Abstract
Oncostatin M (OSM) is an interleukin (IL)-6 family cytokine that we have previously shown can synergize with a number of proinflammatory cytokines to promote the release of collagen from cartilage in explant culture. However, the effects of this potent cytokine combination in vivo are not known. Using adenoviral gene transfer, we have overexpressed murine IL-1 (AdmIL-1) and murine OSM (AdmOSM) intraarticularly in the knees of C57BL/6 mice. Histological analyses indicated marked synovial hyperplasia and inflammatory cell infiltration for both AdmIL-1 and AdmOSM but not in control joints. This inflammation was even more pronounced for the AdmIL-1+AdmOSM combination with evidence of cartilage and bone destruction. Significant loss of both proteoglycan and collagen was also seen for this combination, and immunohistochemistry revealed an increased expression of matrix metalloproteinases (MMPs) with decreased tissue inhibitor of metalloproteinases (TIMPs) in both articular cartilage and synovium. Similar expression profiles for MMPs/TIMPs were found in IL-1+OSM-stimulated human articular chondrocytes. Taken together, these data confirm that, in vivo, OSM can exacerbate the effects of IL-1 resulting in inflammation and tissue destruction characteristic of that seen in rheumatoid arthritis. We provide further evidence to implicate the up-regulation of MMPs as a key factor in joint pathology.
Rheumatoid arthritis (RA) is an inflammatory disease in which extensive cellular infiltration into the synovium leads to hyperplasia of the tissue, finally resulting in both cartilage and bone destruction. The involvement of proinflammatory cytokines, in particular interleukin (IL)-1 and tumor necrosis factor-α (TNF-α), has been found to play a key role in promoting disease pathogenesis. 1,2 IL-1 is a pleiotropic cytokine with multiple biological actions including the migration of inflammatory cells, synovial cell proliferation, and production of cytokines and matrix metalloproteinases (MMPs). 1 It is overexpressed in RA cartilage and synovial membranes, and raised levels of IL-1 in synovial fluids and sera correlate with disease activity and cartilage/bone destruction in RA. 3-5 Anti-IL-1 therapy in animal models and in humans with RA has been shown to significantly reduce arthritis incidence, inflammation, and joint destruction, 1,6 suggesting that the effector pathways of joint damage are IL-1-mediated.
Oncostatin M (OSM) is an IL-6 cytokine family member that is produced by activated T cells and macrophages. OSM has been localized to the macrophages within RA synovium, 7 and elevated synovial fluid levels of OSM 7,8 correlate with markers of joint inflammation and cartilage destruction in RA. 2 In experimental animals, intraarticular delivery of human OSM or recombinant adenovirus vectors overexpressing murine OSM (AdmOSM) cause synovial inflammation and structural damage. 9-11 Blockade of OSM ameliorates joint inflammation and cartilage damage in collagen-induced arthritis. 12 OSM induces tissue inhibitor of metalloproteinases-1 (TIMP-1), an endogenous inhibitor of MMPs, in synovial fibroblasts and chondrocytes 7,10,13 as well as MMPs, 14 suggesting an important role for this pleiotropic cytokine in RA. Furthermore, growing evidence from in vitro studies suggests that OSM is an important co-factor for other proinflammatory cytokines such as TNF-α and IL-17, 15,16 as well as IL-1 7,17 in mediating cartilage destruction.
Destruction of cartilage and bone is proteinase-mediated, and the MMPs are thought to play a critical role. MMPs are a zinc-dependent family of endopeptidases that collectively degrade all of the components of the extracellular matrix, 18 including bone. 19 Elevated levels of several MMPs have been detected in sera, synovial fluids, and synovial tissues from RA patients at sites of cartilage erosion, and these correlate with disease activity and structural damage. 20-23 IL-1 is a potent inducer molecule of MMP expression in arthritis, promoting the production and activation of MMPs in cartilage and synovium 24 while therapy with either the type II IL-1 decoy receptor or IL-1 receptor antagonist can attenuate the catabolic effects of IL-1. 25,26
Collectively, the TIMPs inhibit all active MMPs 27 and are expressed in joint tissues. 28 In normal cartilage a balance exists between active MMPs and the TIMPs; increased MMPs and concomitantly low levels of TIMPs have been observed in RA synovial tissues, fluids, and sera. 20-23,29 This imbalance is thought to be a key factor in joint destruction, such that the direct modulation of MMP activity has been considered a potential therapeutic target. 30,31 We have previously shown that OSM in combination with IL-1 synergistically induces cartilage proteoglycan and collagen degradation in a cartilage explant culture system. 7 This is thought to occur via the up-regulation of MMPs, especially the collagenases, as well as aggrecanases. 7,32 In this study, we investigate the effects of intraarticular gene transfer of OSM in combination with IL-1 on murine knee joints using recombinant adenovirus.
Materials and Methods
Reagents
Anti-MMP-13, TIMP-1, and TIMP-2 polyclonal antibodies were raised in-house. 7 Anti-MMP-8 polyclonal antibodies and recombinant human OSM were from R&D Systems (Abingdon, UK), anti-MMP-3 polyclonal was a kind gift from H. Nagase (Kennedy Institute, London, UK), while anti-type II collagen was from Southern Biotechnology Associates, Inc. (Birmingham, AL). Human IL-1α and OSM were generous gifts from GlaxoSmithKline (Stevenage, UK) and J. Heath (Birmingham University, Birmingham, UK), respectively. Replication-defective recombinant adenovirus, engineered to overexpress murine IL-1β (AdmIL-1) and murine OSM (AdmOSM), as well as Add170 (used as control), were as described previously. 33-35 VECTASTAIN Elite ABC kits PK 6102 and 6106 were from Vector Laboratories (Burlingame, CA). All other reagents were commercially available analytical grade obtained from Merck (Poole, UK) or Sigma (Dorset, UK).
Animals and Delivery of Adenoviral Vectors
C57BL/6 mice were housed until 12 to 14 weeks of age. Intraarticular injections of adenovirus vectors (1 × 106 or 5 × 106 pfu/joint for each vector) were as previously described. 10 Briefly, anesthesia was maintained with isofluorane, knees were swabbed with 70% ethanol and a 5-μl volume (treatment) was injected into the synovial space. The contralateral knee was treated with Add170 (control). Each treatment included 4 joints and animals were sacrificed 7 days after administration. To compare the effects of the AdmIL-1+AdmOSM combination to either vector alone, Add170 was used such that the total dose of vector was equivalent for each treated knee (ie, a total of 2 × 106 or 1 × 107 pfu/joint). A further 4 mice were injected with PBS (vehicle) only into the right knee and the contralateral knee was left untreated. This group was used to confirm that Add170-treated joints were unchanged from either PBS or untreated joints as found previously. 10,11 Previous studies have validated this adenoviral delivery system as an effective means of cytokine overexpression in synovial tissues. 10,11,34,36
Histology and Immunohistochemistry
Knee joints were dissected separately from the limbs and fixed overnight with 7% formaldehyde in PBS (pH 7.4). Subsequently, joints were decalcified in 10% ethylenediaminetetraacetate (EDTA) in PBS for 10 days, and wax-embedded. Sections (5 μm) were stained with hematoxylin & eosin (H&E), or safranin O (for proteoglycans) with hematoxylin counterstaining, or analyzed using immunohistochemistry. H&E sections were graded based on a pathological scoring system essentially as described. 37
Formalin-fixed paraffin sections were deparaffinized, rehydrated, and treated with 0.1% trypsin at 37°C for 20 minutes or microwave boiling for 5 minutes, followed by 3% H2O2 for 15 minutes. Sections were blocked (1.5% normal sheep or rabbit serum) for 30 minutes, and then incubated with primary antibody for 90 minutes at room temperature. After sequential incubations with biotinylated secondary antibody and avidin-biotin complex (both for 30 minutes), the signal was developed using 3, 3′-diaminobenzidine tetrahydrochloride chromogenic solution (DAKO, Ely, UK) with hematoxylin counterstaining.
Image and Statistical Analyses
The density of safranin O and type II collagen staining in articular cartilage was analyzed by Image-Pro Plus (MEDIA Cybernetics, Silver Spring, MD). Images of stained sections were captured using a JVC 3-CCD color video camera (Victor Company of Japan, Ltd., Tokyo, Japan) and displayed on a computer monitor. For each joint, four measurements of articular cartilage apposition (two on the femoral side and two on the tibial side) were performed in a standardized manner. Bonferroni’s pairwise multiple comparison test or the Mann-Whitney U-test were used where appropriate. Values of P ≤ 0.05 were considered significant.
Cell Culture and Northern Blot Analysis
Chondrocytes were isolated from bovine nasal cartilage and human articular cartilage as previously described. 38 Human tissue was obtained following joint replacement surgery with full ethical approval. Primary cells were incubated in supplemented Dulbecco’s modified Eagle’s medium (DMEM) containing 10% (v/v) fetal calf serum at 1 × 106 cells/T-25 cm2 tissue culture flask. 38 When the cells reached 80% to 90% confluence, the medium was removed and replaced with serum-free medium overnight, and then cultured in DMEM containing test cytokines for the indicated time periods. Total cellular RNA was isolated and purified using the RNeasy kit (Qiagen, Crawley, UK). Equal amounts (15 to 20 μg) of RNA were fractionated on 1% agarose gels as described. 17 Following transfer of RNA to a GeneScreen Plus membrane (Perkin Elmer Life Sciences, Boston, MA) and UV cross-linking, blots were pre-hybridized for 2 to 3 hours and then probed for 18 hours at 42°C with full-length cDNA probes labeled by random priming with α[32P]dCTP. Radio-labeled cDNA-mRNA hybrids were visualized by phosphorimager (Molecular Dynamics, Chesham, UK). The signal for GAPDH was used to normalize for RNA loading.
Results
Intraarticular Gene Transfer of IL-1+OSM Induces Joint Inflammation and Destruction
Sections from all treated joints were stained with H&E and the morphology assessed. The contralateral control sections (Add170 treated) showed no evidence of joint damage (Figure 1A) ▶ as previously described. 10 AdmIL-1 alone caused a mild to moderate inflammatory response in the joints, involving diffuse mononuclear cell infiltration with evidence of moderate synovial hyperplasia, pannus formation and cartilage and bone erosions (Figure 1B) ▶ , similar to that seen for AdmOSM (Figure 1C) ▶ alone. 10 With the AdmIL-1+AdmOSM combination, more severe pathological changes were observed compared to each cytokine alone (see Figure 1D ▶ ). These changes were even more pronounced in animals receiving a five-fold higher adenoviral titer of AdmIL-1+AdmOSM (Figure 2) ▶ . Pronounced synovial hyperplasia and pannus formation were evident, as were indications of cartilage erosion (Figure 2, A and B) ▶ . Severe peri- and intraarticular soft tissue inflammation with a marked (inflammatory) cell infiltration into the synovial space were also seen (Figure 2, C and D) ▶ . Within the inflamed synovium there was evidence of angiogenesis (Figure 2E) ▶ and there appeared to be an accumulation of proliferative cells juxtaposed to the bone surface (Figure 2, F and G) ▶ . Furthermore, marked bone erosions were evident (Figure 2H) ▶ . The pathological changes seen in the AdmIL-1+AdmOSM-treated joints are very similar to those seen in rheumatoid disease. Quantitative evaluations 37 of the extent of inflammation, synovial hyperplasia, pannus formation and cartilage and bone damage further confirmed these observations (Table 1) ▶ .
Figure 1.
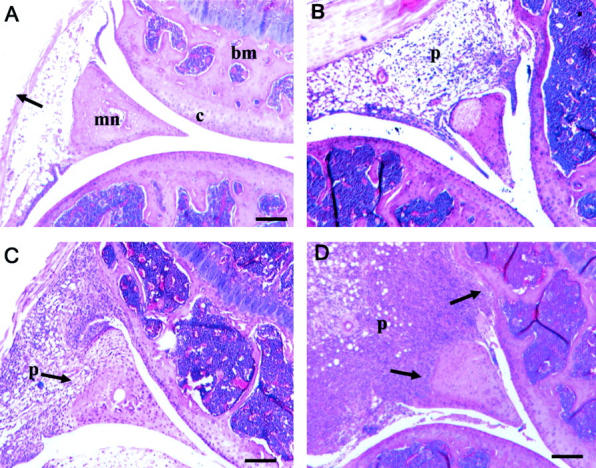
Gene transfer of IL-1+OSM causes tissue damage in murine joints. The right knee joints of mice (n = 4 per treatment group) were injected with AdmIL-1 and/or AdmOSM at 1 × 106 PFU/joint for each vector; all injections had an equivalent viral load as described in Materials and Methods, and all animals were sacrificed at day 7 following administration. Joints were fixed, decalcified, paraffin-embedded, and sections were stained with H&E. Scale bars, 50 μm. A: Add170, control knee showing normal appearance and thickness of the synovial lining (arrow). B: AdmIL-1 treated joint showing some synovial inflammation. C: AdmOSM, with pannus formation and evidence of invasion into the meniscus (arrow). D: AdmIL-1+AdmOSM treated joint. The extent and degree of synovial hyperplasia is considerably enhanced compared to either cytokine alone with erosions of both articular cartilage and meniscus (arrows). b, bone; bm, bone marrow; c, cartilage; mn, meniscus; p, pannus.
Figure 2.
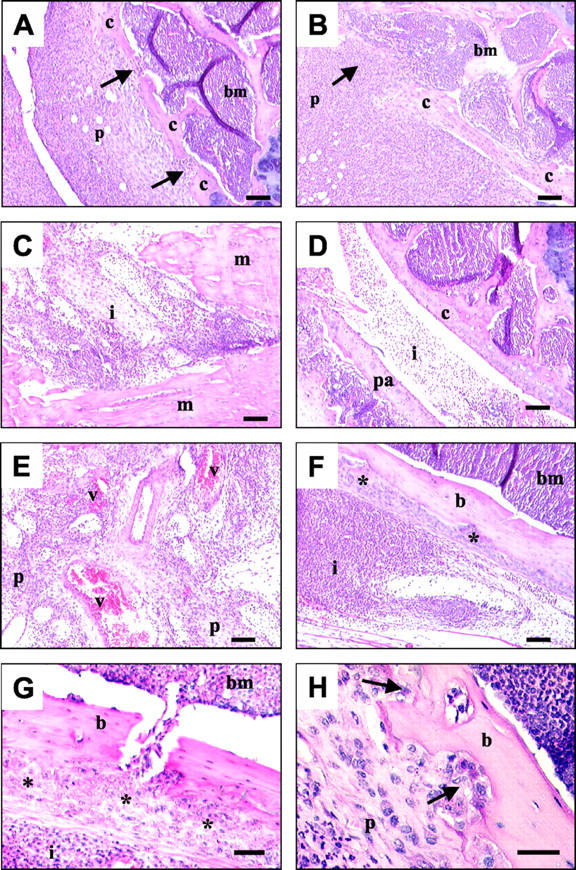
IL-1+OSM overexpression induces marked inflammation, synovial hyperplasia, and cartilage/bone destruction. Murine joints were injected with AdmIL-1+AdmOSM (both at 5 × 106 pfu/joint), and the animals were sacrificed 7 days after administration. Processed sections were stained with H&E. Scale bars, 100 μm (A–F); 50 μm (G); 20 μm (H). A and B: Pronounced synovial hyperplasia and pannus formation with evidence of cartilage erosions (arrows) and infiltration of synovial cells into the bone marrow. C and D: Severe periarticular and intraarticular soft tissue inflammation; a marked joint space exudate of inflammatory cells is evident in contact with both muscle and patellar. E: New blood vessel formation within the pannus. F and G: Inflammatory cells juxtaposed to bone with evidence of periosteal thickening (asterisk). H: Proliferative pannus tissue at sites of bone erosion (arrows). b, bone; bm, bone marrow; c, cartilage; i, inflammatory cells; m, muscle; p, pannus; pa, patellar; v, blood vessel.
Table 1.
Quantitative Analysis of the Morphological Changes in the Knees of Mice Following Intraarticular Injection with AdmIL-1 and/or AdmOSM
| Low viral titer* | High viral titer† | |||||
|---|---|---|---|---|---|---|
| OSM | IL-1 | IL-1+ OSM | OSM | IL-1 | IL-1+ OSM | |
| Tissue inflammation | 1.50 ± 0.29 | 1.50 ± 0.29 | 3.50 ± 0.29 | 3.25 ± 0.25 | 3.25 ± 0.25 | 5.00 ± 0.00 |
| Synovitis | 1.25 ± 0.25 | 1.25 ± 0.29 | 3.50 ± 0.29 | 3.25 ± 0.25 | 3.25 ± 0.25 | 5.00 ± 0.00 |
| Pannus | 1.00 ± 0.41 | 1.25 ± 0.25 | 3.50 ± 0.29 | 3.50 ± 0.29 | 3.25 ± 0.25 | 4.75 ± 0.25 |
| Joint space exudate | 1.00 ± 0.41 | 1.25 ± 0.25 | 3.50 ± 0.29 | 3.50 ± 0.29 | 3.00 ± 0.41 | 5.00 ± 0.00 |
| Cartilage erosion | 1.00 ± 0.41 | 1.00 ± 0.00 | 3.75 ± 0.25 | 3.00 ± 0.00 | 2.75 ± 0.25 | 4.75 ± 0.25 |
| Bone erosion | 0.50 ± 0.29 | 1.00 ± 0.00 | 2.75 ± 0.25 | 3.00 ± 0.00 | 2.75 ± 0.25 | 4.50 ± 0.29 |
| Total Score | 25 ± 0.14 | 29 ± 0.08 | 80 ± 0.12‡ | 78 ± 0.09 | 73 ± 0.13 | 116 ± 0.08‡ |
*1 × 106 pfu/joint for each vector.
†5 × 106 pfu/joint for each vector.
‡Statistical significances were assessed using the Mann-Whitney U-test comparing the AdmIL-1+AdmOSM treatment group with either AdmIL-1 alone or AdmOSM alone, where P = 0.03 for each comparison.
Mice (n = 4 for each treatment group) were injected with AdmIL-1 and/or AdmOSM at low or high titers in the left rear knee. The corresponding final viral titer of Add170 was injected into the contralateral knee of each mouse (either 2 × 106 or 1 × 107 PFU/joint) as described in the Materials and Methods. Mice were sacrificed at day 7, and sections (5 μm) H&E stained. Sections were scored for morphological changes, 37 and the values represent mean ± SEM for each treatment group (the Add170 control scored 0). The total scores are the combined scores of the four mice in each treatment group.
Proteoglycan and Collagen Degradation in AdmIL-1+AdmOSM-Treated Joints
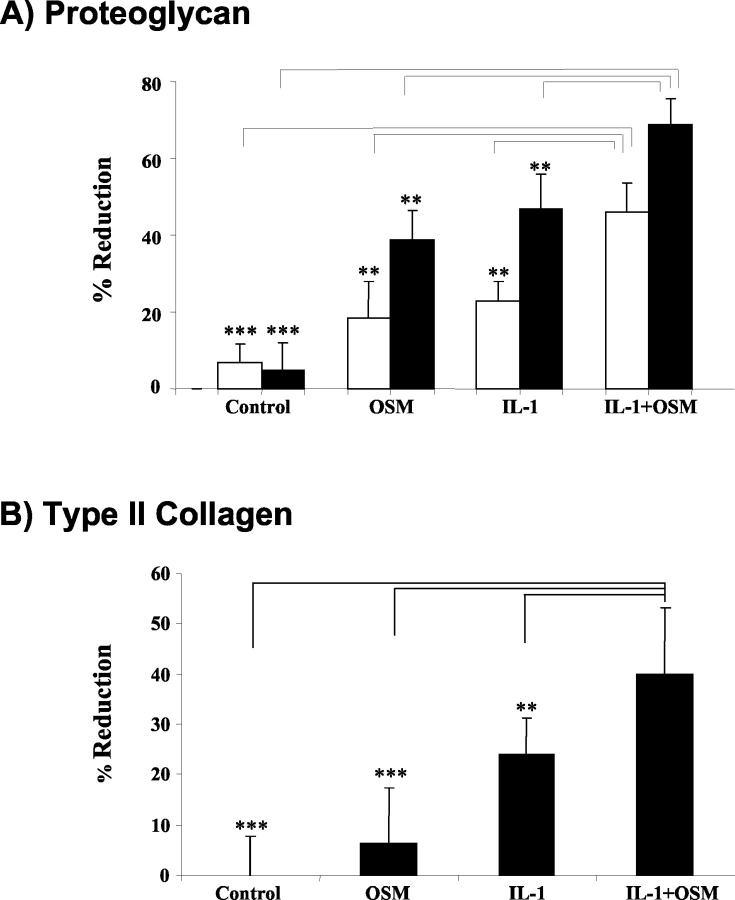
The extent of proteoglycan and collagen loss from the articular cartilage of adenovirus treated joints was assessed using safranin O and immunohistochemical staining, respectively. For the control joints, strong safranin O staining was evident within the articular cartilage (Figure 3) ▶ . AdmIL-1-treated joints had a moderate to severe depletion in staining compared to the more mild reduction for the AdmOSM injected joints. This was more pronounced for the higher AdmIL-1 titer (compare Figure 3, A and B ▶ ). The most significant loss of safranin O staining was seen in the AdmOSM+AdmIL-1-treated joints, with marked depletion in the superficial and middle layers of cartilage resulting in a pronounced margin, even at the lowest adenoviral titer used (Figure 3, A and B) ▶ . The strong type II collagen staining seen in the control joints (Figure 3C) ▶ was also seen for each cytokine alone even at the highest adenoviral titer, while the AdmIL-1+AdmOSM combination induced significant loss of type II collagen from the articular cartilage, especially at the articular surface (Figure 3C) ▶ . No significant collagen loss was seen for the lower adenoviral titer (not shown). Quantitative analysis of the density of cartilage proteoglycan and type II collagen staining further confirmed these findings (Figure 4) ▶ .
Figure 3.
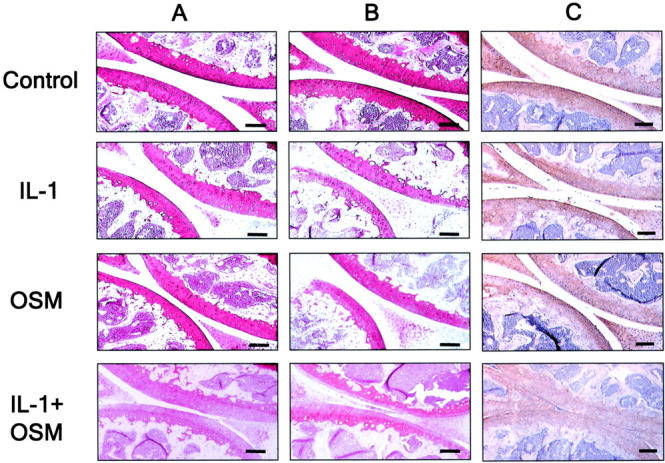
IL1+OSM overexpression induces marked proteoglycan and type II collagen depletion from articular cartilage. Mouse joints (n = 4 per treatment group) were injected with AdmIL-1 and/or AdmOSM at a titer of 1 × 106 PFU/joint (A) or 5 × 106 PFU/joint (B and C) as described in Materials and Methods. A and B: Processed sections were stained with safranin O (red) for proteoglycan. C: Sections were immunostained for type II collagen (brown) with hematoxylin counterstaining. Treatments were as indicated. Scale bars, 50 μm.
Figure 4.
Quantitative analyses of joint sections confirm significant IL-1+OSM-induced matrix depletion. The density of articular cartilage proteoglycan and collagen staining in sections prepared from mice treated with 1 × 106 PFU/joint of each adenoviral vector (open bars), or with 5 × 106 PFU/joint (closed bars), was quantitatively analyzed using Image-Pro Plus software. Results are expressed as a percentage reduction in staining for proteoglycan (A) or type II collagen (B) compared to the PBS-treated control joints (mean ± SD, n = 4). Bonferroni’s multiple comparison test was used to compare IL-1+OSM with control or IL-1 alone or OSM alone, where **P ≤ 0.01, ***P ≤ 0.001.
MMP and TIMP Expression Following IL-1+OSM Treatment in Vivo and in Vitro
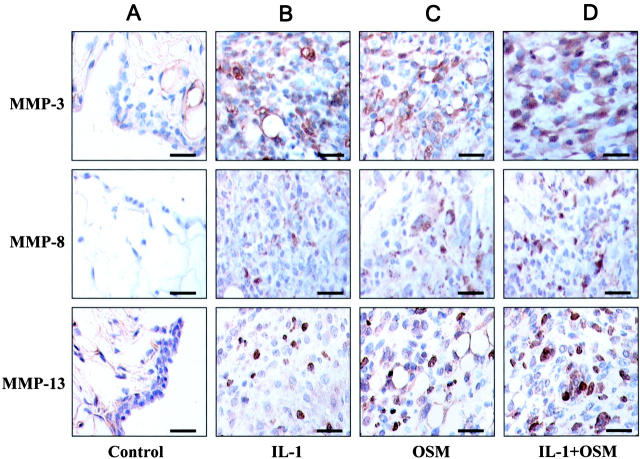
Since MMPs have been strongly implicated in cartilage destruction, 18,20-23 we assessed MMP expression in the adenovirus-treated joints. In the synovia of control mice, weak and diffuse staining for both MMP-3 and -13 were seen with no staining for MMP-8 (Figure 5A) ▶ . For both AdmIL-1- and AdmOSM-treated animals, staining for MMP-3, -8 and -13 was evident (see Figure 5, B and C ▶ ), and this staining was more pronounced for the AdmIL-1+AdmOSM combination (Figure 5D) ▶ . Staining for MMP-1 was not performed since it is not expressed in adult murine tissues. 39
Figure 5.
Intraarticular overexpression of IL-1+OSM induces synovial MMP expression. Synovial sections from joints of mice (n = 4 per treatment group) treated with AdmIL-1 and/or AdmOSM at 5 × 106 PFU/joint were immunolocalized with specific antibodies and counterstained with hematoxylin. Positive signal stains are brown. Treatments were Add170 control (A); AdmIL-1 (B); AdmOSM (C); AdmIL-1+AdmOSM (D). Scale bars, 20 μm.
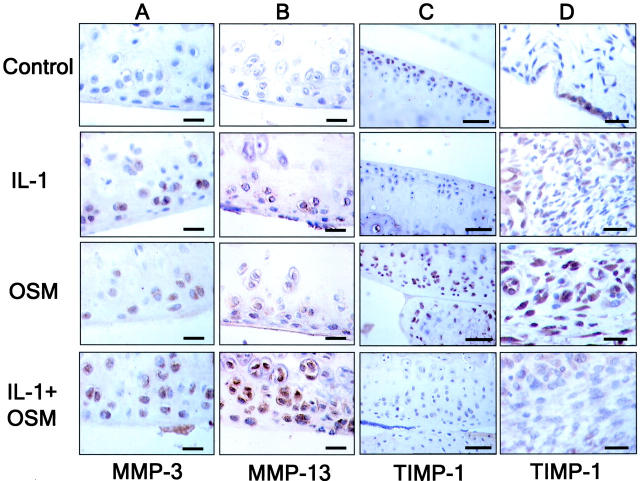
Immunolocalization data from the cartilage revealed, compared to control, that both IL-1 and OSM alone stimulated MMP-3 and -13 positive staining in chondrocytes, and that this staining was more pronounced with the IL-1+OSM combination (Figure 6,A and B) ▶ . No MMP-8 staining was seen in the cartilage of any treated animal (not shown). Although there was some weak staining for MMP-3 in control cartilage, no MMP-13 staining was evident (Figure 6B) ▶ . OSM has been shown to induce TIMP-1, a potent inhibitor of many MMPs. 18 Both cartilage and synovium from control animals showed relatively weak staining for TIMP-1 (Figure 6, C and D) ▶ . AdmIL-1 alone appeared to modestly increase synovial TIMP-1 staining but had not effect on cartilage TIMP-1. In agreement with previous studies, 7,10,17,34 AdmOSM markedly increased TIMP-1 staining in both the cartilage and synovium, and this staining was reduced close to control levels when AdmIL-1 was co-administered with AdmOSM (see Figure 6, C and D ▶ ). None of the cytokines significantly affected the expression of TIMP-2 in either cartilage or synovium (data not shown).
Figure 6.
OSM-induced MMP expression is enhanced by co-expression of IL-1 while TIMP-1 expression is suppressed in murine joints. Sections from the joints of mice (n = 4 per treatment group) treated with AdmIL-1 and/or AdmOSM at 5 × 106 PFU/joint were immunolocalized with specific antibodies and counterstained with hematoxylin. Positive signal stains are brown. Scale bars, 20 μm. A–C: Articular cartilage. D: Synovium.
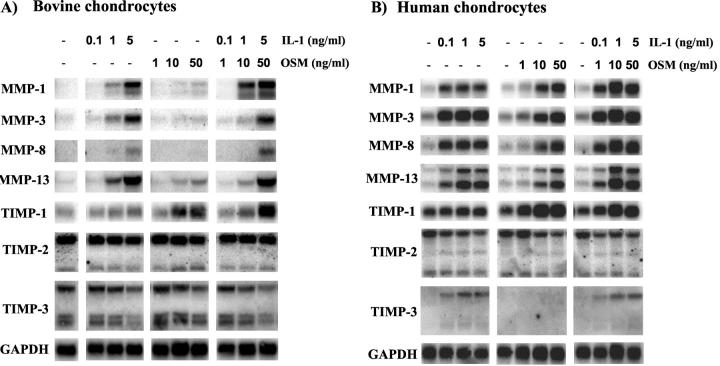
We have previously shown that the combination of IL-1+OSM induces marked proteoglycan and collagen degradation, which is accompanied by high levels of expression, production and activation of collagenases in bovine cartilage. 7,40 The present study has provided clear evidence of MMP induction following overexpression of this cytokine combination in a murine model. For comparison, northern blot analyses were performed on RNA extracted from chondrocytes after stimulation with IL-1 and/or OSM. These analyses indicated that both IL-1 and OSM alone stimulated a dose-dependent enhancement of MMP-1, -3, -8, and -13 expression in both bovine and human chondrocytes which was more pronounced in the human cells (compare Figure 7, A and B ▶ ). When IL-1 and OSM were combined a more marked induction of these genes was seen and was more evident in the human cells (Figure 7B) ▶ . Indeed, the human chondrocytes, appeared to be more responsive to lower cytokine concentrations, although a synergistic induction of MMP-1 was seen in both the human and bovine chondrocytes (Figure 7) ▶ . OSM up-regulated TIMP-1 in a dose-dependent manner, but failed to modulate TIMP-3 expression. Although IL-1 alone did not affect TIMP-1 expression, it did induce modest TIMP-3 expression in human chondrocytes (Figure 7B) ▶ . The expression profiles of TIMP-1 and TIMP-3 were unaffected by the IL-1+OSM combination, while TIMP-2 expression effectively remained unchanged for any of the treatments (Figure 7) ▶ .
Figure 7.
IL-1+OSM markedly induce MMPs in chondrocytes while TIMPs remain unaffected. Primary bovine nasal chondrocytes (A) or human articular chondrocytes (B) were cultured to 80% confluency, serum-starved overnight and then stimulated in serum-free medium with IL-1α (0.1 to 5 ng/ml) or OSM (1 to 50 ng/ml) for 24 hours. Total RNA was isolated and northern blotted. The signal for GAPDH was used to normalize for RNA loading.
Discussion
The involvement of proinflammatory cytokines in joint destruction during RA has been recognized for many years. Both IL-1 and OSM alone have been reported to induce joint inflammation and cartilage destruction in vitro and in vivo, and elevated levels of these two cytokines in synovial fluid of RA patients correlate with disease activity and destruction of cartilage and bone. 2-5,7,8 The present study demonstrates for the first time that IL-1 in combination with OSM induces marked joint inflammation, synovial hyperplasia, pannus formation, and cartilage/bone destruction in a murine model, which are all hallmarks of the pathological changes seen in RA. Specifically, this combination causes dramatic proteoglycan and collagen loss as reported previously in cartilage explant studies, 7,17 and this is associated with elevated levels of MMP expression.
Progressive loss of the cartilage matrix is a major characteristic of RA, and proinflammatory cytokines such as IL-1 and TNF-α play a key role in this process via the stimulation of proteolytic enzymes. Despite the relative success of anti-TNF-α therapy for RA, 41,42 IL-1 is still considered a key target in many patients. 1 OSM is present in RA synovial fluids 2,7,8 and has now been shown to synergize with TNF-α, IL-1, and IL-17, 7,15-17 thus adding to the complexity of RA. Loss of proteoglycan appears to be an early consequence of proinflammatory cytokine stimuli, 7,16,17 and marked loss of safranin O staining was evident in the mice treated with AdmIL-1+AdmOSM compared to either adenoviral vector alone. We have previously reported a chondrocyte membrane-associated metalloproteinase activity that was induced following IL-1+OSM stimulation and had aggrecanase activity. 43 We recently found that IL-1+OSM synergistically induced aggrecanase-1 expression in chondrocytes but not aggrecanase-2. 32 Further studies, therefore, will be required to fully elucidate the mechanisms involved in this proinflammatory cytokine-induced proteoglycan catabolism.
In normal cartilage the degradation of type II collagen is tightly controlled because it represents a key event in cartilage homeostasis; although slow, progressive destruction of the collagen network of cartilage ultimately leads to essentially irreversible joint destruction. 44 We have previously shown that the combination of IL-1+OSM induces marked collagen loss from porcine, bovine, and human cartilage as well as increasing MMP expression in chondrocytes. 7,13 Furthermore, this can be completely blocked by the inclusion of TIMP-1, 45 strongly implicating the involvement of MMPs. In the present study, marked collagen loss occurred only 7 days after administration of AdmIL-1+AdmOSM, and both cartilage and synovium showed strong immunoreactivity for several MMPs. The collagenolytic MMPs (MMP-1, -8 and -13) are considered to be of pivotal importance in the primary cleavage and subsequent denaturation of type II collagen in cartilage, 46 while MMP-3, a known activator of pro-collagenases, 47 has also been implicated in cartilage turnover. 48 We recently demonstrated the synergistic induction of MMP-1, -3, -8 and -13 by IL-1+OSM in chondrocytes 32 and have shown a strong correlation of enhanced active collagenolytic activity in culture media from IL-1+OSM stimulated cartilage explants. 7,17 Elevated immunostaining for these MMPs (except MMP-1 which is not expressed in adult mice) 39 in AdmIL-1+AdmOSM-treated joints combined with markedly elevated mRNA levels in both bovine and human chondrocytes further confirm and extend these observations. Indeed, MMPs are involved in a variety of pathological settings 18,49 where their activation is a critical step. We have shown that IL-1+OSM promotes the activation of procollagenases, 7 and that in cartilage this can be prevented by the inclusion of a serine proteinase inhibitor such as α1-proteinase inhibitor 45 or anti-inflammatory cytokines such as IL-4 or IL-13. 40,50 This ability to activate procollagenases may therefore represent a key mechanism by which this cytokine combination promotes cartilage collagen destruction.
Perturbation of the balance between active MMPs and TIMPs markedly alters cartilage catabolism as is seen in RA, and a reduction in TIMP-1 staining was seen when IL-1 and OSM were combined. Although this reduction was not seen in stimulated chondrocytes (24 hours), we have previously found that IL-1+OSM-induced TIMP-1 is a transient effect while the synergistic induction of MMP-1 is sustained. 17 TIMPs inhibit angiogenesis, 51 which is often a feature of RA tissues. This study clearly reveals the presence of new blood vessel formation, which correlated with the absence of TIMP-1 staining in both cartilage and synovium. Moreover, gene transfer of TIMP-1 has been shown to reduce tissue damage in vivo. 30 Another feature of IL-1+OSM gene transfer was an apparent proliferation of the periosteal lining as has recently been reported for OSM. 11 This phenomenon is known to occur in experimental models of arthritis as well as juvenile idiopathic arthritis and erosive osteoarthritis. 11 The presence of IL-1 did not affect this OSM-mediated bone apposition, although it may be influenced by the presence of other cytokines.
Using a murine model and gene transfer technology we have, for the first time, demonstrated that IL-1 in combination with OSM causes dramatic synovial hyperplasia, inflammation, and cartilage/bone destruction with accompanying high levels of MMPs and low levels of TIMPs. This aggressive phenotype is very similar to RA, and these data further highlight the proinflammatory nature of OSM and confirm a potential role for this cytokine combination in the joint destruction characteristic of inflammatory joint diseases.
Acknowledgments
We thank Dr. Daniel C. Anthony (University of Southampton, UK) for providing the AdmIL-1 vector.
Footnotes
Address reprint requests to Dr. Drew Rowan, Rheumatology, School of Clinical Medical Sciences, University of Newcastle, Newcastle upon Tyne, NE2 4HH, UK. E-mail: A.D.Rowan@ncl.ac.uk.
Supported by the Arthritis Research Campaign, the Anne Coleman Arthritis Fund, the Arthritis Society of Canada, Hamilton Health Sciences Corporation and St. Joseph’s Hospital, Hamilton, Ontario, Canada.
References
- 1.Dayer JM, Bresnihan B: Targeting interleukin-1 in the treatment of rheumatoid arthritis. Arthritis Rheum 2002, 46:574-578 [DOI] [PubMed] [Google Scholar]
- 2.Manicourt DH, Poilvache P, van Egeren A, Devogelaer JP, Lenz ME, Thonar EJ: Synovial fluid levels of tumor necrosis factor-α and oncostatin M correlate with levels of markers of the degradation of crosslinked collagen and cartilage aggrecan in rheumatoid arthritis but not in osteoarthritis. Arthritis Rheum 2000, 43:281-288 [DOI] [PubMed] [Google Scholar]
- 3.Chu CQ, Field M, Allard S, Abney E, Feldmann M, Maini RN: Detection of cytokines at the cartilage/pannus junction in patients with rheumatoid arthritis: implications for the role of cytokines in cartilage destruction and repair. Br J Rheumatol 1992, 31:653-661 [DOI] [PubMed] [Google Scholar]
- 4.Rooney M, Symons JA, Duff GW: Interleukin 1β in synovial fluid is related to local disease activity in rheumatoid arthritis. Rheumatol Int 1990, 10:217-219 [DOI] [PubMed] [Google Scholar]
- 5.Eastgate JA, Symons JA, Wood NC, Grinlinton FM, di Giovine FS, Duff GW: Correlation of plasma interleukin 1 levels with disease activity in rheumatoid arthritis. Lancet 1988, 2:706-709 [DOI] [PubMed] [Google Scholar]
- 6.van de Loo FA, van den Berg WB: Gene therapy for rheumatoid arthritis: lessons from animal models, including studies on interleukin-4, interleukin-10, and interleukin-1 receptor antagonist as potential disease modulators. Rheum Dis Clin North Am 2002, 28:127-149 [DOI] [PubMed] [Google Scholar]
- 7.Cawston TE, Curry VA, Summers CA, Clark IM, Riley GP, Life PF, Spaull JR, Goldring MB, Koshy PJT, Rowan AD, Shingleton WD: The role of oncostatin M in animal and human connective tissue collagen turnover and its localization within the rheumatoid joint. Arthritis Rheum 1998, 41:1760-1771 [DOI] [PubMed] [Google Scholar]
- 8.Hui W, Bell M, Carroll G: Detection of oncostatin M in synovial fluid from patients with rheumatoid arthritis. Ann Rheum Dis 1997, 56:184-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell MC, Carroll GJ, Chapman HM, Mills JN, Hui W: Oncostatin M induces leukocyte infiltration and cartilage proteoglycan degradation in vivo in goat joints. Arthritis Rheum 1999, 42:2543-2551 [DOI] [PubMed] [Google Scholar]
- 10.Langdon C, Kerr C, Hassen M, Hara T, Arsenault AL, Richards CD: Murine oncostatin M stimulates mouse synovial fibroblasts in vitro and induces inflammation and destruction in mouse joints in vivo. Am J Pathol 2000, 157:1187-1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Hooge AS, van de Loo FA, Bennink MB, de Jong DS, Arntz OJ, Lubberts E, Richards CD, van den Berg WB: Adenoviral transfer of murine oncostatin M elicits periosteal bone apposition in knee joints of mice, despite synovial inflammation and up-regulated expression of interleukin-6 and receptor activator of nuclear factor-κ B ligand. Am J Pathol 2002, 160:1733-1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plater-Zyberk C, Buckton J, Thompson S, Spaull J, Zanders E, Papworth J, Life PF: Amelioration of arthritis in two murine models using antibodies to oncostatin M. Arthritis Rheum 2001, 44:2697-2702 [DOI] [PubMed] [Google Scholar]
- 13.Hui W, Rowan AD, Cawston TE: Insulin-like growth factor 1 blocks collagen release and down regulates matrix metalloproteinase-1, -3, -8, and -13 mRNA expression in bovine nasal cartilage stimulated with oncostatin M in combination with interleukin 1α. Ann Rheum Dis 2001, 60:254-261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li WQ, Dehnade F, Zafarullah M: Oncostatin M-induced matrix metalloproteinase and tissue inhibitor of metalloproteinase-3 genes expression in chondrocytes requires Janus kinase/STAT signaling pathway. J Immunol 2001, 166:3491-3498 [DOI] [PubMed] [Google Scholar]
- 15.Hui W, Cawston TE, Rowan AD: Transforming growth factor β1 and insulin-like growth factor 1 block collagen degradation induced by oncostatin M in combination with tumour necrosis factor-α from bovine cartilage. Ann Rheum Dis 2003, 62:172-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koshy PJT, Henderson N, Logan C, Life PF, Cawston TE, Rowan AD: Interleukin-17 induces cartilage collagen breakdown: novel synergistic effects in combination with pro-inflammatory cytokines. Ann Rheum Dis 2002, 61:704-713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rowan AD, Koshy PJT, Shingleton WD, Degnan BA, Heath J, Vernallis AB, Spaull JR, Life PF, Hudson K, Cawston TE: Synergistic effects of gp130 binding cytokines in combination with interleukin-1 on cartilage collagen breakdown. Arthritis Rheum 2001, 44:1620-1632 [DOI] [PubMed] [Google Scholar]
- 18.Cawston TE: Metalloproteinase inhibitors and the prevention of connective tissue breakdown. Pharmacol Ther 1996, 70:163-182 [DOI] [PubMed] [Google Scholar]
- 19.Goto T, Maeda H, Tanaka T: A selective inhibitor of matrix metalloproteinases inhibits the migration of isolated osteoclasts by increasing the life span of podosomes. J Bone Miner Metab 2002, 20:98-105 [DOI] [PubMed] [Google Scholar]
- 20.Ishiguro N, Ito T, Obata K, Fujimoto N, Iwata H: Determination of stromelysin-1, 72 and 92 kDa type IV collagenase, tissue inhibitor of metalloproteinase-1 (TIMP-1), and TIMP-2 in synovial fluid and serum from patients with rheumatoid arthritis. J Rheumatol 1996, 23:1599-1604 [PubMed] [Google Scholar]
- 21.Tetlow LC, Woolley DE: Comparative immunolocalization studies of collagenase 1 and collagenase 3 production in the rheumatoid lesion, and by human chondrocytes and synoviocytes in vitro. Br J Rheumatol 1998, 37:64-70 [DOI] [PubMed] [Google Scholar]
- 22.Yoshihara Y, Nakamura H, Obata K, Yamada H, Hayakawa T, Fujikawa K, Okada Y: Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis 2000, 59:455-461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunnane G, Fitzgerald O, Beeton C, Cawston TE, Bresnihan B: Early joint erosions and serum levels of matrix metalloproteinase 1, matrix metalloproteinase 3, and tissue inhibitor of metalloproteinases 1 in rheumatoid arthritis. Arthritis Rheum 2001, 44:2263-2274 [DOI] [PubMed] [Google Scholar]
- 24.Clegg PD, Carter SD: Matrix metalloproteinase-2 and -9 are activated in joint diseases. Equine Vet J 1999, 31:324-330 [DOI] [PubMed] [Google Scholar]
- 25.Amin AR: Type II interleukin-1β receptor: a candidate for gene therapy in human arthritis. Clin Orthop 2000, 379:S179-S188 [DOI] [PubMed] [Google Scholar]
- 26.Jiang Y, Genant HK, Watt I, Cobby M, Bresnihan B, Aitchison R, McCabe D: A multicenter, double-blind, dose-ranging, randomized, placebo-controlled study of recombinant human interleukin-1 receptor antagonist in patients with rheumatoid arthritis: radiologic progression and correlation of Genant and Larsen scores. Arthritis Rheum 2000, 43:1001-1009 [DOI] [PubMed] [Google Scholar]
- 27.Cawston TE: Matrix metalloproteinases and TIMPs: properties and implications for the rheumatic diseases. Mol Med Today 1998, 4:130-137 [DOI] [PubMed] [Google Scholar]
- 28.Su S, Grover J, Roughley PJ, DiBattista JA, Martel-Pelletier J, Pelletier JP, Zafarullah M: Expression of the tissue inhibitor of metalloproteinases (TIMP) gene family in normal and osteoarthritic joints. Rheumatol Int 1999, 18:183-191 [DOI] [PubMed] [Google Scholar]
- 29.Keyszer G, Lambiri I, Nagel R, Keysser C, Keysser M, Gromnica-Ihle E, Franz J, Burmester GR, Jung K: Circulating levels of matrix metalloproteinases MMP-3 and MMP-1, tissue inhibitor of metalloproteinases 1 (TIMP-1), and MMP-1/TIMP-1 complex in rheumatic disease: correlation with clinical activity of rheumatoid arthritis versus other surrogate markers. J Rheumatol 1999, 26:251-258 [PubMed] [Google Scholar]
- 30.Schett G, Hayer S, Tohidast-Akrad M, Schmid BJ, Lang S, Turk B, Kainberger F, Haralambous S, Kollias G, Newby AC, Xu Q, Steiner G, Smolen J: Adenovirus-based overexpression of tissue inhibitor of metalloproteinases 1 reduces tissue damage in the joints of tumor necrosis factor-α transgenic mice. Arthritis Rheum 2001, 44:2888-2898 [DOI] [PubMed] [Google Scholar]
- 31.Greenwald RA: Thirty-six years in the clinic without an MMP inhibitor: what hath collagenase wrought? Ann NY Acad Sci 1999, 878:413-419 [DOI] [PubMed] [Google Scholar]
- 32.Koshy PJ, Lundy CJ, Rowan AD, Porter S, Edwards DR, Hogan A, Clark IM, Cawston TE: The modulation of matrix metalloproteinase and ADAM gene expression in human chondrocytes by interleukin-1 and oncostatin M: a time-course study using real-time quantitative reverse transcription-polymerase chain reaction. Arthritis Rheum 2002, 46:961-967 [DOI] [PubMed] [Google Scholar]
- 33.Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J: Transient expression of IL-1β induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest 2001, 107:1529-1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kerr C, Langdon C, Graham F, Gauldie J, Hara T, Richards CD: Adenovirus vector expressing mouse oncostatin M induces acute-phase proteins and TIMP-1 expression in vivo in mice. J Interferon Cytokine Res 1999, 19:1195-1205 [DOI] [PubMed] [Google Scholar]
- 35.Bett AJ, Haddara W, Prevec L, Graham FL: An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci USA 1994, 91:8802-8806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goossens PH, Huizinga TW: Adenoviral-mediated gene transfer to the synovial tissue. Clin Exp Rheumatol 2002, 20:415-419 [PubMed] [Google Scholar]
- 37.Staite ND, Richard KA, Aspar DG, Franz KA, Galinet LA, Dunn CJ: Induction of an acute erosive monarticular arthritis in mice by interleukin-1 and methylated bovine serum albumin. Arthritis Rheum 1990, 33:253-260 [DOI] [PubMed] [Google Scholar]
- 38.Shingleton WD, Ellis AJ, Rowan AD, Cawston TE: Retinoic acid combines with interleukin-1 to promote the degradation of collagen from bovine nasal cartilage: matrix metalloproteinases-1 and -13 are involved in cartilage collagen breakdown. J Cell Biochem 2000, 79:519-531 [PubMed] [Google Scholar]
- 39.Balbin M, Fueyo A, Knauper V, Lopez JM, Alvarez J, Sanchez LM, Quesada V, Bordallo J, Murphy G, Lopez-Otin C: Identification and enzymatic characterization of two diverging murine counterparts of human interstitial collagenase (MMP-1) expressed at sites of embryo implantation. J Biol Chem 2001, 276:10253-10262 [DOI] [PubMed] [Google Scholar]
- 40.Cleaver C, Rowan AD, Cawston TE: Interleukin-13 blocks the release of collagen fragments from bovine nasal cartilage treated with proinflammatory cytokines by preventing pro-collagenase activation. Ann Rheum Dis 2001, 60:150-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Catrina A, Lampa J, Ernestam S, af Klint E, Bratt J, Klareskog L, Ulfgren AK: Anti-tumour necrosis factor (TNF)-α therapy (etanercept) down-regulates serum matrix metalloproteinase (MMP)-3 and MMP-1 in rheumatoid arthritis. Rheumatology 2002, 41:484-489 [DOI] [PubMed] [Google Scholar]
- 42.Brennan FM, Browne KA, Green PA, Jaspar JM, Maini RN, Feldmann M: Reduction of serum matrix metalloproteinase 1 and matrix metalloproteinase 3 in rheumatoid arthritis patients following anti-tumour necrosis factor-α (cA2) therapy. Br J Rheumatol 1997, 36:643-650 [DOI] [PubMed] [Google Scholar]
- 43.Billington CJ, Clark IM, Cawston TE: An aggrecan-degrading activity associated with chondrocyte membranes. Biochem J 1998, 336:207-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jubb RW, Fell HB: The breakdown of collagen by chondrocytes. J Pathol 1980, 130:159-167 [DOI] [PubMed] [Google Scholar]
- 45.Milner JM, Elliott SF, Cawston TE: Activation of procollagenases is a key control point in cartilage collagen degradation: interaction of serine and metalloproteinase pathways. Arthritis Rheum 2001, 44:2084-2096 [DOI] [PubMed] [Google Scholar]
- 46.Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O, Tschesche H, Chen J, Van Wart H, Poole AR: Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest 1997, 99:1534-1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki K, Enghild JJ, Morodomi T, Salvesen G, Nagase H: Mechanisms of activation of tissue procollagenase by matrix metalloproteinase 3 (stromelysin). Biochemistry 1990, 29:10261-10270 [DOI] [PubMed] [Google Scholar]
- 48.Saito S, Katoh M, Masumoto M, Matsumoto S, Masuho Y: Involvement of MMP-1 and MMP-3 in collagen degradation induced by IL-1 in rabbit cartilage explant culture. Life Sci 1998, 62:359-365 [DOI] [PubMed] [Google Scholar]
- 49.Nagase H, Woessner JF, Jr: Matrix metalloproteinases. J Biol Chem 1999, 274:21491-21494 [DOI] [PubMed] [Google Scholar]
- 50.Cawston TE, Ellis AJ, Bigg H, Curry V, Lean E, Ward D: Interleukin-4 blocks the release of collagen fragments from bovine nasal cartilage treated with cytokines. Biochim Biophys Acta 1996, 1314:226-232 [DOI] [PubMed] [Google Scholar]
- 51.Chang C, Werb Z: The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol 2001, 11:S37-S43 [DOI] [PMC free article] [PubMed] [Google Scholar]