Abstract
We report a novel method that allows the culture of highly differentiated gastric surface mucous cells. Isolated mouse gastric epithelial cells and fibroblasts were co-cultured in a three-dimensional collagen gel system, and the reconstructed mucosal surface treated with an air-liquid interface. Cultured cells were examined by histology, immunohistochemistry, and electron microscopy. Isolated epithelial cells were positive for MUC5AC, and showed immature mucous cell features (pre-pit cell stage) on cell-free collagen gel. However, when given fibroblastic support, the epithelial cells differentiated into mature surface mucous cells (pit cell stage), and showed a tall columnar cell shape, basal round nuclei, and mucus-filled cytoplasm. In the fine structure, the cells showed junctional complexes, basal lamina, and glycogen and secretary granules. Further treatment by the air-liquid interface environment modified the differentiated state of the pit cells (pit top cell stage); resulting in the expression of cathepsin E, the disappearance of glycogen granules and the apical accumulation of secretory granules along with an increase in apoptotic cells. This culture model should provide a useful tool for studying gastric epithelial cell biology and various diseases of the gastric mucosa.
Epithelial cells of gastric mucosa are organized in vertical tubular structures consisting of an apical pit region, an isthmus just below the pit, and a gland region forming the lower part of the vertical unit. 1 The gland consists of a neck and a base. A single-cell-thick epithelium covers the whole surface and lines the pit, isthmus, and gland. The gastric units contain various cells; mucus-producing surface mucous, mucous neck, acid-producing parietal, pepsinogen-producing chief, and endocrine cells. Gastric epithelial renewal is an asymmetric process. The progenitor cells of the gastric unit are located in the region of the isthmus, in the middle of the tubular unit, and give rise to all of the gastric epithelial cell types that migrate either up or down from this point. One type, called the pit cell lineage, migrates up toward the luminal surface and differentiates into surface mucous cells. Other cell lineages migrate downward, slowly differentiating into mucous neck, parietal, chief, and endocrine cells. These bidirectional processes are known as foveolar and glandular differentiation, respectively.
A simple columnar epithelium, consisting of surface mucous cells, lines the surface of the stomach and the pit walls. 2 After being produced in the isthmus, pre-pit cells migrate in the direction of the pit while differentiating, and as they enter this region become pit cells. The pit cells continue ascending the pit wall in an outward direction toward the gastric surface. After the cells have reached the free surface, they eventually culminate into necrosis or apoptosis. The cells degenerating in this manner are phagocytosed by a neighbor or simply extruded into the gastric lumen. In the course of this travel, the cells develop to maturity characterized by the appearance of mucous granules. Pre-pit cells have a small amount of secretory granules scattered in the cytoplasm. Pit cells are divided into two phases: a maturation phase, the pit cell stage, during the ascension along the pit wall when the cells produce many secretory granules and glycogen granules in the apical cytoplasm, and a terminal differentiation phase, the pit top cell stage, at the pit top when disappearance of glycogen granules and the accumulation of secretory granules beneath the apical membrane is apparent. Although this process is well understood from in vivo labeling studies, little is known about the factors controlling growth and differentiation. 3-6
Cellular interaction between epithelial and stromal cells is a key determinant in the morphogenesis, proliferation, and cytodifferentiation of various organs. 7 Developmental studies have shown the importance of interactions between the mesenchyme and endoderm during intestinal organogenesis. 8 A specialized mesenchymal cell, the pericryptal fibroblast, continues to interact with cytodifferentiation of crypt stem cells even at the adult stage. 9 Because the gastric epithelium undergoes continuous renewal of its component cell lineages while maintaining regional differentiation like the intestinal epithelium, 5 gastric fibroblasts ought to exert an influence on the differentiation of gastric epithelial cells derived from the stem cells. In fact, several studies have reported that gastric stromal cells play an important role in glandular differentiation of gastric mucosa. 8,10 However, the significance of whether fibroblasts influence the differentiation of gastric surface mucous cells, namely foveolar differentiation, remains unclear. To understand the role of fibroblasts in foveolar differentiation, we developed a new in vitro model that utilizes type I collagen, the major component of the interstitium, as a matrix for cell proliferation and differentiation.
The air-liquid interface (ALI) culture method is known to be useful in promoting the specific differentiation of several epithelial cell types. 11-17 However, little is known about the application of the ALI culture technique to gastrointestinal epithelial cells. We have tried to examine the effect of ALI culture on gastric epithelial cells in primary culture.
In this paper, we demonstrate a novel method that allows the culture of highly differentiated gastric surface mucous cells. Our results show that stromal fibroblasts promote the differentiation of gastric surface mucous cells, and that ALI conditions allow for the modification of the state of differentiation of pit cells.
Materials and Methods
Isolation of Gastric Epithelial Cells and Fibroblasts from Newborn Mice
On postnatal day 1, the glandular stomach of C57BL/6 mice was minced and digested in Dispase I solution (bacterial neutral protease: 1000 protease U/ml; Goudoh-Shusei, Tokyo, Japan) for 50 minutes at 37°C. The cell suspension was dispersed in phosphate-buffered saline (PBS) and centrifuged. Isolated gastric epithelial cells were obtained as sediment. To obtain isolated gastric fibroblasts, a primary monolayer culture was first established. Digested tissue fragments as described above were seeded on culture dishes and maintained in Ham’s F12 supplemented with 10% fetal calf serum (FCS, Sigma, St. Louis, MO) and 50 μg/ml gentamicin at 37°C in a humidified atmosphere of 5% CO2 in air. Fibroblasts with spindle shapes grew from the fragments and within 3 weeks became a confluent monolayer. These fibroblasts were subcultured for 5 to 8 passages and used as gastric fibroblasts, which were positive for vimentin and negative for cytokeratin (data not shown).
Reconstruction Culture of the Gastric Surface Mucosa
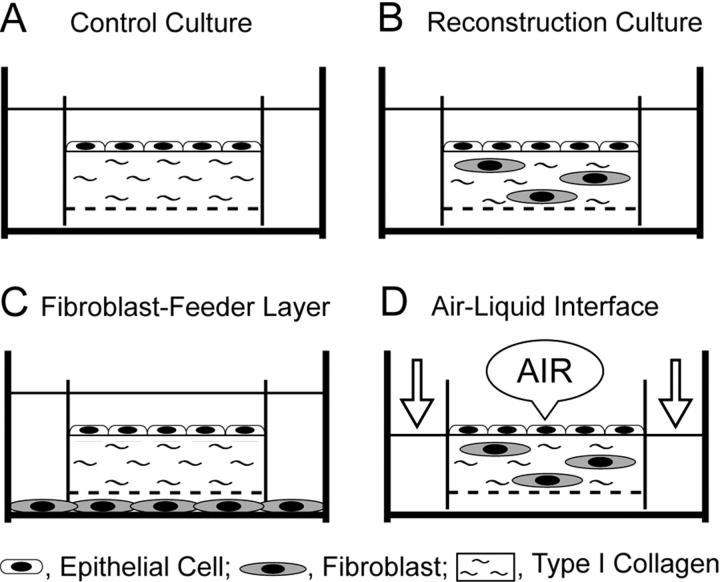
We developed a double-dish culture system, a modification of our previous method. 11 In the control culture, isolated epithelial cells were cultured on a cell-free type I collagen gel with a nitrocellulose bottom (Figure 1A) ▶ . The cells were maintained in complete medium, which was a 1:1 mixture of Eagle’s minimum essential medium (MEM) and Ham’s F12 medium supplemented with 20% FCS and 50 μg/ml gentamicin. To reconstruct the gastric surface mucosa, type I collagen gel solution was mixed with isolated fibroblasts at a concentration of 2.5 × 105 cells per ml. The fibroblast-containing collagen gel layers were compatible with gastric lamina propria in vivo. The epithelial cells were cultured on the reconstructed lamina propria under immersion conditions (Figure 1B) ▶ . To examine the effects of fibroblast-derived soluble factors, isolated gastric epithelial cells were cultured on a cell-free collagen gel with fibroblast-conditioned medium, which was elaborated by monolayer-cultured fibroblasts in the outer dish (Figure 1C) ▶ . Finally, the reconstructed gastric mucosa was treated as an ALI as previously described. 12 As shown in Figure 1D ▶ , the apical media of the inner dish was removed and the outer media was aspirated to the level of the surface epithelial cells. That is, the apical surfaces of the cells were exposed to humidified atmospheric air supplemented with 5% CO2, while the cells were supplied with nutrients from the basal side. Since the humidity inside the incubator was above 95%, the surface of the cells was maintained in a moist condition. The culture assembly was carried out over a period of 7 days, and the medium was changed every other day.
Figure 1.
Schematic representation of the double-dish culture system. A: Control culture. The epithelial cells are cultured on cell-free type I collagen gels. B: Reconstruction culture for gastric surface mucosa. Isolated gastric epithelial cells are maintained on fibroblast-containing collagen gel layer under immersion conditions. C: Effects of fibroblast-derived soluble factors on epithelial differentiation. Isolated epithelial cells are cultured on the cell-free collagen gel layer with conditioned medium elaborated by monolayer-cultured fibroblasts in the outer dish. D: Air-liquid interface (ALI) culture. The reconstructed gastric mucosa was treated with an ALI environment.
Examination of Culture Cells
Culture cells were examined as described below.
Histology
Culture cells were fixed with Carnoy’s solution at 4°C for 2 hours, routinely processed and then vertically embedded in paraffin. Deparaffinized cross sections were stained with hematoxylin and eosin (H&E).
Histochemistry
To detect mucous substances, sections were stained with periodic acid-Schiff (PAS). To identify zymogen granules in chief cells, sections were stained using a modified Bowie’s method. 18 The sections were hydrolyzed in preheated 1 mol/L HCl at 60°C for 10 minutes, immersed in 2% Schiff’s reagent for 10 minutes, washed with 2.5% sodium thiosulfate in 0.5 mol/L HCl, rinsed with distilled water and immersed in 20% alcohol for 5 minutes, placed in Bowie’s solution for 15 hours at room temperature, differentiated with 98% acetone, and dehydrated with 100% acetone, cleared with xylene and mounted. To detect neuroendocrine granules in endocrine cells, sections were stained with Grimelius’ method. 19
Immunohistochemistry
The antibodies used are listed below. A rat polyclonal antibody cathepsin E (Wako Pure Chemicals, Osaka, Japan) was used to detect the gastric surface mucous cells. 20 A mouse monoclonal antibody 45M1 (Novocastra, Newcastle, UK) was used to detect the gastric mucin MUC5AC. 21 A mouse monoclonal antibody HIK1083 (M-GGMC-1, Kanto Kagaku, Tokyo, Japan) was used to detect the gastric mucous neck cells. 22 Mouse monoclonal anti-H+/K+-ATPase was from Affinity Bioreagents (Golden, CO). Mouse monoclonal antibodies MUC2 and CD10 (Novocastra, Newcastle, UK) were used to identify the intestinal goblet cells and absorptive cells, respectively. Mouse monoclonal anti-cytokeratin was from Nichirei (Tokyo, Japan), and mouse monoclonal anti-vimentin was from Dako (Kyoto, Japan). Deparaffinized sections were immunostained by the avidin-biotin complex immunoperoxidase (ABC) method as described previously. 23 As a positive control, normal mouse stomach and intestine was applied to immunohistochemistry. The controls always gave positive results in a cell type-specific manner. As a negative control, PBS or normal rabbit IgG was used instead of primary antibodies. The controls always gave negative results.
Transmission Electron Microscopy
Culture cells were fixed with 2.5% glutaraldehyde and 1% osmic acid, dehydrated with alcohol, and embedded in epoxy resin. For the detection of mucosubstances, thin sections were stained using periodic acid-thiocarbohydrazide-silver proteinate (PA-TCH-SP) method. 24 The sections were observed by electron microscopy (JME-1210, JEOL, Tokyo, Japan).
Morphometric Analysis
To estimate the degree of epithelial cytodifferentiation, the vertical cell height, vertical nuclear height, and nuclear vertical-horizontal ratio were measured. A total of 500 cells in each of the culture conditions were counted in at least 20 randomly chosen non-contiguous fields (high power view, ×40 objective) of the H&E sections from five independent experiments.
Cell Proliferation
Bromodeoxyuridine (BrdU, 10 mg/ml) was added to the culture medium for 6 hours. Deparaffinized sections from the culture assembly were immunostained with anti-BrdU antibody in accord with the procedures of the cell proliferation kit’s manufacturer (Amersham, Buckinghamshire, UK). To obtain the rate of nuclear BrdU intake, 1000 cells were counted and the percentage of BrdU-positive nuclei calculated.
Apoptosis
In accord with the Apop Tag manual, using an in situ apoptosis detection kit and peroxidase (Oncor Inc., Gaithersburg, MD), apoptotic cells were labeled by the terminal deoxynucleotidyl transferase-mediated deoxy-UTP nick end labeling (TUNEL) method. 25 To obtain the index of apoptosis, 1000 cells were counted and the percentage of apoptotic cells calculated.
Reagents
In this study, the following growth factors were added to culture medium to determine whether they could reproduce epithelial differentiation as induced by gastric fibroblasts. Human recombinant hepatocyte growth factor (HGF), epidermal growth factor (EGF), and transforming growth factor-β1 (TGF-β1) were from R&D Systems (Minneapolis, MN). Human recombinant basic fibroblast growth factor (bFGF), keratinocyte growth factor (KGF), insulin-like growth factor I (IGF-I), insulin-like growth factor II (IGF-II) and platelet-derived growth factor (PDGF) were from Pepro Tech Inc. (Rocky Hill, NJ).
Statistical Analysis
Data obtained from five to six independent experiments were analyzed by Mann-Whitney’s U-test. Results were expressed as means ± SEM and were considered significant with P values of <0.05.
Results
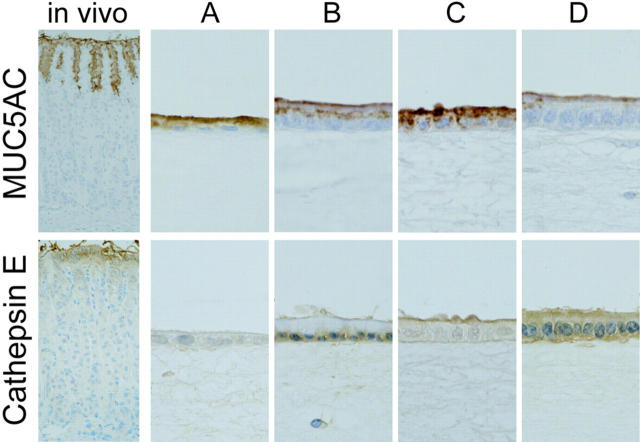
In the control culture, the epithelial cells showed immature features on a cell-free collagen gel (Figure 1A) ▶ . The cells had a wide flat shape, flattened nuclei, and a small amount of PAS-positive mucus (Figure 2A) ▶ . Cell height was 5.1 ± 0.2 μm, mean nuclear height was 2.0 ± 0.1 μm and the nuclear vertical-horizontal ratio was 0.26 ± 0.16. Cells were positive for MUC5AC, and had very little staining with cathepsin E (Figure 3A) ▶ . These results suggest that the isolated epithelial cells were derived from a pit cell lineage, and that the cells showed immature features on the cell-free collagen gels without fibroblastic support. Next, we reconstructed the gastric surface mucosa in a three-dimensional co-culture system. The epithelial cells were cultured on a fibroblast-containing collagen gel corresponding to the lamina propria in immersion conditions (Figure 1B) ▶ . Under these conditions, highly polarized epithelium was observed. Cultured epithelial cells showed a tall columnar shape, basally situated round nuclei, and clear cytoplasm that was almost entirely filled with mucus (Figure 2B) ▶ . The intracytoplasmic mucus stained strongly with PAS. Cell height was 11.7 ± 0.5 μm, mean nuclear height was 4.6 ± 0.2 μm, and the nuclear vertical-horizontal ratio was 1.08 ± 0.07. Immunohistochemically, these epithelial cells were positive for gastric surface mucous cell maker, MUC5AC, and the cytoplasm of the cells stained with cathepsin E (Figure 3B) ▶ . Less than 5% of the epithelial cells had mucus that reacted with the mucous neck cell maker, HIK1083 (data not shown). Neither Bowie’s method, H+/K+-ATPase, nor Grimelius’ method had any reactivity in the cultured cells (data not shown), indicating that none of these epithelial cells showed glandular differentiation; such as to chief, parietal, or endocrine cells. The cells also showed no expression of intestinal goblet cell marker MUC2 and absorptive cell marker CD10 (data not shown). In the fine structure, cultured epithelial cells (Figure 4A) ▶ were almost identical to mouse gastric surface mucous cells just after birth (Figure 4B) ▶ . They showed stubby microvilli protruding from the apical membrane. Many secretory granules in the apical cytoplasm and many glycogen granules in the supra- and infra-nucleus region appeared. Using the PA-TCH-SP method, the secretory granules and glycogen granules were seen to contain large amounts of the reaction product (Figure 4C) ▶ . The lateral membranes of each cell attached to those of their neighbors by junctional complexes at their apical regions (Figure 4D) ▶ , and below by desmosomes, which often connected with well-developed intercellular digitations (Figure 4E) ▶ . An electron-dense band, the basal lamina, followed the contours of the basal surface of these epithelial cells (Figure 4F) ▶ . These results suggest that the cultured epithelial cells differentiate into gastric surface mucous cells in the pit cell stage.
Figure 2.
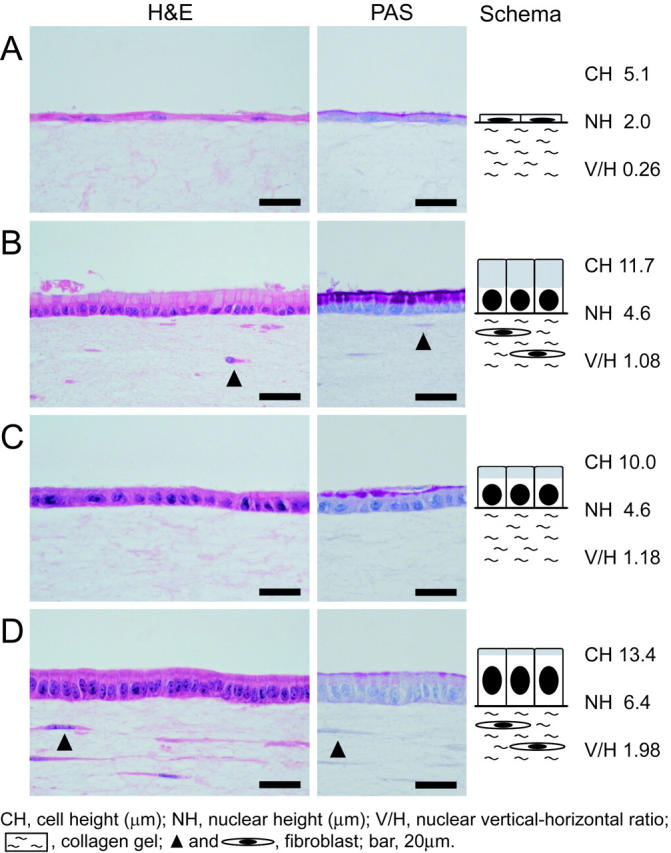
Light micrographs of the reconstructed gastric mucosa. A: In the control culture, the epithelial cells appear flat on the cell-free gel. A small amount of mucus is stained with PAS. B: Reconstructed gastric surface mucosa as schematically illustrated in Figure 1B ▶ . These epithelial cells have a tall columnar shape, basally situated round nuclei, and mucus-filled cytoplasm. The intracytoplasmic mucus is stained with PAS. C: The epithelial cells have a columnar shape on cell-free collagen gel with conditioned medium elaborated by fibroblast-feeder layer. D: Under the air-liquid interface condition, the epithelial cells show a tall columnar shape and oval nuclei on fibroblast-containing collagen gel. The epithelial cells have a small amount of PAS-positive mucus beneath the apical membrane.
Figure 3.
Immunohistochemistry in vivo and in vitro. Note that MUC5AC is stained with mucus of a pit cell lineage unrelated to their differentiated stage. On the other hand, cathepsin E is stained gradually along with their maturity. The gastric surface epithelium, pit top cells, stain strongly with cathepsin E. A: Control culture. The epithelial cells have very little reactivity. B: The reconstructed gastric mucosa. The epithelial cells show cytoplasmic staining. C: Fibroblast-feeder layer. The cells have very little staining. D: ALI culture. The epithelial cells show strong positive like the gastric surface epithelium in vivo.
Figure 4.
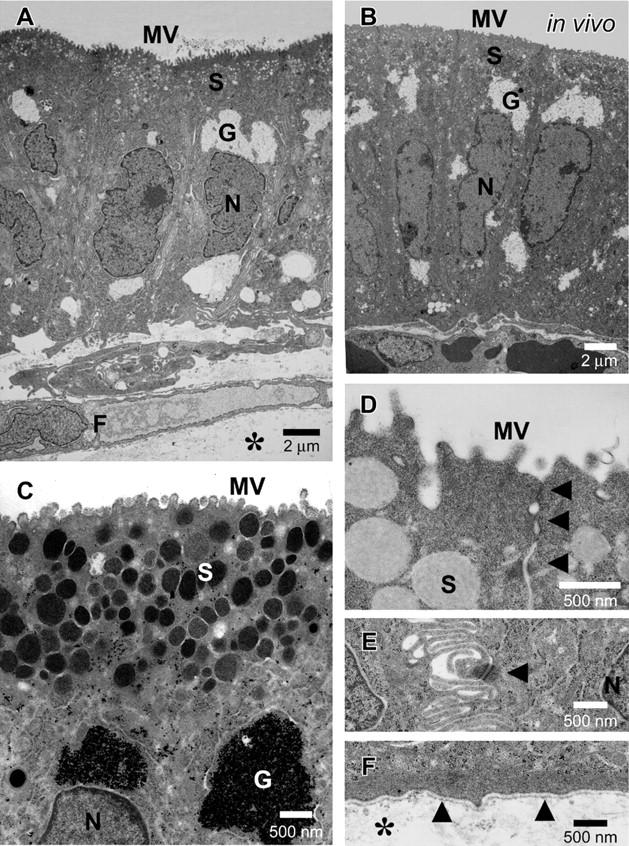
Electron micrographs of the reconstructed gastric mucosa in immersion conditions. A: The epithelial cells are maintained on fibroblast-containing collagen gel under immersion conditions. Highly polarized, tall columnar epithelial cells have many secretory granules (S) and many glycogen granules (G) in their cytoplasm. B: Mouse gastric surface mucous cells just after birth in vivo. C: Secretory granules (S) and glycogen granules (G) contain a large amount of the reaction product in vitro. Stubby microvilli (MV) protrude from the apical surface. PA-TCH-SP method. D: The cultured epithelial cells are typically joined together in their apical regions by junctional complexes (arrows). E: Lateral membranes of cultured epithelial cells are highly interdigitated and interconnected by desmosomes (arrow). F: Cultured epithelial cells organize the basal lamina (arrows) at the contact side with collagen gel (*). *, collagen gel; F, fibroblast; G, glycogen granules; S, secretory granules; MV, microvilli; N, nuclei.
In medium conditioned by a fibroblast-feeder layer (Figure 1C) ▶ , the polarized epithelial cells on the cell-free collagen gel showed a columnar shape, round nuclei, and the production of PAS-positive mucus (Figure 2C) ▶ . Mean cell height was 10.0 ± 0.3 μm, mean nuclear height 4.6 ± 0.2 μm, and the nuclear vertical-horizontal ratio 1.18 ± 0.10. These epithelial cells were positive for MUC5AC; however, as they had very little staining, the results could not conclusively show that they were specific for the expression of cathepsin E (Figure 3C) ▶ . The results, though, did suggest that the fibroblasts play an important role in the differentiation mechanisms of gastric surface mucous cells. To assess whether well-characterized growth/differentiation factors might mediate the foveolar differentiation, isolated gastric epithelial cells were cultured on the cell-free collagen gel either with EGF (10 ng/ml), bFGF (10 ng/ml), IGF-I (10 ng/ml), IGF-II (10 ng/ml), PDGF (10 ng/ml), KGF (10 ng/ml), HGF (10 ng/ml), or TGF-β1 (10 ng/ml). However, none of these treatments satisfactorily promoted pit cell differentiation (data not shown).
In the next series of experiments, we examined the effects of an ALI on gastric surface mucous cells in the reconstructed gastric mucosa (Figure 1D) ▶ , because ALI culture conditions promote the differentiation of various epithelial cell types. 11-17 When the epithelial cells were treated with an ALI environment, they showed a tall columnar shape, central oval nuclei, and considerably lesser PAS-positive mucus just under the apical membrane (Figure 2D ▶ compared to Figure 2B ▶ ). Mean cell height was 13.4 ± 0.2 μm, mean nuclear height 6.4 ± 0.1 μm, and the nuclear vertical-horizontal ratio 1.98 ± 0.05. Cells were positive for MUC5AC in the apical cytoplasm (Figure 3D) ▶ . The cultured epithelial cells strongly stained with cathepsin E like the gastric mucosal surface in vivo (Figure 3D) ▶ . Figure 5B ▶ shows the fine structure of pit top cells in mouse gastric mucosa at 7 days after birth. Note the secretory granules in the superficial cytoplasm and the disappearance of glycogen granules. Interestingly, cultured epithelial cells closely resembled the pit top cells in the fine structure (Figure 5A) ▶ . The secretory granules accumulated just beneath the apical membrane and glycogen granules disappeared in their cytoplasm. These epithelial cells also displayed microvilli at their apical surface, junctional complexes on the lateral surface, and basal lamina at the basal side. Surprisingly, these cells released the contents of the secretory granules by exocytosis (Figure 5C) ▶ . On the other hand, cytodifferentiation was not observed in cultured epithelial cells on fibroblast-free collagen gel in the ALI environment (data not shown). These results suggest that the ALI environment modified the state of differentiation of the pit cells.
Figure 5.
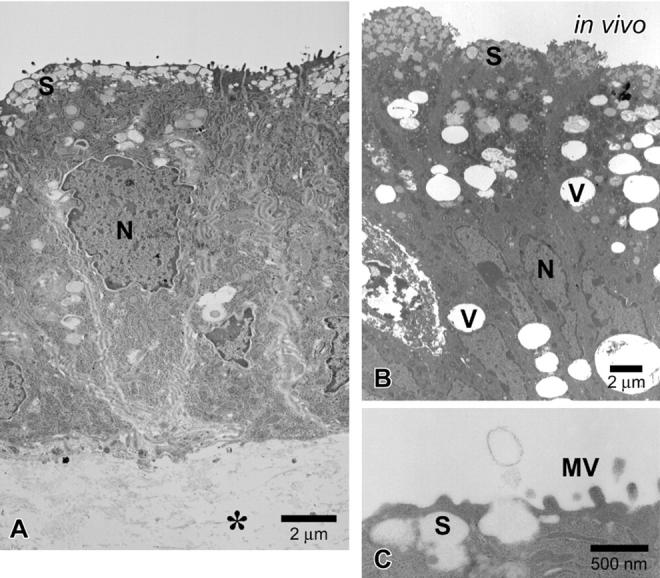
Electron micrographs of the reconstructed gastric mucosa at an air-liquid interface environment. A: The epithelial cells are maintained on a fibroblast-containing collagen gel with an ALI treatment. Tall, columnar epithelial cells accumulate many secretory granules (S) beneath the apical membrane in vitro. Glycogen granules disappear from the cytoplasm. B: Pit top cells in mouse gastric surface mucosa at 7 days after birth. The epithelial cells have secretory granules (S) just under the apical membrane. C: The secretory granule appears to be opening and releasing its contents by exocytosis. *, collagen gel; S, secretory granules; MV, microvilli; N, nuclei; V, vacuole.
We evaluated cell proliferation and apoptosis of these differentiated epithelial cells in both immersion and ALI culture conditions. The BrdU intake of the cells showed no significant differences between immersion and ALI cultures (0.31 ± 0.17 and 0.98 ± 0.55, respectively; P = 0.20). The index of apoptotic cells in the ALI culture was higher than that in the immersion culture (1.39 ± 0.37 and 0.47 ± 0.05, respectively; P = 0.02). These results suggest that an ALI environment might cause the acceleration of foveolar differentiation, and then degeneration as the final fate of gastric surface mucous cells in vitro.
Discussion
In the present study, we established a new in vitro method that reconstructed gastric surface mucosa. When given fibroblastic support, cultured epithelial cells differentiated into a mature mucous secreting cell phenotype. Mammalian gastric mucosa has two types of mucous secreting cells, surface mucous and mucous neck cells. In the cytoplasm of these two types, mucins differ from each other in their different sugar structures. In mouse glandular stomach, surface mucous and mucous neck cells typically react with anti-gastric mucin monoclonal antibodies, MUC5AC 26 and HIK1083. 22 The gastric luminal surface epithelium, namely pit top cells, expresses the acid proteinase, cathepsin E. 26 Isolated gastric epithelial cells are positive for MUC5AC, and epithelial cells with fibroblastic support strongly expressed cathepsin E under ALI conditions. None of the cells showed glandular differentiation, such as to chief, parietal, or endocrine cells. They could also be distinguished from intestinal goblet or absorptive cells by the absence of any expression of the intestinal cell markers, MUC2 and CD10. Thus we identified the cultured epithelial cells as gastric surface mucous cells, not only by their morphological features but also by their functional characteristics.
Primary cultures of gastric epithelial cells have been established in conventional culture dishes. 27-30 Compared with those conventional cultures, our in vitro method creates a physiological environment: the epithelial cells are supported by type I collagen as extracellular matrix, supplied with nutrients from the basal side, interact closely with stromal fibroblasts, and are exposed to luminal air at the apical surface. When given fibroblastic support, isolated epithelial cells differentiated into mature surface mucous cells, suggesting that epithelial-mesenchymal interaction is important for foveolar differentiation. This phenomenon, fibroblast-induced cytodifferentiation, is consistent with other surface-covering epithelium such as skin, 11 cornea, 13 oral cavity, 14 and urinary bladder mucosa. 31 Although some papers have argued that glandular differentiation of the gastric mucosa requires glucocorticoids, 10 our preliminary experiments have not confirmed glandular differentiation in this in vitro method from adding glucocorticoids. Healing of a gastric ulcer requires the proliferation of immature pit cells in the isthmus region that migrate from the ulcer margin onto the granulation tissue and cover the ulcer bed. 3-5 In this process, fibroblasts in the granulation tissue probably play an important role in the progressively foveolar differentiation of the regenerative cells. Chronic gastritis is defined as the presence of chronic mucosal inflammatory changes leading eventually to foveolar hyperplasia and glandular atrophy. These mucosal alterations may be influenced not only by various inflammatory cytokines but also by stromal fibroblasts. Further studies to identify the fibroblast-derived soluble factors and the expressions of their receptors on epithelial cells are currently being undertaken in our laboratories.
The ALI culture method is known to be useful in promoting the specific differentiation of several epithelial cell types. 11-17 Additionally, our recent studies have demonstrated that an ALI environment induces invasive growth of squamous cell carcinoma cells, 32 the active proliferation of fibroblasts involving a mitogen-activated protein kinase cascade, 23 and the long-term preservation of thyroid follicles with C cells in vitro. 33 Although the precise mechanisms of ALI-modified cytodifferentiation remain unclear, we believe that the ALI technique is close to the physiological environment for surface epithelial cells in that the cells are supplied with nutrients from the basal side and are not immersed in the apical media. These conditions may provide the epithelial cells with a suitable environment to retain their correct polarity. Several studies have shown that ALI culture conditions improved oxygenation with both increased oxygen consumption and ATP contents, and decreased lactate production in cells; in contrast, conventional immersion cultures hindered adequate oxygenation with increases in glycolytic enzyme synthesis and lactate production. 34-37 Surface-covering epithelial cell types may use the aerobic environment to maintain homeostasis, and thus protect subepithelial organs against air. We hypothesize that the ALI environment might be involved in the differentiation mechanisms of gastric surface mucous cells in vivo. Further studies to elucidate the mechanism of ALI-induced cytodifferentiation are currently underway in our laboratories.
This in vitro method should be useful to construct various gastric disease models, eg, Helicobacter pylori infection, ulcer healing, and cancer invasion. The inner and outer culture dishes could be regarded as the gastric lumen and body sides, respectively. A pathogenic agent, for instance, may be mixed with the inner medium as if it were infused into the stomach. An agent, including other cell types, could be injected into the outer medium and may be construed as coming from blood or interstitial fluid. Furthermore, our in vitro method could be applied to reconstruct the mucosa of the alimentary tract and open new ways for studying the gastric epithelial cell biology and various diseases of the gastric mucosa.
Acknowledgments
We thank Drs. Ryuichi Iwakiri, Lisa Filippi, Hiroyoshi Ota, Shigehisa Aoki, and Sherif M. Karam for discussions. We also thank Hiroyuki Ideguchi, Shinichi Nakahara, Toshimi Tabata, Fumihiro Mutoh, and Asako Fushihara for their excellent technical assistance.
Footnotes
Address reprint requests to Akifumi Ootani, M.D., Departments of Pathology and Internal Medicine, Saga Medical School, 5–1-1 Nabeshima, Saga 849-8501, Japan. E-mail: ohtani@post.saga-med.ac.jp.
References
- 1.Karam SM, Leblond CP: Dynamics of epithelial cells in the corpus of the mouse stomach: I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec 1993, 236:259-279 [DOI] [PubMed] [Google Scholar]
- 2.Karam SM, Leblond CP: Dynamics of epithelial cells in the corpus of the mouse stomach: II. Outward migration of pit cells. Anat Rec 1993, 236:280-296 [DOI] [PubMed] [Google Scholar]
- 3.Johnson LR: Regulation of gastrointestinal mucosal growth. Physiol Rev 1988, 68:456-502 [DOI] [PubMed] [Google Scholar]
- 4.Allen A, Flemstrom G, Garner A, Kivilaakso E: Gastroduodenal mucosal protection. Physiol Rev 1993, 73:823-857 [DOI] [PubMed] [Google Scholar]
- 5.Gordon JI: Understanding gastrointestinal epithelial cell biology: lessons from mice with help from worms and flies. Gastroenterology 1993, 105:315-324 [DOI] [PubMed] [Google Scholar]
- 6.Montgomery RK, Mulberg AE, Grand RJ: Development of the human gastrointestinal tract: twenty years of progress. Gastroenterology 1999, 116:702-731 [DOI] [PubMed] [Google Scholar]
- 7.Gumbiner BM: Epithelial morphogenesis. Cell 1992, 69:385-387 [DOI] [PubMed] [Google Scholar]
- 8.Yasugi S: Role of epithelial-mesenchymal interactions in differentiation of epithelium of vertebrate digestive organs. Dev Growth Differ 1993, 35:1-9 [DOI] [PubMed] [Google Scholar]
- 9.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB: Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol 1999, 277:C183-C201 [DOI] [PubMed] [Google Scholar]
- 10.Tsukada S, Ichinose M, Yahagi N, Matsubara Y, Yonezawa S, Shiokawa K, Furihata C, Miki K, Fukamachi H: Induction of precocious pepsinogen synthesis by glucocorticoids in fetal rat gastric epithelium in organ culture: importance of mesenchyme for epithelial differentiation. Differentiation 1998, 62:239-247 [DOI] [PubMed] [Google Scholar]
- 11.Sugihara H, Toda S: Reconstruction culture of the skin. Cell and Tissue Culture: Laboratory Procedures. Doyle A Griffiths JB Newell DG eds. 1994:pp 3A. 2.1-3A. 2.12 John Wiley and Sons, Chichester
- 12.Ootani A, Toda S, Fujimoto K, Sugihara H: An air-liquid interface promotes the differentiation of gastric surface mucous cells (GSM06) in culture. Biochem Biophys Res Commun 2000, 271:741-746 [DOI] [PubMed] [Google Scholar]
- 13.Minami Y, Sugihara H, Oono S: Reconstruction of cornea in three-dimensional collagen gel matrix culture. Invest Ophthalmol Vis Sci 1993, 34:2316-2324 [PubMed] [Google Scholar]
- 14.Yamaguchi T, Shin T, Sugihara H: Reconstruction of the laryngeal mucosa: a three-dimensional collagen gel matrix culture. Arch Otolaryngol Head Neck Surg 1996, 122:649-654 [DOI] [PubMed] [Google Scholar]
- 15.Delcourt-Huard A, Corlu A, Joffre A, Magloire H, Bonnaure-Mallet M: Reconstituted human gingival epithelium: non-submerged in vitro model. In Vitro Cell Dev Biol Anim 1997, 33:30-36 [DOI] [PubMed] [Google Scholar]
- 16.Whitcutt MJ, Adler KB, Wu R: A biphasic chamber system for maintaining polarity of differentiation of cultured respiratory tract epithelial cells. In Vitro Cell Dev Biol 1998, 24:420-428 [DOI] [PubMed] [Google Scholar]
- 17.Kohsa K, Yamada H, Sugihara H: Influence of air exposure treatment on alveolar type II epithelial cells cultured on extracellular matrix. Cell Struct Funct 1996, 21:81-89 [DOI] [PubMed] [Google Scholar]
- 18.Hayama M, Katsuyama T, Nakayama J, Akamatsu T, Honda T: A combined stain for identifying epithelial cells of the gastric mucosa. Stain Technol 1987, 62:35-40 [DOI] [PubMed] [Google Scholar]
- 19.Grimelius L: A silver nitrate stain for α-2 cells in human pancreatic islets. Acta Soc Med Ups 1968, 73:243-270 [PubMed] [Google Scholar]
- 20.Sakai H, Saku T, Kato Y, Yamamoto K: Quantitation and immunohistochemical localization of cathepsins E and D in rat tissues and blood cells. Biochim Biophys Acta 1989, 991:367-375 [DOI] [PubMed] [Google Scholar]
- 21.Bara J, Chastre E, Mahiou J, Singh RL, Forgue-Lafitte ME, Hollande E, Godeau F: Gastric M1 mucin, an early oncofetal marker of colon carcinogenesis, is encoded by the MUC5AC gene. Int J Cancer 1998, 75:767-773 [DOI] [PubMed] [Google Scholar]
- 22.Ishihara K, Kurihara M, Goso Y, Urata T, Ota H, Katsuyama T, Hotta K: Peripheral α-linked N-acetylglucosamine on the carbohydrate moiety of mucin derived from mammalian gastric gland mucous cells: epitope recognized by a newly characterized monoclonal antibody. Biochem J 1996, 318:409-416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toda S, Yokoi F, Yamada S, Yonemitsu N, Nishimura T, Watanabe K, Sugihara H: Air exposure promotes fibroblast growth with increased expression of mitogen-activated protein kinase cascade. Biochem Biophys Res Commun 2000, 270:961-966 [DOI] [PubMed] [Google Scholar]
- 24.Thiery JP: Mise en evidence des polysaccharides sur coupes fines en microscopie electronique. J Microsc 1967, 6:987-1018 [Google Scholar]
- 25.Gavrieli Y, Sherman Y, Ben-Sasson SJ: Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992, 119:493-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiocca R, Villani L, Tenti P, Cornaggia M, Finzi G, Riva C, Capella C, Bara J, Samloff IM, Solcia E: The foveolar cell component of gastric cancer. Hum Pathol 1990, 21:260-270 [DOI] [PubMed] [Google Scholar]
- 27.Takahashi M, Ota S, Shimada T, Hamada E, Kawabe T, Okudaira T, Matsumura M, Kaneko N, Terano A, Nakamura T, Omata M: Hepatocyte growth factor is the most potent endogenous stimulant of rabbit gastric epithelial cell proliferation and migration in primary culture. J Clin Invest 1995, 95:1994-2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terano A, Ivey KJ, Stachura J, Sekhon S, Hosojima H, McKenzie WN, Krause WJ, Wyche JH: Cell culture of rat gastric fundic mucosa. Gastroenterology 1982, 83:1280-1291 [PubMed] [Google Scholar]
- 29.Chen MC, Lee AT, Soll AH: Mitogenic response of canine fundic epithelial cells in short-term culture to transforming growth factor α and insulin-like growth factor I. J Clin Invest 1991, 87:1716-1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rokutan K, Yamada M, Torigoe J, Saito T: Transforming growth factor-β inhibits proliferation and maturation of cultured guinea pig gastric pit cells. Am J Physiol 1998, 275:G526-G533 [DOI] [PubMed] [Google Scholar]
- 31.Fujiyama C, Masaki Z, Sugihara H: Reconstruction of the urinary bladder mucosa in three-dimensional collagen gel culture: fibroblast-extracellular matrix interactions on the differentiation of transitional epithelial cells. J Urol 1995, 153:2060-2067 [PubMed] [Google Scholar]
- 32.Yamada S, Toda S, Shin T, Sugihara H: Effects of stromal fibroblasts and fat cells and an environmental factor air exposure on invasion of laryngeal carcinoma (HEp-2) cells in a collagen gel invasion assay system. Arch Otolaryngol Head Neck Surg 1999, 125:424-431 [DOI] [PubMed] [Google Scholar]
- 33.Toda S, Watanebe K, Yokoi F, Matsumura S, Suzuki K, Ootani A, Aoki S, Koike N, Sugihara H: A new organotypic culture of thyroid tissue maintains three-dimensional follicles with C cells for a long term. Biochem Biophys Res Commun 2002, 294:906-911 [DOI] [PubMed] [Google Scholar]
- 34.Stevens KM: Oxygen requirements for liver cells in vitro. Nature 1965, 206:199. [DOI] [PubMed] [Google Scholar]
- 35.Dickman KG, Mandel LJ: Glycolytic and oxidative metabolism in primary renal proximal tubule cultures. Am J Physiol 1989, 257:C333-C340 [DOI] [PubMed] [Google Scholar]
- 36.Kondo M, Tamaoki J, Sakai A, Kameyama S, Kanoh S, Konno K: Increased oxidative metabolism in cow tracheal epithelial cells cultured at air-liquid interface. Am J Respir Cell Mol Biol 1997, 16:62-68 [DOI] [PubMed] [Google Scholar]
- 37.Bebok Z, Tousson A, Schwiebert LM, Venglarik CJ: Improved oxygenation promotes CFTR maturation and trafficking in MDCK monolayers. Am J Physiol 2001, 280:C135-C145 [DOI] [PubMed] [Google Scholar]