Abstract
Recent analyses of head and neck squamous cell carcinomas revealed frequent infections by oncogenic human papillomavirus (HPV) type 16 in tonsillar carcinomas. Concerning involvement of risk factors, clinical course of the disease, and prognosis there are strong indications arguing that the HPV-positive tonsillar carcinomas may represent a separate tumor entity. Looking for a surrogate marker, which in further epidemiological studies could replace the laborious and expensive HPV detection and typing we analyzed p16 protein expression in 34 tonsillar carcinoma for correlation to HPV status and load of viral DNA. p16 has been shown to be of diagnostic value for clinical evaluation of cervical dysplasia. We found 53% of the tested tonsillar carcinomas to be HPV-positive. Fifty-six percent of all tumors tested were immunohistochemically positive for the p16 protein. In 16 of 18 of the HPV-positive carcinomas diffuse p16 expression was observed. In contrast, only one of the HPV-negative carcinomas showed focal p16 staining (P < 0.001). As determined by laser-assisted microdissection and quantitative real-time polymerase chain reaction, p16 expression correlated with the presence of HPV-DNA in the individual tumor specimens. Clinical outcome analysis revealed significant correlation of p16 expression with increased disease-free survival (P = 0.02). These data indicate that p16 is a technically simple immunohistological marker, applicable for routine pathological histology, and its prognostic value for survival is fully equivalent to HPV-DNA detection.
The p16 protein, encoded by the CDKN2A (MTS1, INK4A) tumor suppressor gene on chromosome 9p21, inactivates the function of cdk4- and cdk6-cyclin D complexes. One of the critical substrates of the G1-specific cdk complexes is the release of E2F through phosphorylation of the retinoblastoma (pRb)-E2F protein. p16 negatively regulates cell proliferation by suppression of hyperphosphorylation and functional inactivation of pRb. 1-3 The observation that cell lines with a defect in pRb overexpress p16 led to further studies demonstrating that pRb acts as negative regulator of p16 expression at the transcriptional level. 4 It was shown that p16 is involved in the regulation of the cellular life span and accumulates in senescent cells. 5
Loss of this cell-cycle inhibitor by homozygous deletion of the gene, mutation, or promoter hypermethylation has been demonstrated in a wide variety of tumors. 6 In contrast, in squamous cell carcinomas of the cervix mutations or homozygous deletions of p16 were absent. 7 Interestingly, however, strong p16 overexpression has been observed by immunohistochemical analysis in squamous cell carcinomas of the cervix infected by human papillomaviruses (HPVs). 8 p16 was therefore postulated as a specific biomarker, allowing the identification of dysplastic cervical epithelia in sections of cervical biopsy samples or cervical smears. 9 These observations are in line with the hypothesis that the viral oncoprotein E7 inactivates the pRb protein, which would otherwise inhibit p16 transcription. However, because enhancement of p16 mRNA expression is much stronger than expected only by pRb impairment it has been speculated that there are other yet unknown mechanisms of p16 up-regulation by HPV infection. 10
For head and neck squamous cell carcinomas (HNSCCs), representing the sixth most prevalent cancer worldwide, high frequency of p16 inactivation has been reported. 11,12 However there is a controversy in the literature about the predominant way of inactivation. Chromosome 9p21 abnormalities were reported as early events in the development of HNSCC. 13 Poi and colleagues 14 observed sequence alterations of this locus in 27% of a series of HNSCCs. Other reports favor transcriptional silencing of the p16 promoter by hypermethylation as a dominant mechanism of inactivation of this tumor suppressor in HNSCCs. 15,16 Loss of p16 expression as determined by immunohistochemical analysis could be noted in 54 17 to 82% 12 of the tumors and were associated with decreased survival. 12
Cancer of the oropharynx has emerged as the second type of mucosal cancer to be associated with HPV infection. We recently reported on the prevalence of HPV-DNA in a series of carefully stratified HNSCCs. 18 These and previous results 19-23 show that DNA of oncogenic HPVs is present in ∼20% of all HNSSCs and in nearly 60% of tonsillar cancers. Infection with oncogenic HPV types is associated with increased risk of HNSSC. 24-26 In tonsillar carcinomas quantification of HPV-DNA by laser-assisted microdissection and real-time quantitative polymerase chain reaction (PCR) revealed HPV-DNA consistently located in the tumor cells with HPV 16 loads comparable to other HPV-associated cancers. 18 Furthermore HPV status has been correlated with less differentiated and basaloid histological appearance, 18,23 but better prognosis. 19,27,28 Additionally, HPV-positive cases seem to be less dependent on known risk factors for HNSSC such as smoking and alcohol abuse. 18,19 Altogether, there is accumulating evidence that HPV-positive tonsillar carcinomas may represent a separate tumor entity. 29 In the present study we demonstrate the correlation between p16 expression and HPV infection in a series of tonsillar carcinomas and discuss its clinical relevance.
Materials and Methods
Patients and Materials
Thirty-four patients with newly diagnosed and histologically confirmed tonsillar squamous cell carcinomas were enrolled. HPV16-positive cervical carcinoma specimens were used as a positive control. Tumor specimens were obtained during surgery and stored at −80°C until further processing. Tumor staging was assessed according to the 2002 American Joint Committee on Cancer staging criteria. 30 Histological grading was performed in a blinded manner following the World Health Organization criteria for squamous cell carcinomas of the oral mucosa. 31 Patient’s age ranged from 41 to 81 years (median, 61 years). Twenty-five (74%) patients were males and nine (26%) were females. Three percent of the patients presented at stage I, 21% at stage II, 21% at stage III, 44% at stage IVa, and 12% at stage IVb (see Table 2 ▶ ). No patient had distant metastasis (stage IVc) at the time of diagnosis. Of the patients with stage IVb disease no patient had unresectable tumors and there was no R2 resection in our series. Because all stage IV patients underwent additional postoperative chemoradiotherapy we decided to include R1 patients (n = 4) in our data analysis.
Table 2.
Tumor Stage According to HPV Status/p16 Status and Events
| Stage | All cases | HPV-positive/p16-positive | HPV-negative/p16-negative | HPV-positive/p16-negative | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | R* | D* | Total | R | D | Total | R | D | Total | R | D | |
| All | 34 (100%) | 15/34 (44%) | 9/34 (27%) | 16 (100%) | 3/16 (19%) | 4/16 (25%) | 16 (100%) | 10/16 (63%) | 4/16 (25%) | 2 (100%) | 2/2 (100%) | 1/2 (50%) |
| I | 1 (3%) | 1 | 0 | 0 (0%) | 0 | 0 | 1 (6%) | 1 | 0 | 0 (0%) | 0 | 0 |
| II | 7 (21%) | 3 | 1 | 3 (19%) | 1 | 1 | 4 (25%) | 2 | 0 | 0 (0%) | 0 | 0 |
| III | 7 (21%) | 3 | 1 | 3 (19%) | 1 | 1 | 3 (19%) | 1 | 0 | 1 (50%) | 1 | 0 |
| IVa | 15 (44%) | 5 | 6 | 8 (50%) | 0 | 2 | 6 (38%) | 4 | 3 | 1 (50%) | 1 | 1 |
| IVb | 4 (12%) | 3 | 1 | 2 (13%) | 1 | 0 | 2 (13%) | 2 | 1 | 0 (0%) | 0 | 0 |
*R, recurrence; D, deaths. There were no HPV-negative/p16-positive tumors. No patient had stage IVc disease. Immunoreactivity of more than 25% of the tumor cells were scored as p16-positive, less than 25% as p16-negative.
Sample Preparation, Polymerase Chain Reaction, and HPV Typing
Tissues were processed as previously described. 18 After confirming integrity of DNA by β-globin gene PCR, 32 HPV sequences were detected by highly sensitive nested PCR protocols with degenerate primers A10/A5-A6/A8 for HPV group A (genital/mucosal) HPVs 33 and CP62/70-CP65/69a for group B1 (cutaneous/EV) HPVs. 34 PCR products (10 μl) were separated in 2% agarose gels and visualized by ethidium bromide staining. HPV typing was performed as previously described. 18
Immunohistochemical Staining
Five-μm sections of formalin-fixed and paraffin-embedded biopsy samples were processed by the avidin-biotin-peroxidase (ABC) method (Vectastain-Elite-ABC Kit; Vector Laboratories, Burlingame, CA). Sections were dewaxed by passage through xylene and rehydrated by a graded series of ethanol, followed by microwave treatment in 10 mmol/L of citrate buffer (pH 6.0) for 15 minutes at 650 W. Endogenous peroxidase was inactivated by 3% H2O2 in phosphate-buffered saline (PBS, pH 7.4) for 10 minutes. After rinsing with PBS, nonspecific binding was blocked with 10% rabbit serum (1:10 dilution; DAKO, Hamburg, Germany) in PBS for 20 minutes. Sections were incubated at room temperature for 90 minutes with two different anti-p16 mouse monoclonal antibodies (16P04 and 16P07; NeoMarkers, Fremont, CA), respectively. After rinsing thoroughly with PBS the slides were incubated with biotinylated goat anti-mouse antibody (1:200 dilution, DAKO) for 30 minutes. After washing with PBS sections were incubated with the ABC kit (1:50, Vector Laboratories) for 30 minutes. Visualization was performed with diaminobenzidine tetrahydrochloride (Vectastain-ABC Kit, Vector Laboratories) and sections were counterstained with hematoxylin. Strong nuclear staining as well as strong cytoplasmic staining was considered positive for p16 expression. Immunostaining was graded and scored on whole sections according to Klaes and colleagues 9 as follows: negative (<1% of the cells were positive), sporadic (isolated cells were positive, but <5%), focal (small cell clusters, but <25% of the cells were positive), and diffuse positive (>25% of the cells were stained). For survival analyses immunoreactivity of >25% of the tumor cells were scored as p16-positive, <25% as p16-negative.
Laser-Assisted Microdissection
To compare p16 staining and viral load serial sections were used for microdissection. Five-μm sections of formalin-fixed and paraffin-embedded tissue samples from patients with HPV-positive tonsillar carcinoma were mounted on glass slides covered with a 1.35-μm thin polyethylene membrane (PALM, Wolfratshausen, Germany) and coated with 1% poly-l-lysine. Slides were deparaffinized and stained with hematoxylin. Microdissection was performed using the Robot-MicroBeam system (PALM). 35 Samples were incubated for at least 3 hours at 55°C in 15 μl of PCR buffer (Life Technologies, Karlsruhe, Germany) containing proteinase K (400 μg/ml). Proteinase K activity was inactivated at 95°C for 10 minutes.
Determination of HPV-DNA Load by Real-Time Quantitative PCR
Viral load of HPV-DNA was determined by real-time fluorescence PCR in the hybridization probe format with the LightCycler System (Roche Molecular Biochemicals, Mannheim, Germany) and expressed as HPV16 DNA copies per β-globin gene copy. 18,36 Negative controls (human placental DNA or water) were included in duplicate in each run and never yielded fluorescence signals above the background.
Statistical Analysis
p16 and HPV status were analyzed using cross-tabulations and Fisher’s exact test with the SPSS Base System, version 10.0.7 (SPSS, Chicago, IL). Disease-free survival and overall survival rates were estimated using the Kaplan-Meier algorithm for incomplete observations. The overall survival time was defined as the interval between the date of diagnosis and the last date when the patient was known to be alive (censored) or date of death for any reason (uncensored). The disease-free survival was measured as the period of time between the date of diagnosis and the date of the last follow-up examination, in which the patient was disease-free (censored) or the date of first recurrence independently if it was a local, regional, or distant recurrence (uncensored). All patients were treated according to the same protocol and all patients were included in disease-free survival analysis. The log rank test was used to test for differences between subgroups. All P values were considered statistically significant if less than or equal to 0.05.
Results
HPV Status and HPV Typing
HPV sequences were detected in DNA preparations of 18 of 34 tonsillar carcinoma cases (53%). Of these 17 (96%) carried HPV16 and 1 (6%) HPV33 sequences. In two tonsillar cancers (12%) double infection was identified by HPV5/HPV16 and ADX1/HPV16, respectively.
Expression of p16 in Tonsillar Carcinomas
Immunohistochemical staining was performed on paraffin-embedded samples of 34 tonsillar carcinomas using two anti-p16 mouse monoclonal antibodies (16P04 and 16P07; NeoMarkers, Fremont, CA). Results for both antibodies were equal. According to the criteria of Klaes and colleagues, 9 89% (16 of 18) of the HPV-positive carcinomas showed diffuse p16 expression (Table 1) ▶ . In contrast 94% (15 of 16) of the HPV-negative cancers lacked any p16 immunoreactivity (Figure 1B) ▶ . The correlation of p16 expression and HPV status was highly significant (P < 0.001).
Table 1.
Distribution of p16 Expression in Relation to the HPV Status
| Number of cases | Diffuse* no. (%) | Focal† | Sporadic‡ | Negative§ | |
|---|---|---|---|---|---|
| HPV-positive | 18 | 16 (89) | 1 (6) | 1 (6) | 0 (0) |
| HPV-negative | 16 | 0 (0) | 1 (6) | 0 (0) | 15 (94) |
| Total | 34 | 16 (47) | 2 (6) | 1 (3) | 15 (44) |
*More than 25% of the cells were stained.
†Less than 25% or small clusters were positive.
‡Isolated cells, but less than 5% were positive.
§Less than 1% of the cells were positive, overall percentage more than 100 result from rounding.
Figure 1.
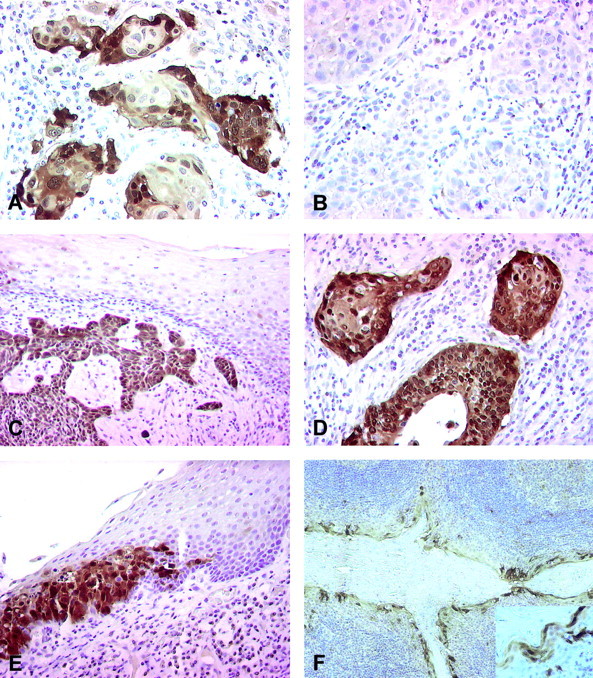
Immunohistochemical staining for p16 (antibody 16P04). A: Clear and distinct positive staining for p16 is seen in nuclei and cytoplasm of tumor cells of a HPV-positive tonsillar carcinoma. B: In the HPV-negative tonsillar carcinoma all tumor cells are p16-negative. C: Strong positive staining of tumor cells of a HPV-positive carcinoma is surrounded by p16-negative nondysplastic epithelium. D: The pattern of the p16 staining of the tumor cells of the cervical carcinoma is similar to the HPV-positive tonsillar carcinoma. E: In cases with HPV-positive tonsillar carcinomas areas with dysplastic cell epithelium were p16-positive distinct to normal epithelium. F: Crypt epithelium adjacent to HPV-positive tumor shows p16 immunoreactivity in the superficial crypt epithelium (inset: higher magnification of the crypt epithelium).
In the lesions graded diffusely positive almost all neoplastic cells showed strong immunoreactivity for p16 in the nuclei and many also in the cytoplasm (Figure 1A) ▶ . This pattern of p16 immunoreactivity was only found in neoplastic cells of HPV-positive tonsillar cancers. In general, surrounding mesenchymal cells showed no p16 immunoreactivity, but some weak staining was found occasionally in lymphocytes and salivary glands. Nondysplastic, peritonsillar squamous cell epithelium was p16-negative and served as internal negative control (Figure 1C) ▶ . The pattern of p16 immunoreactivity of the HPV-positive tonsillar carcinomas was similar to that observed in HPV-positive cervical carcinomas, which were used as a positive control (Figure 1D) ▶ . Only in the HPV-positive cases did the dysplastic peritonsillar epithelium show marked p16 expression and the border between dysplastic and normal squamous epithelium was clear and distinct (Figure 1E) ▶ . In contrast, the superficial crypt epithelium showed p16 immunoreactivity regardless of the HPV status of the carcinoma (Figure 1F) ▶ . The p16 immunoreactivity pattern of the HPV33-positive carcinoma was similar to the HPV16-positive cases.
Laser-Assisted Microdissection and Real-Time Quantitative PCR
To compare p16 staining and viral load laser-assisted microdissection followed by real-time quantitative PCR was performed for 12 HPV16-positive cases to determine HPV-DNA load in p16-positive neoplastic cells. For reliable determination of HPV16 DNA load only samples with at least 10 β-globin genes were accepted. Viral load in lesions with strong and diffuse p16-positive expression ranged from 1 to 408 HPV16 copies per β-globin gene copy, respectively. In contrast, only focal p16 immunoreactivity was observed in one case judged to be HPV16-positive on the base of HPV16 sequences detected by nested PCR in DNA preparations from whole tumor sections. After microdissection quantitative HPV16-PCR was negative in this case. The viral load of the DNA preparations from whole tumor section was 6.6 × 10−4 HPV16 copy per β-globin copy. Among cases with diffuse p16 staining no significant correlation between the extent of the p16 staining and viral load could be observed.
Survival Analysis
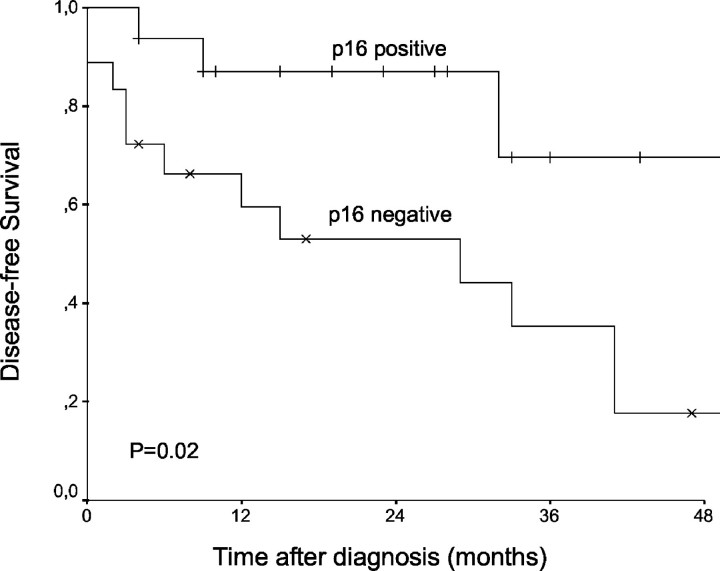
To determine the predictive value of the HPV status and p16 immunoreactivity for prognosis we analyzed our 34 cases for disease-free survival and overall survival. The tumor stages of all cases in relation to p16 status/HPV status and events are given in Table 2 ▶ . The distribution of tumor stages was similar between HPV-positive/p16-positive and HPV-negative/p16-negative cases. Twenty-seven percent of all patients died and 44% had a tumor recurrence. Deaths were observed in equal proportions in the HPV-positive/p16-positive and HPV-negative/p16-negative group (25%). However, recurrences were observed significantly more frequently in HPV-negative/p16-negative patients than in the HPV-positive/p16-positive group (19% versus 63%, P = 0.028; Fishers exact test). The two patients with HPV-positive/p16-negative tumors suffered from a tumor recurrence. The median follow-up period was 33 months with a maximum of 57 months; 76% of the patients were followed for at least 12 months. Using the Kaplan-Meier algorithm for the complete study sample the 4-year disease-free and overall survival rates were 33% and 62%, respectively. Stratification for HPV status revealed a trend toward better prognosis for HPV-positive patients compared to HPV-negative cases, however the difference in the disease-free survival curves between the two groups was not significant (log rank test, P = 0.06). The 4-year overall survival rates among HPV-positive and HPV-negative cases were 71% versus 51%, and for disease-free survival 45% versus 22%. Using p16 immunoreactivity for stratification revealed a significant difference for disease-free survival curves between p16-positive and p16-negative patients (P = 0.02, log rank test; Figure 2 ▶ ). The 4-year disease-free survival rates were 70% for p16-positive and 18% for p16-negative cases. Analysis of the overall survival reached neither significant differences for HPV nor for p16 as predictor (P = 0.97 and P = 0.77, log rank test).
Figure 2.
Disease-free survival curves were generated by the Kaplan-Meier method and compared using the log-rank test. Probability of the disease-free survival was significantly better in p16-positive patients (immunoreactivity of >25% of the tumor cells were scored as p16-positive, <25% as p16-negative).
Discussion
With regard to biological behavior and prognosis HNSCCs are a heterogeneous group of malignancies. 37 Although many clinical and biological parameters have been discussed to predict prognosis, no clear marker could be defined so far. We and others demonstrated in recent studies that ∼60% of tonsillar carcinomas are infected by oncogenic HPV, 18 predominantly HPV16. Measured viral loads were comparable to those observed in cervical carcinomas (SJ Weissenborn and colleagues, personal communication). Furthermore, patients with HPV-positive carcinomas tend to have significantly lower exposure to known risk factors for HNSSC. 18,27 Also serological studies have shown that HPV-16 sero-positive patients have an increased risk for the development of a tonsillar carcinoma. 24
There is now increasing evidence that HPV-associated tonsillar carcinomas represent a different tumor entity, distinct from other HNSCCs in regards to risk factors and tumor biology. 29 Moreover, improved survival of HPV-positive HNSCCs has been shown, 28 which might be the result of a higher sensitivity to radiation therapy. 27 This is in accordance with the observation that HPV status and p53 mutations are inversely correlated 38 and tumors with intact p53 are more susceptible to radiation-induced apoptosis. 39 There are, however, conflicting observations concerning physical status and expression of viral sequences in HNSCCs. 38,40 To identify this subgroup of head and neck cancers molecular approaches as PCR-based techniques are hardly applicable in a routine pathology because of technical obstacles and extensive costs.
Functional loss of p16 has been reported for many human cancers, whereas in HPV-associated cervical carcinomas p16 overexpression has been observed. As the HPV E7 oncogene product inhibits the activity of the pRb protein, p16 is up-regulated via the loss of the negative feedback control of pRb expression. It could be demonstrated that p16 is a specific biomarker that can routinely be used to identify dysplastic cervical epithelia. 9 Furthermore p16 immunostaining has been shown to identify lesions with relevant viral oncogene expression. 41 In this study we demonstrate that p16 immunohistochemical staining of neoplastic cells may be used as a surrogate marker for HPV status of tonsillar carcinomas. Using previously described grading criteria to evaluate p16 immunoreactivity 9 revealed a highly significant correlation of p16 and HPV status. To use p16 immunoreactivity as predictor for survival we scored all lesions with diffuse p16 immunoreactivity as positive and all other staining patterns as negative. According to this grading all HPV-negative tonsillar cancers were considered to be negative for p16 immunoreactivity. Of the HPV-positive cases 16 of 18 (89%) had diffuse p16 expression in the neoplastic cells (Figure 1, A and C) ▶ . One exceptional case with only focal p16 immunoreactivity turned out to have a very low HPV16 viral load, suggesting that the oncogenic role of HPV16 in this tumor may be questionable. HPV-DNA load determination using microdissected tumor cells demonstrated that neoplastic cells with clear p16 overexpression contain 1 to 408 HPV16 copies per β-globin copy. Viral loads are comparable to loads that were measured in cervical carcinomas (SJ Weissenborn and colleagues, personal communication). In HPV-positive carcinomas strong p16 expression could be shown in dysplastic lesions whereas the adjacent normal epithelium was p16-negative. In contrast, in the HPV-negative carcinomas, dysplastic epithelium showed no p16 immunoreactivity. p16 expression in crypt epithelium was seen in both HPV-positive and -negative cases, which implicates other mechanisms of p16 activation in this specialized epithelium.
p16 inactivation has been reported for HNSCCs, 11,12 but was not correlated to HPV status. Furthermore, previous studies on p16 expression in HNSCCs did not focus on tonsillar carcinomas, now known to be preferably associated with oncogenic HPV. In our study HPV-positive/p16-positive tumors had a lower recurrence rate than the HPV-negative/p16-negative cases. The survival analysis of tonsillar carcinoma patients revealed a trend for better prognosis for the HPV-positive cases as compared to the HPV-negative cases. Immunostaining of p16 predicted disease-free survival, but not overall survival in our series. In view of the rather small number of cases and the relatively short follow-up time, the true predictive power of p16 immunostaining will have to be elucidated for larger cohorts of patients with longer clinical follow-up.
In conclusion, p16 expression is highly correlated with HPV status in tonsillar cancers. p16 immunoreactivity is likely to result from transcriptionally active HPV infection thus underlining the hypothesis that HPV-positive tonsillar carcinomas represent a biologically different tumor entity. If our results will be confirmed in future studies p16 could be a discriminator that is easily applicable in a routine pathology and become the basis of treatment decisions based on histological features rather than on staging alone.
Footnotes
Address reprint requests to Jens P. Klussmann, Department of Oto-Rhino-Laryngology, Head and Neck Surgery, Joseph-Stelzmann-Str. 9, Köln, Germany D-50924. E-mail: peter.klussmann@uni-koeln.de.
Supported by the Jean Uhrmacher Foundation, the Köln Fortune-Programme of the Faculty of Medicine, University of Cologne (grant 93/2001 to J. P. K.), and the Cologne Center for Molecular Medicine (ZMMK to H. J. P. and P. G. F.).
Presented in part at the 20th International Papillomavirus Conference, October 4–9, 2002, Paris, France.
References
- 1.Kamb A, Gruis NA, Weaver-Feldhaus J, Liu Q, Harshman K, Tavtigian SV, Stockert E, Day RS, III, Johnson BE, Skolnick MH: A cell cycle regulator potentially involved in genesis of many tumor types. Science 1994, 264:436-440 [DOI] [PubMed] [Google Scholar]
- 2.Serrano M, Hannon GJ, Beach D: A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 1993, 366:704-707 [DOI] [PubMed] [Google Scholar]
- 3.Nobori T, Miura K, Wu DJ, Lois A, Takabayashi K, Carson DA: Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature 1994, 368:753-756 [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Nichols MA, Shay JW, Xiong Y: Transcriptional repression of the D-type cyclin-dependent kinase inhibitor p16 by the retinoblastoma susceptibility gene product pRb. Cancer Res 1994, 54:6078-6082 [PubMed] [Google Scholar]
- 5.Hara E, Smith R, Parry D, Tahara H, Stone S, Peters G: Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol Cell Biol 1996, 16:859-867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roussel MF: The INK4 family of cell cycle inhibitors in cancer. Oncogene 1999, 18:5311-5317 [DOI] [PubMed] [Google Scholar]
- 7.Kim JW, Namkoong SE, Ryu SW, Kim HS, Shin JW, Lee JM, Kim DH, Kim IK: Absence of p15INK4B and p16INK4A gene alterations in primary cervical carcinoma tissues and cell lines with human papillomavirus infection. Gynecol Oncol 1998, 70:75-79 [DOI] [PubMed] [Google Scholar]
- 8.Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T: Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol 1998, 153:1741-1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klaes R, Friedrich T, Spitkovsky D, Ridder R, Rudy W, Petry U, Dallenbach-Hellweg G, Schmidt D, von Knebel DM: Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer 2001, 92:276-284 [DOI] [PubMed] [Google Scholar]
- 10.Nakao Y, Yang X, Yokoyama M, Ferenczy A, Tang SC, Pater MM, Pater A: Induction of p16 during immortalization by HPV 16 and 18 and not during malignant transformation. Br J Cancer 1997, 75:1410-1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reed AL, Califano J, Cairns P, Westra WH, Jones RM, Koch W, Ahrendt S, Eby Y, Sewell D, Nawroz H, Bartek J, Sidransky D: High frequency of p16 (CDKN2/MTS-1/INK4A) inactivation in head and neck squamous cell carcinoma. Cancer Res 1996, 56:3630-3633 [PubMed] [Google Scholar]
- 12.Ambrosch P, Schlott T, Hilmes D, Ruschenburg I: p16 alterations and retinoblastoma protein expression in squamous cell carcinoma and neighboring dysplasia from the upper aerodigestive tract. Virchows Arch 2001, 438:343-349 [DOI] [PubMed] [Google Scholar]
- 13.Mao L, Lee JS, Fan YH, Ro JY, Batsakis JG, Lippman S, Hittelman W, Hong WK: Frequent microsatellite alterations at chromosomes 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. Nat Med 1996, 2:682-685 [DOI] [PubMed] [Google Scholar]
- 14.Poi MJ, Yen T, Li J, Song H, Lang JC, Schuller DE, Pearl DK, Casto BC, Tsai MD, Weghorst CM: Somatic INK4a-ARF locus mutations: a significant mechanism of gene inactivation in squamous cell carcinomas of the head and neck. Mol Carcinog 2001, 30:26-36 [DOI] [PubMed] [Google Scholar]
- 15.El Naggar AK, Lai S, Clayman G, Lee JK, Luna MA, Goepfert H, Batsakis JG: Methylation, a major mechanism of p16/CDKN2 gene inactivation in head and neck squamous carcinoma. Am J Pathol 1997, 151:1767-1774 [PMC free article] [PubMed] [Google Scholar]
- 16.Akanuma D, Uzawa N, Yoshida MA, Negishi A, Amagasa T, Ikeuchi T: Inactivation patterns of the p16 (INK4a) gene in oral squamous cell carcinoma cell lines. Oral Oncol 1999, 35:476-483 [DOI] [PubMed] [Google Scholar]
- 17.Gruttgen A, Reichenzeller M, Junger M, Schlien S, Affolter A, Bosch FX: Detailed gene expression analysis but not microsatellite marker analysis of 9p21 reveals differential defects in the INK4a gene locus in the majority of head and neck cancers. J Pathol 2001, 194:311-317 [DOI] [PubMed] [Google Scholar]
- 18.Klussmann JP, Weissenborn S, Wieland U, Dries V, Kolligs J, Jungehuelsing M, Eckel HE, Dienes HP, Pfister H, Fuchs P: Prevalence, distribution and viral load of human papillomavirus 16 DNA in tonsillar carcinomas. Cancer 2001, 92:2875-2884 [DOI] [PubMed] [Google Scholar]
- 19.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D: Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 2000, 92:709-720 [DOI] [PubMed] [Google Scholar]
- 20.Snijders PJ, Cromme FV, van den Brule AJ, Schrijnemakers HF, Snow GB, Meijer CJ, Walboomers JM: Prevalence and expression of human papillomavirus in tonsillar carcinomas, indicating a possible viral etiology. Int J Cancer 1992, 51:845-850 [DOI] [PubMed] [Google Scholar]
- 21.Paz IB, Cook N, Odom-Maryon T, Xie Y, Wilczynski SP: Human papillomavirus (HPV) in head and neck cancer. An association of HPV 16 with squamous cell carcinoma of Waldeyer’s tonsillar ring. Cancer 1997, 79:595-604 [DOI] [PubMed] [Google Scholar]
- 22.Brandsma JL, Abramson AL: Association of papillomavirus with cancers of the head and neck. Arch Otolaryngol Head Neck Surg 1989, 115:621-625 [DOI] [PubMed] [Google Scholar]
- 23.Wilczynski SP, Lin BT, Xie Y, Paz IB: Detection of human papillomavirus DNA and oncoprotein overexpression are associated with distinct morphological patterns of tonsillar squamous cell carcinoma. Am J Pathol 1998, 152:145-156 [PMC free article] [PubMed] [Google Scholar]
- 24.Mork J, Lie AK, Glattre E, Hallmans G, Jellum E, Koskela P, Moller B, Pukkala E, Schiller JT, Youngman L, Lehtinen M, Dillner J: Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med 2001, 344:1125-1131 [DOI] [PubMed] [Google Scholar]
- 25.Klussmann JP, Weissenborn S, Fuchs PG: Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med 2001, 345:376. [PubMed] [Google Scholar]
- 26.Smith EM, Hoffman HT, Summersgill KS, Kirchner HL, Turek LP, Haugen TH: Human papillomavirus and risk of oral cancer. Laryngoscope 1998, 108:1098-1103 [DOI] [PubMed] [Google Scholar]
- 27.Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM: Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer 2001, 92:805-813 [DOI] [PubMed] [Google Scholar]
- 28.Mellin H, Friesland S, Lewensohn R, Dalianis T, Munck-Wikland E: Human papillomavirus (HPV) DNA in tonsillar cancer: clinical correlates, risk of relapse, and survival. Int J Cancer 2000, 89:300-304 [PubMed] [Google Scholar]
- 29.Klussmann JP, Weissenborn SJ, Wieland U, Eckel HE, Pfister HJ, Fuchs PG: HPV positive tonsillar carcinomas: a different tumor entity? Med Microbiol Immunol, in press [DOI] [PubMed]
- 30.Greene FL, Page DL, Fleming ID, Fritz AG, Blach CM, Haller DG, Morrow M: American Joint Committee on Cancer Staging Manual, ed 6 2002. Springer, Berlin
- 31.Pindbord JJ, Reichart PA, Smith CJ, van der Waal I: Histological Typing of Cancer and Precancer of the Oral Mucosa. 1997:pp 11-12 Springer, Berlin
- 32.de Roda Husman AM, Snijders PJ, Stel HV, van den Brule AJ, Meijer CJ, Walboomers JM: Processing of long-stored archival cervical smears for human papillomavirus detection by the polymerase chain reaction. Br J Cancer 1995, 72:412-417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wieland U, Ritzkowsky A, Stoltidis M, Weissenborn S, Stark S, Ploner M, Majewski S, Jablonska S, Pfister HJ, Fuchs PG: Communication: papillomavirus DNA in basal cell carcinomas of immunocompetent patients: an accidental association? J Invest Dermatol 2000, 115:124-128 [DOI] [PubMed] [Google Scholar]
- 34.Boxman IL, Berkhout RJ, Mulder LH, Wolkers MC, Bouwes Bavinck JN, Vermeer BJ, ter Schegget J: Detection of human papillomavirus DNA in plucked hairs from renal transplant recipients and healthy volunteers. J Invest Dermatol 1997, 108:712-715 [DOI] [PubMed] [Google Scholar]
- 35.Schutze K, Lahr G: Identification of expressed genes by laser-mediated manipulation of single cells. Nature Biotechnol 1998, 16:737-742 [DOI] [PubMed] [Google Scholar]
- 36.Wittwer CT, Ririe KM, Andrew RV, David DA, Gundry RA, Balis UJ: The LightCycler: a microvolume multisample fluorimeter with rapid temperature control. Biotechniques 1997, 22:176-181 [DOI] [PubMed] [Google Scholar]
- 37.Forastiere A, Koch W, Trotti A, Sidransky D: Head and neck cancer. N Engl J Med 2001, 345:1890-1900 [DOI] [PubMed] [Google Scholar]
- 38.van Houten VM, Snijders PJ, van den Brekel MW, Kummer JA, Meijer CJ, van Leeuwen B, Denkers F, Smeele LE, Snow GB, Brakenhoff RH: Biological evidence that human papillomaviruses are etiologically involved in a subgroup of head and neck squamous cell carcinomas. Int J Cancer 2001, 93:232-235 [DOI] [PubMed] [Google Scholar]
- 39.Peltenburg LT: Radiosensitivity of tumor cells. Oncogenes and apoptosis. Q J Nucl Med 2000, 44:355-364 [PubMed] [Google Scholar]
- 40.Wiest T, Schwarz E, Enders C, Flechtenmacher C, Bosch FX: Involvement of intact HPV16 E6/E7 gene expression in head and neck cancers with unaltered p53 status and perturbed pRb cell cycle control. Oncogene 2002, 21:1510-1517 [DOI] [PubMed] [Google Scholar]
- 41.von Knebel DM: New molecular tools for efficient screening of cervical cancer. Dis Markers 2001, 17:123-128 [DOI] [PMC free article] [PubMed] [Google Scholar]