Abstract
Platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31) is a 130-kd member of the immunoglobulin superfamily of proteins, expressed on endothelial cells, leukocytes, and platelets. Antibody-blocking studies have implicated it in modulating leukocyte transmigration and angiogenesis. However, the generation of the PECAM-1 knockout mouse has shown that its function can be compensated for by similarly acting proteins because most acute inflammatory models proceed in a comparable manner in wild-type and knockout animals. We decided to examine the function of PECAM-1 in the chronic process of foreign body inflammation. We show that PECAM-1-deficient mice exhibit attenuated neutrophil infiltration in and around a subcutaneous polyvinyl acetyl implant. Bone marrow engraftment studies indicate that the lack of CD31 expression on the endothelium determines the diminished leukocyte accumulation in the knockout implants. Specifically, we find that decreased angiogenesis (as manifested by lower vessel density, decreased hemoglobin content, and less laminin deposition) correlates with lower neutrophil accumulation in the knockout animals. This study indicates that the absence of endothelial PECAM-1 results in decreased angiogenesis and therefore in diminished delivery of leukocytes to the foreign body implants.
Platelet endothelial cell adhesion molecule-1 (PECAM-1 or CD31) is a 130-kd single-span, type I transmembrane glycoprotein and a member of the immunoglobulin (Ig) superfamily because of its six extracellular C2 Ig domains. It is expressed on endothelial cells, platelets, neutrophils, monocytes, and subsets of lymphocytes. 1-4 It has been implicated in facilitating leukocyte transmigration through endothelial monolayers, in promoting angiogenesis, in modulating vascular permeability, and in serving as a β-catenin reservoir in endothelial cells. 5-10 Most early in vitro and in vivo studies have used PECAM-1-blocking antibodies to extrapolate its function. The generation of a PECAM-1 knockout (KO) animal has demonstrated the redundancy of its function during development and in transient acute inflammatory models. 11 Clearly, the sudden blocking of a protein with an antibody does not allow the cells time to up-regulate any similarly functioning mechanisms, whereas a developing KO animal may change its phenotype to accommodate for the loss of PECAM-1.
The mechanisms of PECAM-1 functions have not been fully elucidated. PECAM-1 participates in cell-cell adhesion by binding homophilically to Ig domains of other CD31 proteins and by binding heterophilically to glycosaminoglycans and CD38 and integrin αvβ3 in cis. 12-17 PECAM-1 is also a signaling molecule. Its 118-amino acid cytoplasmic domain binds SHP-2, β- and γ-catenin, as well as STAT family members, and thus participates in transduction of signals through the cell. 18-21 Its signaling pathways have been implicated in the processes of apoptosis and endothelial cell migration. 22-24
We decided to use the KO mouse in the chronic inflammatory process of foreign body inflammation. This type of inflammation is a reaction to a sterile and seemingly inert object, such as a prosthetic implant or a catheter cuff in a clinical setting. It is a subtle and most likely a nonspecific type of inflammation, which may result in leukocyte accumulation, neovascularization, and fibrosis around the implant. 25 Because its key players are neutrophils, monocytes/macrophages, and endothelial cells, PECAM-1 could be playing a crucial role in its progression. Additionally, this process can be monitored throughout a number of days, rather than throughout a few hours, as were most inflammation models used to study this protein so far.
Angiogenesis, the formation of new blood vessels from pre-existing ones, can be a beneficial process during development, wound healing, the menstrual cycle, life at high altitudes, and regular exercise. 26,27 It can also be destructive when it occurs as a complication of chronic inflammation, diabetes, or arthritis or when it aids in tumor growth. 27,28 Most models implicating PECAM-1 in the progression of angiogenesis have done so by blocking its function with antibodies. 5,6,9 No differences in angiogenesis have been shown between PECAM-1 wild-type (WT) and KO animals. The KO mice develop a functional vascular system that does not seem different from that of their WT counterparts. 11 Our study demonstrates that angiogenesis activated during a chronic inflammatory process to promote repair is impaired in the KO mice. The decreased neovascularization results in lower blood cell delivery to and accumulation in the KO implants. This study, therefore, indicates that PECAM-1 serves different functions during developmental and inflammatory angiogenic processes. The differences could lie in the sources signaling endothelial cell activation, migration, or organization, and/or in the ability of endothelial cells to up-regulate compensatory mechanisms during development.
Materials and Methods
Cells and Cell Culture
The endothelioma cell lines were a gift from Dr. Britta Engelhardt at the Max-Planck Institute for Vascular Biology, Münster, Germany, and were isolated as described previously. 7,29 PECAM-1-negative (luEnd.PECAM-1.1 KO) cells were isolated from a KO mouse, and PECAM-1-positive (luEnd.PECAM-1.1 RC) cells were isolated from a KO and reconstituted with CD31. A line that was reconstituted with PECAM-1, but lost the protein expression was also used (RCNE). These lines were immortalized by retroviral transduction with polyoma virus middle T-oncogene. They were cultured on tissue culture plastic in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, 1% l-glutamine, 1% nonessential amino acids, 1% sodium pyruvate, 10−5 mol/L β-mercaptoethanol, and 10 U/ml penicillin-streptomycin. One μg/ml of puromycin (Sigma Chemical Co, St. Louis, MO) was added to the PECAM-1-positive cell media.
Mice
C57BL/6 PECAM-1-positive (WT) mice were purchased from Charles River Laboratories (Wilmington, MA). Animals were housed in our facility for periods of 1 to 3 weeks before the start of experiments. PECAM-1-deficient mice (KO) were originally a gift from Dr. Tak Mak (AMGEN Institute, University of Toronto, Toronto, Ontario, Canada) and were generated as described previously. 11 A breeding colony was then maintained at Yale University Animal Resource Center in accordance with established protocols. PECAM-1 expression by the mice was assessed using fluorescence-activated cell sorting (FACS) of peripheral blood as described previously. 29
Polyvinyl Acetyl (PVA) Sponge Implantation
Mice were anesthetized with ether (J.T. Baker Inc., Phillipsburg, NJ), and a 10-mm mid-line incision was made under sterile conditions on the dorsal side. Two air pockets were created on either side of the incision, and two sterile water-moistened PVA sponges (M-Pact Worldwide Management Corp., Eudora, KS) of 10-mm diameter and 4-mm thickness were inserted subcutaneously 5 to 10 mm away from the incision, to minimize the effects of the incision-repair process on the foreign body reaction. The incision was closed with 9-mm stainless steel Autoclip wound clips (Becton Dickinson Primary Care Diagnostics, Circle Sparks, MD). The animals were monitored for signs of wound dehiscence or infection at the Yale Animal Resource Center. All implantation studies were performed at least three times using three to five animals per each experimental group.
Myeloperoxidase (MPO) Assay on the PVA Sponges
The MPO assay was modified from previously described methods. 30,31 At timed intervals, mice bearing PVA implants were sacrificed and the sponges removed and dissected of any surrounding tissue. The fluid from the implants was isolated by mechanical squeezing. Twenty-five μl of fluid was set aside for a hemoglobin assay and the remainder spun at 8000 rpm. The resulting cell pellet was resuspended in 250 μl of 50 mmol/L KH2PO4 (Mallinckrodt Inc., St. Louis, MO) buffer, pH 6.0, with 0.5% hexadecyltrimethylammonium bromide (Sigma Chemical Co.). The samples were then sonicated for 20 seconds and incubated at 60°C for 2 hours. Next, the tubes were spun at 12,000 rpm for 10 minutes and the supernatant retained for analysis. Immediately before a spectrophotometric analysis, 100 μl of each sample was added to 2.9 ml of the above KH2PO4 buffer supplemented with 0.167 mg/ml of o-dianisidine dihydrochloride (Sigma Chemical Co.) and 0.005% hydrogen peroxide (J.T. Baker). The samples were prepared in duplicate and were read at time 0 and at 3 minutes after fluid addition at 490 nm. The change was recorded as MPO activity in mU/3 minutes.
Bone Marrow Engraftment
A previously described method used in our laboratory was followed. 29 At least 8 weeks after engraftment, PECAM-1 expression in total peripheral blood cell population was analyzed by FACS, using a phycoerythrin-conjugated anti-PECAM-1 antibody (MEC13.3; Pharmingen, San Diego, CA) as described previously. 29
Analysis of peripheral blood neutrophils, specifically, was performed after obtaining the blood via cardiac puncture and isolating cells as described in Neutrophil Transmigration Assay. The cells were stained with phycoerythrin-conjugated CD31 and fluorescein isothiocyanate-conjugated Gr-1 (both from BD Pharmingen, San Diego, CA) antibodies for 30 minutes at 4°C, which was preceded and followed by three washes with FACS staining buffer [1× phosphate-buffered saline (PBS) with 10% fetal bovine serum and 0.01% sodium azide]. All analyses were performed on a FACScalibur using Cell Quest software (Becton Dickinson, Mountain View, CA).
Hemoglobin Concentration Assay
The fluid for the assay was obtained as for the above Myeloperoxidase Assay. Hemoglobin concentration was determined with a Plasma Hemoglobin kit (Sigma Diagnostics Inc.). Briefly, 10 μl of sponge fluid or hemoglobin standard was added to the tetramethylbenzidine/acetic acid buffer, followed by timed hydrogen peroxide addition. Ten minutes after hydrogen peroxide addition, each sample was read on a spectrophotometer at 600 nm, and hemoglobin concentration calculated using a provided equation: test-blank/standard-blank × 30 = mg/dl. Peripheral hemoglobin concentration analysis was performed by Antech Diagnostics Laboratories (New York) on the blood samples obtained by cardiac puncture.
Immunohistochemistry
Paraffin tissue blocks were prepared after fixing the removed PVA implants in 4% paraformaldehyde (Sigma Chemical Co.) in PBS. Sectioning and hematoxylin and eosin (H&E) staining was performed in the Yale Research Histology facility of the Department of Pathology. For the Mac-3-based monocyte/macrophage analysis and the ICAM-2-based vessel quantification, three animals were used in each experimental group, and six sponge sections per animal were cut from the center of the implant, 50 μm apart, resulting in 18 sections per experimental group. For laminin staining, three animals were used in each group, and four sponge sections were cut from the implant center, resulting in 12 sections per group.
Alkaline phosphatase or diaminobenzidine immunostaining was performed after deparaffinizing the sections. The sections were blocked with 0.3% bovine serum albumin (Life Technologies Inc., Grand Island, NY) in PBS for 1 hour. Primary ICAM-2 antibody, primary Mac-3 antibody (BD Pharmingen), or a laminin antibody generated in our laboratory 32 at a concentration of 1:250 was incubated with the slides overnight at 4°C. The following day, a 1-hour incubation with a secondary biotinylated donkey anti-rat antibody for ICAM-2 and Mac-3 or biotinylated goat anti-rabbit (both from Jackson ImmunoResearch Laboratories Inc., West Grove, PA) at a concentration of 1:500 was followed by incubation with either an avidin-biotin-peroxidase complex-alkaline phosphatase or an avidin-biotin-peroxidase complex kit reagent (Vector Laboratories, Burlingame, CA) for 30 minutes. Nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate substrate with levamisole (both from Vector Laboratories) was prepared according to the manufacturer’s instructions as was diaminobenzidine substrate. Color development was monitored under the microscope and the reaction stopped with substrate buffer. The slides were coverslipped using gelvatol and glycerol mounting medium.
Thirty light microscopy images taken from the sponge edge at ×20 magnification for ICAM-2, or eight images at ×6.3 for laminin, were analyzed blindly and quantified with NIH Image.
Matrigel Assay
BD Matrigel matrix was used to coat tissue culture dishes according to the manufacturer’s instructions (BD Biosciences, San Jose, CA). PECAM-1-negative (KO), reconstituted (RC), and reconstituted that lost PECAM-1 expression (RCNE) endothelioma cells were plated onto the matrix at a density of 5 × 105 cells per 30-mm plate, and allowed to grow for 72 hours. At that time point, light microscopy images were taken and analyzed.
Statistics
Statistical analysis was performed using Student’s paired t-test. The values were considered significant at P < 0.05.
Results
Decreased Neutrophil Infiltration of the KO Implants
To study the function of PECAM-1 in a chronic foreign body reaction, we subcutaneously implanted PVA sponges into WT and PECAM-1 KO mice. The sponges were removed at various time points and processed for morphological and biochemical analysis. Our first observation was that paraffin-embedded, H&E-stained sponges exhibit different morphologies in the two types of animals. Figure 1 ▶ demonstrates cross-sections of sponges removed at days 7 and 14 after implantation. In Figure 1, A and B ▶ (day 7), under low magnification (×6.3), the epidermis with hair follicles is visible, and underneath, below a subdermal muscle layer, granulation tissue is forming. The sponges harvested from the WT animals are infiltrated with many more cells than the KO implants. The sponge material is light and porous and the gaps are filled with infiltrating cells. Figure 1C ▶ shows that at day 7, in the WT animals, the majority of the infiltrating cells are neutrophils, identifiable by their characteristic polymorphic nuclei documented in the inset. Figure 1D ▶ indicates that the KO sponges are infiltrated by significantly fewer neutrophils. Figure 1, E to J ▶ , shows the morphology of 14-day implants. At that time point, WT implants show deeper penetration by a cellular infiltrate (Figure 1, E and F) ▶ . Figure 1, G and H ▶ , shows that the majority of the invading cells are elongated and spindle-shaped, most likely endothelial cells and fibroblasts, both components of the granulation tissue. Figure 1I ▶ shows red blood cells inside the newly formed vessels of the WT implants. Figure 1J ▶ illustrates the lack of vessels in the KO sponge. The insets in I and J show that both WT and KO implants contain foreign body giant cells, fused multinucleated macrophages, and hallmark cells of this type of inflammation.
Figure 1.
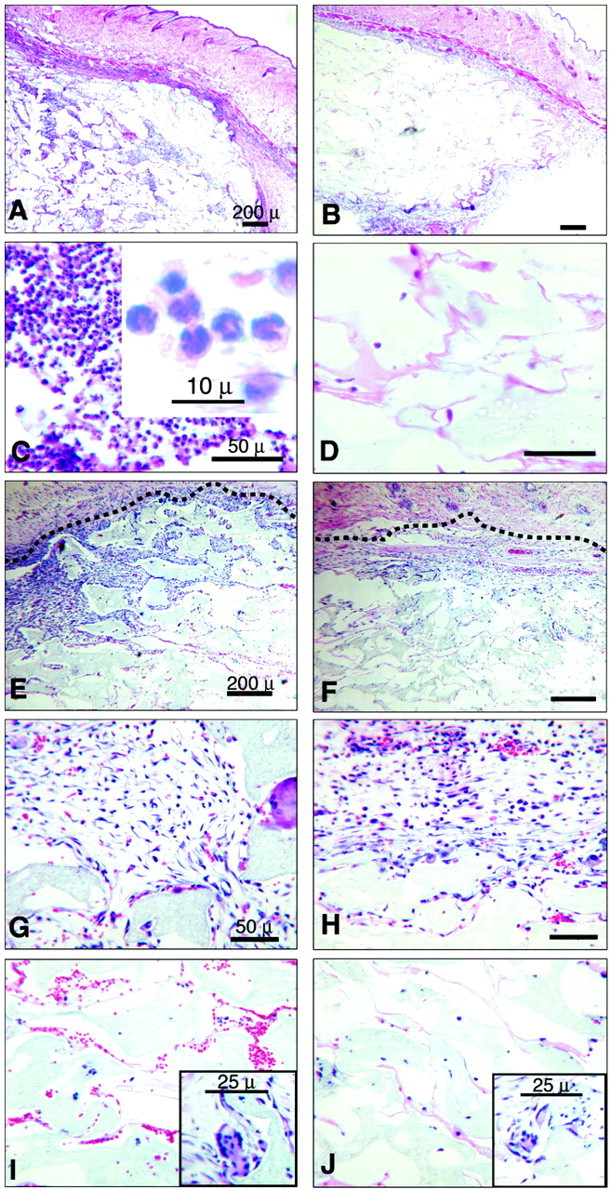
Light micrographs of H&E-stained PVA sponges at days 7 and 14 after implantation, harvested from WT (A, C, E, G, and I) and KO (B, D, F, H, and J) mice. A: Low-power micrograph of a representative WT sponge cross-section at day 7. Note that the implant interstices are infiltrated with cells. B: Low-power micrograph of a representative KO sponge cross-section at day 7. Note that the implant interstices exhibit much less cell infiltration. C: High-power micrograph of a WT sponge at day 7 illustrating that the predominant cell type is the neutrophil. The inset illustrates the polylobated nuclei of the polymorphonuclear leukocytes. D: High-power micrograph of a KO sponge at day 7 demonstrating the paucity of cellular infiltration. E: Low-power micrograph of a WT sponge on day 14 illustrating the deeper level of cellular infiltration into the implant when compared with the KO. The dashed line denotes the rim of the sponge. F: Low-power micrograph of a KO sponge at day 14 showing a modest cellular infiltration. G: High-power micrograph of a WT sponge at day 14 illustrates the granulation tissue comprised of endothelial cells and fibroblasts. H: High-power micrograph of a KO sponge on day 14 demonstrates only a modest formation of granulation tissue at the rim of an implant. I: High-power micrograph of a WT sponge at day 14 shows newly formed microvessels that contain red blood cells and that are found deep into the implant. J: High-power micrograph of a KO sponge at day 14 shows fewer vascular structures and red blood cells. Insets in I and J show the presence of foreign body giant cells in both WT and KO implants. Scale bars: 200 μ (A, B, E, F); 50 μ (C, D, G, H, I, J); 10 μ (inset in C); 25 μ (insets in I and J). These micrographs are representative of at least three independent experiments comprised of groups of three to five animals in each experimental group.
Histological observations were also made at earlier time points, but no significant differences can be observed in and around the sponges before day 7.
In light of these morphological differences, we decided to quantify the level of neutrophil infiltration. MPO is a cytotoxic enzyme highly expressed by neutrophils and used to quantify their number. 30,31 Figure 2 ▶ shows the enzyme activity at various time points. As expected, there is a significant difference in MPO activity at day 7 after implantation. There is some activity at days 2 and 7, but it is lower and not significantly different between the WT and the KO animals. The activity found at day 7 diminishes by day 14, as the inflammation shifts into a chronic phase.
Figure 2.
MPO activity in the sponges at various time points after implantation in the WT and PECAM-1 KO mice. Day 2: WT, n = 6; KO, n = 5. Day 4: WT, n = 8; KO, n = 3. Day 7: WT, n = 4; KO, n = 4. Day 14: WT, n = 4; KO, n = 4. *, P = 0.003 WT versus KO at day 7. Data are expressed as means. Vertical lines denote standard errors.
Monocyte/Macrophage Infiltration of the KO Implants
Immunohistochemical observations were performed to assess the relative numbers of monocytes/macrophages present in the granulation tissues forming around and infiltrating the interstices of sponges implanted in WT and KO animals. 33 As illustrated in Figure 3 ▶ , at days 7 (Figure 3A, B, E, and F) ▶ and 14 (Figure 3, D and H) ▶ monocytes/macrophages are present in approximately equal densities in the granulation tissues forming around and infiltrating into the sponges implanted in WT and KO mice (Figure 3 compare A, B, and D, with E, F, and H) ▶ . However, similar to our observations on neutrophil infiltration into the sponge interstices (Figures 1 and 2) ▶ ▶ , we observed a paucity of monocytes/macrophages in the sponge interstices in the KO mice compared to the WT mice at day 7 after implantation (Figure 3 ▶ , compare C with G).
Figure 3.

Light micrographs of immunohistochemically stained PVA sponges at days 7 (A–C, E–G) and 14 (D and H) after implantation, harvested from WT (A–D) and KO (E–H) mice. A, B, E, and F: Micrographs of Mac-3-stained granulation tissues infiltrating into sponges harvested from WT (A and B) and KO (E and F) mice at 7 days after implantation illustrating the presence of brown-stained monocytes/macrophages in all four panels. D and H: Micrographs of Mac-3-stained granulation tissues infiltrating into sponges harvested from WT (D) and KO (H) mice at 14 days after implantation illustrating the presence of brown-stained monocytes/macrophages in both panels. C and G: Micrographs of Mac-3-stained sponges harvested from WT (C) and KO (G) mice at 7 days after implantation illustrating a paucity of infiltrating neutrophils and monocytes/macrophages (stained brown) in the KO sections (G) compared to the intense neutrophil and monocyte/macrophage infiltrate observed in the WT sections (C). Scale bars: 50 μ (A–H); 25 μ (insets in C and G). These micrographs are representative of at least three independent experiments comprised of groups of three to five animals in each experimental group.
PECAM-1 Expression on the Endothelium Drives Leukocyte Infiltration
Because PECAM-1 is expressed on neutrophils and endothelial cells, we began investigating whether one cell type or both are responsible for the increased inflammation in the WT animals. We generated chimeric mice by bone marrow transplantation. We ablated the cell populations in the bone marrow of the recipients with irradiation. We then injected the recipients intravenously with donor bone marrow precursor populations. We tested the resulting phenotype and performed experiments no earlier than 9 weeks after transplantation to ensure a complete marrow engraftment. The results of a representative transplantation are shown in Figure 4 ▶ . Figure 4A ▶ represents peripheral blood FACS analysis for PECAM-1 expression. Cells analyzed include platelets, lymphocytes, and granulocytes. Any erythrocytes present are always PECAM-1-negative. The three groups studied are labeled WT->KO (WT bone marrow transplanted into KO animals), KO->WT (KO bone marrow transplanted into WT animals), and WT->WT control. It is clear that PECAM-1 peripheral blood cell expression follows the donor phenotype. Because Figure 4A ▶ represents averaged animal histograms for each group, there is some variation in expression.
Figure 4.

Evaluation of bone marrow engraftment and MPO activity in the chimeric mice. A: FACS analysis of PECAM-1 expression on peripheral blood cells in the bone marrow-engrafted mice. C and WT are negative and positive controls, respectively. WK (WT marrow transplanted into KO animals) have a high PECAM-1 expression, whereas KW (KO marrow transplanted into WT animals) shows low levels. B: A representative double-label FACS analysis of the entire blood cell population of a WW animal analyzed for the expression of PECAM-1 (y axis) and GR-1 (x axis). The R1 gate indicates the neutrophil population presented in C. C: FACS analysis of PECAM-1 expression on peripheral blood neutrophils harvested from WW-, KW-, KK-, and WK-engrafted animals, showing that only animals engrafted with PECAM-1-positive marrow exhibit PECAM-1-positive neutrophil staining. D: MPO activity at 8 and 11 days after implantation in the engrafted mice. The activity is significantly increased in the animals with WT vasculature. Day 8: WW, n = 3; WK, n = 5; KW, n = 4. Day 11: WW, n = 9; WK, n = 6; KW, n = 7. *, P = 0.008 WK versus WW; *, P = 0.04 WK versus KW. Data are expressed as means. Vertical lines denote standard errors. E, F, and G: Representative photomicrographs of 5-μ sections H&E-stained 11-day sponges harvested from WT->WT (E), WT->KO (F), and KO->WT (G) animals illustrating relative neutrophil infiltrations. The insets illustrate the polylobated nuclei of the polymorphonuclear leukocytes. Scale bar, 50 μ; inset scale bar, 10 μ.
To more precisely quantify PECAM-1 expression on circulating neutrophils, we performed a two-color FACS analysis on a purified neutrophil population using PECAM-1 and Gr-1 (neutrophil marker). Figure 4C ▶ represents PECAM-1-only expression on neutrophil populations identified from a Gr-1 gate on a dot plot (a representative plot is shown in Figure 3B ▶ ). The composite overlay shows that WT->WT and WT->KO groups show positivity in the same range, and KO->KO and KO->WT show similar level of low staining.
Having established uniform neutrophil profiles, we implanted PVA sponges into the engrafted mice. Figure 4C ▶ demonstrates MPO activity in the implants. Initially, at days 7 or 8, we could detect only low levels of neutrophil infiltration in the sponges. Only at day 11 after implantation does the MPO activity rise, and only in the animals with PECAM-1-positive vasculature. WT->WT and KO->WT animals show high levels of MPO, which are raised significantly more than the WT->KO group. As observed in Figures 1 and 2 ▶ ▶ , there is a good correlation between the levels of MPO activity and the histological appearance of neutrophils in the sponges implanted in the WT->WT, WT->KO, and KO->WT engrafted mice. Figure 4, E, F, and G ▶ , shows representative histological sections illustrating the presence of abundant neutrophils in the interstices of the sponges harvested from WT->WT (Figure 4E) ▶ and KO->WT (Figure 4G) ▶ animals 11 days after sponge implantation compared to the paucity of neutrophils in the WT->KO (Figure 4F) ▶ animals, consistent with our MPO activity data (Figure 4D) ▶ . Thus, our original observation of increased severity of foreign body inflammation correlates with the presence of PECAM-1 on the endothelium, regardless of neutrophil PECAM-1 expression. The delay in detectable inflammation from day 7 to day 11 is likely because of radiation injury of the recipients. To achieve complete bone marrow ablation, the animals were subjected to two 500-rad doses of radiation, a lethal amount. Thus, the animals are likely to be suffering from radiation injury, which compromises a number of organ systems, including the vasculature. Irradiation inhibits in vitro angiogenesis, endothelial cell proliferation, attachment, migration, and differentiation; 34,35 it also induces a temporary growth arrest followed by a growth delay in endothelial cells, smooth muscle cells, and fibroblasts. 36
PECAM-1 KO Animals Exhibit a Lower Rate of Angiogenesis
Because the bone marrow transplantation study also indicated an endothelial defect in the KO mice, we began to investigate which vascular functions may be compromised. We first assessed the rate of angiogenesis, formation of new blood vessels from pre-existing ones.
It is apparent, with H&E staining, that the sponges do become vascularized. Figure 1I ▶ shows the blood vessels filled with red blood cells in the interstices of the sponge. Quantitation of the vascular networks was accomplished using ICAM-2 (specific for endothelial cells and lymphocytes), on 7-day and 14-day implants. Table 1 ▶ shows the quantitation results. Using ×20 magnification images, we measured the following parameters: the number of lumen-like structures, the number of unconnected vascular tendrils, the area of the lumens, the perimeter of the lumens, and the length of the tendrils. The organization of the ICAM-2-stained networks shows that WT sponges have more and larger luminal structures, whereas the KO implants are filled with long tendrils having little luminal organization.
Table 1.
Quantification of Vessel Organization by ICAM-2 Stain in 7- and 14-Day Sponges
| Lumens/×20 field | Tendrils/×20 field | Lumen area (μm2) | Lumen perimeter (μm) | Tendril length (μm) | |
|---|---|---|---|---|---|
| Day 7 | |||||
| WT | 15.0 ± 0.7 | 6.3 ± 0.4 | 975.3 ± 59.7 | 157.2 ± 10.8 | 174.9 ± 6.2 |
| KO | 5.4† ± 0.3 | 14.6† ± 0.7 | 634.2* ± 86.4 | 107.9† ± 5.9 | 220.8† ± 7.7 |
| Day 14 | |||||
| WT | 18.3 ± 0.8 | 8.8 ± 1.6 | 734 ± 40.0 | 131.2 ± 3.2 | 144.8 ± 5.5 |
| KO | 6.9† ± 0.3 | 12.5† ± 2.3 | 475.9† ± 43.2 | 101.1† ± 4.4 | 203.7† ± 6.1 |
*P < 0.01,
†P < 0.001. Data are means ± SE.
To confirm the morphological data, we measured hemoglobin content of the implants. Hemoglobin content has been used extensively as a surrogate marker of angiogenesis 6,37-40 and has been found to correlate significantly with vessel density. 40 Hemoglobin concentration in the sponges at various time points is show in Figure 5A ▶ . We observed a significant difference in the sponge red blood cell content between WT and KO animals at both days 7 and 14. It is possible that the hemoglobin concentration may correspond to hemorrhagic events in and around the implant. However, morphological examination of the implants reveals that the vast majority of the red blood cells are inside the blood vessels. Also, the timing of the hemoglobin increase makes hemorrhage an unlikely event. Very low levels of hemoglobin are detected at days 2 and 4, when one might expect some hemorrhage because of surgical injury. The hemoglobin concentration increases only after 7 days and remains steady through day 14. It is also possible that the KO mice are anemic and possess an intrinsically lower amount of red blood cells. Blood hemoglobin content was analyzed in WT and KO mice and the results were identical between two groups at baseline and after implantation (Figure 5B) ▶ . Furthermore, the analysis of sponge hemoglobin levels in the bone marrow engrafted mice (Figure 5C) ▶ corresponds to the MPO trend (Figure 2C) ▶ . At day 8 after implantation, the levels are low in all groups. The concentration increases at day 11 in WT->WT and KO->WT groups but not in the WT->KO set. Again, the observed delay (when compared to the mice that did not undergo the engraftment) in this aspect of inflammation, is most likely related to radiation injury.
Figure 5.

Hemoglobin concentrations in the sponges of WT and PECAM-1 KO mice at various time points after implantation. A: Hemoglobin concentration in the sponges increases significantly in the WTs at day 7 and remains high for as long as 2 weeks. Day 2: WT, n = 6; KO, n = 5. Day 4: WT, n = 8; KO, n = 3. Day 7: WT, n = 11; KO, n = 11. Day 14: WT, n = 13; KO, n = 11. *, P = 0.003. B: Hemoglobin concentration in peripheral blood at baseline and 7 days after implantation in WT and KO animals. C: Hemoglobin concentration in the bone marrow-engrafted mice. The levels are significantly increased in the animals with WT vasculature (WW and KW) at day 11 after implantation. Day 8: WW, n = 3; WK, n = 5; KW, n = 4. Day 11: WW, n = 8; WK, n = 6; KW, n = 7. *, P = 0.02 WK versus WW; *, P = 0.007 WK versus KW. Vertical lines denote standard errors. Data are expressed as means. D, E, and F: Representative photomicrographs of 5-μ sections H&E-stained 11-day sponges harvested from WT->WT (D), WT->KO (E), and KO->WT (F) animals illustrating relative angiogenesis (arrows). Scale bar, 50 μ (inset).
Figure 5, D, E, and F ▶ , shows representative histological sections illustrating the presence of robust (albeit less extensive) angiogenesis in the granulation tissue bordering and infiltrating the interstices of the implants harvested from WT->WT (Figure 5D) ▶ and KO->WT (Figure 5F) ▶ animals 11 days after sponge implantation compared to the paucity of angiogenesis in the WT->KO (Figure 5E) ▶ animals, consistent with our hemoglobin data (Figure 5C) ▶ . Again, the delay in angiogenesis observed in these irradiated engrafted animals is likely because of the effects of radiation injury on the endothelial cells and stromal cells of the bone marrow-engrafted recipients. 34-36
Along with the endothelial organization, extracellular matrix remodeling and deposition is a crucial part of neovascularization. Laminin deposition (Table 2) ▶ can be considered another indicator of angiogenesis. 41 Indeed, staining of this basement membrane component was significantly decreased in the sponges in KO animals. On day 7 after implantation, an average area of each laminin network in a ×6.3 field was calculated (using NIH Image software). Because of the density of laminin deposition on day 14, individual networks were difficult to separate. Thus, the results from day 14 represent the average of the entire areas of laminin deposition in ×6.3 fields analyzed. Based on the above experiments, we conclude that decreased rate of angiogenesis is the primary determinant of attenuated foreign body inflammation in the KO animals. It is logical that an increased number of microvessels in the WTs allows for a greater delivery of blood and therefore of leukocytes to the affected area.
Table 2.
Quantification of Laminin Deposition in the 7- and 14-Day Sponges
| Average area of a laminin network at day 7 (μm2) | Total area of laminin deposition in a ×6.3 field at day 14 (μm2) | |
|---|---|---|
| WT | 14,577 ± 2499 | 598,268 ± 31,010 |
| KO | 3928* ± 711 | 440,082* ± 23,272 |
*P < 0.05 with respect to WT. Data are means ± SE.
We were also able to assess the function of PECAM-1 in an in vitro angiogenesis model and do so without the use of blocking antibodies. We observed the organization of immortalized lung microvascular endothelial cells on the Matrigel matrix 72 hours after plating the cells. Figure 6 ▶ shows that PECAM-1-negative cells (Figure 6A) ▶ form small round aggregates on the matrix. In contrast, PECAM-1-positive (reconstituted) cells elongate and organize with neighboring cells into cord-like structures, consistent with the early stages of tube formation (Figure 6B) ▶ . The cells that were reconstituted with PECAM-1, but lost its expression, revert to the phenotype of round aggregates, with minimal cellular extensions Figure 6C ▶ ). These observations further implicate PECAM-1 in modulating endothelial cell organization during angiogenesis and confirm our earlier in vitro findings 6 and the current in vivo findings.
Figure 6.

Organization of immortalized lung microvascular endothelial cells on a Matrigel matrix. A: PECAM-1-negative (KO) cells form round aggregates and are essentially devoid of network formation. B: PECAM-1-reconstituted (RC) cells exhibit robust network formation consisting of interconnecting cords of endothelial cells typical of in vitro angiogenesis. C: Reconstituted cells that have been taken out of antibiotic selection and have lost PECAM-1 expression (RCNE) revert to forming aggregates with minimal network formation. Scale bar, 200 μ.
Discussion
We have made a novel observation regarding the role of PECAM-1 in the development of foreign body inflammation. Our experiments indicate that the high neutrophil accumulation in the WT implants depends primarily on increased angiogenesis, which in turn, depends on endothelial PECAM-1 expression.
During inflammation, endothelial cells play a variety of roles. They must respond to the acute inducers of permeability, such as histamine, released from resident mast cells after foreign invasion. Such inducers cause disruption of cell-cell contacts and leakage of plasma proteins and blood cells into the interstitium. The endothelial cells themselves up-regulate a number of adhesive molecules to allow for tighter binding and eventual transmigration of leukocytes toward the invaded area. If the inflammatory agent persists, endothelial cells begin a process of angiogenesis; they proliferate, migrate, and organize into new microvessels to deliver more leukocytes for destruction of the invader. 42 Our data are consistent with the concept that angiogenesis is the primary process modulating the level of neutrophil infiltration in this model of foreign body inflammation. Our experiments reveal a greater number of lumen-like structures, increased red blood cell accumulation, more laminin deposition, and therefore a more robust angiogenesis in the WT animals. Because ours is a chronic model, angiogenesis is expected in and around the implant. Increased angiogenesis allows for greater blood delivery to the area, resulting in increased neutrophil accumulation in the implant.
Because PECAM-1 KO animals develop what appears to be a normal and a functional vascular system, we conclude that the angiogenic processes of adult inflammation and embryonic development are mechanistically different and that PECAM-1 serves different functions in each process. The concept of distinct mechanisms driving physiological and pathological angiogenesis is currently under investigation. 27 The differences could stem from the environment of the endothelial cells. During development, angiogenesis occurs subsequently to vasculogenesis to remodel a primitive vascular network into a functional system, and the endothelial cells are continuously under the influence of local growth factors and proteases. 27 In our model, the angiogenic signals influencing the stable quiescent skin vasculature may come from the cells infiltrating the implant in the early stages of inflammation. The components of the acute phase include neutrophils and monocytes, which will differentiate into macrophages. Whereas neutrophils apoptose within hours of extravasation out of the vessels, the macrophages fuse with each other to form foreign body giant cells and take stable residence in the implant. 25 The function of the macrophages is not only the phagocytosis of the invading object, but also the release of cytokines (interleukins-1, -6, -8, and 12, and tumor necrosis factor-α) that activate endothelial cells, increase vascular permeability, and act as chemotactic factors for leukocytes. 43 The decreased angiogenesis in the PECAM-1 KO could result from either impaired cytokine release by the macrophages or from the inability of endothelial cells to respond to chemical signals. The roles of PECAM-1 in modulating cytokine expression in monocytes/macrophages and in regulating cytokine responsiveness in endothelial cells are currently unknown and fertile areas for investigation.
A crucial finding affecting bone marrow transplantation studies is the presence of an adult pool of hematopoietic precursor cells capable of contributing to the neovasculature. 44 Even though we have analyzed the leukocyte populations for the persistence of a recipient phenotype, it is possible that some of the neovasculature in our implants derives from the precursors in the transplanted bone marrow. A study using bone marrow engraftments and a PVA sponge implantation model, has shown that between 8.3% and 11.2% of endothelial cells in the new sponge vessels after 1 month of implantation are derived from circulating hematopoietic progenitors. 45 This low percentage indicates that the majority of the endothelium is of the recipient phenotype and that in our model the presence or absence of PECAM-1 on that fraction of the cell population modulates the neutrophil infiltration. Also, our analysis of donor-derived endothelium in areas of skin wounds, after bone marrow transplantation, revealed negligible endothelial engraftment (S Mahooti and JA Madri, unpublished data).
We have shown that the absence of PECAM-1 can attenuate neovascularization, extracellular matrix deposition and leukocyte accumulation in and around these sterile, inert PVA implants. Although PVA sponges have been retired from their use as human arterial grafts, 46 they are widely used to study cellular components of foreign body reaction: angiogenesis, granuloma formation, and extracellular matrix deposition. 45,47,48
It will be of great interest to pursue potential clinical applications of this finding, including the use of local PECAM-1-blocking reagents (anti-sense oligonucleotides, PECAM-1 antibodies, recombinant PECAM-1 peptides) coupled to synthetic prosthetic devices or local delivery of such reagents to the site(s) of prosthetic implants to ameliorate the fibrotic complications that result in functional compromise of prosthetic devices including cardiac and vascular patches and arteriovenous shunt prosthetics. 49-51
Acknowledgments
We thank Dr. Neta Ilan for his help with animal handling; Dr. Jordan Pober for insightful scientific advice and discussion; Sandy Davis for superb technical assistance; and Dr. Britta Engelhardt at the Max-Planck Institute for Vascular Biology, Munster, Germany, for the generous gift of the immortalized PECAM-1 KO and RC endothelial cell lines.
Footnotes
Address reprint requests to Joseph A. Madri, Ph.D., M.D., Department of Pathology, Yale University School of Medicine, 310 Cedar St., LH 115, New Haven, CT 06520. E-mail: joseph.madri@yale.edu.
Supported in part by the United States Public Health Service (grants R37-HL-28373 and PO 1 DK 38797 to J. A. M.) and the American Heart Association (postdoctoral fellowship to P. B.).
References
- 1.Albelda SM, Oliver PD, Romer LH, Buck CA: EndoCAM: a novel endothelial cell-cell adhesion molecule. J Cell Biol 1990, 110:1227-1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newman PJ, Berndt MC, Gorski J, White II GC, Lyman S, Paddock C, Muller WA: PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science 1990, 247:1219-1222 [DOI] [PubMed] [Google Scholar]
- 3.Stockinger H, Gadd SJ, Eher R, Majdic O, Schreiber W, Kasinrerk W, Strass B, Schnabl E, Knapp W: Molecular characterization and functional analysis of the leukocyte surface protein CD31. J Immunol 1990, 145:3889-3897 [PubMed] [Google Scholar]
- 4.Simmons DL, Walker C, Power C, Pigott R: Molecular cloning of CD31, a putative intercellular adhesion molecule closely related to carcinoembryonic antigen. J Exp Med 1990, 171:2147-2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao G, O’Brien CD, Zhou Z, Sanders SM, Greenbaum JN, Makrigiannakis A, DeLisser HM: Involvement of human PECAM-1 in angiogenesis and in vitro endothelial cell migration. Am J Physiol 2002, 282:C1181-C1190 [DOI] [PubMed] [Google Scholar]
- 6.DeLisser HM, Christofidou-Solomidou M, Strieter RM, Burdick MD, Robinson CS, Wexler RS, Kerr JS, Garlanda C, Merwin JR, Madri JA, Albelda SM: Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol 1997, 151:671-677 [PMC free article] [PubMed] [Google Scholar]
- 7.Graesser D, Solowiej A, Bruckner M, Osterweil E, Juedes A, Davis S, Ruddle NH, Engelhardt B, Madri JA: Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J Clin Invest 2002, 109:383-392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller WA, Weigl SA, Deng X, Phillips DM: PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med 1993, 178:449-460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Z, Christofidou-Solomidou M, Garlanda C, DeLisser H: Antibody against murine PECAM-1 inhibits tumor angiogenesis in mice. Angiogenesis 1999, 3:181-188 [DOI] [PubMed] [Google Scholar]
- 10.Ilan N, Mahooti S, Rimm DL, Madri JA: PECAM-1 (CD31) functions as a reservoir for and a modulator of tyrosine-phosphorylated beta-catenin. J Cell Sci 1999, 112:3005-3014 [DOI] [PubMed] [Google Scholar]
- 11.Duncan GS, Andrew DP, Takimoto H, Kaufman SA, Yoshida H, Spellberg J, Luis de la Pompa J, Elia A, Wakeham A, Karan-Tamir B, Muller WA, Senaldi G, Zukowski MM, Mak TW: Genetic evidence for functional redundancy of platelet/endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J Immunol 1999, 162:3022-3030 [PubMed] [Google Scholar]
- 12.Albelda SM, Muller WA, Buck CA, Newman PJ: Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol 1991, 114:1059-1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeLisser HM, Yan HC, Newman PJ, Muller WA, Buck CA, Albelda SM: Platelet/endothelial cell adhesion molecule-1 (CD31)-mediated cellular aggregation involves cell surface glycosaminoglycans. J Biol Chem 1993, 268:16037-16046 [PubMed] [Google Scholar]
- 14.Deaglio S, Mallone R, Baj G, Donati D, Giraudo G, Corno F, Bruzzone S, Geuna M, Ausiello C, Malavasi F: Human CD38 and its ligand CD31 define a unique lamina propria T lymphocyte signaling pathway. EMBO J 2001, 15:580-582 [DOI] [PubMed] [Google Scholar]
- 15.Sun J, Williams J, Yan HC, Amin KM, Albelda SM, DeLisser HM: Platelet endothelial cell adhesion molecule-1 (PECAM-1) homophilic adhesion is mediated by immunoglobulin-like domains 1 and 2 and depends on the cytoplasmic domain and the level of surface expression. J Biol Chem 1996, 271:18561-18570 [DOI] [PubMed] [Google Scholar]
- 16.Piali L, Hammel P, Uherek C, Bachmann F, Gisler RH, Dunon D, Imhof BA: CD31/PECAM-1 is a ligand for alpha v beta 3 integrin involved in adhesion of leukocytes to endothelium. J Cell Biol 1995, 130:451-460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong CW, Wiedle G, Ballestrem C, Wehrle-Haller B, Etteldorf S, Bruckner M, Engelhardt B, Gisler RH, Imhof BA: PECAM-1/CD31 trans-homophilic binding at the intercellular junctions is independent of its cytoplasmic domain; evidence for heterophilic interaction with integrin alphavbeta3 in Cis. Mol Biol Cell 2000, 11:3109-3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu TT, Barreuther M, Davis S, Madri JA: Platelet endothelial cell adhesion molecule-1 is phosphorylatable by c-Src, binds Src-Src homology 2 domain, and exhibits immunoreceptor tyrosine-based activation motif-like properties. J Biol Chem 1997, 272:14442-14446 [DOI] [PubMed] [Google Scholar]
- 19.Ilan N, Cheung L, Pinter E, Madri JA: Platelet-endothelial cell adhesion molecule-1 (CD31), a scaffolding molecule for selected catenin family members whose binding is mediated by different tyrosine and serine/threonine phosphorylation. J Biol Chem 2000, 275:21435-21443 [DOI] [PubMed] [Google Scholar]
- 20.Ilan N, Cheung L, Miller S, Mohsenin A, Tucker A, Madri JA: Pecam-1 is a modulator of stat family member phosphorylation and localization: lessons from a transgenic mouse. Dev Biol 2001, 232:219-232 [DOI] [PubMed] [Google Scholar]
- 21.Masuda M, Osawa M, Shigematsu H, Harada N, Fujiwara K: Platelet endothelial cell adhesion molecule-1 is a major SH-PTP2 binding protein in vascular endothelial cells. FEBS Lett 1997, 408:331-336 [DOI] [PubMed] [Google Scholar]
- 22.Ilan N, Mohsenin A, Cheung L, Madri JA: PECAM-1 shedding during apoptosis generates a membrane-anchored truncated molecule with unique signaling characteristics. EMBO J 2001, 15:362-372 [DOI] [PubMed] [Google Scholar]
- 23.Kim CS, Wang T, Madri JA: Platelet endothelial cell adhesion molecule-1 expression modulates endothelial cell migration in vitro. Lab Invest 1998, 78:583-590 [PubMed] [Google Scholar]
- 24.Schimmenti LA, Yan HC, Madri JA, Albelda SM: Platelet endothelial cell adhesion molecule, PECAM-1, modulates cell migration. J Cell Physiol 1992, 153:417-428 [DOI] [PubMed] [Google Scholar]
- 25.Anderson JM: Inflammatory response to implants. ASAIO Trans 1988, 34:101-107 [DOI] [PubMed] [Google Scholar]
- 26.Hudlicka O, Brown MD SE: Angiogenesis: Basic Concepts and Methodology. Halliday A Hunt B Poston L Schachter M eds. 1998:3-19 Cambridge University Press Cambridge
- 27.Carmeliet P, Jain RK: Angiogenesis in cancer and other diseases. Nature 2000, 407:249-257 [DOI] [PubMed] [Google Scholar]
- 28.Carmeliet P: Mechanisms of angiogenesis and arteriogenesis. Nat Med 2000, 6:389-395 [DOI] [PubMed] [Google Scholar]
- 29.Mahooti S, Graesser D, Patil S, Newman P, Duncan G, Mak T, Madri JA: PECAM-1 (CD31) expression modulates bleeding time in vivo. Am J Pathol 2000, 157:75-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gumina RJ, el Schultz J, Yao Z, Kenny D, Warltier DC, Newman PJ, Gross GJ: Antibody to platelet/endothelial cell adhesion molecule-1 reduces myocardial infarct size in a rat model of ischemia-reperfusion injury. Circulation 1996, 94:3327-3333 [DOI] [PubMed] [Google Scholar]
- 31.Parsey MV, Kaneko D, Shenkar R, Abraham E: Neutrophil apoptosis in the lung after hemorrhage or endotoxemia: apoptosis and migration are independent of IL-1beta. Clin Immunol 1999, 91:219-225 [DOI] [PubMed] [Google Scholar]
- 32.Roll FJ, Madri JA, Albert J, Furthmayr H: Codistribution of collagen types IV and AB2 in basement membranes and mesangium of the kidney. An immunoferritin study of ultrathin frozen sections. J Cell Biol 1980, 85:597-616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho M-K, Springer TA: Tissue distribution, structural characterization and biosynthesis of Mac-3, a macrophage surface glycoprotein exhibiting molecular weight heterogeneity. J Biol Chem 1983, 258:636-642 [PubMed] [Google Scholar]
- 34.Denham JW, Hauer-Jensen M: The radiotherapeutic injury—a complex ‘wound’. Radiotherapy Oncol 2002, 63:129-145 [DOI] [PubMed] [Google Scholar]
- 35.Rose RW, Grant DS, O’Hara MD, Williamson SK: The role of laminin-1 in the modulation of radiation damage in endothelial cells and differentiation. Radiat Res 1999, 152:14-28 [PubMed] [Google Scholar]
- 36.Bertrand OF, Mongrain R, Thorin E, Lehnert S: In vitro response of human and porcine vascular cells exposed to high dose-rate gamma-irradiation. Int J Radiat Biol 2000, 76:999-1007 [DOI] [PubMed] [Google Scholar]
- 37.Belo AV, Ferreira MA, Bosco AA, Machado RD, Andrade SP: Differential effects of thalidomide on angiogenesis and tumor growth in mice. Inflammation 2001, 25:91-96 [DOI] [PubMed] [Google Scholar]
- 38.Muramatsu M, Katada J, Hayashi I, Majima M: Chymase as a proangiogenic factor. A possible involvement of chymase-angiotensin-dependent pathway in the hamster sponge angiogenesis model. J Biol Chem 2000, 275:5545-5552 [DOI] [PubMed] [Google Scholar]
- 39.Dupont E, Falardeau P, Mousa SA, Dimitriadou V, Pepin MC, Wang T, Alaoui-Jamali MA: Antiangiogenic and antimetastatic properties of Neovastat (AE-941), an orally active extract derived from cartilage tissue. Clin Exp Metastasis 2002, 19:145-153 [DOI] [PubMed] [Google Scholar]
- 40.Ishihara K, Hayash I, Yamashina S, Majima M: A potential role of bradykinin in angiogenesis and growth of S-180 mouse tumors. Jpn J Pharmacol 2001, 87:318-326 [DOI] [PubMed] [Google Scholar]
- 41.Form DM, Pratt BM, Madri JA: Endothelial cell proliferation during angiogenesis: in vitro modulation by basement membrane components. Lab Invest 1986, 55:521-530 [PubMed] [Google Scholar]
- 42.Hunt BJ, Jurd KM: Endothelial Function in Inflammation, Sepsis, Reperfusion and the Vasculitides. Hunt BJ Poston L Schachter M Halliday A eds. 1998:pp 225-247 Cambridge University Press, Cambridge
- 43.Janeway C, Travers P, Hunt S, Walport M: Immunobiology. Current Biology Ltd. 1997:9:11-9:14 Garland Publishing Inc. London
- 44.Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP: Evidence for circulating bone marrow-derived endothelial cells. Blood 1998, 92:362-367 [PubMed] [Google Scholar]
- 45.Crosby JR, Kaminski WE, Schatteman G, Martin PJ, Raines EW, Seifert RA, Bowen-Pope DF: Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res 2000, 87:728-730 [DOI] [PubMed] [Google Scholar]
- 46.Fitch EA, Denman FR, Waldron GW: The obituary of ivalon arterial grafts. Arch Surg 1960, 81:824-833 [DOI] [PubMed] [Google Scholar]
- 47.Bitar MS, Farook T, Wahid S, Francis IM: Glucocorticoid-dependent impairment of wound healing in experimental diabetes: amelioration by adrenalectomy and RU 486. J Surg Res 1999, 82:234-243 [DOI] [PubMed] [Google Scholar]
- 48.Andrade SP, Fan TP, Lewis GP: Quantitative in-vivo studies on angiogenesis in a rat sponge model. Br J Exp Pathol 1987, 68:755-766 [PMC free article] [PubMed] [Google Scholar]
- 49.Clowes AW, Gown AM, Hanson SR, Reidy MA: Mechanisms of arterial graft failure. 1. Role of cellular proliferation in early healing of PTFE prostheses. Am J Pathol 1985, 118:43-54 [PMC free article] [PubMed] [Google Scholar]
- 50.Weiss MF, Scivittaro V, Anderson JM: Oxidative stress and increased expression of growth factors in lesions of failed hemodialysis access. Am J Kidney Dis 2001, 37:970-980 [DOI] [PubMed] [Google Scholar]
- 51.Salzman DL, Kleinert LB, Berman SS, Williams SK: Inflammation and neovascularization associated with clinically used vascular prosthetic materials. Cardiovasc Pathol 1999, 8:63-71 [DOI] [PubMed] [Google Scholar]



