Abstract
Uterine fibroids (leiomyomas) are a major women’s health problem. Currently, the standard for treatment remains hysterectomy, because no other treatment modalities can reduce both symptoms and recurrence. As leiomyomas are a hyperproliferation of smooth muscle cells, we sought to understand the regulation of uterine smooth muscle cell mitogenesis by the glycosaminoglycan heparin, which has been extensively studied as an anti-proliferative molecule in vascular smooth muscle cells. Using matched pairs of human myometrial and leiomyoma smooth muscle cells from the same uterus, we demonstrate that the proliferation and motility of both cell types are inhibited by heparin. We report that the decrease in cell number seen in the presence of heparin is not because of cell death. Interestingly, there is significant patient-to-patient variability in the proliferation response but not in the motility response to heparin. Furthermore, nonanticoagulant and anticoagulant heparin were equally effective at inhibiting leiomyoma and myometrial smooth muscle cell proliferation. These results warrant further investigation into the possibility that heparin might be useful in the treatment of uterine fibroids.
Uterine fibroids (leiomyomas) are the most common pelvic tumor in women. 1,2 Leiomyomas are a major cause of abnormal uterine bleeding and also cause pelvic pressure, pain, and impaired fertility. 3 They are responsible for nearly 200,000 hysterectomies annually in the United States. 4 The prevalence of clinically apparent uterine leiomyomas is 20 to 25% in the overall population, 1,2 and black women have an incidence rate that is at least three times higher than that of white women. 5,6
Although these tumors affect a large population of women and are a major women’s health problem, the precise pathophysiology of uterine fibroids remains unclear. Currently, the only definitive treatment option available is hysterectomy, as other treatment options reduce symptoms only temporarily and do not significantly alter recurrence. 7 Although it clearly would be advantageous to discover new treatment options for women wishing to maintain an intact uterus without the risk of a high recurrence rate, achieving this goal requires a better understanding of the cellular and molecular mechanisms controlling the pathogenesis of uterine fibroids.
Because leiomyomas are benign tumors of smooth muscle cells (SMCs), one approach to developing therapeutic rationales is to elucidate the mechanisms that regulate human uterine SMC (HUtSMC) proliferation and to search for molecules that can inhibit HUtSMC mitogenesis. One such candidate molecule is the glycosaminoglycan heparin, which has been extensively studied as an anti-proliferative and anti-motility molecule in vascular SMCs in vivo and in cell culture. 8 SMCs from other organs are also sensitive to the anti-proliferative action of heparin, as are glomerular mesangial cells. 9-13 Both anticoagulant (AC) and nonanticoagulant (NAC) forms of heparin are equally effective as inhibitors of vascular SMC proliferation. 14-16 Recently, indirect evidence suggesting that AC heparin can inhibit myometrial and leiomyoma SMC proliferation was reported. 17 Though intriguing, it is unclear from this study how many patient samples were analyzed, or whether the leiomyoma and myometrial SMCs were from the same uterus, leaving open the important question of patient-to-patient variability.
In this study, we provide the first direct evidence that both AC and NAC heparin have equipotent anti-proliferative and anti-motility effects on eight matched pairs of human myometrial and leiomyoma SMCs. The effect on these cell functions is not because of apoptosis. Furthermore, we investigate the important parameters of inherent variability between patients and the cell-cycle dependence of heparin’s effect on HUtSMCs.
Materials and Methods
All tissue culture plastic was obtained from Costar (Cambridge, MA) and Falcon (BD Biosciences, Franklin Lakes, NJ). Fetal calf serum (FCS) (characterized) was purchased from HyClone (Logan, UT). Dulbecco’s modified Eagle’s medium (DMEM), trypsin-ethylenediaminetetraacetic acid, glutamine, and penicillin-streptomycin were purchased from Invitrogen (Grand Island, NY). NAC heparin was obtained from Glycomed (Alameda, CA). AC heparin was obtained from Pharmacia & Upjohn (Kalamazoo, MI).
Patients
Fibroid and myometrial tissues were obtained from eight premenopausal women with symptomatic uterine fibroids at the time of elective hysterectomy (patients A, B, C, G, and H in Tables 1 ▶ and 2 ▶ ; age, 45 to 47 years) or myomectomy (patients D, E, and F in Tables 1 and 2 ▶ ; age, 33 to 38 years) and who were not receiving any type of hormonal or drug therapy. Collection of tissues was obtained under a consent for use of discarded human tissue in accordance with the Human Research Committee of the Brigham and Women’s Hospital (Boston, MA). Four of the patients (patients A, B, C, and G) were in the proliferative phase of the menstrual cycle at the time of surgery and one was in the secretory phase (patient H); the menstrual phase of the myomectomy patients was not available.
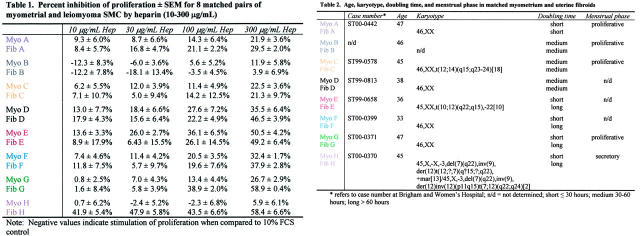
Table 1.
Percent Inhibition of Proliferation ± SEM for 8 Matched Pairs of Myometrial and Leiomyoma SMCs by Heparin (10-300 μg/mL)
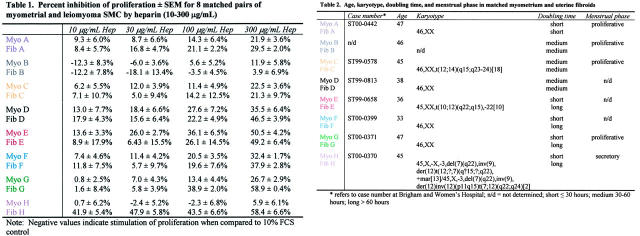
Table 2.
Age, karyotype, doubling time, and menstrual phase in matched myometrium and uterine fibroids
Cell Culture
Fibroid and myometrial tissues were minced into 1- to 2-mm explants and placed in 10% FCS/DMEM containing 200 U/ml of collagenase (Invitrogen). Myometrial tissue was digested for 8 to 10 hours, and fibroid tissue for 18 to 20 hours in a 37°C incubator in 5% CO2. Disaggregated cells were then centrifuged at 300 × g for 5 minutes, the resulting cell pellet was resuspended in 10% FCS/DMEM, and the cells were plated into 75-ml tissue-culture flasks. Cultures were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2. The purity of the cells was assessed as described in our earlier studies. 18 Normal growth medium consisted of 10% FCS/DMEM supplemented with 4 mmol/L of l-glutamine, 100 U/ml of penicillin G sodium, and 100 μg/ml of streptomycin sulfate. Cells were growth-arrested by placing them in 0.25 to 0.5% FCS/DMEM for 3 days. Cells were used in experiments at passages 4 to 9.
Proliferation Assay
Myometrial and leiomyoma SMCs were plated into 24-well tissue culture plates (well area, 1.9 cm2) at a density of 1.1 to 1.3 × 104 cells/cm2 for exponentially growing cells and 2.6 to 3.2 × 103 cells/cm2 for cells to be growth-arrested, and allowed to attach overnight. The following day, the exponentially growing cells were treated with either 10% FCS/DMEM or 10% FCS/DMEM with heparin at different concentrations (10 to 300 μg/ml). Cells plated at lower density were growth-arrested as discussed above. After 3 days, cells were released from growth-arrest by adding 10% FCS/DMEM or 10% FCS/DMEM with heparin at different concentrations (10 to 300 μg/ml). Exponentially growing cells were counted after 2 days of heparin treatment and growth-arrested cells were counted after 2 to 4 days using a Coulter Counter (Beckman Coulter, Fullerton, CA). At least two independent experiments were performed in duplicate for each data point shown. Growth inhibition was calculated as follows:
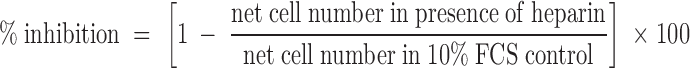 |
Migration Assay
Myometrial and leiomyoma SMCs were plated onto two-chamber tissue culture slides (growth area, 4 cm2) at a density of 4.5 to 5 × 104 cells/cm2 in 10% FCS/DMEM and 5 mmol/L of hydroxyurea (Sigma, St. Louis, MO) and were allowed to attach overnight. The following day, the confluent cell layer was scraped using a P200 pipette tip, creating a wound ∼300 μm wide. The cells were rinsed five times in 10% FCS/DMEM to wash away cells scraped off in the wound, and heparin was added (10 to 500 μg/ml) in the presence of 5 mmol/L of hydroxyurea. Digital images (×100 magnification) were taken of the initial wound and at various times until the 10% FCS/DMEM control wound was confluent (ie, until the cells had fully migrated into the wound from the edges), typically 22 hours. All cells were fixed in 1% paraformaldehyde (Fisher Scientific, Pittsburgh, PA) when the 10% FCS/DMEM control was confluent. Hoechst 33258 (Sigma)-containing mounting medium was added to visualize cell nuclei, and the wounds were analyzed by fluorescence microscopy. Fluorescent images were taken (magnification ×100), and the number of cells migrating into the wound was quantitated using Optimas imaging software (Media Cybernetics, Silver Spring, MD). At least two, and usually many more, independent experiments were performed in duplicate for each data point shown. Inhibition of cell migration was calculated as above.
Caspase-3 Assay
Myometrial and leiomyoma SMCs were grown in 175-cm2 tissue-culture flasks containing 10% FCS/DMEM until ∼60% confluence. At this point, either 10% FCS/DMEM or 10% FCS/DMEM containing 500 μg/ml of heparin was added. Cells were allowed to grow in the presence or absence of heparin for 2 days. As a positive control, cells were treated with 0.5 μmol/L of staurosporine (Sigma) in 10% FCS/DMEM for 20 hours. After these treatments, cells were trypsinized and counted on a Coulter Counter (Beckman Coulter), and 2 × 106 cells were used in a colorimetric caspase-3 assay (Clontech, Palo Alto, CA). The assay was performed as described in the manufacturer’s protocol with the exception that cell pellets were resuspended in 1× phosphate-buffered saline and centrifuged at 400 × g for 10 minutes before freezing at −80°C. Two independent experiments were performed in triplicate for each data point shown.
Statistical Analysis
Data are presented as the mean ± SEM. Significance of difference was assessed using a paired Student’s t-test. Differences were considered significant when P < 0.05.
Results
Growth State Affects Heparin Sensitivity of HUtSMCs
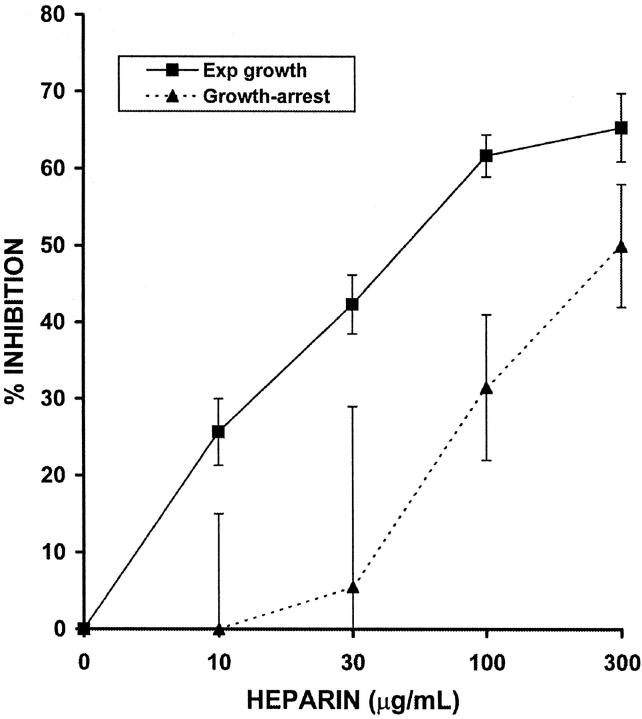
SMCs and closely related cells exhibit variable cell-cycle dependence in their responses to heparin. Vascular SMCs released from quiescence have been shown to be 50- to 100-fold more sensitive to the anti-proliferative effect of heparin than exponentially growing cells. 16 Closely related glomerular mesangial cells, however, are only twofold to fourfold more sensitive to heparin when released from growth-arrest than when exponentially growing. 9
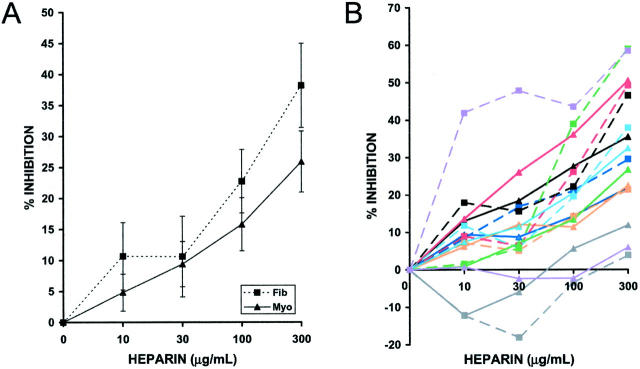
To test the cell-cycle dependence of the anti-proliferative action of heparin on HUtSMCs, we performed direct cell-counting proliferation assays using serum-starved (quiescent) or exponentially growing cells. The myometrial and leiomyoma SMCs used are from the same patient, thus providing a good internal control for any observed differences between the normal and fibroid cells. In contrast to both vascular SMCs and glomerular mesangial cells, we observed that exponentially growing myometrial SMCs are sixfold more sensitive to the anti-proliferative effect of heparin than are cells released from growth-arrest. The IC50 for quiescent cells is 300 μg/ml and the IC50 for exponentially growing cells is 50 μg/ml. The efficacy of heparin was also greater in exponentially growing cells: at 300 μg/ml heparin, exponentially growing cells are inhibited by 65% whereas cells released from quiescence are inhibited by 50% (Figure 1) ▶ . None of the other four SMC cultures tested (two myometrial and two leiomyoma) were more sensitive in the quiescent state, and exponentially growing cells were, on average, fourfold to sixfold more sensitive to the anti-proliferative effect of heparin than cells released from growth-arrest (data not shown). Because of this finding, we chose to use exponentially growing cells in all subsequent experiments.
Figure 1.
Exponentially growing myometrial SMCs are more sensitive to the anti-proliferative effect of heparin than are cells released from quiescence. Exponentially growing SMCs were treated with 10% FCS in the presence or absence of NAC heparin (10 to 300 μg/ml) for 2 days. Quiescent cells were obtained by placing the cells in 0.25% FCS for 3 days. Cells were then released from growth arrest by the addition of 10% FCS in the presence or absence of NAC heparin (10 to 300 μg/ml) and were counted when confluent using a Coulter Counter. The dashed line represents the cells released from growth arrest and the solid line represents exponentially growing cells.
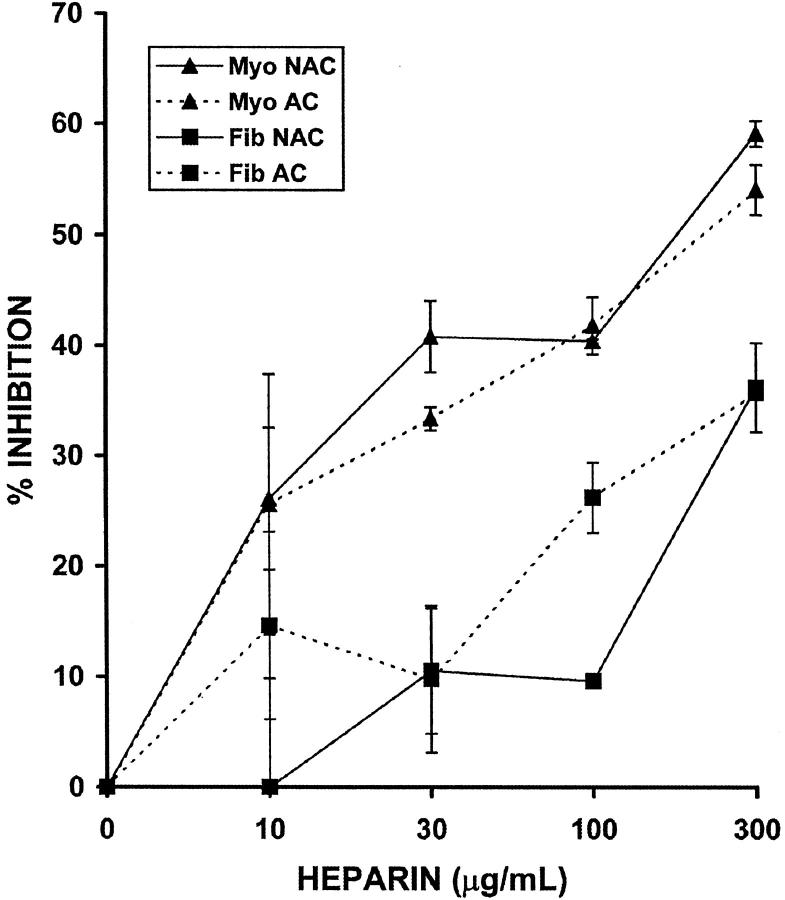
NAC Heparin Is as Effective an Anti-Proliferative Agent as AC Heparin on HUtSMCs
Previous studies have shown that both NAC and AC heparin are equally effective in suppressing vascular SMC proliferation, 14-16 but no such studies are available on HUtSMCs. To compare the anti-proliferative action of AC and NAC heparin on HUtSMCs, we performed proliferation assays on matched pairs of myometrial and leiomyoma SMCs (Figure 2) ▶ . Using direct cell counting methods, we found that the amount of inhibition at 300 μg/ml heparin was 55 to 60% for both AC and NAC heparin for the myometrial SMCs, and 36% for both AC and NAC heparin for the leiomyoma SMCs. Thus, the anti-proliferative response to AC and NAC heparin is virtually identical in myometrial and leiomyoma SMCs.
Figure 2.
NAC and AC heparin are equally effective in the inhibition of myometrial and leiomyoma SMCs. Myometrial (Myo) and leiomyoma (Fib) SMCs were treated with 10% FCS in the presence or absence of NAC or AC heparin (10 to 300 μg/ml) for 2 days. Direct cell counting was performed on a Coulter Counter. Dashed lines represent cells treated with AC heparin and solid lines represent cells treated with NAC heparin. Note that the SEM for Fib NAC at 100 μg/ml heparin is <1%.
The Effect of Heparin on HUtSMC Proliferation Shows Patient-to-Patient Variability
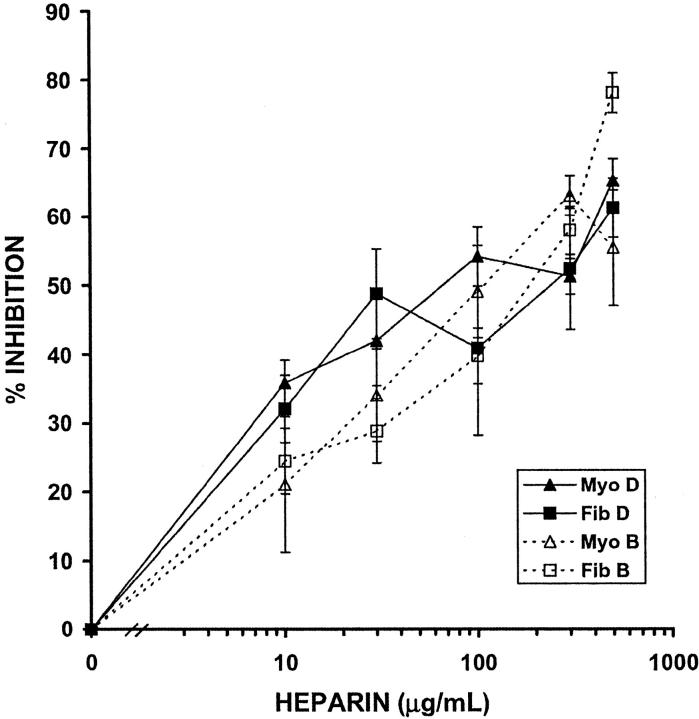
The results described above demonstrate directly that heparin is capable of inhibiting HUtSMC proliferation. However, it is possible that significant variation in heparin responsiveness among individuals in a population may exist. To determine the variability of the anti-proliferative activity within the patient population, we tested matched pairs of myometrial and leiomyoma SMCs from eight patients using a direct cell-counting proliferation assay (Figure 3) ▶ . Exponentially growing cells were treated for 2 days in 10% FCS with or without heparin (10 to 300 μg/ml). Our study showed no difference between the inhibition on day 2 or day 3 for either cell type (data not shown); for simplicity, we have chosen to show 2-day heparin treatments (Figure 3, A and B) ▶ . Both myometrial and leiomyoma SMCs showed a dose-dependent sensitivity to the anti-proliferative activity of heparin (Figure 3A) ▶ that was statistically significant when compared to untreated cells, as indicated by the results of a paired Student’s t-test (P < 0.05). However, the results of a paired Student’s t-test revealed no statistically significant difference in heparin sensitivity between normal and fibroid SMCs (P > 0.05).
Figure 3.
The anti-proliferative response to heparin is variable from patient to patient. Exponentially growing myometrial (Myo) and leiomyoma (Fib) SMCs were treated with 10% FCS in the presence or absence of NAC heparin (10 to 300 μg/ml) for 2 days. Cells were counted on a Coulter Counter. A: Average of data from eight patients. B: Matched pair data from the same eight patients. Solid lines represent myometrial SMCs and dashed lines represent leiomyoma SMCs. Each different color represents a different patient, and the color code for patients is the same as that for Tables 1 and 2 ▶ . SEM for values in B are shown in Table 1 ▶ . Negative values indicate stimulation of proliferation when compared to 10% FCS control.
Although the eight patient average did not indicate a statistically significant difference between myometrial and leiomyoma SMCs, the heparin response among individual patients is highly variable (Table 1 ▶ , Figure 3B ▶ ). The myometrial and leiomyoma SMCs isolated from patient E show a high degree of inhibition with heparin (50% at 300 μg/ml heparin; Table 1 ▶ and Figure 3B ▶ , red lines), whereas cells isolated from patient B show almost no inhibition with heparin (<12% at 300 μg/ml heparin; Table 1 ▶ and Figure 3B ▶ , gray lines). For six of the eight matched pairs tested, there is no statistically significant difference between the heparin response of myometrial and leiomyoma SMCs isolated from the same patient (P > 0.05); however, two interesting cases were noted (Table 1 ▶ and Figure 3B ▶ , patients G and H, green and purple lines). In both patients, the leiomyoma SMCs (∼59% at 300 μg/ml in both) were inhibited by heparin more than the myometrial SMCs (27% at 300 μg/ml for patient G; 6% at 300 μg/ml for patient H). These results indicate that considerable patient-to-patient variability exists in the anti-proliferative response to heparin. However, it should be noted that 81% of the 16 cultures tested show moderate (20 to 40%) or strong (>40%) sensitivity to heparin.
We considered the possibility that menstrual phase, karyotype, doubling time, or age might influence the sensitivity of myometrial and leiomyoma SMCs to heparin. We can rule out menstrual phase as a cause of increased sensitivity to heparin because there appears to be no difference between the anti-proliferative effect of heparin on myometrial or leiomyoma SMCs isolated from the proliferative or secretory phase (Table 2 ▶ , Figure 3B ▶ ). In addition, abnormal karyotype does not appear to influence heparin sensitivity as abnormal karyotypes were seen in both heparin-sensitive and insensitive cells (Table 2 ▶ , Figure 3B ▶ ). Interestingly, although the doubling time of the myometrial SMCs does not appear to affect the response to heparin, an increased doubling time does seem to correlate with increased heparin sensitivity for leiomyoma SMCs (Table 2 ▶ , Figure 3B ▶ ) because four (patients E to H) of the five most heparin-responsive leiomyoma SMC cultures at 300 μg/ml of heparin have high doubling times. Finally, the age of the patient at the time of surgery did appear to influence heparin responsiveness. The three matched pairs isolated from the myomectomy patients who were the youngest in age (patients D to F in Table 2 ▶ ) were the most heparin responsive of the eight matched pairs studied (Figure 3B) ▶ .
Heparin Inhibits HUtSMC Motility
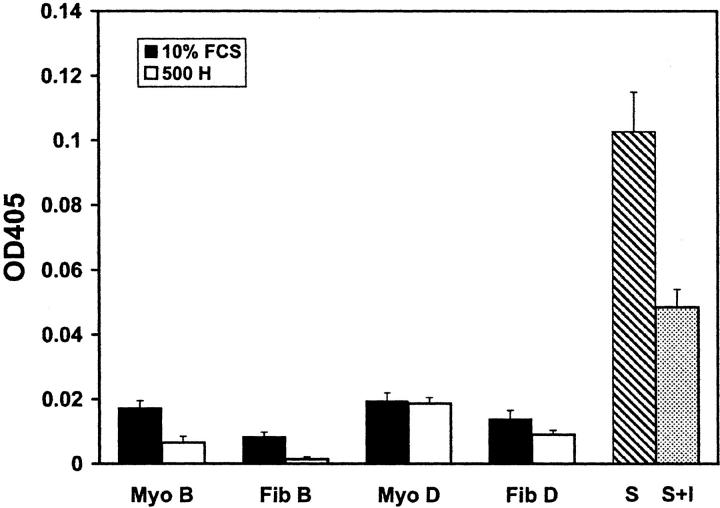
Heparin strongly inhibits motility in vascular SMCs, 19-21 but no information is available on the effect of heparin in uterine SMCs in any species. We therefore chose to use one of the most heparin-responsive (patient D) and the least heparin-responsive (patient B) cell cultures from the proliferation assay to identify whether heparin had the same effect on the motility of these cells (Figures 4 and 5 ▶ and Table 3 ▶ ). Using the scratch wound assay, the inhibition of migration was found to be dose-dependent in all four SMC cultures. Interestingly, however, the SMC culture that showed little response to heparin in a proliferation assay (patient B) demonstrated a robust response to heparin in our motility assay. The motility of the leiomyoma SMCs from patient B (Fib B) was inhibited by 58% at 300 μg/ml, whereas the myometrial SMCs from patient B (Myo B) were inhibited by 63% at 300 μg/ml of heparin (Figure 5 ▶ , Table 3 ▶ ). Similarly, the motility of the leiomyoma SMCs from patient D (Fib D) was inhibited by 52% at 300 μg/ml, and the myometrial SMCs from the same patient (Myo D) were inhibited by 51%. The IC50 for both SMC cultures from both patients was ∼100 to 200 μg/ml. As was the case for the effect of heparin on proliferation, no statistically significant difference was seen between the effects of heparin on the motility of myometrial and leiomyoma SMCs (P > 0.05).
Figure 4.
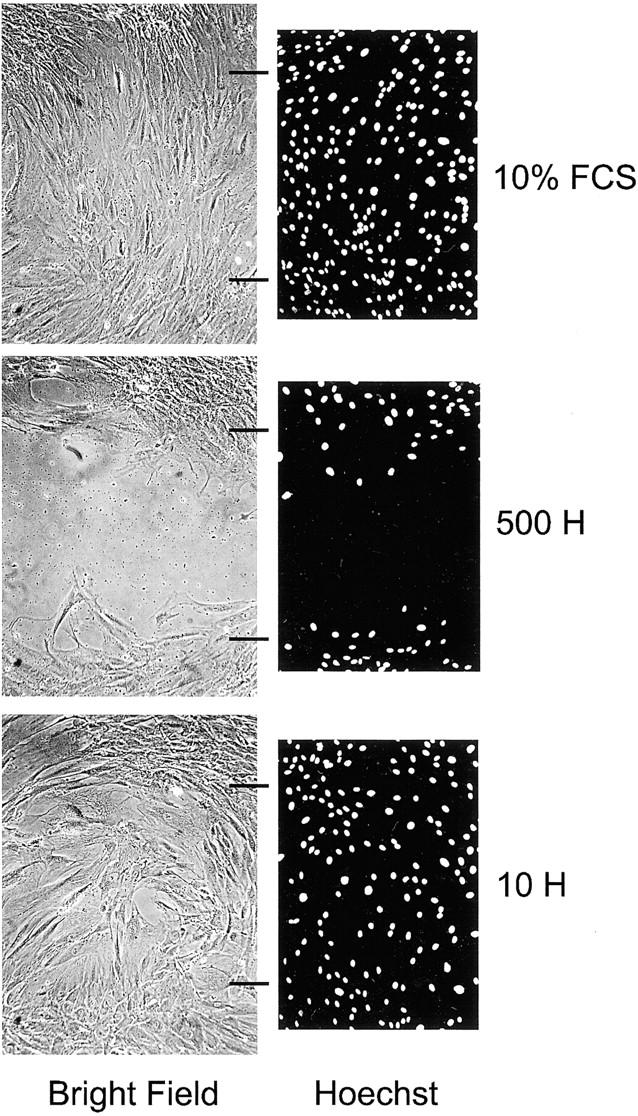
Heparin inhibits the migration of leiomyoma SMCs into a wound. Bright-field and fluorescent microscopy images for 10% FCS control and 500 and 10 μg/ml of heparin (500 H and 10 H). Hoechst-stained images have been converted to black and white images for greater contrast. Wound edges are indicated by horizontal black lines and are the same for both bright-field and fluorescent images. The cells shown are leiomyoma SMCs from patient B (Fib B in Tables 1 and 2 ▶ ). Original magnifications, ×100.
Table 3.
Percent Inhibition of Migration ± SEM for Two Matched Pairs of Myometrial and Leiomyoma SMCs by Heparin (10 to 500 μg/mL)
| 10 μg/mL Hep | 30 μg/mL Hep | 100 μg/mL Hep | 300 μg/mL Hep | 500 μg/mL Hep | |
|---|---|---|---|---|---|
| Myo D | 35.9 ± 3.3% | 42.0 ± 6.5% | 54.2 ± 4.3% | 51.3 ± 2.6% | 65.3 ± 3.2% |
| Fib D | 32.1 ± 4.9% | 48.8 ± 6.5% | 40.9 ± 12.6% | 52.4 ± 8.8% | 61.3 ± 4.3% |
| Myo B | 21.1 ± 9.9% | 34.1 ± 6.7% | 49.1 ± 6.7% | 63.1 ± 2.9% | 55.5 ± 8.4% |
| Fib B | 24.5 ± 4.8% | 28.9 ± 4.7% | 39.8 ± 4.0% | 58.0 ± 3.5% | 78.1 ± 2.9% |
Figure 5.
Heparin inhibits the motility of myometrial and leiomyoma SMCs. Cells were plated to confluence and a wound was created using a P200 pipette tip. 10% FCS in the presence or absence of NAC heparin (10 to 500 μg/ml) was added and cells were fixed when the 10% FCS control wound was confluent. Hoechst 33258-containing medium was added to the fixed cells and fluorescent images were taken. The number of cells migrating into the wound was determined using image analysis software. Solid lines represent myometrial (Myo) and leiomyoma (Fib) SMCs from patient D, and dashed lines represent SMCs from patient B (Tables 1 and 2) ▶ . SEM are shown in Table 3 ▶ .
We considered the possibility that cell proliferation might confound our motility data interpretation. Therefore, we repeated these experiments in the presence of 5 mmol/L of hydroxyurea to inhibit cell proliferation. 19 No difference was seen in the number of cells that migrated into the wound in the presence or absence of heparin (Table 4) ▶ . In addition, the number of cells with mitotic figures was <1% (data not shown).
Table 4.
Percent Inhibition ± SEM in Heparin Migration Experiments in the Presence or Absence of Hydroxyurea
| Heparin concentration | HU | No HU |
|---|---|---|
| 10 | 46 ± 5 | 36 ± 3 |
| 30 | 35 ± 10 | 42 ± 7 |
| 100 | 42 ± 7 | 54 ± 4 |
| 300 | 67 ± 1 | 51 ± 3 |
| 500 | 58 ± 3 | 65 ± 3 |
HU, hydroxyurea.
Number of cells migrating into the wound in the absence of heparin was 188 in the presence of HU; 217 in the absence of HU.
Heparin Does Not Induce Apoptosis in HUtSMCs
Early work on heparin as an anti-proliferative molecule found that the inhibitory effect of heparin on vascular SMCs and glomerular mesangial cells was reversible and therefore unlikely to be cytotoxic. 9,16 In addition, previous studies have shown that heparin does not induce apoptosis in pulmonary vascular pericytes or in porcine aortic SMCs. 22 The demonstrated differences in heparin responsiveness among different SMC types and the need to rigorously establish the role, if any, of heparin-induced apoptosis in HUtSMCs led us to undertake an examination of programmed cell death in myometrial and leiomyoma SMCs.
Apoptosis was analyzed using a colorimetric caspase-3 assay on the most and least heparin-responsive SMC cultures to proliferation (patients D and B, respectively). We chose to use this assay because caspase-3 is central to the caspase cascade and is activated by many apoptotic stimuli. 23 The four SMC cultures were treated with 10% FCS in the presence or absence of 500 μg/ml of heparin for 2 days, at which time cells were lysed and caspase-3 activity was analyzed. Staurosporine (0.5 μmol/L in 10% FCS, 20-hour treatment) was used as a positive control. By light microscopy, >98% of the cells treated with staurosporine had the morphological characteristics of apoptosis, such as cell rounding, shrinkage, and membrane blebbing (Figure 6) ▶ . In contrast, <2% of cells in all four SMC cultures treated with either 10% FCS or 10% FCS containing 500 μg/ml of heparin showed features typical of apoptosis (Figure 6) ▶ .
Figure 6.
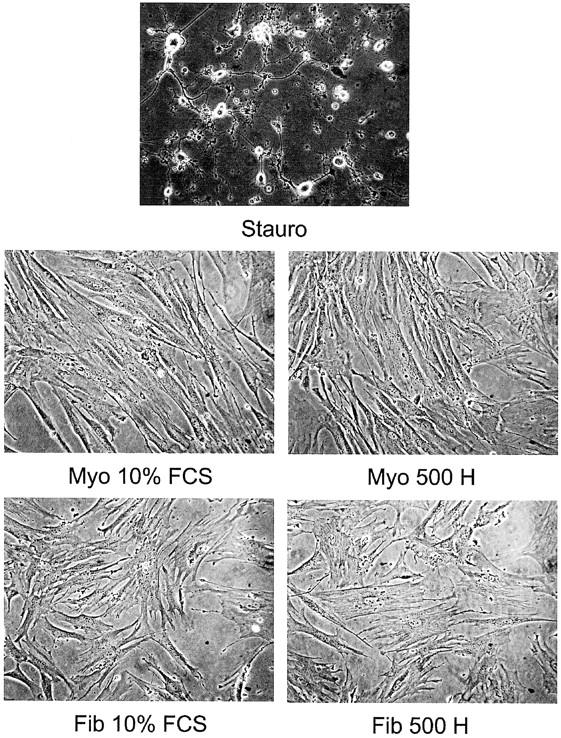
Cells treated with staurosporine but not heparin have distinctive features of apoptosis. Myometrial (Myo) and leiomyoma (Fib) SMCs were treated with 10% FCS in the presence or absence of 500 μg/ml of heparin (500 H) for 2 days. Staurosporine (0.5 μmol/L) (Stauro) was used as a positive control. Cells shown are from patient B (Tables 1 and 2) ▶ . Original magnifications, ×200.
Quantitative analysis of caspase-3 activity indicates that heparin does not appear to induce apoptosis in any of the four SMC cultures tested (Figure 7) ▶ . The positive control with staurosporine had an OD405 of 0.108, whereas the staurosporine-containing caspase-3 inhibitor had an OD405 of 0.049. Any readings below 0.049 were therefore considered background and not because of caspase-3 activity. All of the heparin-treated and untreated matched pairs had OD405 below 0.049, and therefore contain no significant caspase-3 activity. Thus, both light microscopy and biochemical data indicate that the decrease in cell number found in heparin-treated HUtSMCs in the proliferation assays is not because of induction of apoptosis.
Figure 7.
Heparin does not affect apoptosis in myometrial and leiomyoma SMCs. Myometrial (Myo) and leiomyoma (Fib) SMCs were treated with 10% FCS in the presence or absence of 500 μg/ml of heparin (500 H) for 2 days. Staurosporine (0.5 μmol/L) (S) was used as a positive control. The caspase-3 inhibitor DEVD-fmk was used to inhibit the caspase-3 activity induced by staurosporine (S + I). Apoptosis was analyzed using a colorimetric caspase-3 kit. Patients shown are patients B and D from Tables 1 and 2 ▶ .
Discussion
In this communication, we have carefully examined the ability of heparin to regulate important functional properties of HUtSMCs. To our knowledge, this is the first study that systematically examines both proliferation and motility in matched pairs of normal and fibroid SMCs from women. Using the matched pair approach, we have made the following new observations in HUtSMCs: 1) heparin inhibits the motility of both normal myometrial and leiomyoma SMCs; 2) heparin exerts an anti-proliferative effect on both normal and fibroid HUtSMCs; 3) in contrast to their vascular counterparts, HUtSMCs are much more responsive to heparin when exponentially growing than when quiescent; and 4) both AC and NAC heparin species appear to be equally effective anti-proliferative agents on these cell types. Although the ability of heparin to inhibit the proliferation and motility of normal myometrial SMCs might be predictable based on previous studies with vascular SMCs, the anti-proliferative and anti-motility effect of heparin on fibroid SMCs was not predictable, given the neoplastic nature of this cell. Furthermore, the greatly increased sensitivity of exponentially growing uterine SMCs to heparin compared to their vascular counterparts was quite unexpected and represents a major difference in the response of these two SMC types.
Uterine fibroids are a very common problem in women’s health, affecting as many as one in four women, with symptoms that include severe pain, bleeding, and reproductive dysfunction. Current invasive treatments consist of hysterectomy, myomectomy, and uterine artery embolization, but only hysterectomy is capable of reducing both symptoms and recurrence. Medical treatment for uterine fibroids includes anti-progestins, GnRH agonists, androgens, and selective estrogen receptor modulators, but these treatments either cause significant side effects, such as bone loss with GnRH agonists, or cause only a temporary reduction in uterine volume, with an increase in size on cessation of treatment. 1,7 Thus, these pharmacological agents are typically only used preoperatively to reduce fibroid size. Because it is clearly advantageous for women to retain their uterus rather than undergo hysterectomy, a pharmacological agent that reduces fibroid size would be highly desirable. Furthermore, the fibroid SMCs derived from younger women are especially sensitive to heparin, which may bode well for the possible treatment of this group of women for whom retention of reproductive capacity is especially important.
Because leiomyomas are a common cause of abnormal uterine bleeding, the possibility of treating this condition with AC heparin seems unlikely. However, NAC heparin does not have the hemorrhagic side effects of AC heparin, 14 and our data show that NAC and AC heparin are equally effective in the inhibition of HUtSMC proliferation, in agreement with earlier studies on vascular SMCs. 14-16 We considered the possibility that apoptosis might contribute to the reduction in cell number seen in the presence of heparin. However, our data rule this out, corroborating other apoptosis studies in vascular SMCs and pulmonary vascular pericytes. 22,24
Surprisingly, we found that exponentially growing HUtSMCs are slightly more sensitive to the anti-proliferative effect of heparin than HUtSMCs released from growth-arrest. This is in stark contrast to the results observed for other SMC types. In vascular SMCs, cells released from growth-arrest are 50- to 100-fold more sensitive to the anti-proliferative effect of heparin than are exponentially growing cells. 16 To a lesser extent, glomerular mesangial cells, a closely related cell type, are also more sensitive to heparin when released from quiescence; however, the difference is only twofold to fourfold. 9 Thus, in this study, we present for the first time a cell type that is more sensitive to heparin when exponentially growing. The reasons for this difference could be because of intrinsic differences between growth-arrested and exponentially growing uterine SMCs that are not present in vascular SMCs or glomerular mesangial cells. Mechanistically, the reasons for this inherent heparin resistance or sensitivity are not yet known and may include differences in the number or type of cell-surface heparin receptors, 16,25 differences in binding and internalization of heparin, 25,26 presence or absence of heparin-regulated mitogenic signaling pathways, 27 or differences in the activation of heparin-induced genes such as CCN5 (Cop-1). 28 Experiments to examine these possibilities as well as the effect of heparin on the matrix environment in HUtSMCs are underway. The identification of both high and low heparin responders from this study will provide additional insight into molecular mechanism.
Although our direct analysis of the effect of heparin on HUtSMC proliferation agrees with an earlier finding, 17 we find that a high degree of variability exists between patients. Even though we have found that 13 of 16 of the cultures tested (including 7 of 8 leiomyoma SMC cultures) display a moderate-to-strong heparin response (>20% inhibition), we identify 3 of 16 cultures with little or no response (<20% inhibition). This variability is important because our data suggest that not all patients would respond positively to heparin-based therapy. However, the possibility that a pharmacological intervention could potentially inhibit leiomyoma growth in many patients wishing to maintain an intact uterus would make it an attractive alternative to surgery.
Regarding the heterogeneity in heparin response among the different patients, it should be noted that the response of individual fibroids to GnRH agonist therapy (the only nonsurgical therapy for fibroids at this time) is also highly variable, with reduction in volumes ranging from 0 to 100% by 6 months of treatment. 29-32 It is unclear what produces the variation in response, although calcification of fibroids does appear to inhibit shrinkage. 29 It has been suggested that the heterogeneity of leiomyoma composition, differences in steroid receptor content, intramyoma steroid metabolism, or growth factor content may contribute to the wide variation of myoma response to a hypoestrogenic state. 29,30,32 Variation exists among uterine fibroids in their karyotypes and has been correlated with tumor size 33,34 and uterine location. 34 This might also lead to large variations in phenotype and biological function between tumors. 35,36
The issue of variability in response to heparin remains an intriguing one. The availability of highly characterized human cell cultures with a wide range of heparin sensitivities should provide an excellent model system for testing putative mechanisms of action of heparin. The existence of heparin-resistant vascular SMCs is well established, and San Antonio and colleagues 37 have proposed a definition of heparin-resistant vascular SMCs as those that exhibit ≤25% inhibition by heparin at 100 of μg/ml heparin under standard assay conditions that include growth-arrest. This is a useful definition for vascular SMCs, but as noted by these authors it may be difficult to apply to other cell types under different culture or assay conditions. For example, HUtSMCs are more sensitive when exponentially growing, thus the standard conditions for vascular SMCs do not apply. The range of heparin sensitivities intrinsic to HUtSMCs suggests that it might be more useful to characterize cultures of this cell type as sensitive (≥40% inhibited by heparin), moderately sensitive (20 to 39% inhibited by heparin), and resistant (<20% inhibited by heparin). It is also difficult to define fixed heparin concentrations at which the comparison should be made among different cell types, because so many factors affect the efficacy of heparin, in particular high variability in heparin preparations from different suppliers and serum concentration in the assay. Taken together, our data warrants further investigation into the possibility that heparin, especially NAC species, might be useful in the treatment of fibroids. We note with caution, however, that our study has raised the important question of how to determine which individuals have heparin-sensitive cells before undergoing treatment. Ideally, one would want to assess heparin sensitivity before starting treatment, and a practical means of accomplishing this is required. In addition, leiomyomas are considered clonal tumors; 38 thus, for those women with multiple tumors within the same uterus, the idea of heparin resistance and sensitivity poses the question of whether heparin treatment would only inhibit proliferation in sensitive tumors and have no effect on other tumors.
Although we observed patient-to-patient variation in proliferation response, we did not find the same variation in our motility studies. The reason for this is not clear. We considered the possibility that cell proliferation may confound the motility results, but we can rule this out in our experiments for three reasons. First, we observed no difference in the number of cells migrating into the wound in the presence or absence of heparin when the motility experiments were repeated using 5 mmol/L of hydroxyurea to inhibit cell proliferation. Second, because the wound is created from confluent cells in a quiescent growth state, the number of mitotic figures in the wounded cultures was <1%. Significant cell division is not observed until 36 to 40 hours after wounding, at which time three of the four cultures tested had already filled in the wound. Finally, analysis of the wounds at 18 hours also shows substantial inhibition of motility (data not shown). Thus, our data suggest that different pathways may be used for the inhibition of proliferation and motility by heparin, or that heparin may interfere with multiple steps in the signaling pathways for motility and mitogenesis, one or more of which are not present in insensitive SMCs.
To our knowledge, we present the first report of a quantitative in vitro motility assay using primary cultures of HUtSMCs. One study has been published using an in vitro migration assay on uterine leiomyoma cells derived from the Eker rat. 39 Our finding that heparin can inhibit HUtSMC motility agrees with other motility studies in vascular SMCs in which heparin had an anti-motility effect. 19-21 Motility studies on uterine leiomyomas are interesting in light of benign metastasizing leiomyoma, a condition in which leiomyoma cells migrate and seed other locations, most commonly the lung, where they can cause significant morbidity and even death. 40 As little is known about this rare condition, examining the motility of leiomyoma cells from patients with benign metastasizing leiomyoma could lead to clues regarding its pathophysiology.
In summary, our data indicate that matched pairs of HUtSMCs can be used successfully in the rigorous analysis of the mechanisms regulating the pathogenesis of fibroids. Furthermore, it indicates that heparin can inhibit two HUtSMC functions thought to be important in the pathogenesis of leiomyomas: proliferation and motility. This culture system should be highly useful in future studies of molecular mechanism and should provide a useful system for examining the efficacy of putative therapeutic agents designed for the treatment of fibroids.
Acknowledgments
We thank Jen Wubben for help in maintaining cell cultures and David Neskey for assistance in collecting tissue samples. We are also deeply indebted to Andrew Lake for his many helpful discussions.
Footnotes
Address reprint requests to John J. Castellot, Jr., Department of Anatomy and Cellular Biology, Tufts University School of Medicine, 136 Harrison Ave., Boston, MA 02111. E-mail: john.castellot@tufts.edu.
Supported by the National Institutes of Health (grants HL49973 to J. J. C., HD35148 to R.A.N., and CA78895 to C. C. M.).
References
- 1.Nowak RA: Fibroids: pathophysiology and current medical treatment. Baillieres Best Pract Res Clin Obstet Gynaecol 1999, 13:223-238 [DOI] [PubMed] [Google Scholar]
- 2.Nowak RA: Identification of new therapies for leiomyomas: what in vitro studies can tell us. Clin Obstet Gynecol 2001, 44:327-334 [DOI] [PubMed] [Google Scholar]
- 3.Stewart EA: Uterine fibroids. Lancet 2001, 357:293-298 [DOI] [PubMed] [Google Scholar]
- 4.Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB: Hysterectomy in the United States, 1988–1990. Obstet Gynecol 1994, 83:549-555 [DOI] [PubMed] [Google Scholar]
- 5.Cramer DW: Epidemiology of myomas. Semin Reprod Endocrinol 1992, 10:320-324 [Google Scholar]
- 6.Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, Willett WC, Hunter DJ: Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol 1997, 90:967-973 [DOI] [PubMed] [Google Scholar]
- 7.Moorehead ME, Conard CJ: Uterine leiomyoma: a treatable condition. Ann NY Acad Sci 2001, 948:121-129 [PubMed] [Google Scholar]
- 8.Mishra-Gorur K, Delmolino LM, Castellot JJ, Jr: Biological functions of heparan sulfate and heparan sulfate proteoglycans. Trends Glycosci Glycotechnol 1998, 10:193-210 [Google Scholar]
- 9.Castellot JJ, Jr, Hoover RL, Harper PA, Karnovsky MJ: Heparin and glomerular epithelial cell-secreted heparin-like species inhibit mesangial-cell proliferation. Am J Pathol 1985, 120:427-435 [PMC free article] [PubMed] [Google Scholar]
- 10.Coffey AK, Karnovsky MJ: Heparin inhibits mesangial cell proliferation in habu-venom-induced glomerular injury. Am J Pathol 1985, 120:248-255 [PMC free article] [PubMed] [Google Scholar]
- 11.Graham MF, Drucker DE, Perr HA, Diegelmann RF, Ehrlich HP: Heparin modulates human intestinal smooth muscle cell proliferation, protein synthesis, and lattice contraction. Gastroenterology 1987, 93:801-809 [DOI] [PubMed] [Google Scholar]
- 12.Johnson PR, Armour CL, Carey D, Black JL: Heparin and PGE2 inhibit DNA synthesis in human airway smooth muscle cells in culture. Am J Physiol 1995, 269:L514-L519 [DOI] [PubMed] [Google Scholar]
- 13.Kilfeather SA, Tagoe S, Perez AC, Okona-Mensa K, Matin R, Page CP: Inhibition of serum-induced proliferation of bovine tracheal smooth muscle cells in culture by heparin and related glycosaminoglycans. Br J Pharmacol 1995, 114:1442-1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyton JR, Rosenberg RD, Clowes AW, Karnovsky MJ: Inhibition of rat arterial smooth muscle cell proliferation by heparin. In vivo studies with anticoagulant and nonanticoagulant heparin. Circ Res 1980, 46:625-634 [DOI] [PubMed] [Google Scholar]
- 15.Hoover RL, Rosenberg R, Haering W, Karnovsky MJ: Inhibition of rat arterial smooth muscle cell proliferation by heparin. II. In vitro studies. Circ Res 1980, 47:578-583 [DOI] [PubMed] [Google Scholar]
- 16.Castellot JJ, Jr, Addonizio ML, Rosenberg R, Karnovsky MJ: Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol 1981, 90:372-379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horiuchi A, Nikaido T, Ya-Li Z, Ito K, Orii A, Fujii S: Heparin inhibits proliferation of myometrial and leiomyomal smooth muscle cells through the induction of alpha-smooth muscle actin, calponin h1 and p27. Mol Hum Reprod 1999, 5:139-145 [DOI] [PubMed] [Google Scholar]
- 18.Nowak RA, Rein MS, Heffner LJ, Friedman AJ, Tashjian AH, Jr: Production of prolactin by smooth muscle cells cultured from human uterine fibroid tumors. J Clin Endocrinol Metab 1993, 76:1308-1313 [DOI] [PubMed] [Google Scholar]
- 19.Majack RA, Clowes AW: Inhibition of vascular smooth muscle cell migration by heparin-like glycosaminoglycans. J Cell Physiol 1984, 118:253-256 [DOI] [PubMed] [Google Scholar]
- 20.Clowes AW, Clowes MM: Kinetics of cellular proliferation after arterial injury. IV. Heparin inhibits rat smooth muscle mitogenesis and migration. Circ Res 1986, 58:839-845 [DOI] [PubMed] [Google Scholar]
- 21.Kohno M, Yokokawa K, Yasunari K, Minami M, Kano H, Mandal AK, Yoshikawa J: Heparin inhibits human coronary artery smooth muscle cell migration. Metabolism 1998, 47:1065-1069 [DOI] [PubMed] [Google Scholar]
- 22.Pelisek J, Armeanu S, Nikol S: Quiescence, cell viability, apoptosis and necrosis of smooth muscle cells using different growth inhibitors. Cell Prolif 2001, 34:305-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porter AG, Janicke RU: Emerging roles of caspase-3 in apoptosis. Cell Death Differ 1999, 6:99-104 [DOI] [PubMed] [Google Scholar]
- 24.Khoury J, Langleben D: Heparin-like molecules inhibit pulmonary vascular pericyte proliferation in vitro. Am J Physiol 2000, 279:L252-L261 [DOI] [PubMed] [Google Scholar]
- 25.Letourneur D, Caleb BL, Castellot JJ, Jr: Heparin binding, internalization, and metabolism in vascular smooth muscle cells: I. Upregulation of heparin binding correlates with antiproliferative activity. J Cell Physiol 1995, 165:676-686 [DOI] [PubMed] [Google Scholar]
- 26.Barzu T, Herbert JM, Desmouliere A, Carayon P, Pascal M: Characterization of rat aortic smooth muscle cells resistant to the antiproliferative activity of heparin following long-term heparin treatment. J Cell Physiol 1994, 160:239-248 [DOI] [PubMed] [Google Scholar]
- 27.Mishra-Gorur K, Castellot JJ, Jr: Heparin rapidly and selectively regulates protein phosphorylation in vascular smooth muscle cells. J Cell Physiol 1999, 178:205-215 [DOI] [PubMed] [Google Scholar]
- 28.Delmolino LM, Stearns NA, Castellot JJ, Jr: COP-1, a member of the CCN family, is a heparin-induced growth arrest specific gene in vascular smooth muscle cells. J Cell Physiol 2001, 188:45-55 [DOI] [PubMed] [Google Scholar]
- 29.Maheux R, Guilloteau C, Lemay A, Bastide A, Fazekas AT: Luteinizing hormone-releasing hormone agonist and uterine leiomyoma: a pilot study. Am J Obstet Gynecol 1985, 152:1034-1038 [DOI] [PubMed] [Google Scholar]
- 30.West CP, Lumsden MA, Lawson S, Williamson J, Baird DT: Shrinkage of uterine fibroids during therapy with goserelin (Zoladex): a luteinizing hormone-releasing hormone agonist administered as a monthly subcutaneous depot. Fertil Steril 1987, 48:45-51 [DOI] [PubMed] [Google Scholar]
- 31.Healy DL, Lawson SR, Abbott M, Baird DT, Fraser HM: Toward removing uterine fibroids without surgery: subcutaneous infusion of a luteinizing hormone-releasing hormone agonist commencing in the luteal phase. J Clin Endocrinol Metab 1986, 63:619-625 [DOI] [PubMed] [Google Scholar]
- 32.Friedman AJ, Barbieri RL, Benacerraf BR, Schiff I: Treatment of leiomyomata with intranasal or subcutaneous leuprolide, a gonadotropin-releasing hormone agonist. Fertil Steril 1987, 48:560-564 [PubMed] [Google Scholar]
- 33.Rein MS, Powell WL, Walters FC, Weremowicz S, Cantor RM, Barbieri RL, Morton CC: Cytogenetic abnormalities in uterine myomas are associated with myoma size. Mol Hum Reprod 1998, 4:83-86 [DOI] [PubMed] [Google Scholar]
- 34.Brosens I, Deprest J, Dal Cin P, Van den BH: Clinical significance of cytogenetic abnormalities in uterine myomas. Fertil Steril 1998, 69:232-235 [DOI] [PubMed] [Google Scholar]
- 35.Ligon AH, Morton CC: Genetics of uterine leiomyomata. Genes Chromosom Cancer 2000, 28:235-245 [PubMed] [Google Scholar]
- 36.Ligon AH, Morton CC: Leiomyomata: heritability and cytogenetic studies. Hum Reprod Update 2001, 7:8-14 [DOI] [PubMed] [Google Scholar]
- 37.San Antonio JD, Verrecchio A, Pukac LA: Heparin sensitive and resistant vascular smooth muscle cells: biology and role in restenosis. Connect Tissue Res 1998, 37:87-103 [DOI] [PubMed] [Google Scholar]
- 38.Gross KL, Morton CC: Genetics and the development of fibroids. Clin Obstet Gynecol 2001, 44:335-349 [DOI] [PubMed] [Google Scholar]
- 39.Irani C, Goncharova EA, Hunter DS, Walker CL, Panettieri RA, Krymskaya VP: Phosphatidylinositol 3-kinase but not tuberin is required for PDGF-induced cell migration. Am J Physiol 2002, 282:L854-L862 [DOI] [PubMed] [Google Scholar]
- 40.Parenti DJ, Morley TF, Giudice JC: Benign metastasizing leiomyoma. A case report and review of the literature. Respiration 1992, 59:347-350 [DOI] [PubMed] [Google Scholar]