Abstract
Studies have shown that joint bleeding leads to cartilage degradation independent of concurrent synovitis. We hypothesized that the blood-induced cartilage damage is because of increased chondrocyte apoptosis after short-term exposure of whole blood or isolated mononuclear cells plus red blood cells to cartilage. Human cartilage tissue samples were co-cultured for 4 days with whole blood (50% v/v) or with mononuclear cells plus red blood cells (50% v/v equivalents). Cartilage matrix proteoglycan synthesis (35SO42− incorporation) was determined after 4 days as well as at day 16 (after a 12-day recovery period in the absence of any additions). To test the involvement of apoptosis a specific caspase-3 inhibitor (acDEVDcho, 0 to 500 μmol/L) as well as a pan-caspase inhibitor (zVADfmk, 0 to 500 μmol/L) were added. Chondrocyte apoptosis was evaluated by immunohistochemical staining of single-strand DNA and by terminal dUTP nick-end labeling. Cartilage co-cultured with whole blood as well as mononuclear cells plus red blood cells induced a long-term inhibition of proteoglycan synthesis (74% and 78% inhibition on day 16, respectively). Immunohistochemistry showed a threefold increase in apoptotic chondrocytes in cultures with 50% whole blood as well as with mononuclear cells plus red blood cells. Both the specific caspase-3 inhibitor and the pan-caspase inhibitor partially restored proteoglycan synthesis in the cartilage after blood exposure. This effect was accompanied by a decrease in the number of apoptotic chondrocytes. These data suggest that a single joint hemorrhage (a 4-day exposure of cartilage to 50% v/v blood) results in induction of chondrocyte apoptosis, responsible for the observed inability of the chondrocytes to restore the proteoglycan synthesis during recovery from a short-term exposure to blood. This reduced restoration could eventually lead to cartilage degeneration and ultimately joint destruction.
Recurrent joint hemorrhages often occur in hemophilia despite prophylactic treatment with clotting factors and result in irreparable joint damage, primarily by destruction of articular cartilage followed by synovial inflammation. 1,2 In vitro and animal in vivo studies provide evidence that not only repeated but also a relative short exposure of cartilage to blood results in long-lasting changes in chondrocyte metabolism that eventually may lead to cartilage destruction. 3,4 Such a short bleeding episode may be the first in a series in hemophiliacs but may also contribute to cartilage damage as the result of a sports injury or a single trauma, eg, ligament rupture. 5 After the initiation phase progression of joint damage in humans is slow. Therefore the causality between a joint bleeding and final joint damage remains difficult to prove.
Currently little is known about the pathogenesis of this blood-induced cartilage damage, although several mechanisms have been proposed. It has been suggested that accumulation of iron from red blood cells (RBCs), ie, hemosiderine, in synovial tissue results in the production of proteolytic enzymes. Also the production of proinflammatory cytokines released by synoviocytes and recruited phagocytes are thought to be primarily responsible for cartilage damage and joint destruction. 6,7 However, it became clear that, although these processes do contribute significantly to joint damage after repeated bleeding as in hemophilia, they are not the first to occur. 2-4
We showed that a short intra-articular bleeding episode in vivo or a short exposure of cartilage to blood in vitro induces lasting degenerative changes in cartilage independent of synovial inflammation and proinflammatory cytokine production. 4,8 Synthesis and release of matrix components such as proteoglycans are inhibited by blood in a concentration-dependent manner. It seems that these changes are the result of the combined presence of mononuclear cells (MNCs) and RBCs, whereas both cell types individually do not result in lasting changes. 3,8 The changes in cartilage after a relatively short exposure to blood were degenerative in nature and showed similarities to osteoarthritis, ie, a centrifugal pattern of cartilage damage, loss of cartilage matrix components, as well as chondrocyte clustering. 2 Although primarily degenerative in nature, the exact mechanisms behind the induction of these long-lasting alterations in chondrocyte activity and cartilage damage are not yet understood.
Apoptosis in chondrocytes has been shown to adversely affect cartilage matrix turnover and chondrocyte apoptosis is associated with osteoarthritis. 9-11 Similar adverse degenerative changes in cartilage matrix turnover are also observed after blood exposure. This suggests chondrocyte apoptosis might be involved in the prolonged disturbed cartilage matrix turnover after joint hemorrhage.
Therefore we evaluated the hypothesis that exposure of cartilage to blood results within days in chondrocyte apoptosis, without the interference of synovial inflammation. We tested this in an in vitro co-culture system of human cartilage with blood.
Materials and Methods
Cartilage Culture Technique
Healthy full-thickness articular cartilage tissue was obtained postmortem from human humoral heads within 24 hours after death of the donor. The donors (n = 18; mean age, 69.8 ± 14.3 years; 12 males and 6 females) had no known history of joint disorders. Macroscopic analysis revealed completely intact articular cartilage that previously has been shown to represent healthy cartilage. 12 When histology was performed in our experiments (see below), data corroborated that the cartilage used was intact and healthy. Slices of cartilage were cut aseptically from the articular surface, excluding the underlying bone, and kept in phosphate-buffered saline (PBS, pH 7.4). Cartilage was treated and handled as described. 12 Briefly, within 1 hour of dissection the slices were cut into square pieces and weighed aseptically (range, 5.0 to 15.0 mg) and each sample was individually put into culture in 96-well round-bottom microtiter plates (200 μl of culture medium, 5% CO2 in air, 37°C, pH 7.4). The culture medium consisted of Dulbecco’s modified Eagle’s medium supplemented with glutamine (2 mmol/L), penicillin (100 IU/ml), streptomycin sulfate (100 μg/ml), ascorbic acid (85 μmol/L), and 10% heat-inactivated pooled human male AB+ serum.
Preparation of Blood (Components)
For each individual experiment fresh blood from healthy donors (n = 12; mean age, 30.0 ± 7.1 years; six males and six females) was collected into heparinized vacutainer tubes (143 USP Li-heparin/10 ml, Becton Dickinson). To obtain a concentration of 50% v/v whole blood was diluted in culture medium.
MNCs were isolated by density centrifugation, using Ficoll-Paque (Pharmacia, Uppsala, Sweden), and washed twice in ice-cold Dulbecco’s modified Eagle’s medium. RBCs of the same donor were isolated by filtration (PALL, The Netherlands). MNCs and RBCs were redistributed in culture medium to their original cell number before isolation, based on cell counts before and after isolation (Coulter Counter). The amounts of cells used are equivalent to a concentration of 50% v/v whole blood, which previously has been proven to evoke the same lasting changes in cartilage matrix turnover. 3
Experimental Design
Cartilage samples were cultured, without a preculture period, for 4 days in the presence of whole blood or MNCs plus RBCs, with or without additions. Inhibition of proteoglycan synthesis was determined as a measure of inhibition of cartilage matrix synthesis. To measure the reversibility of the inhibition, after a 4-day exposure, the cartilage was washed twice (200 μl of culture medium, 45 minutes, under culture conditions) and cultured for an additional 12 days (referred to as recovery period) in the absence of blood cells. The culture medium was changed every 4 days. On both days, day 4 and 16, proteoglycan synthesis was measured. In addition, cartilage samples after 4 and 16 days of culture were fixed in methanol/PBS (6:1) and used for immunohistochemical detection of apoptosis. For each experiment a separate cartilage donor and blood donor was used, the n values represent the number of these experiments.
Proteoglycan Synthesis Inhibition
Proteoglycan synthesis was measured as the rate of sulfate incorporation during the last 4 hours of the 4- and 16-day culture period. At the end of both time points Na235SO4 (37 MBq/ml, NEX-041-H, carrier-free; DuPont, The Netherlands) was used as a tracer and added in 10-μl aliquots (∼74 kBq) to the 200 μl of culture medium. In the case of the 4-day culture, cartilage samples were placed in fresh medium before addition of 35SO42− without blood or isolated cells (to prevent interference of the blood cells with the incorporation rate). Procedures were followed as described previously. 13 In short, after 4 hours, the tissue samples were rinsed twice in ice-cold PBS, digested with 2% papain (Sigma, The Netherlands), and the glycosaminoglycans were precipitated with cetylpyridinium chloride (Sigma). The amount of 35SO42− incorporated was analyzed by liquid scintillation analysis. The 35SO42− incorporation rate was normalized to the specific activity of the medium and is expressed as nmol of sulfate incorporated per hour per gram wet weight of cartilage tissue.
Interleukin (IL)-1β and Tumor Necrosis Factor (TNF)-α Production
Supernatants from control cartilage and cartilage co-cultured with whole blood or MNCs plus RBCs were harvested and rendered cell-free by centrifugation (500 × g, 10 minutes). Concentrations of IL-1β and TNF-α were measured by using an enzyme-linked immunosorbent assay according to the manufacturer’s instructions (Medgenix, The Netherlands). Detection limits were 20 pg/ml for both IL-1β and TNF-α.
Apoptosis Induction
The role of chondrocyte apoptosis during co-culture of cartilage with whole blood or MNCs plus RBCs was investigated by addition of caspase inhibitors. acDEVDcho (0 to 500 μmol/L; BioSource), a caspase-3-specific inhibitor, and zVADfmk (0 to 500 μmol/L; Enzyme Systems Products), a pan-caspase inhibitor, were added during the first 4 days of culture when whole blood or MNCs plus RBCs were present. As a positive control, and to compare the effects of apoptosis on the proteoglycan synthesis, cartilage samples were incubated during the first 4 days of culture with the apoptogen staurosporine (10 μmol/L; BioMol). 14
For immunohistochemical detection of apoptotic chondrocytes, human cartilage samples harvested after 4 and 16 days of (co)culture, were fixed in methanol/PBS (6:1) for 3 days at −20°C. Fixed samples were dehydrated with absolute methanol, prepared with xylene, and embedded in paraffin. Sections (4 μm) were cut and deparaffinized. Apoptotic chondrocytes were either detected with anti-single-strand DNA monoclonal antibody (clone F7–26/apostain; Alexis Corporation, The Netherlands) or by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL; Roche, Germany). Both techniques had hematoxylin counterstaining to detect total DNA content at the chondrocyte locations. The amount of red-positive cells was calculated as a percentage of the total amount of chondrocytes per slice, excluding empty lacunae.
The histological sections used for ssDNA staining were prepared as described before. 15 Briefly, cartilage sections were rehydrated in PBS, followed by incubation with PBS (with 5 mmol/L of MgCl2) containing 0.2% Triton X-100 for 5 minutes. After transfer of the slides in plastic tubes containing PBS supplemented with 5 mmol/L of MgCl2, the slides were heated in a water bath at 99°C for 7 minutes to denature unstable DNA. Immediately after heating, the sections were placed in tubes containing ice-cold PBS. The sections were incubated with the anti-ssDNA monoclonal antibody and stained with alkaline phosphatase conversion.
For the TUNEL assay slices were rehydrated in PBS and slides were placed in 200 ml of 0.1 mol/L citrate buffer (pH 6), heated in a microwave oven (750 W, 1 minute) and rapidly cooled by immediately adding 80 ml of distilled water (room temperature). Slides were transferred in PBS and preincubated with 3% bovine serum albumin/Tris-HCl (pH 7.5) and normal bovine serum (20% v/v). Then slides were rinsed with PBS at room temperature and TUNEL mixture was applied during 1 hour, subsequently sheep anti-fluorescein isothiocyanate-alkaline phosphatase antibody was added, which converted alkaline phosphatase-substrate into red detectable spots.
Chondrocyte Apoptosis and Inhibition of Proteoglycan Synthesis
In a separate set of experiments the effect of both caspase inhibitors was tested on cartilage explants exposed to blood and MNCs plus RBCs, quantifying in the same experiment apoptosis and inhibition of proteoglycan synthesis. Conditions and techniques were identical as described above. The concentrations of acDEVDcho and zVADfmk were 500 μmol/L.
Calculations and Statistical Analyses
Each experiment in which proteoglycan synthesis was measured was performed with cartilage of a single donor with at least 10 tissue samples for each culture condition, each taken at random and handled individually. The mean value of the 10 explants was taken as representative for that condition per experiment. For the co-cultures of cartilage with MNCs plus RBCs repeated experiments were performed with cartilage and blood cells of three different donors of which mean values (±SEM) are given. Previous experiments have shown that the effects of these MNCs plus RBCs are similar to that of whole blood. Nevertheless, co-cultures of cartilage with whole blood were performed to verify the effects of the isolated MNCs plus RBCs. For these cultures averages of the 10 cartilage samples of a single experiment with intra-assay variation (±SEM) are given.
For histological examinations, four slices of eight consecutive slices, taken at approximately a quarter of the edge of each tissue sample, were scored by two independent observers blind to the culture condition of the cartilage. Of each culture condition from each cartilage donor the average values were considered representative for that experiment and used for statistical calculation. Experiments were performed with cartilage and blood cells of three separate donors. Mean values of these three experiments (±SEM) are given.
For the combination experiments (the effects of caspase inhibitors on proteoglycan synthesis and number of apoptotic cells) identical procedures and calculations were used. Mean values (±SEM) for four analyses using cartilage of two different donors are given.
Differences between treatments were statistically analyzed using a nonparametric test for independent samples (Mann-Whitney), using SPSS v9.0 software. P values ≤0.05 were considered statistically significant.
Results
Inhibition of Proteoglycan Synthesis and Reversibility of This Effect
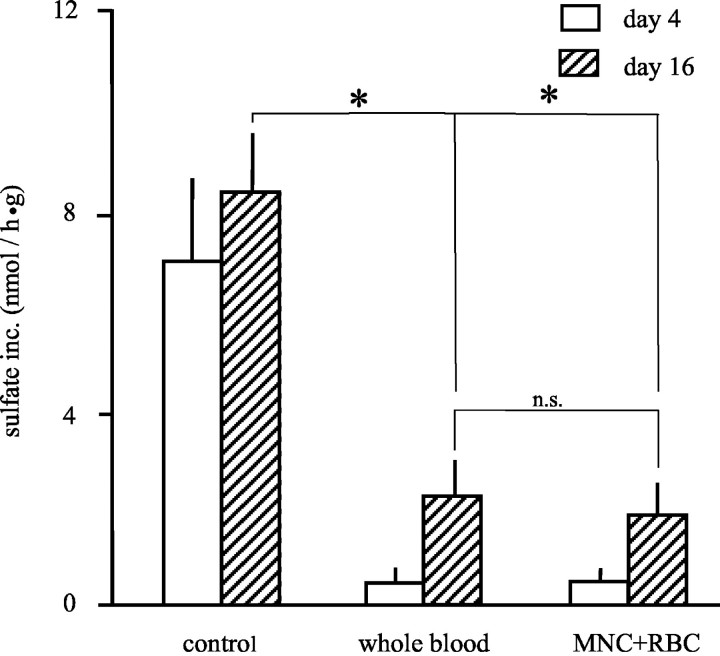
Proteoglycan synthesis was almost completely inhibited after a 4-day in vitro exposure of cartilage to 50% v/v whole blood or equal amounts of isolated MNCs plus RBCs (Figure 1) ▶ . This inhibition of proteoglycan synthesis was still evident (>78% inhibition) after 12 days of recovery (day 16). No significant differences were found between the effects of whole blood and the addition of MNCs plus RBCs added in equivalent amounts as present in whole blood (Figure 1) ▶ . Previous experiments have shown that the addition of the plasma (equivalents of 50% whole blood) or up to 50% serum, did not influence cartilage matrix turnover in any way. 3
Figure 1.
Inhibition of proteoglycan synthesis by whole blood or the combination of MNCs plus RBCs. Sulfate incorporation was used as a measure of proteoglycan synthesis in cartilage. Mean values ± SEM (n = 3 experiments, P < 0.05). Open bars represent the proteoglycan synthesis after 4 days of either controls, co-cultures with 50% whole blood, or MNCs plus RBCs. The figure shows that there is 92% inhibition of proteoglycan synthesis compared to the control synthesis (*, P < 0.05). Shaded bars represent the proteoglycan synthesis after 12 days of additional culture without additions to determine recovery of synthesis by chondrocytes. Proteoglycan synthesis was for both the 50% whole blood and MNC plus RBC co-cultures significantly inhibited (up to 78%; *, P < 0.05). No significant differences in proteoglycan synthesis were found between co-cultures with 50% whole blood and MNCs and RBCs on both days 4 and day 16 (n.s.).
The Role of IL-1β and TNF-α
IL-1β and TNF-α are the most important inflammatory mediators in cartilage destruction. The levels of these cytokines, to establish their role in the induction of prolonged inhibition of proteoglycan synthesis after blood exposure, were measured in supernatants of the co-cultures. Slightly enhanced levels of both proinflammatory cytokines were found at day 4 (Table 1) ▶ . After washing and subsequent culture in the absence of additions, cytokine levels at day 16 were below detection levels. The levels observed after 4 days are known to reduce proteoglycan synthesis. However, these levels have been reported not to be able to induce prolonged irreversible inhibition of proteoglycan synthesis. 8,16,17 Addition of similar amounts of IL-1β and TNF-α as measured on blood cell addition results in comparable inhibition of proteoglycan synthesis. Refreshment of medium, and thus removing the cytokines, results in complete normalization of proteoglycan synthesis (data not shown).
Table 1.
Levels of Proinflammatory Cytokines IL-1β and TNF-α in Supernatant of Co-Cultures of MNCs Plus RBCs or 50% Whole Blood with Human Cartilage
| Control | 50% Whole blood | MNCs plus RBCs | |
|---|---|---|---|
| IL-1β (pg/ml) | <20 | 234 ± 97 | 757 ± 267 |
| TNF-α (pg/ml) | <20 | 245 ± 155 | 619 ± 287 |
Proinflammatory cytokine production, ie., IL-1β and TNF-α, during the first 4 days of co-culture of human cartilage with either 50% whole blood or MNCs plus RBCs. Both IL-1β and TNF-α were elevated after 4 days of co-culture. The levels are all significantly increased compared to control cartilage culture (n = 4, both cartilage and blood donors; P < 0.05), but only to levels that are known to inhibit proteoglycan synthesis in a reversible manner.
Prevention of Apoptosis Prevents Inhibition of Proteoglycan Synthesis
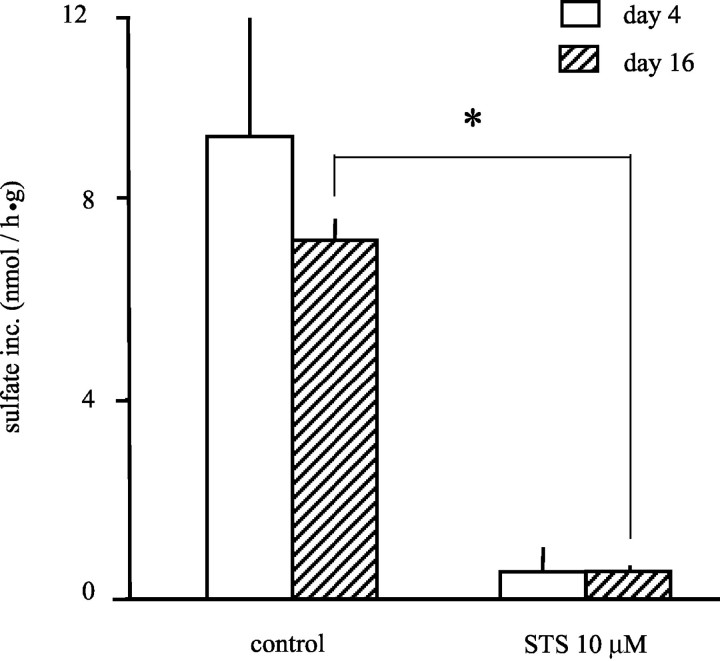
Cartilage cultured in the presence of the apoptogen staurosporine during the first 4 days showed a concentration-dependent inhibition of proteoglycan synthesis (data not shown). At a concentration of 10 μmol/L, staurosporine could inhibit proteoglycan synthesis equal to 4 days of co-culture with whole blood or MNCs plus RBCs. A subsequent culture of 12 days without staurosporine demonstrated lasting inhibition of proteoglycan synthesis (Figure 2) ▶ .
Figure 2.
Inhibition of proteoglycan synthesis after induction of apoptosis through addition of staurosporine (STS). Sulfate incorporation was used as a measure of proteoglycan synthesis in cartilage. The open bars represent the synthesis after 4 days of cartilage culture, in the absence or presence of staurosporine (STS, 10 μmol/L). Shaded bars represent the proteoglycan synthesis in cartilage cultured for an additional 12 days without additions to determine the recovery of synthesis by chondrocytes. Cartilage cultured with STS showed an irreversible 92% inhibition of proteoglycan synthesis through induction of apoptosis (n = 3 experiments; *, P < 0.05).
The inhibition of proteoglycan synthesis seen after 4 days of culture with MNCs plus RBCs was not significantly influenced by the addition of the caspase-3 inhibitor acDEVDcho (Figure 3A) ▶ . Addition of acDEVDcho during the first 4 days, however, reversed the inhibition of proteoglycan synthesis statistically significantly in the highest concentration, as measured after 12 days of recovery (at 500 μmol/L, >39% of control cultures; *, P < 0.05). Almost identical results were found when this caspase inhibitor was added to cartilage cultured with whole blood (Figure 3B) ▶ . At 500 μmol/L a significant although not complete recovery of proteoglycan synthesis was observed (>51% of control cultures; *, P < 0.02). Values with acDEVDcho in the highest concentration in the absence of blood or MNCs plus RBCs had no significant effect on proteoglycan synthesis and were used as control values, put on 100%.
Figure 3.
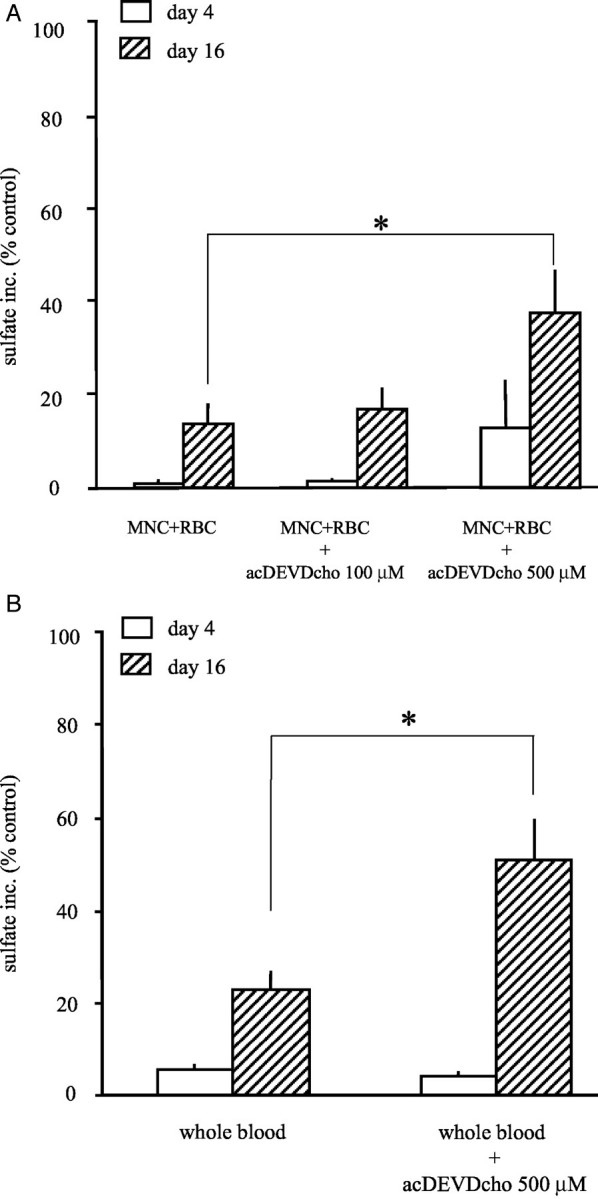
A: Reversibility of the proteoglycan synthesis by preventing apoptosis with acDEVDcho, a caspase-3 inhibitor. The relative sulfate incorporation compared to control cultures (percent of control synthesis ± SEM) is shown. Cartilage samples were co-cultured in the presence of MNCs plus RBCs and acDEVDcho (0 to 500 μmol/L). Open bars show the absence of an effect of acDEVDcho on day 4. Hatched bars show the statistically significant recovery of proteoglycan synthesis on day 16 with increasing concentrations of acDEVDcho (up to 39% of control synthesis, n = 3 experiments; *, P < 0.05). B: Reversibility of the proteoglycan synthesis in cartilage after exposure to 50% whole blood by preventing chondrocyte apoptosis with acDEVDcho, a caspase-3 inhibitor. The relative sulfate incorporation compared to control culture (percent of control synthesis ± SEM) is shown. Cartilage samples were co-cultured in the presence of 50% whole blood and acDEVDcho (500 μmol/L). The open bars show the absence of an effect of acDEVDcho on day 4. Hatched bars show the statistically significant recovery of proteoglycan synthesis on day 16 (up to 51% of control synthesis; *, P < 0.05).
Addition of the pan-caspase inhibitor zVADfmk to the cartilage MNC plus RBC co-cultures was more effective in this respect. At 500 μmol/L resulting in >78% synthesis of control cultures (Figure 4A ▶ ; *, P < 0.01). The recovery of proteoglycan synthesis by adding zVADfmk was concentration-dependent (Figure 4A) ▶ . When added to the cartilage whole blood co-cultures effects were similar; at 500 μmol/L a recovery up to >83% synthesis of control culture was reached (Figure 4B ▶ ; *, P < 0.01), a synthesis rate not significantly different from control (=100%) anymore. For the control zVADfmk was used in the highest concentration in the absence of blood or MNCs plus RBCs, which had no significant effect on proteoglycan synthesis.
Figure 4.
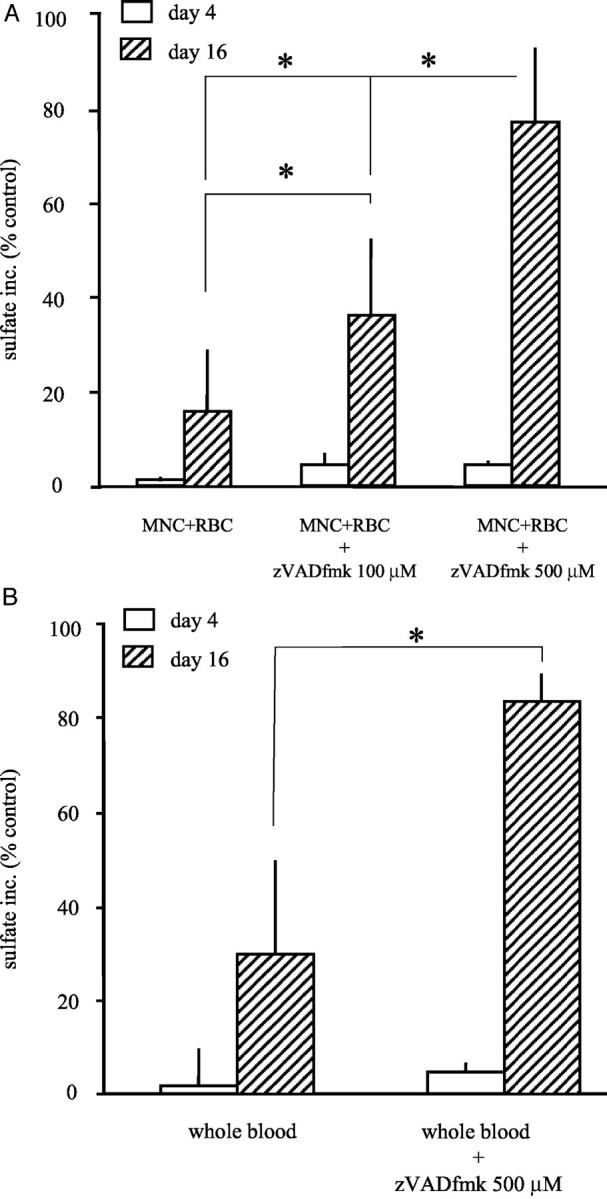
A: Reversibility of the proteoglycan synthesis by preventing apoptosis with zVADfmk, a pan-caspase inhibitor. The relative sulfate incorporation compared to control cultures (percent of control synthesis ± SEM) is shown. Cartilage samples were co-cultured in the presence of MNCs plus RBCs and zVADfmk (0 to 500 μmol/L). Open bars show the absence of an effect of zVADfmk on day 4. Hatched bars show the statistically significant recovery of proteoglycan synthesis on day 16 with increasing concentrations of zVADfmk (up to 78% of control synthesis, n = 3 experiments; *, P < 0.05). B: Reversibility of the proteoglycan synthesis in cartilage after exposure to 50% whole blood by preventing chondrocyte apoptosis with zVADfmk, a pan-caspase inhibitor. The relative sulfate incorporation compared to control culture (percent of control synthesis ± SEM) is shown. Cartilage samples were co-cultured in the presence of 50% whole blood and zVADfmk (500 μmol/L). The open bars show the absence of an effect of zVADfmk on day 4. Hatched bars show the statistically significant recovery of proteoglycan synthesis on day 16 (up to 83% of control synthesis; *, P < 0.05).
Interestingly, the use of both caspase inhibitors had no effect on the inhibition of proteoglycan synthesis obtained directly after exposure to blood or blood components (namely at day 4); only recovery from the exposure to blood during the following 12 days in culture without blood was found.
Blood Exposure Induced Chondrocyte Apoptosis
Immunohistochemical staining of cartilage after a 4-day exposure to MNCs plus RBCs or whole blood with the anti-ssDNA monoclonal antibody as an early marker of apoptosis revealed a significant increase of apoptotic chondrocytes after 4 days of co-culture (Table 2) ▶ . Apoptosis was equally distributed throughout the entire cartilage sample. After 12 days of recovery, the number of ssDNA-positive cells observed were not statistically significantly different between the control cultures and the blood-exposed cartilage anymore. As shown in Figure 5A ▶ , slices of control cartilage from a 4-day culture period showed hematoxylin staining and very little staining of ssDNA. When cartilage was co-cultured during 4 days in the presence of 50% v/v whole blood or MNCs plus RBCs (50% equivalents of whole blood) the cartilage slices showed a twofold to threefold increase in apoptotic ssDNA-positive cells (Table 2 ▶ ; both P < 0.05 and illustrated by Figure 5, B and C ▶ , respectively).
Table 2.
Quantification of Anti-ssDNA and TUNEL Staining in Apoptotic Chondrocytes in Healthy Control Cartilage and Healthy Cartilage Incubated with 50% Whole Blood or Isolated Mononuclear Cells Plus Red Blood Cells
| ssDNA | TUNEL | |||
|---|---|---|---|---|
| Day 4 | Day 16 | Day 4 | Day 16 | |
| Control | 3 ± 1% | 7 ± 1% | 18 ± 5% | 14 ± 4% |
| MNCs plus RBCs | 7 ± 2%* | 7 ± 1% | 30 ± 5%* | 36 ± 6%* |
| 50% Whole blood | 8 ± 2%* | 9 ± 2% | 33 ± 2%* | 35 ± 5%* |
* A statistically significant increase in number of apoptotic chondrocytes in cartilage that has been exposed to either 50% whole blood or MNCs plus RBCs during 4 days and after a 12-day recovery period (P < 0.05). Cartilage tissue (n = 5 donors) was scored by two independent observers, blind to the culture condition of cartilage. At day 16, no significant effect of blood or MNCs plus RBCs was found on ssDNA staining.
Figure 5.
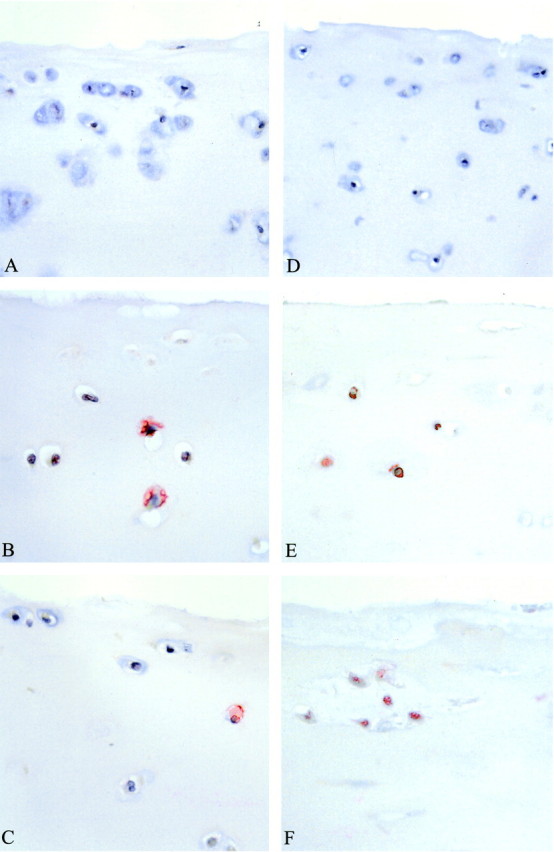
Representative micrographs of immunohistologically stained human cartilage tissue (original magnifications, ×200). The left panels show representatives of cartilage stained for ssDNA after 4 days of co-culture, the right panels are stained with TUNEL after 12 days of recovery period without any additions to the culture medium. A: Control cartilage after 4 days of culture without blood or isolated blood cells. B: Increased staining for ssDNA directly after co-culture of human cartilage with 50% whole blood (day 4). C: Increased staining for ssDNA directly after co-culture of human cartilage with MNCs plus RBCs (day 4). D: Control cartilage stained by TUNEL after a 12-day recovery period showing no apoptotic chondrocytes. E: Increased staining by TUNEL after 12 days of recovery of human cartilage that was co-cultured for 4 days with 50% whole blood. F: Increased staining by TUNEL after 12 days of recovery of human cartilage that was co-cultured for 4 days with MNCs plus RBCs. For average values of several experiments see Table 2 ▶ .
The TUNEL staining method reflects a more advanced stage apoptosis. After 12 days of recovery, on day 16 the increase in apoptotic chondrocytes was still evident (Table 2) ▶ . By using this staining an increase of apoptotic chondrocytes could also be found at day 4, for both the whole blood as well as the MNC plus RBC co-cultured cartilage samples (both P < 0.05). As shown in Figure 5D ▶ , slices of control cartilage from a 16-day culture period showed hematoxylin staining and little TUNEL staining. When cartilage was co-cultured during 4 days in the presence of 50% v/v whole blood or MNCs plus RBCs and an additional 12-day recovery was allowed, the cartilage slices showed a significant increase in TUNEL-positive cells (Figure 5, E and F) ▶ . Quantification of apoptosis revealed that there was an increase from 14% (control cartilage) up to 36% for both whole blood co-cultures as well as MNC plus RBC co-cultures, respectively (Table 2 ▶ ; both P < 0.05).
Chondrocyte Apoptosis Inhibits Proteoglycan Synthesis
When in the same experiment proteoglycan synthesis and chondrocyte apoptosis were measured, similar results as for the separated experiments were observed (Table 3) ▶ . A strong inhibition in proteoglycan synthesis induced by MNCs plus RBCs or by blood (Table 3 ▶ , left column) was accompanied by a significant increase in the percentage of apoptotic cells as measured by ssDNA and TUNEL staining at day 4. After a 12-day recovery period proteoglycan synthesis was still significantly inhibited and chondrocyte apoptosis still evident as measured by TUNEL staining. However, the number of ssDNA-stained cells observed was not statistically significant different from controls anymore (Table 3 ▶ , ssDNA, day 16; see also Table 2 ▶ ). When the caspase inhibitors (DEVD or zVAD) were added during exposure to blood or MNCs plus RBCs, proteoglycan synthesis could partly be restored during the successive 12-day recovery period. Because inhibition of proteoglycan synthesis by cytokines is present during the first 4 days of culture no significant effects of the caspase inhibitors on day 4 were observed. However, the ability to regain matrix synthesis was accompanied by a significantly lower percentage of ssDNA- or TUNEL-positive chondrocytes at day 4 and still evident at day 16 when measured by TUNEL staining (Table 3 ▶ , right column). Effects in this respect were not different between MNC plus RBC-exposed cartilage or whole blood-exposed cartilage.
Table 3.
Inhibition of Proteoglycan Synthesis and Percentage of Apoptotic Cells under the Influence of MNCs plus RBCs and Whole Blood with or without Caspase Inhibitors
| PG synthesis | ssDNA | TUNEL | ||||
|---|---|---|---|---|---|---|
| Day 4 | Day 16 | Day 4 | Day 16 | Day 4 | Day 16 | |
| Control | 15.5 ± 5.0 | 14.1 ± 6.0 | 3 ± 1 | 5 ± 2 | 12 ± 2 | 16 ± 1 |
| MNC + RBC | 1.2 ± 0.1* | 4.5 ± 2.4* | 8 ± 2* | 5 ± 2 | 33 ± 1* | 28 ± 3* |
| +DEVD | 1.0 ± 0.5 | 8.9 ± 4.6† | 4 ± 1† | 1 ± 1 | 20 ± 3† | 18 ± 1† |
| +zVAD | 1.4 ± 0.6 | 10.4 ± 6.7† | 4 ± 1† | 2 ± 1 | 16 ± 3† | 18 ± 1† |
| Whole blood | 0.8 ± 0.1* | 3.5 ± 1.7* | 8 ± 2* | 11 ± 4 | 38 ± 2* | 26 ± 3* |
| +DEVD | 0.8 ± 0.2 | 6.1 ± 2.8† | 5 ± 1† | 3 ± 1 | 26 ± 4† | 21 ± 2† |
| +zVAD | 0.7 ± 0.2 | 6.3 ± 2.8† | 4 ± 1† | 3 ± 1 | 25 ± 3† | 26 ± 3† |
Cartilage tissue of two donors was scored each by two independent observers, blind to the culture condition of cartilage.
*A statistically significant decrease in proteoglycan synthesis and a significant increase in number of apoptotic chondrocytes in cartilage that has been exposed for 4 days to either 50% whole blood or MNCs plus RBCs, at day 4 and after a 12-day recovery period (P < 0.05). Note that at day 16, no significant effect of blood or MNCs plus RBCs was found on ssDNA staining.
†A statistically significant recovery of proteoglycan synthesis (increase) and percentage of apoptotic chondrocyte (decrease).
Discussion
Several bleeding episodes as in hemophilia result in permanent joint damage. Furthermore, we also know that a single bleeding episode as a result of sports injury or after trauma, or the first bleeding during hemophilia, might induce long-lasting changes in cartilage matrix turnover. Throughout the years such changes are expected to lead to clinically diagnosable joint damage. 3,4 Unfortunately, little is known about the mechanisms that are responsible for these blood-induced adverse alterations in cartilage matrix turnover. Knowledge about these mechanisms is of importance in view of treatment of joint hemorrhages.
It has previously been shown that the combination of MNCs plus RBCs, as present in whole blood, is responsible for the effect as observed for whole blood. 3 The present results confirm these findings. By what mechanism these two cell populations influence chondrocyte activity, however, is unknown and is subject of this study.
It was hypothesized that the permanent loss of chondrocyte activity as a result of blood or MNC plus RBC exposure is the result of chondrocyte apoptosis. When chondrocytes enter apoptosis, cartilage matrix turnover and possible repair activity on damage is impaired. This will inevitably lead to a disturbed integrity of the cartilage matrix and ultimately results in degradation of cartilage as seen in osteoarthritis. 2,11,14
Our in vitro results show that when cartilage is co-cultured with whole blood or isolated MNCs plus RBCs proteoglycan synthesis is inhibited. Similar inhibition is found when cartilage is incubated with staurosporine, which is a direct stimulus to the induction of chondrocyte apoptosis. 18,19 Staurosporine is a protein kinase inhibitor that which ultimately prevents the action of topoisomerase II and hence induces permanent DNA defects. 20
When the specific caspase-3 inhibitory peptide, acDEVDcho, was added during a 4-day co-culture of cartilage tissue with whole blood or MNCs plus RBCs, the cartilage proteoglycan synthesis as measured after 12 days of recovery, could partially be restored. This effect was even more pronounced when the nonselective caspase inhibitor zVADfmk was used, suggesting a protective effect on chondrocytes by preventing apoptosis and with that increased survival rate of chondrocytes. The fact that zVADfmk is more effective than the specific caspase-3 inhibitory peptide acDEVDcho may be because of the fact that zVADfmk acts more upstream in the caspase cascade (ie, caspase-8 and -9), thereby preventing broad activation of the caspase cascade. By preventing the activation of upstream caspases, zVADfmk also prevents the activation of procaspase-3 into caspase-3. 21
These biochemical data suggest chondrocyte apoptosis after blood exposure, which is confirmed by immunohistochemical staining with ssDNA monoclonal antibody and TUNEL staining. Single-strand DNA staining, namely DNA instability, was found shortly after blood exposure (day 4). At day 16 the effect of blood exposure was not represented by an increase in number of ssDNA-positive cells anymore. There was only a culture-induced increase in early apoptotic cells as observed in the control samples. The TUNEL assay on the other hand was able to detect DNA damage, namely DNA strand breaks, after 12 days of recovery (day 16), and was significantly enhanced in the blood and MNC plus RBC-exposed samples.
Interestingly, at day 4, adding caspase inhibitors could not prevent proteoglycan synthesis inhibition. At day 4 the apoptosis was already evident as observed by immunohistochemical staining. This suggests an additional pathway involved in the primary inhibition of proteoglycan synthesis after blood exposure, as observed at day 4. Despite prevention of apoptosis by caspase inhibitors, there are still increased levels of proinflammatory cytokines, ie, IL-1β and TNF-α, produced in the culture system during the first 4 days of co-culture. It is known that IL-1β and TNF-α can inhibit cartilage matrix synthesis at very low concentrations (<100 pg/ml). 22,23 However, it is described that the levels of IL-1β and TNF-α as measured in our co-culture system do not lead to lasting adverse changes, ie, permanent inhibition of cartilage matrix synthesis during 4 days of culture. 8,16,17,24 This implicates that production of both cytokines, to concentrations reached in our co-culture system, does not lead to apoptosis of chondrocytes, which is consistent with other studies. 25 It does not rule out the possibility that IL-1β and TNF-α lead to breakdown of cartilage matrix, but it shows that these cytokines do not induce permanent loss of chondrocyte activity as observed after a 12-day recovery period on a 4-day exposure to blood. The fact that addition of caspase inhibitors prevents the increase in numbers of apoptotic chondrocytes, accompanied by a recovery of proteoglycan synthesis inhibition, strongly suggests the association between apoptosis and persisting inhibition of cartilage matrix synthesis.
These data do suggest that apoptosis plays an important role in the pathophysiology of blood-induced cartilage damage, however, the follow-up is only 16 days. But because of the observed apoptosis and the low or absent proliferation of chondrocytes in adult articular cartilage, it is to be expected that the inhibition of proteoglycan synthesis is long-lasting. 26 In time, it could be that the remaining chondrocytes take over the synthetic activity of the lost chondrocytes. If so, this could result in normalization of proteoglycan synthesis. But even then the potency of cartilage repair will remain impaired, unless chondrocyte proliferation takes place.
Reconsideration of therapeutic interventions for intra-articular bleeding in hemophiliacs may be indicated. 27,28 The present data suggest that new treatment strategies to prevent joint destruction may be directed toward prevention of apoptosis. The use of nonselective caspase inhibitors might be an approach in this respect. In an in vivo experimental animal model for myocardial infarct such a strategy, ie, the use of nonselective caspase inhibitors, has been demonstrated to reduce the infarct size. 29 This finding might initiate studies to the effectiveness of treatment strategies with caspase inhibitors, treating blood-induced joint damage in an in vivo experimental setting as well.
In conclusion, apoptosis occurs in chondrocytes in vitro after a 4-day exposure to 50% whole blood or an equivalent amount of MNCs plus RBCs. This would imply that the induction of apoptosis in vivo after a single bleeding episode impairs cartilage repair activity and thus will contribute to cartilage damage in time.
Footnotes
Address reprint requests to F. P. J. G. Lafeber, Rheumatology and Clinical Immunology, F02.127, University Medical Center Utrecht, PO Box 85500, 3508 GA Utrecht, The Netherlands. E-mail: f.lafeber@azu.nl.
Supported by a grant from Aventis Behring.
References
- 1.Madhok R, Bennett D, Sturrock RD, Forbes CD: Mechanisms of joint damage in an experimental model of hemophilic arthritis. Arthritis Rheum 1988, 31:1148-1155 [DOI] [PubMed] [Google Scholar]
- 2.Roosendaal G, van Rinsum AC, Vianen ME, van den Berg HM, Lafeber FP, Bijlsma JW: Haemophilic arthropathy resembles degenerative rather than inflammatory joint disease. Histopathology 1999, 34:144-153 [DOI] [PubMed] [Google Scholar]
- 3.Roosendaal G, Vianen ME, van den Berg HM, Lafeber FP, Bijlsma JW: Cartilage damage as a result of hemarthrosis in a human in vitro model. J Rheumatol 1997, 24:1350-1354 [PubMed] [Google Scholar]
- 4.Roosendaal G, TeKoppele JM, Vianen ME, van den Berg HM, Lafeber FP, Bijlsma JW: Blood-induced joint damage: a canine in vivo study. Arthritis Rheum 1999, 42:1033-1039 [DOI] [PubMed] [Google Scholar]
- 5.Myers SL, Brandt KD, O’Connor BL, Visco DM, Albrecht ME: Synovitis and osteoarthritic changes in canine articular cartilage after anterior cruciate ligament transection. Effect of surgical hemostasis. Arthritis Rheum 1990, 33:1406-1415 [DOI] [PubMed] [Google Scholar]
- 6.Mainardi CL, Levine PH, Werb Z, Harris ED, Jr: Proliferative synovitis in hemophilia: biochemical and morphologic observations. Arthritis Rheum 1978, 21:137-144 [DOI] [PubMed] [Google Scholar]
- 7.Stein H, Duthie RB: The pathogenesis of chronic haemophilic arthropathy. J Bone Joint Surg [Br ] 1981, 63B:601-609 [DOI] [PubMed] [Google Scholar]
- 8.Roosendaal G, Vianen ME, Marx JJ, van den Berg HM, Lafeber FP, Bijlsma JW: Blood-induced joint damage: a human in vitro study. Arthritis Rheum 1999, 42:1025-1032 [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto S, Ochs RL, Komiya S, Lotz M: Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum 1998, 41:1632-1638 [DOI] [PubMed] [Google Scholar]
- 10.Mollenhauer J, Mok MT, King KB, Gupta M, Chubinskaya S, Koepp H, Cole AA: Expression of anchorin CII (cartilage annexin V) in human young, normal adult, and osteoarthritic cartilage. J Histochem Cytochem 1999, 47:209-220 [DOI] [PubMed] [Google Scholar]
- 11.Kim HA, Lee YJ, Seong SC, Choe KW, Song YW: Apoptotic chondrocyte death in human osteoarthritis. J Rheumatol 2000, 27:455-462 [PubMed] [Google Scholar]
- 12.Lafeber FP, Vander Kraan PM, Van Roy JL, Huber-Bruning O, Bijlsma JW: Articular cartilage explant culture; an appropriate in vitro system to compare osteoarthritic and normal human cartilage. Connect Tissue Res 1993, 29:287-299 [DOI] [PubMed] [Google Scholar]
- 13.Lafeber FP, van der Kraan PM, van Roy HL, Vitters EL, Huber-Bruning O, van den Berg WB, Bijlsma JW: Local changes in proteoglycan synthesis during culture are different for normal and osteoarthritic cartilage. Am J Pathol 1992, 140:1421-1429 [PMC free article] [PubMed] [Google Scholar]
- 14.Lotz M, Hashimoto S, Kuhn K: Mechanisms of chondrocyte apoptosis. Osteoarthritis Cartilage 1999, 7:389-391 [DOI] [PubMed] [Google Scholar]
- 15.Frankfurt OS, Robb JA, Sugarbaker EV, Villa L: Monoclonal antibody to single-stranded DNA is a specific and sensitive cellular marker of apoptosis. Exp Cell Res 1996, 226:387-397 [DOI] [PubMed] [Google Scholar]
- 16.Nietfeld JJ, Wilbrink B, den Otter W, Huber J, Huber-Bruning O: The effect of human interleukin 1 on proteoglycan metabolism in human and porcine cartilage explants. J Rheumatol 1990, 17:818-826 [PubMed] [Google Scholar]
- 17.Wilbrink B, Nietfeld JJ, den Otter W, Van Roy JL, Bijlsma JW, Huber-Bruning O: Role of TNF alpha, in relation to IL-1 and IL-6 in the proteoglycan turnover of human articular cartilage. Br J Rheumatol 1991, 30:265-271 [DOI] [PubMed] [Google Scholar]
- 18.Jacobsen MD, Weil M, Raff MC: Role of Ced-3/ICE-family proteases in staurosporine-induced programmed cell death. J Cell Biol 1996, 133:1041-1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nuttall ME, Nadeau DP, Fisher PW, Wang F, Keller PM, DeWolf WE, Jr, Goldring MB, Badger AM, Lee D, Levy MA, Gowen M, Lark MW: Inhibition of caspase-3-like activity prevents apoptosis while retaining functionality of human chondrocytes in vitro. J Orthop Res 2000, 18:356-363 [DOI] [PubMed] [Google Scholar]
- 20.Lassota P, Singh G, Kramer R: Mechanism of topoisomerase II inhibition by staurosporine and other protein kinase inhibitors. J Biol Chem 1996, 271:26418-26423 [DOI] [PubMed] [Google Scholar]
- 21.Earnshaw WC, Martins LM, Kaufmann SH: Mammalian caspases: structure, activation, substrates and functions during apoptosis. Annu Rev Immunol 1999, 68:383-424 [DOI] [PubMed] [Google Scholar]
- 22.Pratta MA, Di Meo TM, Ruhl DM, Arner EC: Effect of interleukin-1-beta and tumor necrosis factor-alpha on cartilage proteoglycan metabolism in vitro. Agents Actions 1989, 27:250-253 [DOI] [PubMed] [Google Scholar]
- 23.Dingle JT, Horner A, Shield M: The sensitivity of synthesis of human cartilage matrix to inhibition by IL-1 suggests a mechanism for the development of osteoarthritis. Cell Biochem Funct 1991, 9:99-102 [DOI] [PubMed] [Google Scholar]
- 24.Dingle JT, Parker M: NSAID stimulation of human cartilage matrix synthesis. Clin Drug Invest 1997, 14:353-362 [Google Scholar]
- 25.Fischer BA, Mundle S, Cole AA: Tumor necrosis factor-alpha induced DNA cleavage in human articular chondrocytes may involve multiple endonucleolytic activities during apoptosis. Microsc Res Tech 2000, 50:236-242 [DOI] [PubMed] [Google Scholar]
- 26.Buckwalter JA, Mankin HJ: Articular cartilage: tissue design and chondrocyte-matrix interactions. Instr Course Lect 1998, 47:477-486 [PubMed] [Google Scholar]
- 27.Fischer K, Van Der Bom JG, Mauser-Bunschoten EP, Roosendaal G, Beek FJ, de Kleijn P, Grobbee DE, van den Berg HM: Endogenous clotting factor activity and long-term outcome in patients with moderate haemophilia. Thromb Haemost 2000, 84:977-980 [PubMed] [Google Scholar]
- 28.van den Berg HM, Fischer K, Mauser-Bunschoten EP, Beek FJ, Roosendaal G, Van Der Bom JG, Nieuwenhuis HK: Long-term outcome of individualized prophylactic treatment of children with severe haemophilia. Br J Haematol 2001, 112:561-565 [DOI] [PubMed] [Google Scholar]
- 29.Huang JQ, Radinovic S, Rezaiefar P, Black SC: In vivo myocardial infarct size reduction by a caspase inhibitor administered after the onset of ischemia. Eur J Pharmacol 2000, 402:139-142 [DOI] [PubMed] [Google Scholar]