Clinical Perspective
Venous intimal hyperplasia (VIH) leads to stenosis in veins used for bypass grafting in patients with atherosclerotic vascular disease or in the venous side of arteriovenous fistulae in hemodialysis patients. In the hemodialysis population, estimated at over 230,000, 1 VIH results in vascular access failure, a major cause of patient morbidity. In the United States, the yearly health care costs for vascular access-related morbidity is well over one billion dollars. 2,3 Therefore, suitable models to study the pathogenesis of this process are timely and will potentially lead to novel therapeutic strategies to inhibit VIH. The purpose of this commentary is to reflect on the functional significance of the inflammatory gene expression changes in the model for VIH described in this issue of The American Journal of Pathology by Nath and colleagues. 4
The Animal Model
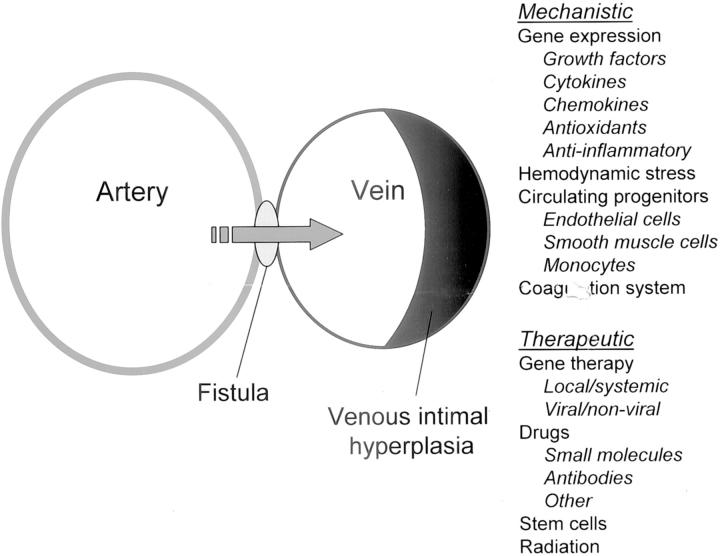
The ideal animal model should be inexpensive, readily available, and easy to implement while mimicking the human pathology but developing lesions in a short period of time. Both rodent and large animal models of VIH have been developed that mimic the human lesion. 5-9 However, most of these models require microsurgical expertise, are expensive and are confounded by additional variables such as the presence of foreign materials (suture or synthetic grafts) that could influence the development of the intimal lesion. In this issue, Nath and colleagues 4 have described the pathology and proinflammatory changes in gene expression in a rat aorto-caval model of an arteriovenous fistula. The model was first described by Garcia and Diebold 10 and as the authors point out, has been widely used to study cardiac hypertrophy and cardiac failure, 11,12 features that are not uncommonly seen in hemodialysis patients with peripheral arteriovenous fistulae. 13 This model demonstrates neointimal hyperplasia in the venous limb of the fistula, a process that is clinically relevant and a significant problem in patients undergoing venous bypass grafts for coronary artery disease as well as vascular access in hemodialysis patients. More importantly, the simplicity of this model and the absence of the use of artificial materials such as sutures or synthetic grafts, provides great potential for the use of this model to explore mechanistic and therapeutic interventions for VIH as summarized in Figure 1 ▶ . Several factors such as the profile of gene expression as described by the authors, 4 hemodynamic stress, circulating progenitor cells, the coagulation system as well as other factors that address the mechanisms involved in VIH could be explored. From a therapeutic standpoint, the potential for gene therapy approaches, pharmacological agents, stem cells and radiation are some avenues that could be investigated using this model.
Figure 1.
Schematic representation of the aorto-caval fistula model of venous intimal hyperplasia. This model will allow for further investigation of both the mechanistic as well as therapeutic strategies aimed at inhibiting venous intimal hyperplasia.
Gene Expression
The findings of alterations in gene expression, specifically upregulation of monocyte chemoattractant protein-1 (MCP-1), plasminogen activator inhibitor type-1 (PAI-1) and endothelin-1 (ET-1) and down-regulation of transforming growth factor- β1 (TGF-β1), 4 before the development of histological changes of neointimal hyperplasia, represent novel observations with potential therapeutic implications in developing strategies to inhibit vascular restenosis. One could speculate that a therapeutic intervention to cause a reversal of these changes ie, down-regulation of these and other pro-inflammatory cytokines or adhesion molecules and early activation of the TGF-β1 pathway may be beneficial in retarding the progression of the intimal lesion. For example, intercellular adhesion molecule-1 (ICAM-1) is upregulated early in vein interposition grafts in wild-type mice. The functional significance of such upregulation was shown by the findings that ICAM-1-deficient mice have significantly less intimal hyperplasia. 14
The Lesion
The pathology of VIH is characterized by smooth muscle cell migration and proliferation, monocyte/macrophage infiltration, extracellular matrix deposition and microvessel formation with varying degrees of thrombosis and calcification. 3,15 The process is a response to increased pressure from arterial blood flow. The demonstration of regression of VIH following reimplantation of vein grafts into the venous circulation suggest that exposure to the arterial environment is critical in lesion development and progression. 16 The mechanical force of arterial blood pressure induces activation of adhesion molecules, growth factors, cytokines, reactive oxygen species and matrix proteins. 3,14,17-19 Mechanical stress also activates signaling cascades (eg, mitogen activated protein kinases), that are essential stimuli for cell proliferation. 20 While most of these studies were performed in vitro or in isolated vascular segments, the results of Nath and colleagues demonstrate changes in inflammatory gene expression in vivo in the aorto-caval fistula model. A prominent feature in human arteriovenous fistulae and in murine models of VIH is the presence of abundant mononuclear cell infiltration in the early stages of the lesion, findings that correlate with the early upregulation of MCP-1 described in the rat model. 4
Circulating Progenitor Cells
A significant body of recent literature has focused on the contribution of circulating progenitor cells, derived from the bone marrow, in neointimal proliferation. 21-24 It has been recognized that these progenitor blood cells give rise to endothelial and smooth muscle cells at sites of injury to blood vessels. While most of these studies have been done in neointimal proliferation seen in the arterial circulation either following balloon injury or transplant rejection, limited work exists in VIH. Diao et al 25 have performed a venous interposition surgery in an irradiated wild-type mouse transplanted with bone marrow from a transgenic green fluorescent protein (GFP) mouse in which a segment of the internal jugular vein is used to repair a defect created in the carotid artery. Twenty days following the procedure, prominent VIH is observed resulting in >50% narrowing of the lumen. Approximately, 10 to 15% of both endothelial and smooth muscle cells within the intimal lesion were GFP positive suggesting a contribution of bone marrow derived cells to the neointimal lesion. Hu et al 26 have provided evidence that both donor and recipient derived cells contribute to smooth muscle cell accumulation in a transplant vein graft model of VIH. However, the contribution of donor-derived smooth muscle cells in VIH is controversial. 27
Where Do Recipient-Derived Cells in a Transplant Vein Graft Model of VIH Come From?
The site of origin for the recipient derived cells is not entirely clear, but migration of medial smooth muscle cells from the adjacent artery or circulating progenitor cells are possible sources. The contribution of recipient derived factors to the propagation of the intimal lesion may explain why some local therapeutic interventions aimed at the vessel wall have not been successful. However, while the presence of bone marrow derived cells in neointimal lesions has been shown in transplant-related or balloon/wire injury-induced neointimal proliferation, the actual contribution and functional significance of such cells in modifying the pathological process remains to be determined. 28
Venous versus Arterial Neointimal Proliferation
It is important to differentiate between venous and the more well studied arterial neointimal proliferation. As discussed in a recent elegant review by Roy-Chaudhury et al, 3 several factors including anatomical (poorly defined internal elastic lamina in veins), physiological (low levels of nitric oxide and prostacyclin, increased vasconstrictor sensitivity), hemodynamic (compliance, turbulence and shear stress) and dialysis-related factors (presence of uremia, needle trauma) contribute to the more aggressive venous lesion compared to arterial neointimal hyperplasia. These differences could also account for the relatively poor response and high restenosis rates following angioplasty in VIH. 3
Aorto-Caval Fistula in the Mouse
Guzman et al 29 have recently reported the feasibility of the aorto-caval fistula model in mice to evaluate the hemodynamic changes and medial cell activation in the mouse aorta. However, the inferior vena cava was not examined in these studies. Given the availability of several inbred lines of transgenic and knockout mice and the inflammatory gene expression changes observed, 4 taking the described rat model to mice will allow for evaluating specific genes involved in the pathogenesis of VIH. Combining a remnant kidney model with the aorto-caval fistula will allow for investigating the effects of uremia on the progression of VIH.
Therapeutic Implications
While significant progress has been made in our understanding of the pathology and the pathogenesis of the neointimal lesion, little success has been achieved with regards to therapy. Most studies have focused on arterial neointimal proliferation in the setting of restenosis following balloon injury wherein the use of drug-coated stents, gene therapy and radiation have shown promise. A large number of pharmacological agents have been shown to reduce stenosis in animal models, although no systemic agent has yet proven effective in humans. Limited studies have explored the use of therapeutic interventions besides angioplasty and surgical repair in venous intimal hyperplasia for dialysis access failure. External support with stenting devices reduce VIH in vein grafts in animal models, 30 however studies using external cuffs in human arteriovenous grafts have not shown encouraging results. The incidence of thrombotic occlusions is reduced but the patency rate is not improved. 31
VIH causing venous stenosis and access dysfunction is a major clinical problem in hemodialysis patients. Despite this complication, the native arteriovenous fistula provides the optimal vascular access for hemodialysis patients and serves as a “life-line” for these patients. 31 The changes in inflammatory gene expression in the model described reproduces many of the features of human VIH and will be useful for our understanding of the mechanism of VIH and to evaluate the effects of potential therapeutic interventions.
Footnotes
Address reprint requests to Anupam Agarwal, M.D., Associate Professor of Medicine, Biochemistry and Molecular Biology, Box 100224, University of Florida, 1600 SW Archer Road, Gainesville, FL 32610. E-mail: agarwal@nersp.nerdc.ufl.edu.
References
- 1.: US Renal Data System: USRDS 2001 annual data report. 2001. The National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Bethesda, MD
- 2.Feldman HI, Kobrin S, Wasserstein A: Hemodialysis vascular access morbidity. J Am Soc Nephrol 1996, 7:523-535 [DOI] [PubMed] [Google Scholar]
- 3.Roy-Chaudhury P, Kelly BS, Zhang J, Narayana A, Desai P, Melhem M, Duncan H, Heffelfinger SC: Hemodialysis vascular access dysfunction: from pathophysiology to novel therapies. Blood Purif 2003, 21:99-110 [DOI] [PubMed] [Google Scholar]
- 4.Nath KA, Kanakiriya SKR, Grande JP, Croatt AJ, Katusic ZS: Increased venous proinflammatory gene expression and intimal hyperplasia in an aorto-caval fistula model in the rat. Am J Pathol 2003, 162:2079-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou Y, Dietrich H, Hu Y, Metzler B, Wick G, Xu Q: Mouse model of venous bypass graft arteriosclerosis. Am J Pathol 1998, 153:1301-1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly BS, Heffelfinger SC, Whiting JF, Miller MA, Reaves A, Armstrong J, Narayana A, Roy-Chaudhury P: Aggressive venous neointimal hyperplasia in a pig model of arteriovenous graft stenosis. Kidney Int 2002, 62:2272-2280 [DOI] [PubMed] [Google Scholar]
- 7.Johnson MS, McLennan G, Lalka SG, Whitfield RM, Dreesen RG: The porcine hemodialysis access model. J Vasc Interv Radiol 2001, 12:969-977 [DOI] [PubMed] [Google Scholar]
- 8.Lemson MS, Daemen MJ, Kitslaar PJ, Tordoir JH: A new animal model to study intimal hyperplasia in arteriovenous fistulas. J Surg Res 1999, 85:51-58 [DOI] [PubMed] [Google Scholar]
- 9.Kohler TR, Kirkman TR: Dialysis access failure: a sheep model of rapid stenosis. J Vasc Surg 1999, 30:744-751 [DOI] [PubMed] [Google Scholar]
- 10.Garcia R, Diebold S: Simple, rapid, and effective method of producing aortocaval shunts in the rat. Cardiovasc Res 1990, 24:430-432 [DOI] [PubMed] [Google Scholar]
- 11.Huang M, Hester RL, Guyton AC, Norman RA, Jr: Hemodynamic studies in DOCA-salt hypertensive rats after opening of an arteriovenous fistula. Am J Physiol 1992, 262:H1802-H1808 [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Ren B, Liu S, Sentex E, Tappia PS, Dhalla NS: Characterization of cardiac hypertrophy and heart failure due to volume overload in the rat. J Appl Physiol 2003, 94:752-763 [DOI] [PubMed] [Google Scholar]
- 13.Ori Y, Korzets A, Katz M, Erman A, Weinstein T, Malachi T, Gafter U: The contribution of an arteriovenous access for hemodialysis to left ventricular hypertrophy. Am J Kidney Dis 2002, 40:745-752 [DOI] [PubMed] [Google Scholar]
- 14.Zou Y, Hu Y, Mayr M, Dietrich H, Wick G, Xu Q: Reduced neointima hyperplasia of vein bypass grafts in intercellular adhesion molecule-1 deficient mice. Circ Res 2000, 86:434-440 [DOI] [PubMed] [Google Scholar]
- 15.Motwani JG, Topol EJ: Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation 1998, 97:916-931 [DOI] [PubMed] [Google Scholar]
- 16.Davies MG, Fulton GJ, Svendsen E, Hagen PO: Time course of the regression of intimal hyperplasia in experimental vein grafts. Cardiovasc Pathol 1999, 8:161-168 [DOI] [PubMed] [Google Scholar]
- 17.West NEJ, Guzik TJ, Black E, Channon KM: Enhanced superoxide production in experimental venous bypass graft intimal hyperplasia. Role of NAD(P)H oxidase. Arterioscler Thromb Vasc Biol 2001, 21:189-194 [DOI] [PubMed] [Google Scholar]
- 18.Roy-Chaudhury P, Kelly BS, Miller MA, Reaves A, Armstrong J, Nanayakkara N, Heffelfinger SC: Venous neointimal hyperplasia in polytetrafluoroethylene dialysis grafts. Kidney Int 2001, 59:2325-2334 [DOI] [PubMed] [Google Scholar]
- 19.Weiss MF, Scivittaro V, Anderson JM: Oxidative stress and increased expression of growth factors in lesions of failed hemodialysis access. Am J Kidney Dis 2001, 37:970-980 [DOI] [PubMed] [Google Scholar]
- 20.Hu Y, Bock G, Wick G, Xu Q: Activation of PDGF receptor α in vascular smooth muscle cells by mechanical stress. EMBO J 1998, 12:1135-1142 [DOI] [PubMed] [Google Scholar]
- 21.Hillebrands JL, Klatter FA, van den Hurk BM, Popa ER, Nieuwenhuis P, Rozing J: Origin of neointimal endothelium and α-actin-positive smooth muscle cells in transplant arteriosclerosis. J Clin Invest 2001, 107:1411-1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell JH, Han CL, Campbell GR: Neointimal formation by circulating bone marrow cells. Ann NY Acad Sci 2001, 947:18-24 [DOI] [PubMed] [Google Scholar]
- 23.Shimizu K, Sugiyama S, Aikawa M, Fukumoto Y, Rabkin E, Libby P, Mitchell RN: Host bone-marrow cells are a source of donor intimal smooth-muscle-like cells in murine aortic transplant arteriopathy. Nat Med 2001, 7:738-741 [DOI] [PubMed] [Google Scholar]
- 24.Zalewski A, Shi Y, Johnson AG: Diverse origin of intimal cells: smooth muscle cells, myofibroblasts, fibroblasts and beyond? Circ Res 2002, 91:652-655 [DOI] [PubMed] [Google Scholar]
- 25.Diao Y, Grant M, Guthrie S, Scott E, Segal MS: Role of circulating progenitor cells in the development of venous intimal hyperplasia. J Am Soc Nephrol 2002, 13:58-59A [Google Scholar]
- 26.Hu Y, Mayr M, Metzler B, Erdel M, Davison F, Xu Q: Both donor and recipient origins of smooth muscle cells in vein graft atherosclerotic lesions. Circ Res 2002, 91:e13-e20 [DOI] [PubMed] [Google Scholar]
- 27.Redwood AJ, Tennant M: Cellular survival in rat vein-to-artery grafts: extensive depletion of donor cells Cell Tissue Res 2001, 306:251-256 [DOI] [PubMed] [Google Scholar]
- 28.Hillebrands JL, Klatter FA, Dijk WDV, Rozing J: Bone marrow does not contribute substantially to endothelial-cell replacement in transplant arteriosclerosis. Nat Med 2002, 8:194-195 [DOI] [PubMed] [Google Scholar]
- 29.Guzman RJ, Krystkowiak A, Zarins CK: Early and sustained medial cell activation after aortocaval fistula creation in mice. J Surg Res 2002, 108:112-121 [DOI] [PubMed] [Google Scholar]
- 30.Lardenoye JH, De Vries MR, Grimbergen JM, Havekes LM, Knaapen MW, Kockx MM, van Hinsbergh VW, van Bockel JH, Quax PH: Inhibition of accelerated atherosclerosis in vein grafts by placement of external stent in apoE*3-Leiden transgenic mice. Arterioscler Thromb Vasc Biol 2002, 22:1433-1438 [DOI] [PubMed] [Google Scholar]
- 31.Lemson MS, Tordoir JH, van Det RJ, Welten RJ, Burger H, Estourgie RJ, Stroecken HJ, Leunissen KM: Effects of a venous cuff at the venous anastomosis of polytetrafluoroethylene grafts for hemodialysis vascular access. J Vasc Surg 2000, 32:1155-1163 [DOI] [PubMed] [Google Scholar]