is given in the bars.
Figure 2.
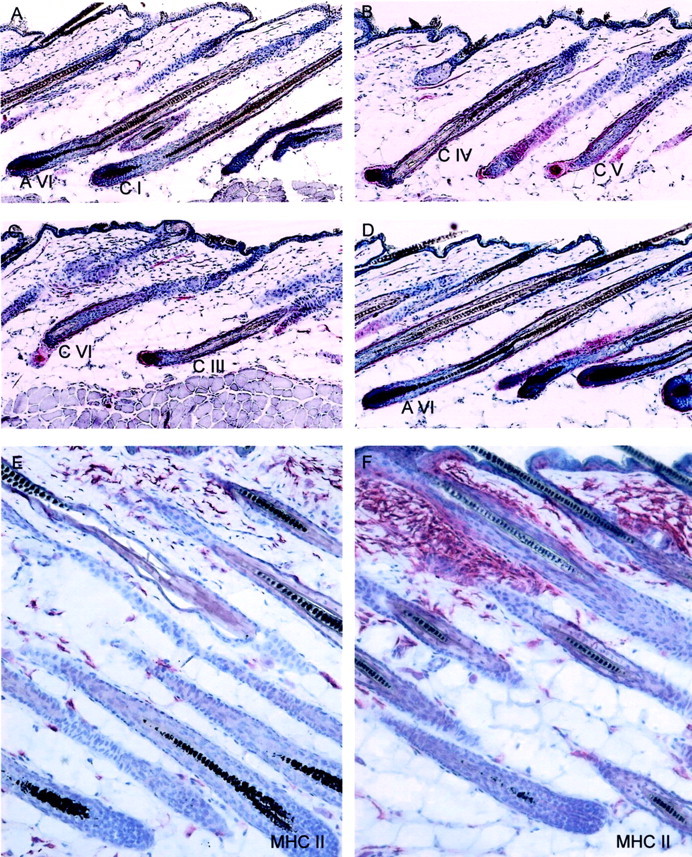
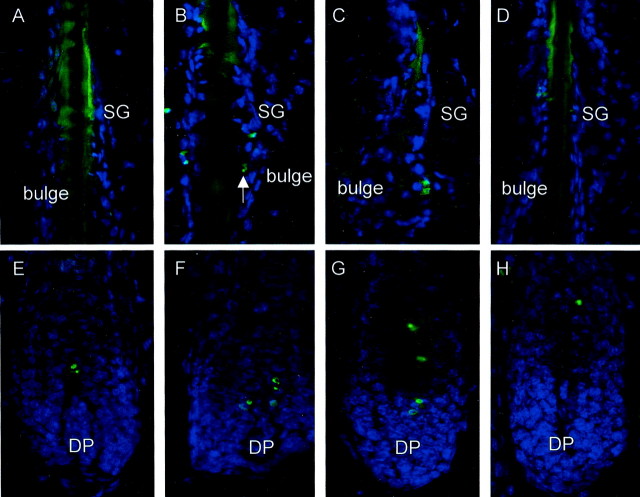
The effect of stress on the hair cycle stage is depicted in A–D. A: A representative area of control mice 16 days after depilation with the majority of HFs in anagen VI (AVI). B mirrors the effect of stress on the hair cycle stage on day 16 after depilation with HFs in catagen IV (CIV) or catagen V (CV). C: HF of stressed mice that received injection of SP, which mimicked the effect of stress on the vulnerability of HFs toward catagen progression with HFs in catagen III to VI (CIII-VI). D: A representative example of mice exposed to stress and treated with the NK1-RA, in which the majority of HFs were scored as anagen VI, similar to the nonstressed control group. E–F: The effect of stress on immunocytes in murine skin. E: Distribution of MHC-II+ cells in parafollicular dermis of nonstressed mice or in similar distribution in stressed mice that received the NK1-RA (bright red staining), compared to F, increased number of MHC-II+ cells in the bulge region, forming cluster after stress exposure. Similar staining patterns were present after injection of SP. Original magnifications, ×200.
Stress Increases Intrafollicular Apoptosis
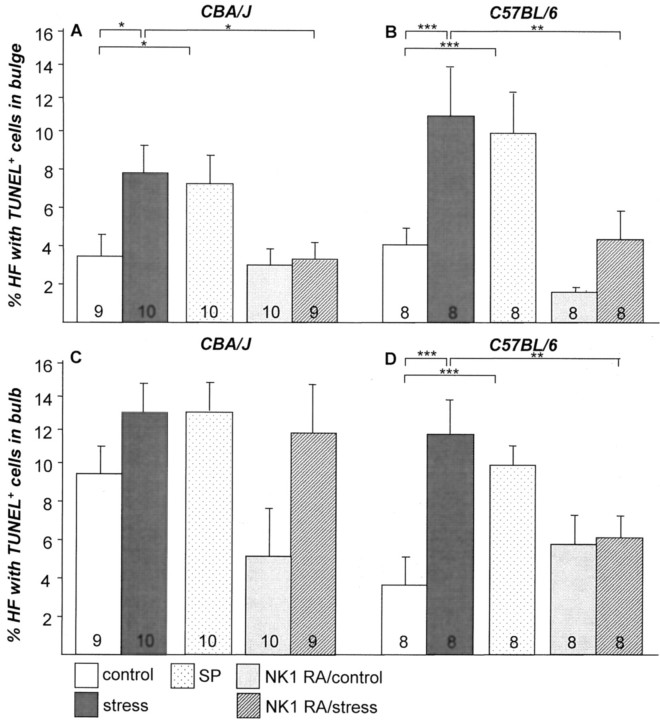
This premature onset of catagen after application of stress was independently confirmed by measuring the intrafollicular patterns of apoptosis, because the massive, yet temporospatially highly controlled occurrence of intraepithelial apoptosis in the HFs is a hallmark of catagen development. 48 As shown in Figure 3 ▶ , exposure to stress 2 days before the assessment of HFs on day 16 after depilation resulted in a significantly increased percentage of HF units with signs of apoptosis (TUNEL+ cells) in the bulge region of both CBA/J mice (Figure 3A) ▶ and C57BL/6 mice (Figure 3B) ▶ , which exceeded substantially the physiological signs of apoptosis in catagen HFs, as previously indicated by the so-called apoptomap. 48 Because this HF region harbors the crucial population of epithelial stem cells of the HF, 49,50 these stress-induced up-regulations of TUNEL+ cells in this sensitive HF compartment is of particular biological importance. Similar observations of stress-triggered increase of TUNEL+ cells were detectable in the bulb area (Figure 3, C and D) ▶ , where levels of significance were reached only in C57BL/6 mice exclusively (Figure 3D) ▶ . Absolute numbers of TUNEL+ cells in bulge and bulb region are given in Table 1 ▶ , supporting the data on percentages given in the figures. Figure 4 ▶ shows representative examples of apoptotic cells in HF bulge (Figure 4A) ▶ and bulb (Figure 4E) ▶ of nonstressed control C57BL/6 mice, compared to HFs with high numbers of apoptotic cells in bulge (Figure 4B) ▶ and bulb (Figure 4F) ▶ in mice of the same strain exposed to stress.
Figure 3.
The effect of stress on the percentage of HFs with TUNEL+ cells in bulge (A) and bulb (C) in CBA/J mice and TUNEL+ cells in bulge (B) and bulb (D) in C57BL/6 mice (mean ± SEM). Exposure to stress 2 days before assessment led to a significant increase of apoptotic cells/HF bulge (*, P < 0.05 for CBA/J; ***, P < 0.001 for C57BL/6) and bulb (***, P < 0.001 for C57BL/6). The effect of stress on HF apoptosis in bulge could be mimicked by injection of SP in both strains of nonstressed mice (A and B) and abrogated by treatment with NK1-RA in the bulge region of stressed mice (A and B). The number of mice per group is given in the bars.
Table 1.
Absolute Cell Counts in CBA/J and C57BL/6 Mice (Mean ± SEM)
| Control | Stress | SP | Control NK1-RA | Stress NK1-RA | |
|---|---|---|---|---|---|
| CBA/J mice | |||||
| TUNEL+ cells | |||||
| Bulge* | 3.4 ± 1.1 | 7.9 ± 1.7↑ | 6.9 ± 1.1↑ | 3.4 ± 1.1 | 2.8 ± 0.9 ↓ |
| Bulb† | 9.7 ± 2.6 | 13.7 ± 4.7 | 6.9 ± 2.9 | 2.6 ± 0.7 | 5.0 ± 1.7 |
| MHC II+‡ | 2.0 ± 0.3 | 7.0 ± 1.1 ↑ | 8.3 ± 1.2 ↑ | 3.3 ± 0.3 | 2.7 ± 0.3 ↓ |
| Mast cells | |||||
| Dermis total/ | 7.6 ± 0.5/ | 8.6 ± 0.7/ | 7.8 ± 0.4/ | ND | ND |
| Degranulated§ | 2.2 ± 0.3 | 4.1 ± 0.3 ↑ | 3.3 ± 0.3 ↑ | ||
| Subcutis total/ | 3.6 ± 0.4/ | 4.5 ± 0.5/ | 3.7 ± 0.4/ | ND | ND |
| Degranulated§ | 1.0 ± 0.2 | 1.9 ± 0.2 ↑ | 1.3 ± 0.2 | ||
| C57BL/6 mice | |||||
| TUNEL+ cells | |||||
| Bulge* | 6.7 ± 1.8 | 15.2 ± 1.7 ↑ | 21.7 ± 3 ↑ | 2.1 ± 0.4 | 7.5 ± 2.1 ↓ |
| Bulb† | 6.2 ± 1.1 | 14.8 ± 5.0 ↑ | 22.0 ± 3 ↑ | 6.4 ± 1.1 | 8.2 ± 1.3 ↓ |
| MHC II+‡ | 4.7 ± 0.6 | 11.8 ± 0.8 ↑ | 8.5 ± 0.9 ↑ | 7.0 ± 1.8 | 5.3 ± 0.5 ↓ |
| Mast cells | |||||
| Dermis total/ | 6.8 ± 0.4/ | 6.7 ± 0.9/ | 5.7 ± 0.4/ | ND | ND |
| Degranulated§ | 0.7 ± 0.1 | 4.1 ± 0.9 ↑ | 2.5 ± 0.3 ↑ | ||
| Subcutis total/ | 2.9 ± 0.3/ | 3.0 ± 0.2/ | 2.5 ± 0.3/ | ND | ND |
| Degranulated§ | 0.3 ± 0.1 | 1.8 ± 0.2 ↑ | 1.8 ± 0.2 ↑ |
*Bulge area of anagen VI/catagen I follicles covers 40-μm2 tissue.
†Bulb area of anagen VI/catagen I follicles covers 60-μm2 tissue.
‡Number of cell clusters per 12.5-mm epidermal length.
§Classified as degranulated when eight or more granules were seen outside the cell membrane.
ND, Not determined.
↑, Significant increase compared to nonstressed control (P ≤ 0.05).
↓, Significant decrease compared to stressed, non-NK1 RA-treated group (P ≤ 0.05).
Figure 4.
The effect of stress on HF keratinocyte apoptosis in bulge (A–D) and bulb (E and F) region in dermis of C57BL/6 mice. A and E: TUNEL staining in dermis of control mice, very few apoptotic cells can be observed in bulge (A) or bulb (E). HF keratinocyte nuclei appear in bright blue, resulting from DAPI counterstaining. B and F: Stress exposure 2 days before TUNEL staining caused an increase in apoptotic cells in the bulge (B) and bulb (F) region of the HF, as depicted by green fluorescence and pointed out in one case with an arrow. C and G: Injection of SP simulated the effect of stress in nonstressed C57BL/6 mice by causing an increase in apoptotic cells in the bulge (C) and bulb (G) region of the HF, as depicted by green fluorescence. D and H: HF from a stressed C57BL/6 mouse treated with NK1-RA. No signs of apoptosis in bulge (D) and bulb (H).
Stress Increases Perifollicular Macrophage Clusters
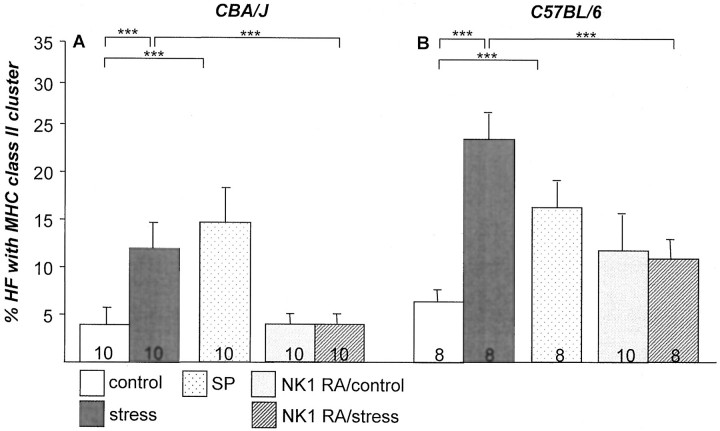
We had previously shown that the exposure to stress significantly boosts the occurrence of perifollicular MHC class II+ cell clusters. These MHC class II+ cell clusters represent primarily activated macrophages and are associated with a substantially higher risk of HFs to be immunosurgically eliminated by a process termed “programmed organ deletion.” 40 The current study revealed a significant increase of HFs that displayed prominent perifollicular MHC class II+ cell clusters (>5 cells) in both stressed strain of mice (CBA/J, Figure 5A ▶ ; C57BL/6, Figure 5B ▶ ). Absolute numbers of MHC class II+ cells per 12.5-mm epidermal length are given in Table 1 ▶ , also indicating the significant increase in actual cell cluster numbers positive for MHC class II around the HF. Figure 2E ▶ shows representative immunohistochemistry of control HFs with low numbers of perifollicular MHC II+ cells in nonstressed C57BL/6 mice. For comparison, an example of high numbers of perifollicular MHC II+ cells in stressed C57BL/6 mice is depicted in Figure 2F ▶ .
Figure 5.
The effect of stress on the percentage of HFs with MHC-II+ perifollicular cell cluster in CBA/J (A) and C57BL/6 (B) mice (mean ± SEM). Exposure to stress 2 days before assessment led to a significant increase of MHC-II+ cells cluster (***, P < 0.001 for both strains). Injection of SP in nonstressed mice mirrored the effect of stress in both strains and injection of NK1-RA abrogated the effect of stress on number of MHC-II+ cells cluster in CBA/J (A) and C57BL/6 (B) mice (***, P < 0.001 for both strains). The number of mice per group is given in the bars.
Stress Stimulates Mast Cell Degranulation
Mast cells have been shown to play a key role in the neuroimmunological linkage of stress to defined inflammatory events. 51-53 We therefore evaluated activated (degranulated) mast cells in the current stress model. Interestingly, a significantly increased percentage of degranulated mast cells was seen in the dermis (Figure 6A) ▶ and in the subcutis (Figure 6C) ▶ of stressed CBA/J mice. Similar observations were made with C57BL/6 (Figure 6, B and D) ▶ after exposure to stress. Absolute numbers of total and degranulated mast cells in dermis and subcutis are given in Table 1 ▶ , indicating that the total number of mast cells did not significantly differ among the groups.
Figure 6.
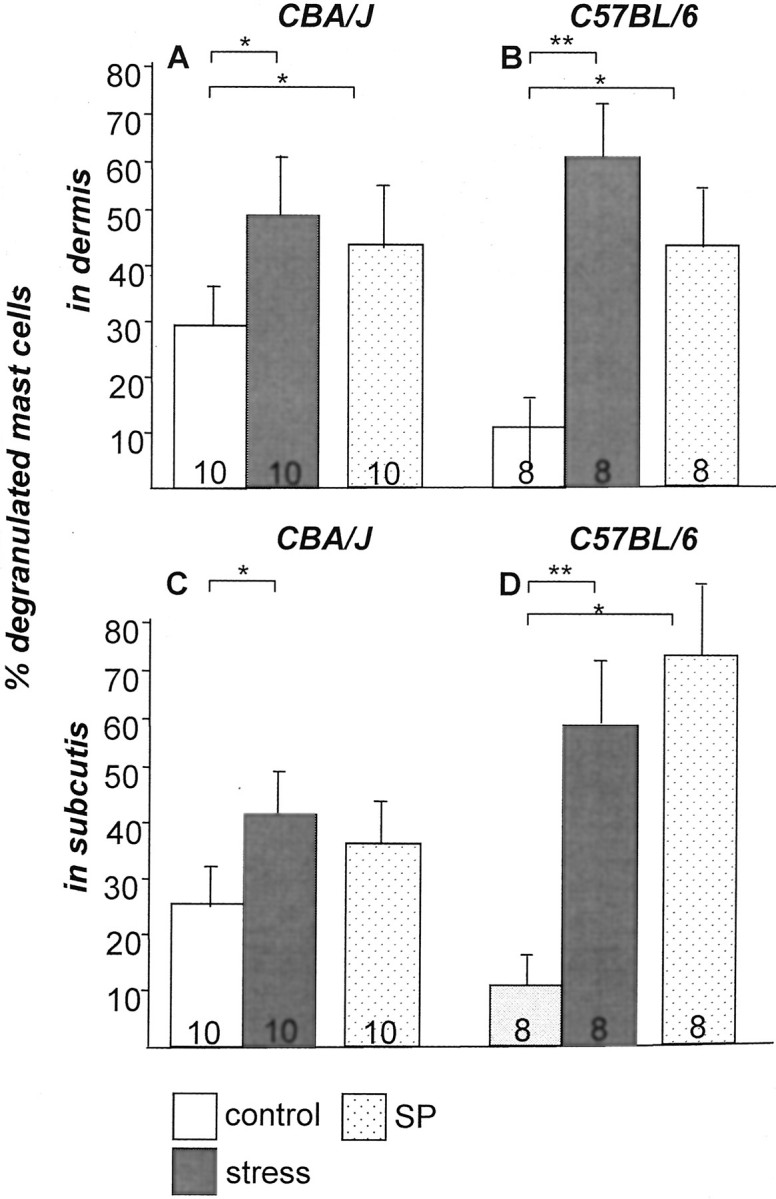
The effect of stress on the percentage of degranulated (= activated) mast cells calculated from the total number of mast cells (mean ± SEM) in dermis (A and B) and subcutis (C and D) in CBA/J (A and C) or C57/BL6 (B and D) mice. Exposure to stress 2 days before assessment led to a significant increase of degranulated mast cells in murine dermis and subcutis in both strains. Injection of SP mimicked the effect of stress in nonstressed control mice. The number of mice per group is given in the bars.
Figure 7A ▶ and, at a higher magnification in B, show a representative example of nondegranulated (nonactivated) mast cells (white arrow) in the dermis near a HF in the skin of control C57BL/6 mice, compared to the degranulated (activated) mast cells in stressed C57BL/6 mice (purple arrow), where extracellular granules can be seen in the dermis (Figure 7, C and D) ▶ .
Figure 7.
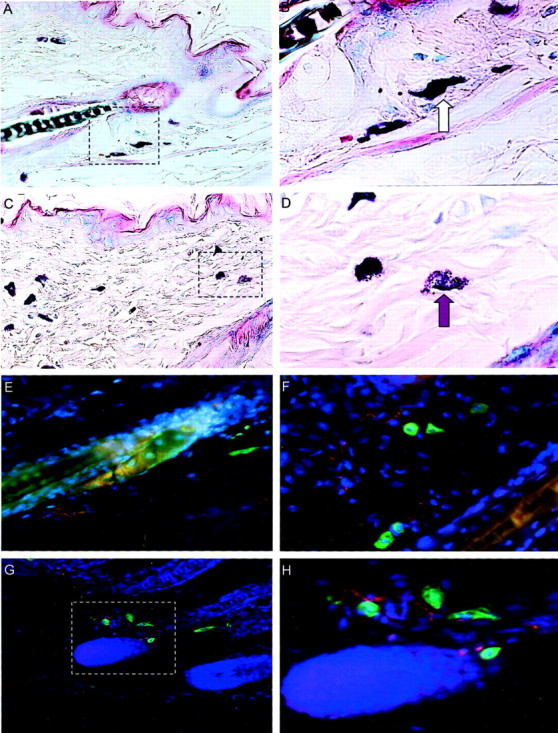
A: Mostly nonactivated mast cells in dermis of nonstressed C57BL/6 mice (dark purple staining). B: A magnification of the region marked in A with the stippled line. No extracellular granules are present in the vicinity of the resting mast cell, as pointed out by the white arrow. C: Stress exposure caused an increase in activated (degranulated) mast cells in dermis. The region magnification indicated in C and presented in D shows a representative example of an activated, degranulated mast cell with more than eight extracellular granules, as indicated by the purple arrow. E–H: Fluorescence immunohistochemistry of SP nerve fibers (delicate red lines) in the dermis (bulge area) of nonstressed mice is presented in E. A mast cell appears in bright green. F: SP nerve fibers in the dermis (bulge area) of a mouse exposed to stress. In this section, an increase in mast cell number can also be observed. Interestingly, we frequently observed SP-positive nerve fibers in the direct vicinity (<2 μm) of mast cells in dermis and subcutis (G) of stressed mice. H: Higher magnification of the stippled square in G.
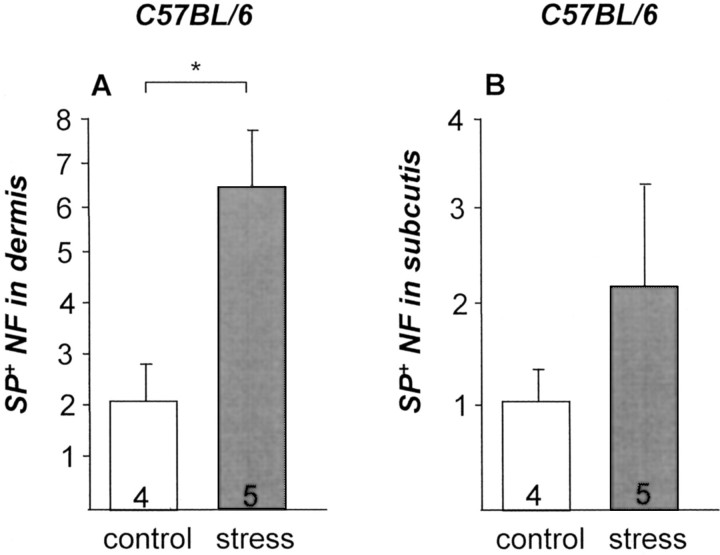
Stress Exposure Up-Regulates Protein Expression of SP Nerve Fibers in Dermis
To directly prove that exposure to stress affects the density and expression pattern of neuropeptide in skin, we included perfusion-fixed mice and demonstrated that stress indeed triggered a significant increase of SP-positive nerve fibers in dermis and in subcutis, whereby in subcutis, levels of significance could not be reached (P = 0.064) (Figure 8) ▶ . Fluorescence immunohistochemistry of SP nerve fibers (delicate red lines) in the dermis (bulge area) of nonstressed mice is presented in Figure 7 ▶ . A mast cell appears in bright green. Figure 7H ▶ depicts SP nerve fibers in the dermis (bulge area) of a mouse exposed to stress. In this section, an increase in mast cell number can also be observed. Interestingly, we frequently observed SP-positive nerve fibers in the direct vicinity (<2 μm) of mast cells in dermis and subcutis (Figure 7G) ▶ of stressed mice. Figure 7H ▶ shows the higher magnification of the stippled square in Figure 7G ▶ .
Figure 8.
A: Stress exposure significantly up-regulates number of SP nerve fibers/visual field in dermis of C57BL/6 mice. B: A tendency of up-regulation could also be observed in the subcutis.
Stress-Induced Premature Catagen Development, Intrafollicular Apoptosis, and Deleterious Inflammatory Events around the HF Are Reproduced by Injection of SP
Next, we wished to determine whether the effects of stress on premature catagen development and intrafollicular apoptosis could be reproduced by systemic injection of one key modulator implicated in stress responses, the neuropeptide SP. CBA/J or C57BL/6 mice received a single intraperitoneal injection of recombinant SP 2 days before skin harvesting. As presented in Figure 1, A and B ▶ , a significant hair cycle progression toward catagen could be induced by injection of SP in both strains of mice in absence of the experimental stressor, thus mimicking the effect of stress. Histology of a representative area of catagen III to IV HF is given in Figure 2C ▶ .
As shown in Figure 3, A and B ▶ , a significant increase of TUNEL+ HFs was indeed seen after SP injection in the bulge region in both (nonstressed) mouse strains, simulating the highly significant increase of apoptotic cells triggered by stress. The effect of SP injection on apoptotic cells in the bulb region showed similar tendencies, however, levels of significance were only reached in C57BL/6 mice (Figure 3, C and D) ▶ . Representative bulge or bulb areas of SP-injected C57BL/6 mice are shown in Figure 4, C and G ▶ , respectively.
SP mimicked the effect of stress exposure on the percentage of HFs with MHC II+ cell cluster in CBA/J and C57BL/6 mice (Figure 5, A and B) ▶ and further simulated the effect of stress on the activation status of mast cells in dermis in both strains (Figure 6, A and B) ▶ and in subcutis for C57BL/6 mice (Figure 6D) ▶ .
NK1 Receptor Antagonist Normalizes Most Stress-Induced Alterations
Further, we investigated whether co-administration of a SP receptor antagonist in stressed mice would abrogate the effects of sound stress on catagen induction and intrafollicular apoptosis. Injection of the highly specific NK1-RA significantly down-regulated the effects of stress on the premature progression of catagen in both mouse strains (Figure 1, A and B ▶ , and Figure 2D ▶ for histology). The same was true for intrafollicular apoptosis in the bulge (Figure 3, A and B) ▶ of both strains and in bulb region of C57BL/6 mice (Figure 3D) ▶ . Representative histology is shown in Figure 4, D and H ▶ .
The effect of stress exposure on the percentage and number of MHC II+ cell clusters could be significantly abrogated by NK1-RA in both mouse strains (Figure 5, A and B) ▶ . Because we previously demonstrated that murine skin mast cells appear not to express the NK1 receptor, 12 we did not investigate the effect of the NK1-RA in the present study.
Discussion
Extending our previous pilot study in telogen mouse skin, 12 we show here for the first time that a well-defined psychoemotional stressor indeed alters actual HF cycling in vivo, ie, prematurely terminates the normal duration of hair growth in mice and up-regulates apoptosis and deleterious inflammatory events in and around the murine HFs. This provides the first solid pathophysiological mechanism for how stress may actually cause telogen effluvium and/or may even be involved in triggering the development of alopecia areata: 1) induction of premature catagen induction; 2) up-regulation of keratinocyte apoptosis in the hair bulb and bulge, the most damage-sensitive epithelial component of the HF; 3) induction of mast cell degranulation; 4) attraction of potentially damaging perifollicular infiltrates of activated macrophages home in on the bulge, the site of HF epithelial stem cells, thus reflecting an immunoactivation.
Furthermore, we show that most hair growth-inhibitory effects of stress can be imitated by the prototypic stress-related neuropeptide, SP, and, with respect to premature catagen onset, increased apoptosis and MHC class II+ cell infiltrates, can be counteracted effectively by co-administration of a specific SP receptor antagonist.
To date, SP has been implicated in diverse pathophysiologies, especially diseases of the central nervous system, which have been examined in the greatest detail. 21,54,55 Although SP is best known as a pain-mediating neuropeptide, it also controls various behavioral, neurochemical, and cardiovascular responses to stress 26,27,55-58 and exerts a wide range of immunomodulating properties. 55,59-61 The development of small molecule antagonists of the SP-preferring tachykinin NK1 receptor during the past decade 32,49 offers an important opportunity to exploit these molecules as novel therapeutic agents for hair growth disorders. In addition, recent clinical trials have confirmed the efficacy of NK1 receptor antagonists to alleviate depression and emesis but, surprisingly, not pain. 62 In the future, clinical trials, targeted to appropriate patient populations, ie, patients with telogen effluvium or alopecia areata, may establish the therapeutic potential of novel neuropeptide ligands in such hair growth disorders.
In the present study we compared a well-established CBA/J mouse model for stress-related research (based on activation of the immune system and hypothalamo-pituitary-adrenal axis), 16,23 with the in hair research widely used mouse strain C57BL/6. 14,40,41,47 Published data indicate that different strains of mice react differently to various methods of stress application, ie, restraint stress affects C3H/HeJ, however, CBA/J or A/J mice proved resistant to restraint stress. 16 By contrast, noise stress would affect CBA/J mice and C57BL/6 mice, both of which have been used in the present study. 16,63 Comparing different strains in stress models, one might further question the hearing ability of the genetically different strains of mice, particularly when an audio stressor has been used. In our present study we observed that both strains, CBA/J and C57BL/6, showed high similarities in their response to stress/SP and skin immune environment. This is supported by published data indicating that C57BL/6 mice at the age of 6 weeks, as used in the present study, showed the same susceptibility to noise than CBA/J mice at the same age. 64
An important focus of research on stress-induced hair growth inhibition is to identify stress-vulnerable areas of the HF. One such area might be the residence of stem cell areas within the skin, because stem cells are vital for the homeostasis of self-renewing tissues and their manipulation may have wide-ranging consequences. 49 Rodent HF stem cells have been localized to the follicle bulge, whereas the location of human HF stem cells is less clear, and their characterization has been hampered by a lack of cellular markers for the bulge area. 49 These cells acquire the ability to produce a new hair. 65 The up-regulation of TUNEL+ cells in this key area of the HF, the bulge, by stress, as observed in the present study, is particularly striking because this region is one of the most sensitive targets for hair growth inhibitory agents and an abnormally high number of apoptotic cells in the epithelial stem cell region of the bulge carries the risk of permanent HF loss by programmed organ deletion. 15,40 This observation further supports our previously published data on increased signs of apoptosis in stressed mice with all HFs in resting telogen stage. 12 Thus, we propose that such delicate area as the bulge region of the cycling HF is one key target for immune cells, particularly activated macrophages and mast cells, activated by systemic stress mediators and that have the potential to damage the HF morphology by up-regulation of apoptosis through the secretion of inflammatory cytokines, ie, tumor necrosis factor-α, interleukin-1β, and interferon-γ. 66-69 Longitudinal studies are now required to elucidate if a stress-triggered attack of HF stem cells would be associated with permanent or reversible hair growth inhibition. Further, based on our current data, we cannot differentiate whether the apoptotic cells in the bulge region are really stem cells or may be transient amplifying cells. Therefore we hope that the findings of the present study may foster research with label-retaining cells, 70 which would tell us whether we are dealing with stem cells or not.
Clinical experience has long suggested that psychological factors play a role in triggering or exacerbating hair loss. 1,3,71,72 The present study now reports the first experimental evidence available in the literature that psychoemotional stress indeed negatively affects the cycling activity of HFs as well as their immune environment in vivo, thus strongly suggesting the concept that stress-induced hair loss is a clinical reality that should and can be treated. Once confirmed for the human system, the data presented here together with the recent emergence of NK1 antagonists as promising anti-depressants, SP receptor antagonists might serve a dual clinical role, ie, for alleviating both stress-induced hair loss itself, and secondary the depressive mood that comes with it.
Acknowledgments
We thank R. Pliet and P. Busse for their excellent technical assistance.
Footnotes
Address reprint requests to Petra Arck, M.D., Charité, Campus Virchow Klinikum, Medizinische Klinik/Biomedizinisches Forschungszentrum, Raum 2.0549, Augustenburger Platz 1, 13353 Berlin, Germany. E-mail: petra.arck@charite.de.
Supported by grants from the German Science Foundation (Deutsche Forschungsgemeinschaft, AR232/14-1, Pa 345/11-1) and the Charité (UFF 99-648).
References
- 1.Selye H: The Physiology and Pathology of Exposure to Stress. 1950:p 727 ACTA Inc. Medical Publishers, Montreal
- 2.Trepat L, Petre AJ: ‘Pelada Universal por shock emotiva.’ Semana Méd 1942, 1:65 [Google Scholar]
- 3.Whitlock FA: Rook A eds. Psychophysiological aspects of skin disease. Major Problems in Dermatology 1976, vol 8. Saunders, London
- 4.York J, Nicholson T, Minors P, Duncan DF: Stressful life events and loss of hair among adult women, a case-control study. Psychol Rep 1998, 82:1044-1046 [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Hernandez MJ, Ruiz-Doblado S, Rodriguez-Pichardo A, Camacho F: Alopecia areata, stress and psychiatric disorders: a review. J Dermatol 1999, 26:625-662 [DOI] [PubMed] [Google Scholar]
- 6.Sinclair R: Diffuse hair loss. Int J Dermatol 1999, 38:8-18 [DOI] [PubMed] [Google Scholar]
- 7.MacAlpine I: Is alopecia areata psychosomatic? A psychiatric study. Br J Dermatol 1958, 70:117-131 [DOI] [PubMed] [Google Scholar]
- 8.Van der Steen P, Boezeman J, Duller P, Happle R: Can alopecia areata be triggered by emotional stress? An uncontrolled evaluation of 178 patients with extensive hair loss. Acta Dermatol Venereol 1992, 72:279-280 [PubMed] [Google Scholar]
- 9.Russiello F, Arciero G, Decaminada F, Corona R, Ferrigno L, Fucci M, Pasquini M, Pasquini P: Stress, attachment and skin disease: a case-control study. J Eur Acad Dermatol Venereol 1995, 5:234-239 [Google Scholar]
- 10.Chrousos GP: Stressors, stress, and neuroendocrine integration of the adaptive response. The 1997 Hans Selye Memorial Lecture. Ann NY Acad Sci 1998, 851:311-335 [DOI] [PubMed] [Google Scholar]
- 11.Paus R, Cotsarelis G: The biology of hair follicles. N Engl J Med 1999, 341:491-497 [DOI] [PubMed] [Google Scholar]
- 12.Arck PC, Handjiski B, Hagen E, Joachim R, Klapp BF, Paus R: Indications for a ‘brain-hair follicle axis (BHA)’: inhibition of keratinocyte proliferation and up-regulation of keratinocyte apoptosis in telogen hair follicles by stress and substance P. FASEB J 2001, 5:2536-2538 [DOI] [PubMed] [Google Scholar]
- 13.Chase HB: Physical factors which influence the growth of hair. Montagna W Ellis RA eds. The Biology of Hair Growth. 1953:pp 435-440 Academic Press, New York
- 14.Maurer M, Handjiski B, Paus R: Hair growth modulation by topical immunophilin ligands: induction of anagen, inhibition of massive catagen development, and relative protection from chemotherapy-induced alopecia. Am J Pathol 1997, 150:1433-1441 [PMC free article] [PubMed] [Google Scholar]
- 15.Stenn KS, Paus R: Controls of hair follicle cycling. Physiol Rev 2001, 81:449-494 [DOI] [PubMed] [Google Scholar]
- 16.Clark DA, Banwatt D, Chaouat G: Stress-triggered abortion in mice prevented by alloimmunization. Am J Reprod Immunol 1993, 29:141-147 [DOI] [PubMed] [Google Scholar]
- 17.Aoki E, Shibasaki T, Kawana S: Intermittent foot shock stress prolongs the telogen stage in the hair cycle of mice. Exp Dermatol, in press [DOI] [PubMed]
- 18.Daly TJ: Alopecia areata has low plasma levels of the vasodilator/immunomodulator calcitonin gene-related peptide. Arch Dermatol 1998, 134:1164-1165 [DOI] [PubMed] [Google Scholar]
- 19.Paus R, Heinzelmann T, Schultz KD, Furkert J, Fechner K, Czarnetzki BM: Hair growth induction by substance P. Lab Invest 1994, 71:134-140 [PubMed] [Google Scholar]
- 20.Peters EM, Botchkarev VA, Botchkareva NV, Tobin DJ, Paus R: Hair-cycle-associated remodeling of the peptidergic innervation of murine skin, and hair growth modulation by neuropeptides. J Invest Dermatol 2001, 116:236-245 [DOI] [PubMed] [Google Scholar]
- 21.von Euler US, Gaddum JH: An unidentified depressor substance in certain tissue extracts. J Physiol 1931, 72:74-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rathsack R, Oehme P, Roske I, Hilse H: Substance P-like immunoreactivity in plasma and adrenal medulla of rats with spontaneous hypertension and WKY rats under acute stress. Biomed Biochim Acta 1983, 42:955-958 [PubMed] [Google Scholar]
- 23.Arck PC, Merali FS, Stanisz AM, Stead RH, Chaouat G, Manuel J, Clark DA: Stress-induced murine abortion associated with substance P-dependent alteration in cytokines in maternal uterine decidua. Biol Reprod 1995, 53:814-819 [DOI] [PubMed] [Google Scholar]
- 24.Zhu GF, Chancellor-Freeland C, Berman AS, Kage R, Leeman SE, Beller DI, Black PH: Endogenous substance P mediates cold water stress-induced increase in interleukin-6 secretion from peritoneal macrophages. J Neurosci 1996, 16:3745-3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaupel R, Jarry H, Schlomer HT, Wuttke W: Differential response of substance P-containing subtypes of adrenomedullary cells to different stressors. Endocrinology 1998, 23:2140-2145 [DOI] [PubMed] [Google Scholar]
- 26.Cao YQ, Mantyh PW, Carlson EJ, Gillespie AM, Epstein CJ, Basbaum AI: Primary afferent tachykinins are required to experience moderate to intense pain. Nature 1998, 392:390-394 [DOI] [PubMed] [Google Scholar]
- 27.De Felipe C, Herrero JF, O’Brien JA, Palmer JA, Doyle CA, Smith AJ, Laird JM, Belmonte C, Cervero F, Hunt SP: Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature 1998, 392:394-397 [DOI] [PubMed] [Google Scholar]
- 28.Teichberg VI, Cohen S, Blumberg S: Distinct classes of substance P receptors revealed by a comparison of the activities of substance P and some of its segments. Regul Pept 1981, 1:327-333 [DOI] [PubMed] [Google Scholar]
- 29.Lee C-M, Iversen LL, Hanley MR, Sandberg BEB: The possible existence of multiple receptors for substance P. Naunyn-Schmiedeberg’s Arch Pharmacol 1982, 318:281-287 [DOI] [PubMed] [Google Scholar]
- 30.Buck SH, Burcher E: The tachykinins: a family of peptides with a brood of receptors. Trends Pharmacol Sci 1986, 7:65-68 [Google Scholar]
- 31.Nakanishi S: Mammalian tachykinin receptors. Annu Rev Neurosci 1991, 14:123-136 [DOI] [PubMed] [Google Scholar]
- 32.Gether U, Johansen TE, Snider RM, Lowe AJ, Nakanishi S, Schwartz TW: Different binding epitope for substance P and the non-peptide antagonist, CP-96, 345, on the NK1 receptor. Nature 1993, 362:345-348 [DOI] [PubMed] [Google Scholar]
- 33.Folkers K, Hörig J, Rosell S, Björkroth U: Chemical design of antagonists of substance P. Acta Physiol Scand 1981, 111:505-506 [DOI] [PubMed] [Google Scholar]
- 34.Wang ZY, Tung SR, Strichartz GR, Håkanson R: Investigation of the specificity of FK 888 as a tachykinin NK1 receptor antagonist. Br J Pharmacol 1994, 111:1342-1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garret C, Carruette A, Fardin V, Moussaoui S, Peyronel JF, Blanchard JC, Laduron PM: Pharmacological properties of a potent and selective nonpeptide substance P antagonist. Proc Natl Acad Sci 1991, 88:10208-10212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snider RM, Constantine JW, Lowe JA, III, Longo KP, Lebel WS, Woody HA, Drozda SE, Desai MC, Vinick FJ, Spencer RW: A potent nonpeptide antagonist of the substance P (NK1) receptor. Science 1991, 251:435-437 [DOI] [PubMed] [Google Scholar]
- 37.Betancur C, Azzi M, Rostène W: Nonpeptide antagonists of neuropeptide receptors: tools for research and therapy. Trends Physiol Sci 1997, 18:372-386 [DOI] [PubMed] [Google Scholar]
- 38.Müller-Röver S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, Stenn KS, Paus R: A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol 2001, 117:3-15 [DOI] [PubMed] [Google Scholar]
- 39.Maurer M, Paus R, Czarnetzki BM: Mast cells as modulators of hair follicle cycling. Exp Dermatol 1995, 4:266-271 [DOI] [PubMed] [Google Scholar]
- 40.Eichmüller S, van der Veen C, Moll I, Hermes B, Hofmann U, Müller-Röver S, Paus R: Clusters of perifollicular macrophages in normal murine skin: physiological degeneration of selected hair follicles by programmed organ deletion. J Histochem Cytochem 1998, 46:361-370 [DOI] [PubMed] [Google Scholar]
- 41.Handjiski BK, Eichmuller S, Hofmann U, Czarnetzki BM, Paus R: Alkaline phosphatase activity and localization during the murine hair cycle. Br J Dermatol 1994, 131:303-310 [DOI] [PubMed] [Google Scholar]
- 42.Ljungberg A, Johansson O: Methodological aspects on immunohistochemistry in dermatology with special reference to neuronal markers. Histochem J 1993, 25:735-745 [DOI] [PubMed] [Google Scholar]
- 43.Botchkarev VA, Eichmüller S, Johansson O, Paus R: Hair cycle-dependent plasticity of skin and hair follicle innervation in normal murine skin. J Comp Neurol 1997, 386:379-395 [DOI] [PubMed] [Google Scholar]
- 44.Botchkarev VA, Eichmuller S, Peters EM, Pietsch P, Johansson O, Maurer M, Paus R: A simple immunofluorescence technique for simultaneous visualization of mast cells and nerve fibers reveals selectivity and hair cycle-dependent changes in mast cell-nerve fiber contacts in murine skin. Arch Dermatol Res 1997, 289:292-302 [DOI] [PubMed] [Google Scholar]
- 45.Paus R, Peters EM, Eichmüller S, Botchkarev VA: Neural mechanisms of hair growth control. J Invest Dermatol Symp Proc 1997, 2:61-68 [DOI] [PubMed] [Google Scholar]
- 46.Peters EM, Botchkarev VA, Botchkareva NV, Tobin DJ, Paus R: Hair-cycle-associated remodeling of the peptidergic innervation of murine skin, and hair growth modulation by neuropeptides. J Invest Dermatol 2001, 116:236-245 [DOI] [PubMed] [Google Scholar]
- 47.Paus R, Maurer M, Slominski A, Czarnetzki BM: Mast cell involvement in murine hair growth. Dev Biol 1994, 163:230-240 [DOI] [PubMed] [Google Scholar]
- 48.Lindner G, Botchkarev VA, Botchkareva NV, Ling G, van der Veen C, Paus R: Analysis of apoptosis during hair follicle regression. Am J Pathol 1997, 151:1601-1617 [PMC free article] [PubMed] [Google Scholar]
- 49.Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM: Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell 1989, 57:201-209 [DOI] [PubMed] [Google Scholar]
- 50.Lavker RM, Miller S, Wilson C, Cotsarelis G, Wei ZG, Yang JS, Sun TT: Hair follicle stem cells: their location, role in hair cycle, and involvement in skin tumor formation. J Invest Dermatol 1993, 101:16S-26S [DOI] [PubMed] [Google Scholar]
- 51.Bienenstock J, Denburg J, Scicchitano R, Stead R, Perdue M, Stanisz A: Role of neuropeptides, nerves and mast cells in intestinal immunity and physiology. Monogr Allergy 1988, 24:134-143 [PubMed] [Google Scholar]
- 52.Williams RH, Bienenstock J, Stead RH: Mast cells—the neuroimmune connection. Chem Immunol 1995, 61:208-251 [PubMed] [Google Scholar]
- 53.Theoharides TC, Singh LK, Boucher W, Pang X, Letourneau R, Webster E, Chrousos G: Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its proinflammatory effects. Endocrinology 1998, 139:403-413 [DOI] [PubMed] [Google Scholar]
- 54.Raffa RB: Possible role(s) of neurokinins in CNS development and neurodegenerative or other disorders. Neurosci Biobehav Rev 1998, 22:789-813 [DOI] [PubMed] [Google Scholar]
- 55.Hokfelt T, Pernow B, Wahren J: Substance P: a pioneer amongst neuropeptides. J Intern Med 2001, 249:27-40 [DOI] [PubMed] [Google Scholar]
- 56.Grelot L, Dapzol J, Esteve E, Frugiere A, Bianchi AL, Sheldrick RL, Gardner CJ, Ward P: Potent inhibition of both the acute and delayed emetic responses to cisplatin in piglets treated with GR205171, a novel highly selective tachykinin NK1 receptor antagonist. Br J Pharmacol 1998, 124:1643-1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fukuda H, Koga T, Furukawa N, Nakamura E, Shiroshita Y: The tachykinin NK1 receptor antagonist GR205171 prevents vagal stimulation-induced retching but not neuronal transmission from emetic vagal afferents to solitary nucleus neurons in dogs. Brain Res 1998, 802:221-231 [DOI] [PubMed] [Google Scholar]
- 58.Onuoha GN, Alpar EK, Chukwulobelu R, Nicholls DP: Distributions of VIP, substance P, neurokinin A and neurotensin in rat heart: an immunocytochemical study. Neuropeptides 1999, 33:19-25 [DOI] [PubMed] [Google Scholar]
- 59.Agro A, Stanisz AM: Neuroimmunomodulation: classical and non-classical cellular activation. Adv Neuroimmunol 1995, 5:311-319 [DOI] [PubMed] [Google Scholar]
- 60.Singh VK: Neuropeptides as native immune modulators. Prog Drug Res 1995, 45:9-31 [DOI] [PubMed] [Google Scholar]
- 61.McGillis JP, Mitsuhashi M, Payan DG: Immunomodulation by tachykinin neuropeptides. Ann NY Acad Sci 1990, 594:85-94 [DOI] [PubMed] [Google Scholar]
- 62.Rupniak NM, Kramer MS: Discovery of the antidepressant and anti-emetic efficacy of substance P receptor (NK1) antagonists. Trends Pharmacol Sci 1999, 20:485-490 [DOI] [PubMed] [Google Scholar]
- 63.Spehner V, De Wazieres B, Nicod L, Harraga S, Robert JF, Seilles E: Auditory stress induces changes in membrane functions of mouse peritoneal macrophages. Scand J Immunol 1996, 44:643-647 [DOI] [PubMed] [Google Scholar]
- 64.Ohlemiller KK, Wright JS, Heidbreder AF: Vulnerability to noise-induced hearing loss in ‘middle-aged’ and young adult mice: a dose-response approach in CBA, C57BL, and BALB inbred strains. Hear Res 2000, 149:239-247 [DOI] [PubMed] [Google Scholar]
- 65.Panteleyev AA, Jahoda CA, Christiano CA: Hair follicle predetermination. J Cell Sci 2001, 114:3419-3431 [DOI] [PubMed] [Google Scholar]
- 66.Yeh WC, Hakem R, Woo M, Mak TW: Gene targeting in the analysis of mammalian apoptosis and TNF receptor superfamily signaling. Immunol Rev 1999, 169:283-302 [DOI] [PubMed] [Google Scholar]
- 67.Totpal K, Singh S, Lapushin R, Aggarwal BB: Qualitative and quantitative differences in the cellular responses mediated through Fas antigen and tumor necrosis factor. J Interferon Cytokine Res 1996, 16:259-267 [DOI] [PubMed] [Google Scholar]
- 68.Manna SK, Mukhopadhyay A, Aggarwal BB: IFN-α suppresses activation of nuclear transcription factors NF-κB and activator protein 1 and potentiates TNF-induced apoptosis. J Immunol 2000, 165:4927-4934 [DOI] [PubMed] [Google Scholar]
- 69.Ruckert R, Lindner G, Bulfone-Paus S, Paus R: High-dose proinflammatory cytokines induce apoptosis of hair bulb keratinocytes in vivo. Br J Dermatol 2000, 143:1036-1039 [DOI] [PubMed] [Google Scholar]
- 70.Wilson C, Cotsarelis G, Wei ZG, Fryer E, Margolis-Fryer J, Ostead M, Tokarek R, Sun TT, Lavker RM: Cells within the bulge region of mouse hair follicle transiently proliferate during early anagen: heterogeneity and functional differences of various hair cycles. Differentiation 1994, 55:127-136 [DOI] [PubMed] [Google Scholar]
- 71.Paus R: Stress and the skin. Exp Dermatol 2001, 10:367. [DOI] [PubMed] [Google Scholar]
- 72.Williamson D, Gonzalez M, Finlay AY: The effect of hair loss on quality of life. J Eur Acad Dermatol Venereol 2000, 15:137-139 [DOI] [PubMed] [Google Scholar]